Abstract
Macrophage migration inhibitory factor (MIF) expression is increased by angiotensin II (Ang II) within paraventricular nucleus (PVN) neurons of normotensive rats and acts via its intrinsic thiol protein oxidoreductase (TPOR) to counterregulate the central nervous system-mediated pressor action of Ang II. Considering that the PVN-mediated actions of Ang II are enhanced in spontaneously hypertensive rats (SHRs) and contribute to the development of hypertension in these animals, we investigated this MIF regulatory mechanism in SHRs. Here, we have demonstrated that Ang II failed to increase MIF protein expression in the PVN of SHRs. Furthermore, although basal levels of MIF protein and mRNA were similar in the PVN of SHRs and normotensive rats, immunostaining revealed that MIF was either absent from or diminished in PVN neurons of SHRs. AAV2-mediated increases in MIF expression within PVN neurons of young (8 wk old) SHRs produced a chronic attenuation of hypertension and cardiac hypertrophy. However, similar AAV2-mediated transduction of [C60S]-MIF, which lacks TPOR activity, did not alter the development of hypertension or cardiac hypertrophy in SHRs. Collectively, these findings suggest that a lack of MIF expression within PVN neurons contributes to the development of hypertension and cardiac hypertrophy in SHRs.—Li, H., Gao, Y., Qi, Y., Katovich, M. J., Jiang, N., Braseth, L. N., Scheuer, D. A., Shi, P., Sumners, C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats.
Keywords: angiotensin II, thiol-protein oxidoreductase, hypertension
Evidence indicating the occurrence of neurogenic hypertension has accumulated during the past decade (1,2,3). One hypothesis of the mechanisms of neurogenic hypertension is an interruption of the balance of excitatory and inhibitory influences that determine the excitability of the autonomic neurons in the central nervous system (CNS). Neurons that drive the peripheral sympathetic branch of the autonomic nervous system are found in a limited number of brain nuclei, including the hypothalamic paraventricular nucleus (PVN), which has been recognized as an important site for integrating the neural signals that regulate cardiovascular function. Although there are controversial opinions on the role of PVN neurons in blood pressure control in normotensive animals (4,5,6), it appears that in spontaneously hypertensive rats (SHRs), the PVN does indeed contribute to an increase in blood pressure by enhancing sympathetic tone (4, 7).
It is thus important to understand the regulatory mechanisms within the PVN that control sympathetic outflow and arterial blood pressure (ABP) in both normal rats and SHRs. From a stimulatory perspective, angiotensin II (Ang II) acts via its Ang II type 1 (AT1) receptors in PVN neurons to increase sympathetic activity and ABP (8,9,10,11). There is significant evidence that the PVN-mediated actions of Ang II are enhanced in SHRs and contribute to the development of hypertension observed in these animals (12,13,14,15). Because these PVN actions of Ang II are mediated through changes in neuronal activity, factors that regulate Ang II-induced alterations in neuronal firing should alter the physiological output. Although it has been established that G protein-coupled receptors undergo desensitization and/or down-regulation after ligand binding (16), there is little cellular or physiological evidence to indicate that such a negative regulatory mechanism exists for neuronal AT1 receptors. Therefore, identification of alternative mechanisms that counterregulate AT1 receptor signaling in neurons is of crucial importance, especially in PVN neurons. Previous work from our group has identified macrophage migration inhibitory factor (MIF) as an intracellular counterregulator of the chronotropic actions of Ang II in hypothalamic neurons of normotensive rats in vitro (17, 18). It is also apparent that MIF exerts this inhibitory effect via its intrinsic thiol-protein oxidoreductase (TPOR) activity that is exerted by a C-A-L-C motif that exists at residues 57–60 of the MIF molecule (18, 19). In vivo, MIF is expressed in neurons in the PVN of normotensive rats [Sprague-Dawley (SD) and Wistar-Kyoto (WKY)], and intracerebroventricular (i.c.v.) injection of Ang II increases MIF expression in this cardiovascular control center (20). In addition, transient MIF transduction into the PVN of normotensive rats reduces the pressor response to Ang II stimulation (20). Collectively, this evidence indicates that MIF is a counterregulator of Ang II-induced cardiovascular effects mediated via the PVN in normotensive rats. However, our further in vitro studies indicated that whereas exogenously applied MIF depresses the chronotropic action of Ang II in SHR neurons, Ang II was unable to induce expression of endogenous MIF in these cells (18), suggesting that the MIF counterregulatory mechanism may be absent in SHR neurons. Furthermore, because the AT1 receptor-mediated actions of Ang II in the PVN are enhanced in SHRs, it is possible that a lack of negative regulation by MIF in this area contributes to the high blood pressure observed in these animals.
