Abstract
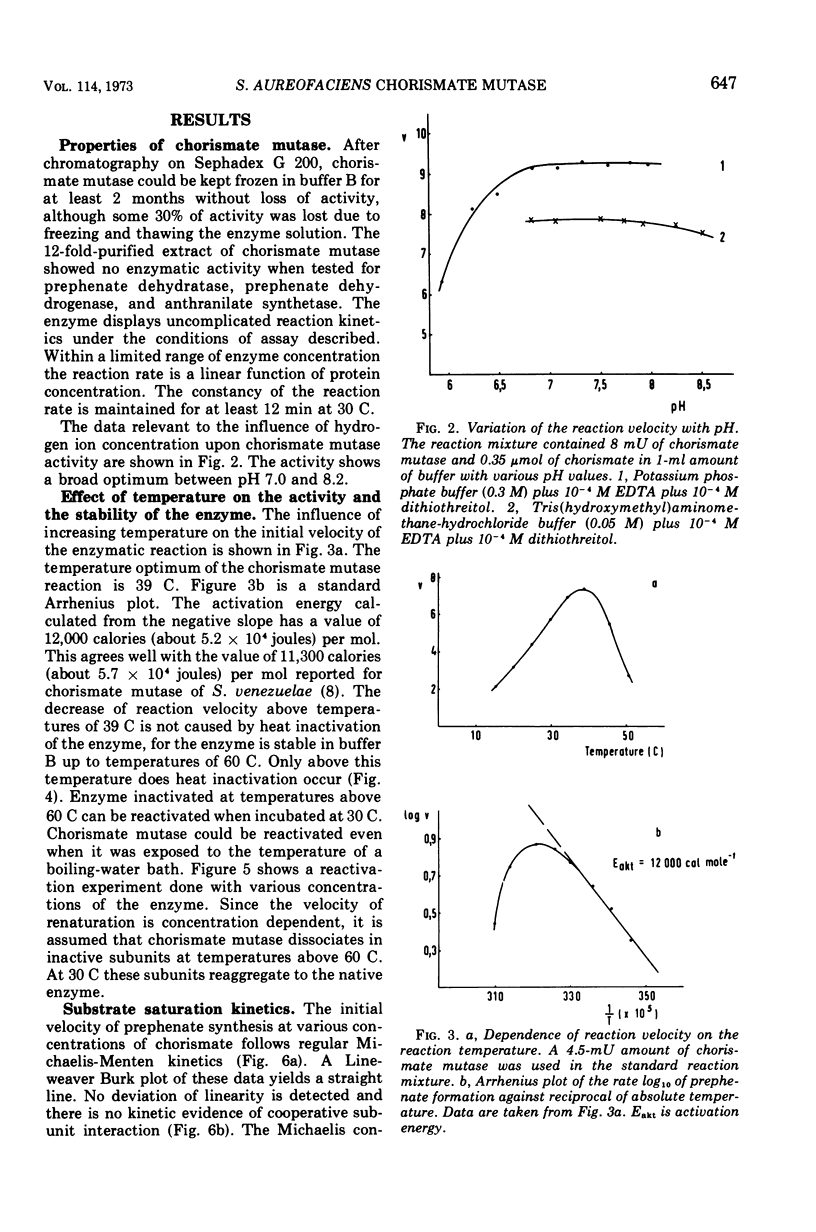
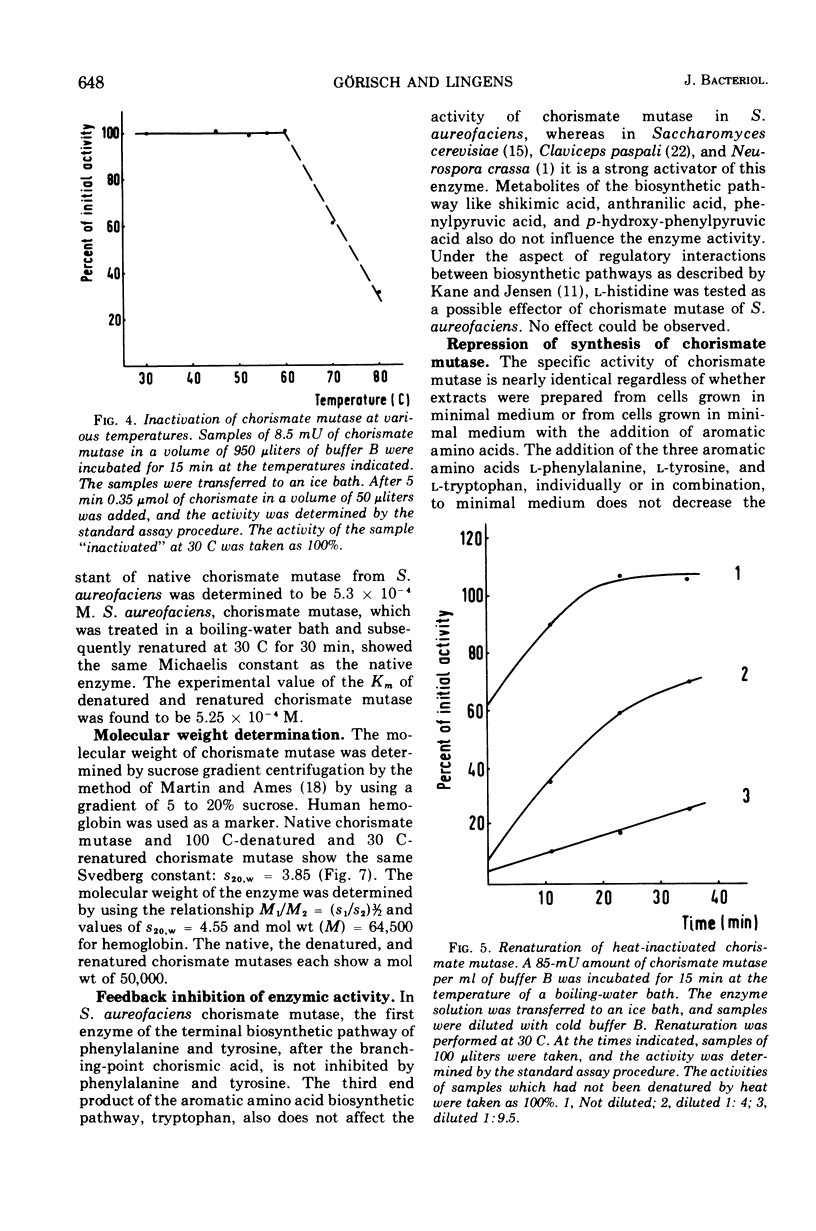
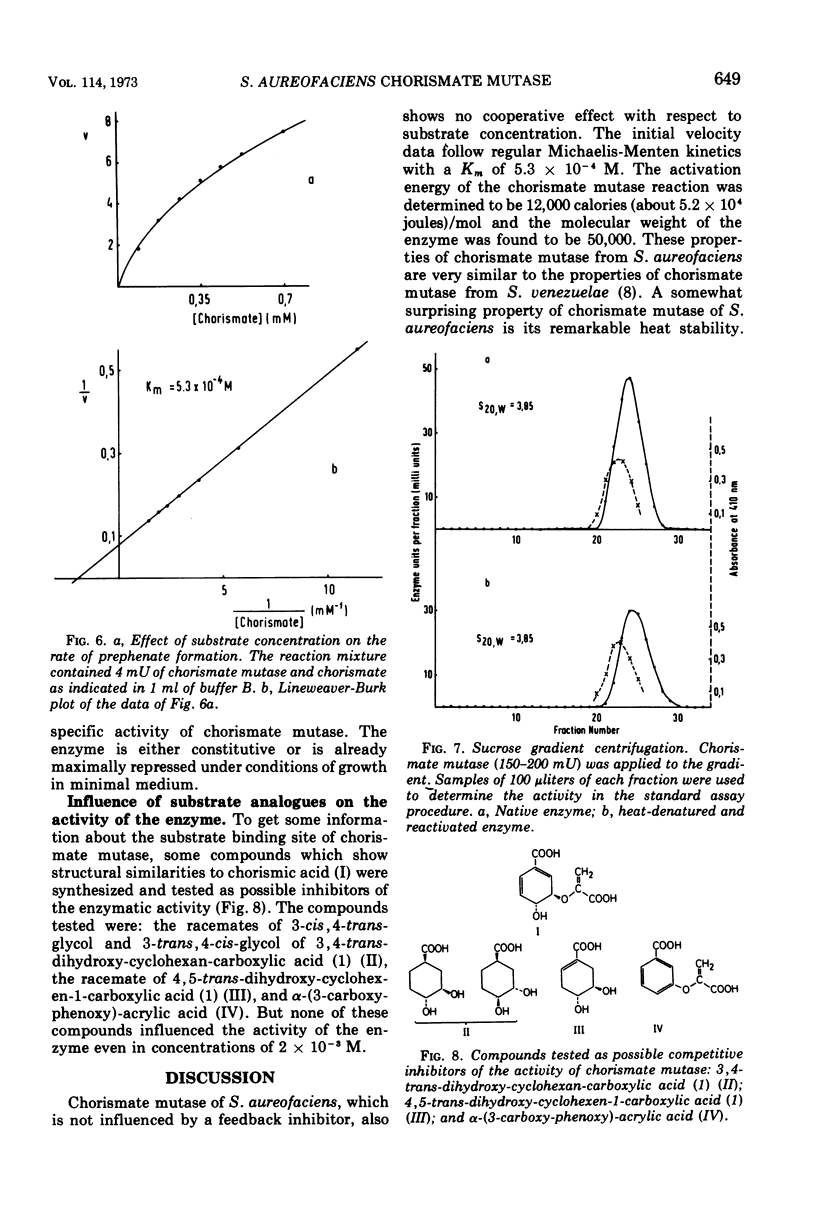
Chorismate mutase from Streptomyces aureofaciens was purified 12-fold. This enzyme preparation did not show any activity when tested for anthranilate synthetase, prephenate dehydrogenase, or prephenate dehydratase. The catalytic activity of chorismate mutase has a broad optimum between pH 7 and 8. The initial velocity data followed regular Michaelis-Menten kinetics with a Km of 5.3 × 10−4 M, and the molecular weight of the enzyme was determined by sucrose gradient centrifugation to be 50,000. Heat inactivation of chorismate mutase, which occurs above temperatures of 60 C, is reversible. The enzyme activity can be restored even when chorismate mutase is treated at the temperature of a boiling-water bath for 15 min. Heat-denatured and renatured enzymes showed the same Michaelis constant and the same molecular weight as the native enzyme. l-Phenylalanine, l-tyrosine, l-tryptophan, and metabolites of the aromatic amino acid pathway were tested as potential modifiers of chorismate mutase activity. The activity of the enzyme was inhibited by none of these substances. Chorismate mutase of S. aureofaciens was not repressed in cells grown in minimal medium supplemented with l-phenylalanine, l-tyrosine, or l-tryptophan.