Abstract
Post operative AF,β-blockade & atrial electrophysiology.
Introduction:
We investigated whether post-cardiac surgery (CS) new-onset atrial fibrillation (AF) is predicted by pre-CS atrial cellular electrophysiology, and whether the anti-arrhythmic effect of β-blocker therapy may involve pre-CS pharmacological remodelling.
Methods and Results:
Atrial myocytes were obtained from consenting patients in sinus rhythm, just prior to CS. Action potentials and ion currents were recorded by whole-cell patch-clamp. Post-CS AF occurred in 53 of 212 patients (25%). Those with post-CS AF were older than those without (67±2 vs 62±1 years, P=0.005). In cells from patients with post-CS AF, the action potential duration at 50 and 90% repolarisation, maximum upstroke velocity and effective refractory period (ERP) were 13±4 ms, 217±16 ms, 185±10 V/s and 216±14 ms, respectively (n=30 cells, 11 patients). Peak L-type Ca2+ current, transient outward and inward rectifier K+ currents, and the sustained outward current, were −5.0±0.5, 12.9±2.4, −4.1±0.4 and 9.7±1.0 pA/pF, respectively (13-62 cells, 7-19 patients). None of these values was significantly different in cells from patients without post-CS AF (P>0.05 for each, 60-279 cells, 29-86 patients), confirmed by multiple and logistic regression. In patients treated >7 days with a β-blocker pre-CS, the incidence of post-CS AF was lower than in non-β-blocked patients (13 vs 27%, P=0.038). Pre-CS β-blockade was associated with a prolonged pre-CS atrial cellular ERP (P=0.001), by a similar degree (∼20%) in those with and without post-CS AF.
Conclusion:
Pre-CS human atrial cellular electrophysiology does not predict post-CS AF. Chronic β-blocker therapy is associated with a reduced incidence of post-CS AF, unrelated to a pre-CS ERP-prolonging effect of this treatment.
Keywords: Atrial fibrillation/atrial arrhythmias, Cellular electrophysiology/electropharmacology, Ion channels and membrane transporters
Introduction
Atrial fibrillation (AF) is common in patients following cardiac surgery (CS). The incidence of post-CS AF ranges from 15-42%,1 with the arrhythmia usually occurring 2-3 days post-CS.1 It is associated with an increased incidence of stroke, poor haemodynamic performance, and increased morbidity and mortality.2 The electrophysiological mechanisms of post-CS AF are not known.
The occurrence of post-CS AF is independently predicted by old age, post-CS withdrawal of beta-adrenoceptor antagonist (β-blocker) treatment, pre-CS AF, valve surgery, P-wave changes, and numerous other clinical factors.1,3,4 Thus, in addition to surgical procedures and drug treatments, the pre-existing (pre-CS) characteristics of patients, and indeed their atria, influence the incidence of this arrhythmia.
It is presently unclear whether pre-CS atrial cellular electrophysiology is predictive of post-CS AF. The pre-CS inward rectifier K+ currents, IK1 and IKACh, were similar in atrial cells from patients who did and did not develop post-CS AF.5 By contrast, the pre-CS L-type Ca2+ current (ICaL) was larger,6 and the transient outward (ITO) and ultra-rapid delayed rectifier K+ (IKur) currents tended to be smaller,7 in cells from those with post-CS AF. In patients, the pre-CS atrial effective refractory period (ERP) was shorter in those who developed post-CS AF,8 suggesting that the action potential duration (APD) might also be shorter in such patients. However, an increase in ICaL, a decrease in ITO and IKur, or both would lengthen, rather than shorten the APD. There are currently no reported studies of pre-CS action potentials or ERP in atrial cells from patients who develop post-CS AF.
Administration of β-blockers pre-CS reduces the incidence of post-CS AF.9,10 Since sympathetic activity is high post-CS,11 it is possible that β-blockers are anti-arrhythmic in this setting as a consequence of their block of this enhanced sympathetic activity. However, we recently demonstrated that atrial myocytes from patients in pre-CS SR who had been given β-blockers, exhibited a prolonged APD and ERP that may be a consequence of pharmacological remodelling.12 It is unknown whether these pre-CS electrophysiological changes confer protection against post-CS AF, but it has been shown that other drugs with Class III anti-arrhythmic action reduce the incidence of post-CS AF.13 It is conceivable that the degree of pre-CS ERP-lengthening produced by pre-CS β-blockade might be greater in the patients who remain in sinus rhythm (SR) post-CS than in those who develop AF.
The aims of this study, therefore, were two-fold. Firstly, to establish whether pre-CS human atrial cell action potential characteristics, ERP and ion currents differ between patients who do and do not develop new-onset post-CS AF, including or excluding patients with co variables expected to influence post-CS AF incidence and/or pre-CS electrophysiology. Secondly, to compare the degree of pre-CS APD- and ERP-prolongation associated with pre-CS β-blockade, between patients who do and who do not develop post-CS AF.
Methods
Right atrial appendage tissue was obtained from 212 consenting patients in SR undergoing cardiac surgery between 1999 and 2005. Procedures were approved by the institutional research ethics committee. Atrial cells were isolated using a method previously described in detail,14 modified from that of Escande et al,15 and often termed the “chunk technique”16 since small chunks of tissue are superfused with enzyme. This technique, whilst having recognised limitations (potential disruption of ion currents, and relatively low yield and depolarisation of myocytes6,15-17) is necessary since atrial appendages cannot be arterially perfused. Briefly, tissue chunks were shaken for 45 min in a low [Ca2+] (50 μM) solution containing protease (4 U/ml). Protease was then replaced by collagenase (400 U/ml), which was renewed (exchanged) 3 times, at 15 min intervals. At each exchange, cells were separated by filtration and centrifugation, washed of residual enzyme in high [K+], low [Ca2+] solution, then placed in a 0.2 mM Ca2+-containing physiological salt solution. The 2nd exchange (30 min collagenase exposure) typically produced the highest yield (5-20%) of Ca2+-tolerant (not exhibiting contracture with 0.2 mM Ca2+), quiescent, striated, rod-shaped cells.