In this study we have demonstrated that MIF is either absent from or its expression is severely reduced in PVN neurons of SHRs and that i.c.v.-injected Ang II fails to increase MIF expression in SHRs. We have also demonstrated that long-term increased expression of MIF within PVN neurons of young SHRs, produced by microinjection of AAV2-CBA-MIF, attenuates the development of hypertension in these animals. In contrast, increased expression of [C60S]-MIF (a mutant that lacks TPOR activity) within the PVN produced no such attenuation of the development of high blood pressure in SHRs. Moreover, AAV2-CBA-MIF-mediated increases in MIF expression within PVN neurons of SHRs decreased the heart weight/body weight ratio and cardiac myocyte diameter compared with [C60S]-MIF-injected rats. Collectively, these findings indicate that a lack of MIF expression within PVN neurons contributes to the development of hypertension and cardiac hypertrophy in SHRs.
MATERIALS AND METHODS
Animals
For the experiments described here, we used a total of 72 adult male SD or WKY rats or SHRs and 60 newborn SD rat pups from Charles River Farms (Wilmington, MA). All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Neuronal cultures
Primary neuronal cultures were prepared from the cerebral cortex of newborn SD rats and grown in plastic tissue culture dishes (Nunc, Napierville, IN, USA) as described previously (17).
AAV2 constructs
Site-directed mutagenesis of the MIF gene and cloning of an isosteric mutant
A rat MIF mutant with the cysteine residue at position 60 substituted with serine was cloned from pShuttle-SYN-MIF (20) with two rounds of polymerase chain reaction (PCR)-based site-specific mutagenesis, essentially according to the methods described by Barik (21). The primers used were as follows: MIF-F, 5′ CTGACGAATTCCCACCATGCCTATGTTCATCGT 3′ (upstream); MIF-R, 5′ AGTATGCGGCCGCTCAAGCGAAGGTGGAACCGTT 3′(downstream); and [C60S]-MIF-R, 5′ ATGCTGTGCAGGCTGCTGAGGGCGCAGGGGTCG 3′.
The primers MIF-F and MIF-R were used to amplify the full coding sequence of rat MIF cDNA (20). The primer [C60S]-MIF-R was designed for the mutant C60S and the underlined nucleotide T, which substituted for the original nucleotide A, caused the Cys → Ser mutation ([C60S]-MIF) of rat MIF. The first PCR reaction was performed with the primers MIF-F and [C60S]-MIF-R under the following conditions: 2 min at 95°C followed by 25 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C, using a Fast Start High Fidelity Kit (Roche Diagnostics Corp., Indianapolis, IN, USA). For the second round of PCR, 0.2 μg of the purified PCR product from the first PCR reaction was used as one of the primers (megaprimer) along with the [C60S]-MIF-R primer. The second round of PCR was performed with the same DNA template pShuttle-SYN-MIF and the same cycle profile as used in the first PCR reaction. PCR products of the expected size were purified by agarose gel electrophoresis and cloned into pCR2.1 vector using TA cloning kits (Invitrogen, Carlsbad, CA, USA). Mutant DNA sequences were confirmed by bidirectional sequencing of the clones and then cloned into an AAV2 vector as described below. Of note, the C60S mutation deleted a PstI restriction site present in wild-type rat MIF cDNA, which benefits identification of the mutation in further subcloning studies.
Construction and transduction of recombinant AAV2 vectors
Three viral vectors, AAV2-CBA-MIF, AAV2-CBA-[C60S]-MIF, and AAV2-CBA-eGFP, were constructed in this study. These constructs contained expression cassettes flanked by the rAAV2 terminal repeats. Expression of enhanced green fluorescent protein (eGFP), MIF, and [C60S]-MIF was driven by a chicken β-actin promoter (CBA) with a human cytomegalovirus (CMV) enhancer. A Woodchuck post-transcriptional regulatory element, which enhanced the expression of transgenes, was present downstream of eGFP, MIF, and [C60S]-MIF. Vectors were propagated in human embryonic kidney (HEK) 293 cells using pDG as helper plasmid and purified with a single-step gravity-flow column (22). Copies of viral genome DNA were determined by quantitative PCR (23). Vector doses were expressed as genome copies (gc).