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. I. Tryptophan: a feedback activator for chorismate mutase from Neurospora. Biochemistry. 1966 Aug;5(8):2654–2657. doi: 10.1021/bi00872a025. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Görisch H., Lingens F. 3-D-eoxy-D-arabino-heptulosonate-7-phosphate synthase of Streptomyces aureofaciens Tü 24. II. Repression and inhibition by tryptophan and tryptophan analogues. Biochim Biophys Acta. 1971 Sep 22;242(3):630–636. doi: 10.1016/0005-2744(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Görisch H., Lingens F. Properties of chorismate mutase of Streptomyces venezuelae. Arch Mikrobiol. 1972;82(2):147–154. doi: 10.1007/BF01890406. [DOI] [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. Metabolic interlock. The influence of histidine on tryptophan biosynthesis in Bacillus subtilis. J Biol Chem. 1970 May 10;245(9):2384–2390. [PubMed] [Google Scholar]
- Koch G. L., Shaw D. C., Gibson F. Tyrosine biosynthesis in Aerobacter aerogenes. Purification and properties of chorismate mutase-prephenate dehydrogenase. Biochim Biophys Acta. 1970 Sep 16;212(3):375–386. doi: 10.1016/0005-2744(70)90243-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lingens F., Goebel W., Uesseler H. Regulation der Biosynthese der aromatischen Aminosäuren in Saccharomyces cerevisiae. 