Action potentials and ion currents were recorded using the whole-cell patch clamp technique, in either the perforated or conventional ruptured patch configuration. Cells were superfused at 35-37 °C, at 1.5-2 ml/min with a physiological salt solution containing (mM): NaCl (130), KCl (4), CaCl2 (2), MgCl2 (1), glucose (10) and HEPES (10); pH 7.4. Cd2+ (0.2 mM) was included to block ICaL, when recording K+ currents from some cells. Glass microelectrodes were pulled and heat polished to 1.5-8 MΩ. With the perforated patch, action potentials were recorded using a K+-based pipette solution, containing (mM): nystatin (0.18), KCl (30.0), HEPES (5.0), MgCl2 (1.0), K methanesulfonic acid (100.0) and NaCl (5.0). ICaL was recorded using a Cs+-based pipette solution (to eliminate outward K+ currents) containing (mM): nystatin (0.18), CsCl (30.0), HEPES (5.0), MgCl2 (1.0), Cs methanesulfonic acid (100.0) and NaCl (5.0). With ruptured patches, action potentials and ion currents were recorded with an aspartate-based pipette solution containing (mM): K-aspartate (110.0), KCl (20.0), MgCl2 (1.0), EGTA (0.15), Na2ATP (4.0), Na2GTP (0.4) and HEPES (5.0). The maximum liquid junction potential was +7 mV (bath relative to pipette), and was compensated prior to seal formation. An Axopatch-1D amplifier (Axon Instruments) and “WinWCP” software (J Dempster, Strathclyde University) was used to stimulate and record electrical activity. Signals were low-pass filtered at 5 kHz prior to digitisation (Digidata 1200, Axon). Capacitative transients were subtracted electronically from the recordings. The voltage drop across the series resistance (Rs) was routinely compensated electronically, by 60-80%. Cell capacity and Rs were 76.7±2.4 pF and 7.4±0.4 MΩ, respectively, in cells from patients with post-CS AF, and not significantly different from those in cells from patients without post-CS AF (78.8±1.0 pF and 8.2±0.3 MΩ; P=0.38 and 0.069, respectively). Action potentials were stimulated with 5 ms current pulses of 1.2 x threshold, with an 8-pulse (S1) conditioning train at 75 beats/min. The resting potential (Vm) was −15±2 and −16±1 mV in cells from patients with and without post-CS AF, respectively (P=0.79), and a small holding current was used to clamp cells to a diastolic potential of −80 mV, as previously described.12,14,17 Action potential restitution was investigated with progressively premature test pulses (S2) following the S1 trains, with S1 and S2 of equal magnitude. The cell's ERP was measured, as previously,12,14 as the longest S1-S2 interval failing to elicit an S2 response of amplitude >80% of the preceding S1. The ICaL voltage-dependent activation was measured from a holding potential (HP) of −40 mV, with 250 ms voltage pulses (0.33 Hz), increasing from −30 to +60 mV in 10 mV steps. Peak ICaL density (at +10 mV) was not significantly different between the perforated- and ruptured-patch techniques (P=0.10), and perforated patch was used in 64% of cells from patients both with and without post-CS AF. The ICaL response to the sympathomimetic isoproterenol (ISO, 0.05 μM; 90 s superfusion) was also measured in some cells. ITO and the sustained outward current, ISUS were stimulated from an HP of −50 mV, with 100 ms pulses (0.33 Hz), increasing from −40 mV to +60 mV in 10 mV steps. ISUS was measured as end-pulse current magnitude, and ITO as peak outward minus end-pulse current. ISUS was considered to reflect mainly IKur,18 but also includes various Ca2+-dependent and -independent currents.15,18,19 The various components of ITO or ISUS were not separated in the present study. IK1 was measured using linear voltage ramps increasing from −120 mV to +50 mV at 24 mV/s, or with 500 ms voltage pulses (0.2 Hz) increasing from −120 mV to +50 mV, from an HP of −50 mV. All currents were normalised to cell capacity.
Details of each patient's clinical characteristics and drug treatments were obtained from the medical records, post-CS, and are shown in Table 1. Sinus rhythm was confirmed from a pre-CS 12 lead ECG. Each patient's cardiac rhythm, heart rate and drug treatments were assessed on the day of surgery, on the preceding day, and post-CS between 1 and 7 days. The early (0-3 day) period after CS was used to compare data between patients with and without post-CS AF whenever sample size permitted, i.e. for all measurements except IK1, ISUS and the effect of ISO on ICaL, for which the 0-7 day period was used. Patients were excluded from the analysis if they had a documented episode of AF at any time pre-CS, if they were taking digoxin, or if their pre-CS β-blocker treatment had started later than 7 days before the day of surgery. Patient and associated cellular electrophysiological data were stored in a database (Access, Microsoft) for subsequent analysis.
Table 1.
Patients' characteristics. Values are numbers of patients (n and % of total) with selected clinical characteristics, except for age and heart rate (mean±SE). ACE, angiotensin converting enzyme; ASD, atrial septal defect; AVR, aortic valve replacement; bpm, beats per min; CABG, coronary artery bypass graft surgery; CCB, calcium channel blocker; f, female; LVSD, left ventricular systolic dysfunction; m, male; MI, myocardial infarction; MVR, mitral valve replacement; post-CS AF, post-cardiac surgery atrial fibrillation; VSD, ventricular septal defect.
| Post-CS AF no | Post-CS AF yes | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patient details | Total (m/f) | 159 (124/35) | (78/22) | 53 (33/20) | (62/38) |
| Age (years) | 61.2±0.8 | - | 66.5±1.3 | - | |
| Heart rate (bpm) | 63.3±1.1 | - | 62.3±2.1 | - | |
| Drug treatments | Lipid lowering | 134 | 84 | 43 | 81 |
| Beta-blocker | 109 | 69 | 32 | 60 | |
| Nitrate | 78 | 49 | 23 | 43 | |
| ACE inhibitor | 82 | 52 | 21 | 40 | |
| CCB | 62 | 39 | 21 | 40 | |
| Diuretic | 43 | 27 | 16 | 30 | |
| Operation type | CABG | 135 | 85 | 43 | 81 |
| AVR | 9 | 6 | 6 | 11 | |
| CABG+AVR | 10 | 6 | 3 | 6 | |
| MVR | 2 | 1 | 1 | 2 | |
| CABG+MVR | 1 | 1 | 0 | 0 | |
| ASD | 1 | 1 | 0 | 0 | |
| VSD | 1 | 1 | 0 | 0 | |
| LVSD | Normal | 98 | 62 | 35 | 66 |
| Mild/moderate | 56 | 35 | 14 | 26 | |
| Severe | 5 | 3 | 3 | 6 | |
| Disease | Angina | 145 | 91 | 48 | 91 |
| Hyperlipidaemia | 124 | 78 | 43 | 81 | |
| Hypertension | 86 | 54 | 31 | 58 | |
| Previous MI | 66 | 42 | 24 | 45 | |
| Diabetes | 19 | 12 | 6 | 11 | |
Statistical methods
Univariate measurements were compared between pairs of various subgroups of patients using 2-sided, 2-sample unpaired Student's t tests. Categorical data were compared using a χ2 test. All univariate electrophysiological data are expressed as cell means±1 standard error (SE) of the mean. All cell means and associated P values (from the t-tests) were confirmed at subject level, by meaning all cell data obtained from each patient prior to meaning patients' data. Multiple linear regression20 was used to further investigate associations between patients' atrial electrophysiology and clinical characteristics or drug treatments. This provided an estimate of the difference in each electrophysiological measurement between the levels of the factor of interest, according to 10 variables considered to be of particular importance. Multiple logistic regression models were used to investigate the influence of both the clinical factors and the electrophysiological covariates on the occurrence of post CS AF. All analyses were performed retrospectively, using SAS 9.1 for Windows software (SAS Institute, Cary, NC, USA). The analyses incorporated all available information. Therefore, the tables and the statistical models are sometimes based on different numbers of subjects, reflecting some missing data for some covariates. Only patients from whom cellular electrophysiological data were obtained were included in the study. No adjustment was made for multiple comparisons. P<0.05 was regarded as statistically significant.