Plasmid transfections
HEK 293 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. For the plasmid transfection, cells (1×105) were seeded into 12-well tissue culture plates (Nunc). On the following day, cells were transfected with plasmids AAV2-CBA-MIF, AAV2- AAV2-CBA-[C60S]-MIF, or AAV2-CBA-eGFP plus Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours later, MIF expression was determined using Western blot as described below.
Immunocytochemistry
The cellular localization of MIF and GFP within the PVN of WKY and SD rats and SHRs or in neuronal cultures was determined by immunocytochemistry. Specific methods were as follows.
PVN
Control, AAV2-CBA-eGFP-, or AAV2-CBA-MIF-injected SHRs or SD or WKY rats were anesthetized with isoflurane, and brains were transcardially perfused in situ with 0.9% saline containing 4% formaldehyde and then cryoprotected in 30% sucrose for 1 wk. The area of the brain containing the PVN was then removed using a brain blocker (David Kopf Instruments, Tujunga, CA, USA), and 30-μm sections were cut using a microtome and were mounted onto glass slides ready for immunostaining. Primary antibodies used were monoclonal anti-NeuN antibody (1:100) and monoclonal anti-glial fibrillary acidic protein (GFAP) (1:100) (both from Chemicon International, Temecula, CA, USA) or rabbit anti-MIF (1:200; Torrey Pines Biolabs, Inc, Houston, TX, USA). Secondary antibodies were Alexa Fluor 594 goat anti-mouse IgG (1:1000) for NeuN or GFAP and Alexa Fluor 488 goat anti-rabbit IgG (1:1000) (all from Molecular Probes, Eugene, OR, USA) for MIF. MIF, NeuN, or GFAP immunofluorescence and green fluorescence (from GFP) were detected using an Olympus BX41 fluorescence microscope(Olympus, Tokyo, Japan).
Neuronal cultures
AAV2-CBA-eGFP-treated cells were washed briefly with Dulbecco’s PBS and then fixed for 10 min with PBS containing 0.1% Tween 20 (PBS/Tween) and 4% formaldehyde solution. The fixed cells were incubated with PBS containing 0.3% Triton X-100 (Sigma-Aldrich Corp., St. Louis, MO.) for 20 min to improve antibody penetration. Immunocytochemistry was then performed on the fixed cells as detailed previously (17), using a neuron-specific primary antibody (monoclonal anti-NeuN antibody, 1:1000), followed by Alexa Fluor 594 goat anti-mouse IgG (1:1000) as the secondary antibody. NeuN immunoreactivity (red) and green fluorescence (from GFP) were detected using an Olympus BX41 fluorescence microscope.
Real-time reverse transcriptase (RT) -PCR
For analyses of endogenous MIF mRNA, the PVN of SHRs and WKY and SD rats was removed by micropunch, and total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA, USA). This kit was also used to isolate total RNA from cerebral cortical neuronal cultures, after treatment with AAV2-CBA-GFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF. MIF mRNA was analyzed via quantitative real-time RT-PCR as detailed by us previously (17). Oligonucleotide primers and TaqMan probes specific for rat MIF were obtained from Applied Biosystems, Inc. (Foster City, CA, USA). Isolated RNA underwent DNase I treatment to remove genomic DNA, and 25 ng of the purified RNA were used to perform RT-PCR in an Applied Biosystems Prism 7000 Sequence Detection System, with the use of One-Step RT-PCR Master Mix Reagents. Data were normalized to 18S RNA or glyceraldehyde-3-phosphate dehydrogenase mRNA.
Identification of MIF or [C60S]-MIF cDNA amplified by RT-PCR using restriction endonuclease digestion
Total RNA was prepared from the AAV2-CBA-GFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF transduced primary cortical neuronal cultures, and RT-PCR was performed using primers MIF-F and MIF-R and a One-Step RT-PCR kit (Qiagen) under the following conditions: 1 cycle of 50°C for 30 min, 95°C for 15 min; 16 cycles of 94°C for 40 s, 55°C for 1 min, 72°C for 1 min, and 1 cycle of 72°C for 8 min. PCR products were separated by electrophoresis on a 2.5% agarose gel and identified by restriction endonuclease (PstI) analysis.