2. Repression, Induktion und Aktivierung. Eur J Biochem. 1967 May;1(3):363–374. doi: 10.1111/j.1432-1033.1967.tb00083.x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nester E. W., Lorence J. H., Nasser D. S. An enzyme aggregate involved in the biosynthesis of aromatic amino acids in Bacillus subtilis. Its possible function in feedback regulation. Biochemistry. 1967 May;6(5):1553–1563. doi: 10.1021/bi00857a042. [DOI] [PubMed] [Google Scholar]
- Nishioka L., Woodin T. Improved assay for phenylpyruvic acid. Anal Biochem. 1972 Feb;45(2):617–623. doi: 10.1016/0003-2697(72)90223-0. [DOI] [PubMed] [Google Scholar]
- Schmit J. C., Zalkin H. Chorismate mutase-prephenate dehydratase. Partial purification and properties of the enzyme from Salmonella typhimurium. Biochemistry. 1969 Jan;8(1):174–181. doi: 10.1021/bi00829a025. [DOI] [PubMed] [Google Scholar]
- Sprössler B., Lingens F. Eigenschaften der Chorismat-Mutase aus verschiedenen Claviceps-Stämmen. Hoppe Seylers Z Physiol Chem. 1970 Apr;351(4):448–458. [PubMed] [Google Scholar]
- Weber H. L., Böck A. Chorismate mutase from Euglena gracilis. Purification and regulatory properties. Eur J Biochem. 1970 Oct;16(2):244–251. doi: 10.1111/j.1432-1033.1970.tb01078.x. [DOI] [PubMed] [Google Scholar]