Results
Post-cardiac surgery atrial fibrillation was predicted by older patient age
Post-CS AF occurred in 53 (25%) of the 212 patients from whom atrial cellular electrophysiological recordings were obtained. The incidence of post-CS AF remained constant throughout the study, eg: at 24% between 1999 and 2002, and 26% between 2003 and 2005. Significantly more patients developed AF within the early (0-3 day) period after CS (36/212: 17%) than within the following 4 day period (17/212: 8%; P=0.005). The patients who had post-CS AF within 3 days were significantly older than those who remained in SR in that time (66.9±1.7 vs 61.7±0.8 years, P=0.005). Older age was confirmed as a significant predictor of post-CS AF, by multiple logistic regression (Table 2), with a 1.4-fold increased risk of developing post-CS AF for a 5 year increment in age (P=0.005).
Table 2.
Multiple logistic regression analysis of clinical factors predictive of post-CS AF. Post-CS period studied, for AF, or initiation or withdrawal of β-blockers =3 days. bpm, beats per minute; CI, confidence intervals; left ventricular systolic dysfunction (LVSD): yes includes mild, moderate and severe LVSD. Number of patients with post-CS AF=27/164. Asterisk=P<0.05. In addition to the covariates listed, the multiple regression model included the covariates listed in Table 4.
| Clinical factor | Level | Odds ratio (95% CI) |
P |
|---|---|---|---|
| Gender | Male vs female | 0.50 (0.21,1.20) | 0.12 |
| Age | 5 years | 1.41 (1.11,1.80) | 0.005 |
| Heart rate | 10 bpm | 1.11 (0.84,1.46) | 0.48 |
| Pre-CS β-blocker | Yes vs no | 0.50 (0.21,1.16) | 0.11 |
| Post-CS β-blocker initiation | Yes vs no | 1.72 (0.17,17.2) | 0.65 |
| Post-CS β-blocker withdrawal | Yes vs no | 0.72 (0.31,1.66) | 0.44 |
| Pre-CS ACE inhibitor | Yes vs no | 0.55 (0.23,1.28) | 0.16 |
| Valve surgery | Yes vs no | 1.67 (0.60,4.65) | 0.33 |
| Hypertension | Yes vs no | 0.47 (0.20,1.11) | 0.08 |
| LVSD | Yes vs no | 0.73 (0.31,1.72) | 0.47 |
Pre-CS atrial Ca2+ current was not predictive of post-CS AF
Figure 1A shows representative examples of atrial ICaL from (upper panel) a patient who remained in SR post-CS and from (lower panel) a patient who developed early post-CS AF. The mean peak ICaL density (at +10 mV), recorded in 62 cells from 19 patients who developed post-CS AF was −5.0±0.5 pA/pF, virtually identical to that in 279 cells from 86 patients who remained in post-CS SR, at −5.0±0.2 pA/pF; P=0.89 (Figure 1B). In a group of 19 patients who did not develop post-CS AF, age matched to the 19 patients who did (age=69.2±2.1 vs 69.7±2.0 years), mean peak ICaL was −5.4±0.5 pA/pF, again, not significantly different from that in the group with post-CS AF, at −5.0±0.5 pA/pF, P=0.58). Of the total 105 patients in which atrial cell ICaL was measured, 50 either had the β-blocker treatment they received pre-CS withdrawn within 3 days post-CS, or were given a β-blocker for the first time within the same period. Exclusion of those patients from the analysis again resulted in a similar mean peak ICaL in the patients who did and did not develop post-CS AF (−4.6±0.6 vs −5.0±0.3 pA/pF, P=0.52). With multiple regression, taking into account co variables considered most likely to affect the incidence of post-CS AF (Table 3), peak ICaL was estimated to be similar in patients who did or did not develop post-CS AF (P=0.70). Logistic regression (Table 4), taking into account all the patient and atrial cell electrophysiological characteristics (detailed in Tables 2 & 4, respectively) confirmed that pre-CS atrial ICaL was not predictive of post-CS AF (odds ratio close to 1, P=0.83). In a subgroup of cells in which ICaL was stimulated with ISO, peak ICaL was −5.5±0.4 and −14.6±0.9 pA/pF in the absence and presence, respectively, of 0.05 μM ISO (n=79 cells, 34 patients; P<0.0001). This was an increase of 9.1±0.8 pA/pF, or 207±22% above control. The increase in ICaL by ISO was similar in cells from patients with and without post-CS AF, at 7.7±1.7 and 9.7±0.8 pA/pF, respectively (n=23 cells, 12 patients and 56 cells, 22 patients, respectively; P=0.25). The corresponding percentage increase in ICaL by ISO also was similar between these patient groups (P=0.95, Figure 1C).
Figure 1. Comparison of pre-CS Ca2+ current between patients without and with post-CS AF.

A, Original, representative, L-type Ca2+ currents (ICaL) recorded from atrial myocytes isolated just prior to cardiac surgery (CS), from a patient in post-CS sinus rhythm, SR (□) and from a patient in post (3 day)-CS AF (■). Superimposed currents shown were evoked by voltage pulses (100 ms, 0.33 Hz, −40 mV holding potential), increasing in 10 mV steps between −30 and +40 mV. B and C, Histograms of peak ICaL density (at +10 mV) and increase in peak ICaL density by 0.05 μM isoproterenol (ISO), respectively, in atrial myocytes from patients in post-CS sinus rhythm (P-CS SR, □) and post-CS AF (P-CS AF, ■). Values are mean±SE. In (B), n=279 cells, 86 patients for P-CS SR, and 62 cells, 19 patients for P-CS AF (3 day). In (C), n=56 cells, 22 patients for P-CS SR, and 23 cells, 12 patients for P-CS AF (7 day). NS=not significant.
Table 3.
Multiple linear regression analysis of the atrial cell L-type Ca2+ current, ICaL. Post-CS period studied=3 days. ICaL estimate=estimated difference in mean ICaL between highest (eg: drug-treatment) and lowest (eg: non-drug-treatment) level of variable, or male minus female. Values are subject (patient) means±standard error (SE). Number of patients with post-CS AF=18/103.
| Variable | ICaL estimate (pA/pF) |
ICaL SEM |
P |
|---|---|---|---|
| Post-CS AF | −0.27 | 0.71 | 0.70 |
| Male | −0.37 | 0.63 | 0.56 |
| Age (5 year increment) | +0.14 | 0.14 | 0.35 |
| Heart rate (10 bpm increment) | −0.17 | 0.20 | 0.40 |
| Pre-CS β-blocker | −0.57 | 0.82 | 0.49 |
| Post-CS β-blocker withdrawal | +0.18 | 0.63 | 0.77 |
| Pre-CS ACE inhibitor | −0.30 | 0.54 | 0.58 |
| Valve surgery | +0.49 | 0.75 | 0.52 |
| Hypertension | +0.41 | 0.49 | 0.41 |
| LVSD | −0.23 | 0.53 | 0.67 |
Table 4.