Analyses of MIF protein
Western immunoblots using a rabbit anti-rat MIF antibody (1:500 dilution; Torrey Pines Biolabs, Inc.) were used to analyze endogenous MIF in the PVN of SHRs or WKY or SD rats or in AAV2-CBA-eGFP or AAV2-CBA-MIF-transduced neuronal cultures, as detailed by us previously (20). MIF protein expression was quantified by densitometry using a calibrated imaging densitometer (model GS710; Bio-Rad Laboratories, Hercules, CA, USA).
Cardiovascular experiments
Implantation of telemetry transducers
Eight-week-old male SHRs or WKY rats were anesthetized with a mixture of O2 (1 L/min) and 4% isoflurane, and anesthesia was maintained for the duration of the surgery using an O2/isoflurane (2%) mixture delivered through a nose cone. Rat telemetry transducers (DSI, St. Paul, MN, USA) were implanted into the abdominal aorta, as detailed by us previously (20). Rats were administered the analgesic (buprenorphine, 0.05 mg/kg s.c.) during the first 24 h after surgery and were left to recover for 10–14 days before PVN microinjections of control solution or AAV2 constructs, as described below.
PVN microinjections
After recovery from implantation of the telemetry transducers, the male SHRs or WKY rats (9.5–10 wk old) were anesthetized using isoflurane as above and placed in a stereotactic frame (David Kopf Instruments). Bilateral microinjections (0.5 μl) of either of the recombinant AAV2-CBA-MIF or AAV2-CBA-eGFP or control solution (PBS) were performed as described by us recently, using the following stereotactic coordinates: −1.6 to 1.8 anteroposterior (bregma), ±0.3 to 0.5 mediolateral, and −7.8 dorsoventral (skull surface) (24). Buprenorphine (0.05 mg/kg s.c.) was administered before the rats awoke from the anesthesia.
Cardiovascular measurements
Measurements of mean arterial pressure (MAP) and heart rate (HR) were made via the telemetry transducers (20) before and for up to 12 wk (depending on the particular protocol) after the PVN microinjections of either AAV2-CBA-MIF, AAV2-CBA-eGFP, AAV2-BBA-[C60S]-MIF, or PBS. Raw data were analyzed initially using Dataquest IV software (DSI).
Cardiac pathology
After the experiments, rats were weighed and then anesthetized with isoflurane as above and euthanized by decapitation. Hearts were removed and weighed, and cross-sections of the ventricles were then fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Myocyte diameters were determined at ×400 using the ImageJ program from the National Institutes of Health (Bethesda, MD, USA). Quantification of diameters was carried out in a blinded fashion. Between 14 and 35 separate images, from different regions of the left ventricle free wall only, were examined. The results for each animal were then averaged for subsequent statistical analysis.
Data analysis
Data are expressed as means ± se. Statistical significance was evaluated with the use of a 1- or 2-way ANOVA as appropriate, followed by the appropriate post hoc test (Bonferroni) to compare individual means. Differences were considered significant at P < 0.05, and individual P values are noted in the figure legends.
RESULTS
Expression of MIF in the PVN of SHRs
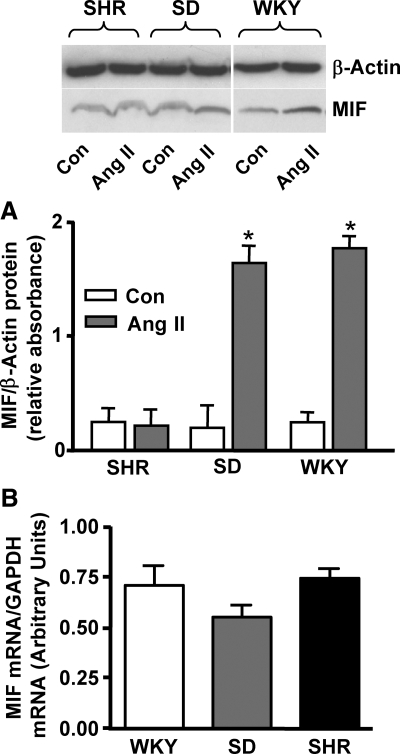
Our previous studies demonstrated that Ang II does not alter MIF protein expression in hypothalamic neurons cultured from SHRs, bringing into question whether the aforementioned MIF negative regulatory mechanism is present in these animals (18). Thus, we first investigated whether Ang II increases the expression of MIF in the PVN of SHRs. The data in Fig. 1A demonstrate that i.c.v. injection of Ang II (10 pmol, 2 μl) into SD or WKY rats elicits a significant increase in MIF protein expression in the PVN, when compared with that in the saline-injected control rats, consistent with our previous findings (20). In contrast, i.c.v. injection of Ang II (10 pmol, 2 μl) into SHRs failed to increase MIF protein expression in the PVN of these animals, compared with that in saline-injected control rats (Fig. 1A), consistent with our in vitro data (18).