Multiple logistic regression analysis of electrophysiological factors predictive of post-CS AF. Post-CS period studied=3 days. Atrial cellular electrophysiological measurements: AP, action potential; APDx, AP duration at the level of x% repolarisation; CI, confidence intervals; ERP, effective refractory period; ICaL, L-type Ca2+ current; Vmax, maximum upstroke velocity. Number of patients with post-CS AF=18/103 for ICaL, 11/79 for all AP measurements, and 9/72 for ERP. In addition to the covariates listed, the multiple regression model included the covariates listed in Table 2.
| Electrophysiological factor |
Units | Odds ratio (95% CI) |
P |
|---|---|---|---|
| ICaL | 3 pA/pF | 0.93(0.50,1.74) | 0.83 |
| AP Vmax | 50 V/s | 1.07(0.54,2.15) | 0.84 |
| AP amplitude | 10 mV | 1.14(0.59,2.22) | 0.70 |
| AP overshoot | 10 mV | 1.20(0.60,2.42) | 0.61 |
| APD50 | 10 ms | 0.93(0.57,1.52) | 0.77 |
| APD75 | 50 ms | 0.89(0.42,1.92) | 0.77 |
| APD90 | 50 ms | 1.06(0.57,1.95) | 0.86 |
| ERP | 50 ms | 0.83(0.40,1.68) | 0.60 |
Pre-CS atrial K+ currents were not predictive of post-CS AF
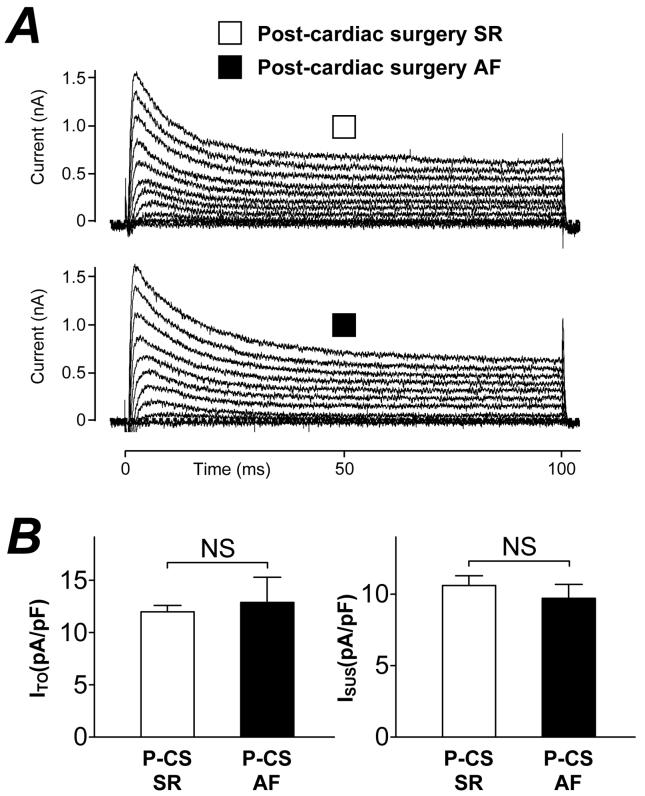
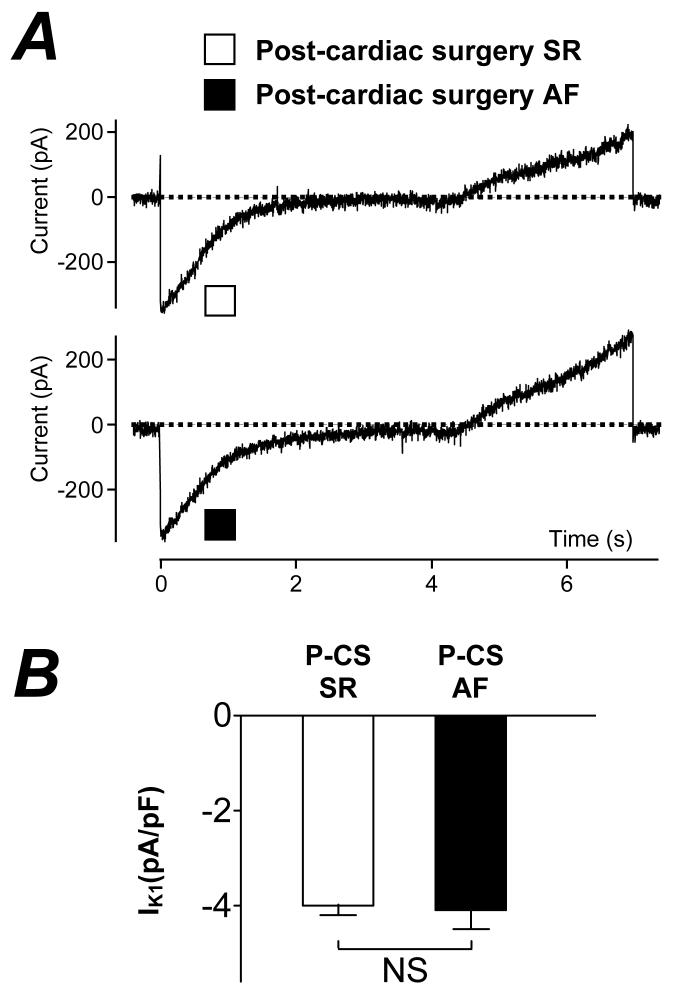
Figure 2A shows representative atrial K+ currents in myocytes from a patient who did not develop post-CS AF (upper panel) and from one who did (lower panel). The initial outward transient peak represents ITO and the steady-state, end-pulse current, the sustained outward current (ISUS). Figure 2B (left panel) shows that the mean peak ITO (at +60 mV) was not significantly different between patients who did and did not develop post-CS AF (12.9±2.4 vs 12.0±0.6 pA/pF, P=0.60). Figure 2B (right panel) shows that the mean peak ISUS (at +60 mV) also was not significantly different between these patient groups (9.7±1.0 vs 10.6±0.7 pA/pF, P=0.49). Figure 3A shows quasi-steady-state current-voltage relationships, with the inward (negative) portion between approximately −95 and −120 mV indicative of the magnitude of IK1. The currents recorded in a cell from a patient without post-CS AF (upper panel) were similar to those in a cell from a patient who developed post-CS AF (lower panel). Figure 3B shows that mean IK1 density at −120 mV was not significantly different between patients who did and who did not develop post CS-AF (−4.1±0.4 vs −4.0±0.2 pA/pF, P=0.83).
Figure 2. Comparison of pre-CS K+ currents between patients without and with post-CS AF.
A, Original transient outward K+ currents (ITO) and sustained outward currents (ISUS) in atrial cells from a patient in post-CS SR (□) and post-CS AF (■). Superimposed currents evoked by voltage pulses (100 ms, 0.33 Hz, −50 mV holding potential) increasing in 10 mV steps between −40 and +60 mV. B, Histograms of peak (at +60 mV) ITO (left hand panel) and ISUS (right hand panel) densities in cells from patients in post-CS SR (□, n=60-84 cells, 29-44 patients) and post-CS AF (■, n=13-21 cells, 7-9 patients). Values are means±SE. NS=not significant.
Figure 3. Comparison of pre-CS inward rectifier K+ current between patients without and with post-CS AF.
A, Original currents recorded in response to a voltage ramp, increasing from −120 to +50 mV at 24 mV/s, in atrial cells from a patient in post-CS SR (□) and post-CS AF (■). B, Histogram of means±SE inward rectifier K+ current density (IK1, measured at −120 mV) in cells from patients in post-CS SR (□, n=111 cells, 48 patients) and post (7 day)-CS AF (■, n=26 cells, 10 patients). NS=not significant.
Pre-CS atrial action potential characteristics and ERP were not predictive of post-CS AF
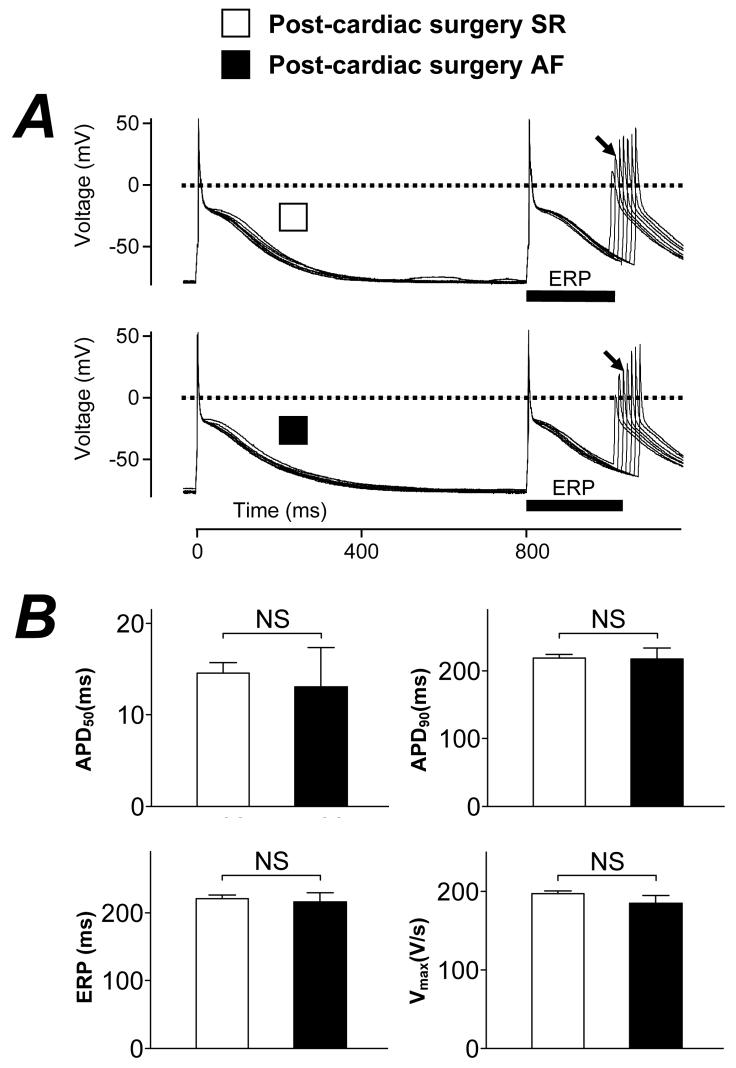
Figure 4A shows original representative action potentials and restitution characteristics in a single atrial cell from a patient who remained in SR post-CS (upper trace) and from one who developed post-CS AF (lower trace). Action potential morphology, including maximum upstroke velocity (Vmax), amplitude, overshoot and APD, and the cellular ERP, were all similar between these patient types, as confirmed by the mean data in Figure 4B. The holding current used was also similar in cells from patients with and without post-CS AF (−0.62±0.09 and −0.71±0.03 pA/pF, respectively; P=0.28). The treatment of patients with a β-blocker for >7 days pre-CS was associated with a significant prolongation in both action potential late repolarisation and ERP: the APD90 and ERP were 193±8 and 192±8 ms, respectively, in the non-β-blocked patients (n=31) vs 230±7 and 234±6 ms, respectively, in the β-blocked patients (n=58, P=0.001 for each). This prolongation was independent of holding current (−0.74±0.07 and −0.68±0.03 pA/pF in non-β-blocked and β-blocked patients, respectively; P=0.38), and was also confirmed by multiple regression analysis, with an estimated increase in ERP by β-blockade of 36 ms (P=0.027). Action potential characteristics and ERP were therefore compared within sub-groups of patients, internally matched for age, who were treated and not-treated pre-CS with a β-blocker, respectively. No significant differences were revealed between patients who did and who did not develop post-CS AF in either sub-group. Furthermore, this outcome was unchanged by the exclusion from the analysis of patients either who had the β-blocker treatment they received pre-CS withdrawn within 3 days post-CS, or were given a β-blocker for the first time within the same post-CS period.
Figure 4. Comparison of pre-CS action potential characteristics between patients without and with post-CS AF.
A, Original, representative, action potentials recorded in atrial myocytes from a patient in post-CS SR (□) and post (3 day)-CS AF (■). Superimposed action potentials shown were stimulated by the 7th and 8th of a train of conditioning current pulses, S1 (rate: 75 beats/min), followed by responses to an increasingly premature test pulse, S2. The cell effective refractory period (ERP, solid bars) was the longest S1-S2 interval failing to elicit an S2 response of amplitude >80% of the preceding S1 action potential. In each case, the S2 response used to measure this interval is labelled (↘). B, Histograms of action potential measurements in atrial cells from patients in post-CS SR (□, n=174-204 cells, 64-69 patients) and post (3 day)-CS AF (■, n=21-30 cells, 9-11 patients). APD50 and APD90=action potential duration at the levels of 50 and 90% repolarisation, respectively. Vmax=action potential maximum phase 0 (upstroke) velocity. Values are means±SE. NS=not significant (P=0.65, 0.94, 0.78 and 0.23 for APD50, APD90, ERP and Vmax, respectively).