Figure 1.
MIF levels in the PVN. A) Ang II increases MIF protein levels in the PVN of WKY and SD rats but not in SHRs. Ang II (10 pmol, 2 μl) or control solution (0.9% saline) was injected i.c.v., and 3 h later rats were euthanized, brains were removed and sectioned, and the PVN was removed bilaterally via micropunch. Top: representative Western blots showing MIF protein in PVN from WKY or SD rats or SHRs after 0.9% saline (Con) or Ang II injections. Bottom: bar graphs show the expression of MIF protein in control and Ang II-injected rats. Data are means ± se from 4 rats (SD and WKY rats) and 6 rats (SHRs). *P < 0.05. B) Comparison of baseline MIF mRNA levels in the PVN of WKY and SD rats and SHRs. Bar graphs are means ± se from 4 rats in each group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
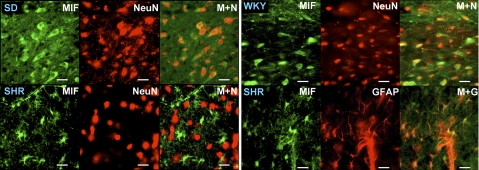
Neither baseline levels of MIF protein nor MIF mRNA were significantly different between the PVN of SD and WKY rats and SHRs (Fig. 1A, B). However, our immunostaining experiments indicated that the cellular location of MIF within the PVN of SHRs is different from that within normotensive rat PVN. The fluorescence micrographs presented in Fig. 2 indicate that in the PVN of SD and WKY rats, immunoreactive MIF (green) is present within neurons, as indicated by colabeling with the neuron-specific marker NeuN (red). However, in the PVN of SHRs the MIF immunoreactivity was not colocalized with NeuN (Fig. 2) but rather was colocalized with the astroglial-specific marker GFAP (Fig. 2). Collectively, these data suggest that MIF is either absent or diminished in neurons in the PVN of SHRs and is not regulated by Ang II in this strain.
Figure 2.
Cellular localization of MIF in the PVN of normotensive rats and SHRs. Fluorescence micrographs show endogenous MIF, NeuN, or GFAP immunoreactivity in the PVN of adult untreated WKY rats, SD rats, and SHRs; ×40 view. Data are representative of 4 rats from each strain. Scale bars = 50 μm. M+N, MIF + NeuN merged; M+G, MIF + GFAP merged.