Three multiple regressions were fitted to further investigate associations between patients' clinical characteristics and action potential shape and ERP. Firstly, taking into account all co variables, ie: as for those in Table 3. Secondly, taking into account only 4 co variables considered the most likely to influence post-CS AF. Thirdly, taking into account only age (shown in Table 2 to be predictive of post-CS AF) and pre-CS β-blockade (shown above to prolong pre-CS APD and ERP). With each of these fits, the APD50, APD90, action potential Vmax, amplitude, overshoot and ERP were estimated to be similar in patients who did and did not develop post-CS AF (P>0.05 for each). A logistic regression analysis, taking into account all the patient and atrial cell electrophysiological characteristics detailed in Tables 2 & 4, confirmed (Table 4) that none of the pre-CS atrial action potential measurements and ERP were significantly predictive of post-CS AF (P>0.05 for each).
Pre-CS β-blockade was associated with decreased incidence of post-CS AF, independently of ERP-lengthening
Figure 5A shows that the incidence of AF which occurred within 3 days post-CS was significantly lower in the patients who received a β-blocker pre-CS, than in the patients who did not receive a β-blocker pre-CS (13% vs 27%, P=0.038). In atrial cells from β-blocked patients, both the ICaL density (−5.1±0.2 pA/pF; n=242 cells, 71 patients) and its increase by 0.05 μM ISO (9.2±0.9 pA/pF; n=61 cells, 25 patients) were similar to those in cells from non β-blocked patients (−4.7±0.3 pA/pF; n=99 cells, 34 patients, P=0.23, and 8.9±1.7 pA/pF; n=18 cells, 9 patients, P=0.88), respectively. Pre-CS β-blockade was associated with a significant prolongation in the pre-CS atrial cellular ERP in the patients both who did and did not develop AF within the 3 day post-CS period (Figure 5B). The degree of ERP-prolongation associated with pre-CS β-blockade, of approximately 20%, occurred similarly in each group, ie: irrespective of whether the patients went on to develop post-CS AF.
Figure 5. Influence of pre-CS beta-blocker therapy on post-CS AF and pre-CS atrial refractory period.
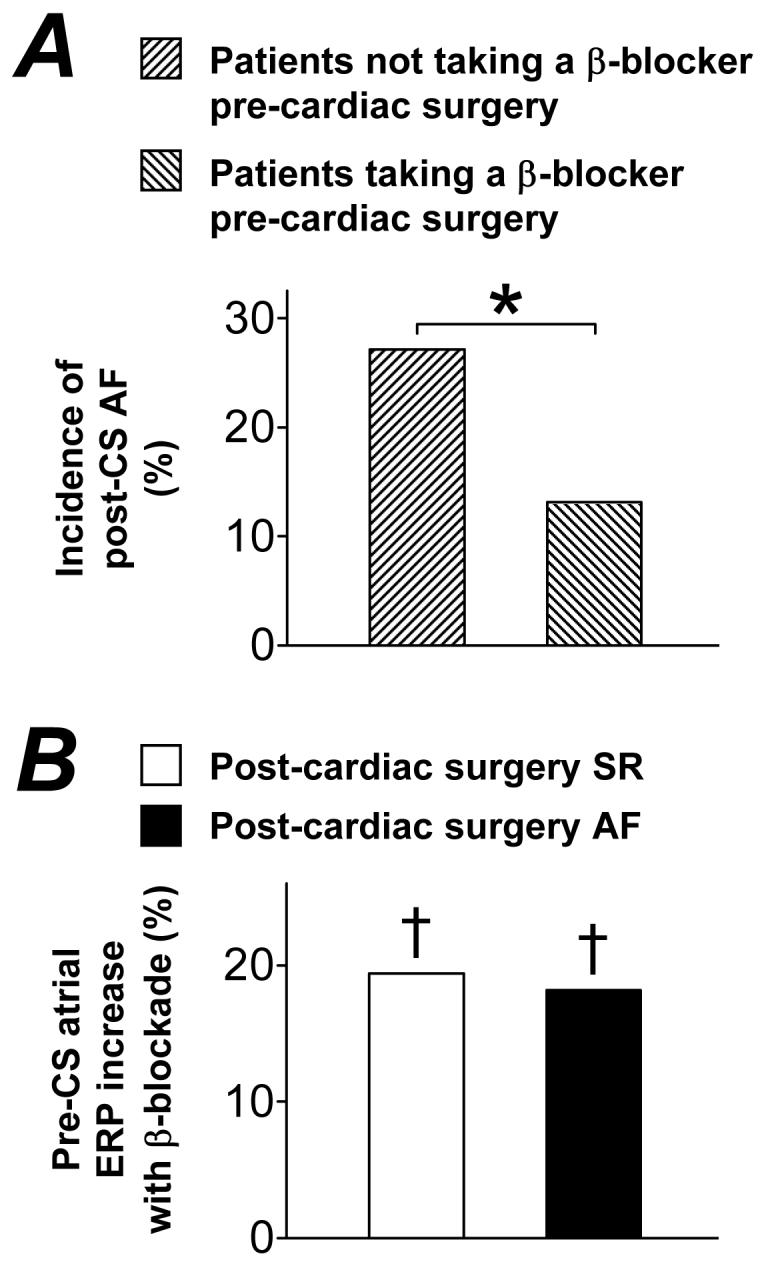
A, Comparison of incidence of post (3 day)-CS AF between patients not treated ( , −BB, n=13/48) and treated (
, −BB, n=13/48) and treated ( , +BB, n=13/99), respectively, for >7 days pre-CS with a beta-blocker. Asterisk denotes P<0.05 between groups. B, Comparison of magnitude of increase in mean pre-CS atrial cellular effective refractory period (ERP) associated with pre-CS treatment (>7 days) of patients with a beta-blocker, between those in post-CS sinus rhythm (□; patient n: −BB=21; +BB=43) and post (3 day)-CS AF (■; patient n: −BB=4; +BB=5). †s denote P<0.05 for the increase in ERP associated with pre-CS β-blockade within each group.
, +BB, n=13/99), respectively, for >7 days pre-CS with a beta-blocker. Asterisk denotes P<0.05 between groups. B, Comparison of magnitude of increase in mean pre-CS atrial cellular effective refractory period (ERP) associated with pre-CS treatment (>7 days) of patients with a beta-blocker, between those in post-CS sinus rhythm (□; patient n: −BB=21; +BB=43) and post (3 day)-CS AF (■; patient n: −BB=4; +BB=5). †s denote P<0.05 for the increase in ERP associated with pre-CS β-blockade within each group.
Discussion
This study is the first, to our knowledge, in which pre-CS human atrial action potentials have been compared between patients who do and do not develop post-CS AF. Earlier studies of changes in patients' pre-CS electrocardiograms associated with post-CS AF showed prolongation of the P-wave duration (Pdur) and/or P-R interval8,21 though a lack of prolongation and an increase in P-wave complexity also were found.4 The present study indicates that APD changes do not contribute to the prolongation of Pdur, which may be due to altered intra-atrial conduction velocity (θ) or atrial size. Reduction in the action potential Vmax may cause θ-slowing, but the present lack of a difference in Vmax suggests that any slowing of θ associated with post-CS AF occurs by other mechanisms, such as changes in expression of gap junction proteins.22 A clinical study demonstrated an association between post-CS AF and a shorter, spatially heterogeneous pre-CS ERP,8 in contrast to the present data which do not support pre-CS ERP-shortening as being predictive of post-CS AF. The measurement of ERP in isolated cells has limitations compared to that made in-vivo, and does not inform about conduction or spatial heterogeneity.23 However, the relevance of this measurement was supported by the demonstration that pre-CS chronic AF was associated with its shortening,14 and by a similar magnitude to that in the right atrial appendage in patients in-vivo.24
The similarity in each of the atrial ion currents measured pre-CS, between groups of patients who did and who did not develop post-CS AF, is consistent with the lack of change in any action potential measurements or the ERP. Our data are also in agreement with a previous report of a lack of difference in human atrial IK1 between such patient groups.5 There is one previous report that pre-CS atrial ICaL was significantly larger in patients who developed post-CS AF, than in those who did not.6 The reasons for this discrepancy are unclear, though they may include differences in the clinical characteristics of the patients studied. For example, those who developed post-CS AF in the earlier study6 were of a similar age to those who remained in SR. It was also noted6 that there was a substantial overlap in the Ca2+ current density data between the groups of patients with and without post-CS AF. It is worth noting that the present and other5-7 analyses of post-CS AF include only patients in pre-CS SR. Pre-CS AF, however, is well established to be accompanied by atrial electrophysiological changes, including decreased ICaL,6,14 ITO7,14 and ERP,14 and increased IK1.5,14 Nevertheless, such changes and, therefore, any potentially associated predisposition to AF, were not found pre-CS in patients in SR who develop post-CS AF.
Long term treatment of patients pre-CS with a β-blocker was associated retrospectively with a significant reduction in the incidence of post-CS AF, in line with previous prospective studies.9,10 Pre-CS chronic β-blockade also was associated with a significant prolongation in the pre-CS atrial cell APD and ERP, consistent with an earlier report from our laboratory.12 Those electrophysiological changes, recorded in the presumed absence of residual β-blocker (since cells had been isolated and washed), and independent of heart rate, reflect an adaptive response, which has been termed pharmacological remodelling.12 This is distinct from reversal of AF-induced remodelling,25 since it occurred in cells from patients in SR. Such β-blocker-induced ERP-prolongation, by lengthening the minimum path length required for atrial re-entry, might contribute to the anti-fibrillatory actions of β-blockers.26 In the present findings, the degree of atrial ERP-prolongation associated with pre-CS chronic β-blockade was virtually identical in the patients who did and did not develop post-CS AF. This suggests that the atrial anti-fibrillatory effects of pre-CS β-blockade were not due to enhanced pharmacological remodelling in the patients who remained in post-CS SR. However, action potential and ion current properties measured pre-CS may not reflect those present in patients in AF post-CS, and an enhanced post-CS ERP-prolongation in the β-blocked patients who maintained SR cannot be excluded. Furthermore, it is conceivable that any such anti-reentrant influence could nevertheless be overcome by a focal arrhythmic mechanism. The present experiments with ISO suggest that such a focal mechanism would not involve any enhancement of the pre-CS ICaL response to catecholamines.
The causes and predictors of post-CS AF are complex and multifactorial. The arrhythmia is re-entrant, with a variety of electrophysiological onset mechanisms, but with the final trigger in the majority of cases being a conducted atrial extrasystole.3 Post-CS AF seems to be caused mainly by the surgical procedure itself, sensitised by the pre-existing influence of age on atrial electrophysiology, and modified by pre- and post-CS drug treatments. The surgical causes are considered to be the effects of circulating catecholamines, imbalances in autonomic tone (including parasympathetic resurgence), transient electrolyte imbalance, myocardial ischaemia, inflammation and mechanical irritation.27-31 Older age may pre-dispose the atrium to re-entry by slowing θ32 as a result of atrial atrophy, dilation and/or fibrosis,33 rather than by slowing atrial cell action potential Vmax. Atrial structure was not examined in the present study, and pre-CS structural changes have been associated with the development of new onset post-CS AF, including atrial enlargement,34 increased fibrosis,33 and up regulation of type I collagen35 and connexin40.22 Persistent AF is accompanied by atrial structural changes which may contribute to the perpetuation of the arrhythmia independently of ERP changes. The precise nature of these structural changes, termed the “second factor” of AF36 remains unclear, but similar changes pre-CS could conceivably promote new onset post-CS AF.
The present data indicate that the occurrence of post-CS AF is not predicted by pre-CS atrial cellular electrophysiology. Furthermore, since pre-CS β-blockade reduced the incidence of this arrhythmia without an involvement of β-blocker effects on the pre-CS ERP, mechanisms independent of pre-CS ERP change, such as attenuation of triggered atrial extrasystoles, are likely to underlie the post-CS atrial anti-arrhythmic effects of β-blockers.
Acknowledgements
We acknowledge the British Heart Foundation for financial support, and Glasgow Royal Infirmary cardiac surgical operating teams for kindly providing atrial tissue.
Acknowledgement of all sources of financial support: British Heart Foundation (BHF) Basic Science Lectureship: BS/2001001 (AJW); BHF project grant: PG/04/084/17400 (DP); BHF Clinical PhD Studentship: FS/02/036 (CJR); BHF Clinical PhD Studentship: FS/04/087 (GEM); BHF Chair holder's Award (JAR).
References
- 1.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 2.Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM. Massumi A: Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AD, Groen JG, Thorn SL, Lewis CT, Marshall AJ. New insights into onset mechanisms of atrial fibrillation and flutter after coronary artery bypass graft surgery. Heart. 2002;88:499–504. doi: 10.1136/heart.88.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gang Y, Hnatkova K, Mandal K, Ghuran A, Malik M. Preoperative electrocardiographic risk assessment of atrial fibrillation after coronary artery bypass grafting. J Cardiovasc Electrophysiol. 2004;15:1379–1386. doi: 10.1046/j.1540-8167.2004.04084.x. [DOI] [PubMed] [Google Scholar]
- 5.Dobrev D, Wettwer E, Kortner A, Knaut M, Schuler S, Ravens U. Human inward rectifier potassium channels in chronic and postoperative atrial fibrillation. Cardiovasc Res. 2002;54:397–404. doi: 10.1016/s0008-6363(01)00555-7. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 7.Brandt MC, Priebe L, Bohle T, Sudkamp M, Beuckelmann DJ. The ultrarapid and the transient outward K+ current in human atrial fibrillation. Their possible role in postoperative atrial fibrillation. J Mol Cell Cardiol. 2000;32:1885–1896. doi: 10.1006/jmcc.2000.1221. [DOI] [PubMed] [Google Scholar]
- 8.Soylu M, Demir AD, Ozdemir O, Soylu O, Topaloglu S, Kunt A, Sasmaz A, Korkmaz S, Tasdemir O. Increased dispersion of refractoriness in patients with atrial fibrillation in the early postoperative period after coronary artery bypass grafting. J Cardiovasc Electrophysiol. 2003;14:28–31. doi: 10.1046/j.1540-8167.2003.02218.x. [DOI] [PubMed] [Google Scholar]
- 9.Lamb RK, Prabhakar G, Thorpe JA, Smith S, Norton R, Dyde JA. The use of atenolol in the prevention of supraventricular arrhythmias following coronary artery surgery. Eur Heart J. 1988;9:32–36. [PubMed] [Google Scholar]
- 10.Crystal E, Healey J, Connolly SJ. Atrial fibrillation after cardiac surgery: update on the evidence on the available prophylactic interventions. Card Electrophysiol Rev. 2003;7:189–192. doi: 10.1023/a:1027432104518. [DOI] [PubMed] [Google Scholar]
- 11.Kaul TK, Swaminathan R, Chatrath RR, Watson DA. Vasoactive pressure hormones during and after cardiopulmonary bypass. Int J Artif Organs. 1990;13:293–299. [PubMed] [Google Scholar]
- 12.Workman AJ, Kane KA, Russell JA, Norrie J, Rankin AC. Chronic beta-adrenoceptor blockade and human atrial cell electrophysiology: evidence of pharmacological remodelling. Cardiovasc Res. 2003;58:518–525. doi: 10.1016/s0008-6363(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 13.Sanjuan R, Blasco M, Carbonell N, Jorda A, Nunez J, Martinez-Leon J, Otero E. Preoperative use of sotalol versus atenolol for atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2004;77:838–843. doi: 10.1016/j.athoracsur.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 15.Escande D, Coulombe A, Faivre JF, Coraboeuf E. Two types of transient outward currents in adult human atrial cells. Am J Physiol. 1987;252:H142–H148. doi: 10.1152/ajpheart.1987.252.1.H142. [DOI] [PubMed] [Google Scholar]
- 16.Yue L, Feng J, Li G-R, Nattel S. Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am J Physiol. 1996;270:H2157–H2168. doi: 10.1152/ajpheart.1996.270.6.H2157. [DOI] [PubMed] [Google Scholar]
- 17.Benardeau A, Hatem SN, Rucker-Martin C, Le Grand B, Mace L, Dervanian P, Mercadier J-J, Coraboeuf E. Contribution of Na+/Ca2+ exchange to action potential of human atrial myocytes. Am J Physiol. 1996;271:H1151–H1161. doi: 10.1152/ajpheart.1996.271.3.H1151. [DOI] [PubMed] [Google Scholar]
- 18.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demion M, Guinamard R, El Chemaly A, Rahmati M, Bois P. An outwardly rectifying chloride channel in human atrial cardiomyocytes. J Cardiovasc Electrophysiol. 2006;17:60–68. doi: 10.1111/j.1540-8167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Draper NR, Smith H. Applied Regression Analysis. 3rd ed. New York: John Wiley & Sons; 1998. [Google Scholar]
- 21.Passman R, Beshai J, Pavri B, Kimmel S. Predicting post-coronary bypass surgery atrial arrhythmias from the preoperative electrocardiogram. Am Heart J. 2001;142:806–810. doi: 10.1067/mhj.2001.118736. [DOI] [PubMed] [Google Scholar]
- 22.Dupont E, Ko Y-S, Rothery S, Coppen SR, Baghai M, Haw M, Severs NJ. The gap-junctional protein connexin40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation. 2001;103:842–849. doi: 10.1161/01.cir.103.6.842. [DOI] [PubMed] [Google Scholar]
- 23.Tse HF, Lau CP, Ayers GM. Heterogeneous changes in electrophysiologic properties in the paroxysmal and chronically fibrillating human atrium. J Cardiovasc Electrophysiol. 1999;10:125–135. doi: 10.1111/j.1540-8167.1999.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu WC, Lee SH, Tai CT, Tsai CF, Hsieh MH, Chen CC, Ding YA, Chang MS, Chen SA. Reversal of atrial electrical remodeling following cardioversion of long-standing atrial fibrillation in man. Cardiovasc Res. 1999;42:470–476. doi: 10.1016/s0008-6363(99)00030-9. [DOI] [PubMed] [Google Scholar]
- 25.Osaka T, Yamazaki M, Yokoyama E, Ito A, Kodama I. Sotalol reverses remodeled action potential in patients with chronic atrial fibrillation but does not prevent arrhythmia recurrence. J Cardiovasc Electrophysiol. 2004;15:877–884. doi: 10.1046/j.1540-8167.2004.03671.x. [DOI] [PubMed] [Google Scholar]
- 26.Gronefeld GC, Hohnloser SH. β-blocker therapy in atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:1607–1612. doi: 10.1046/j.1460-9592.2003.t01-1-00239.x. [DOI] [PubMed] [Google Scholar]
- 27.Kolvekar S, D'Souza A, Akhatar P, Reek C, Garratt C, Spyt T. Role of atrial ischaemia in development of atrial fibrillation following coronary artery bypass surgery. Eur J Cardiothorac Surg. 1997;11:70–75. doi: 10.1016/s1010-7940(96)01095-0. [DOI] [PubMed] [Google Scholar]
- 28.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–1268. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 29.Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, Kanderian A, Pavia S, Hamlin RL, McCarthy PM, Bauer JA, Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:E32–E38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 30.Archbold RA, Curzen NP. Off-pump coronary artery bypass graft surgery: the incidence of postoperative atrial fibrillation. Heart. 2003;89:1134–1137. doi: 10.1136/heart.89.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayaraman P, Ellenbogen KA. Postoperative atrial fibrillation: some more answers, some new questions. J Cardiovasc Electrophysiol. 2003;14:133–134. [PubMed] [Google Scholar]
- 32.Kojodjojo P, Kanagaratnam P, Markides V, Davies DW, Peters N. Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol. 2006;17:120–127. doi: 10.1111/j.1540-8167.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 33.Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, Rocken C. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. 2002;54:390–396. doi: 10.1016/s0008-6363(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 34.Leung JM, Bellows WH, Schiller NB. Impairment of left atrial function predicts post-operative atrial fibrillation after coronary artery bypass graft surgery. Eur Heart J. 2004;25:1836–1844. doi: 10.1016/j.ehj.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Grammer JB, Bohm J, Dufour A, Benz M, Lange R, Bauernschmitt R. Atrial fibrosis in heart surgery patients. Decreased collagen III/I ratio in postoperative atrial fibrillation. Basic Res Cardiol. 2005;100:288–294. doi: 10.1007/s00395-005-0515-x. [DOI] [PubMed] [Google Scholar]
- 36.Todd DM, Fynn SP, Walden AP, Hobbs WJ, Arya S, Garratt CJ. Repetitive 4-week periods of atrial electrical remodeling promote stability of atrial fibrillation: time course of a second factor involved in the self-perpetuation of atrial fibrillation. Circulation. 2004;109:1434–1439. doi: 10.1161/01.CIR.0000124006.84596.D9. [DOI] [PubMed] [Google Scholar]