AAV2-mediated transduction of MIF in vitro and in the PVN
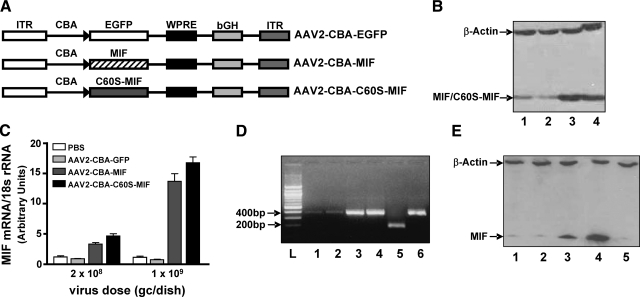
Considering that the PVN-mediated actions of Ang II are enhanced in and contribute to the development of hypertension in SHRs (12,13,14,15), it is possible that the lack of MIF expression/regulation in PVN neurons of SHRs might contribute to the high blood pressure observed in this strain. Therefore, we planned to test whether increasing the expression of MIF within neurons in the PVN of SHRs would reduce basal blood pressure in these animals. To achieve increased neuronal levels of MIF we used an AAV2-based vector system, under the control of a CBA/CMV hybrid promoter (Fig. 3A). As a preliminary step, a MIF recombinant plasmid (pAAV2-CBA-MIF) was constructed. Transfection of this plasmid into HEK 293 cells produced a significant level of MIF protein expression when compared with cells transfected with the control plasmid pAAV2-CBA-eGFP (Fig. 3B). In addition, transfection of HEK 293 cells with the plasmid pAAV2-CBA-[C60S]-MIF ([C60S-MIF] lacks the TPOR activity of native MIF) produced a level of [C60S]-MIF protein expression that was comparable to that observed with MIF (Fig. 3B). The effectiveness of these vectors to produce expression of MIF, [C60S]-MIF, and GFP in the CNS was first tested in primary neurons in culture. In SD rat cerebral cortical neuronal cultures, AAV2-CBA-MIF (2×108 or 1×109 viral gc/dish for 5 days) produced a dose-dependent increase in the expression of MIF mRNA, compared with incubation of cultures with the same doses of AAV2-CBA-eGFP or PBS (Fig. 3B). Incubation of cerebral cortical neuronal cultures with AAV2-CBA-[C60S]-MIF (at 2×108 or 1×109 viral gc/dish for 5 days) elicited dose-dependent increases in the expression of the mutant [C60S]-MIF mRNA, similar to the levels of MIF mRNA obtained with AAV2-CBA-MIF (Fig. 3C). Note that the levels of [C60S]-MIF mRNA were measured using the MIF primers, as there is only a 1-nucleotide mutation in [C60S]-MIF vs. native MIF. Thus, the [C60S]-MIF mRNA data in Fig. 3C are presented as “MIF mRNA.” Confirmation of the identity of the overexpressed MIF and [C60S]-MIF was obtained by performing RT-PCR on total RNA extracted from cerebral cortical neurons transduced as above, separating the PCR products by gel electrophoresis, and then subjecting them to restriction enzyme (PstI) analysis. The representative gel shown in Fig. 3D indicates that DNA fragments of the expected size (385 bp) were amplified from all samples but that much more DNA was amplified in the AAV2-CBA-MIF and AAV2-CBA-[C60S]-MIF transduced neurons than from the AAV2-CBA-eGFP or mock (PBS) transduced samples. Furthermore, the PCR product from the AAV2-CBA-MIF transduced cells was completely digested by PstI to 2 overlapping bands (199 and 188 bp). As expected, the PCR product from the AAV2-CBA-[C60S]-MIF transduced neurons (which does not contain the PstI restriction site due to the C60S mutation) was not digested by PstI. Consistent with the mRNA data in Fig. 3C, incubation of neuronal cultures with AAV2-CBA-MIF also elicited a dose-dependent increase in MIF protein expression compared with AAV2-CBA-eGFP (Fig. 3E).
Figure 3.
AAV2-CBA-eGFP and AAV2-CBA-MIF mediated transduction of eGFP and MIF into neurons in culture. A) AAV2-based vectors used in the present studies. EGFP, enhanced green fluorescent protein; WPRE, Woodchuck hepatitis virus post-transcriptional control element; bGH, bovine growth hormone; ITR, inverted terminal repeat. B) Western blot showing the expression of MIF and [C60S]-MIF in HEK293 cells after transfection with either MIF or [C60S]-MIF recombinant plasmids. Lane 1, mock transfection; lane 2, pAAV2-CBA-eGFP; lane 3, pAAV2-CBA-MIF; and lane 4, pAAV2-CBA-[C60S]-MIF-eGFP. Blotting was performed using a MIF primary antibody. Also shown are the levels of a control protein, β-actin, under each treatment condition. C) Real-time RT-PCR data showing the expression of MIF mRNA in SD rat cerebral cortical neuronal cultures treated with either PBS (pH 7.4), AAV2-CBA-eGFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF at the indicated doses for 3 days. Data are means ± se from 4 experiments. D) Agarose gel electrophoresis confirming the identity of MIF or [C60S]-MIF cDNA amplified by RT-PCR using restriction endonuclease (PstI) digestion. RT-PCR was performed to amplify MIF and/or [C60S]-MIF cDNA in SD rat cerebral cortical neuronal cultures that had been incubated/transduced with either PBS (pH 7.4), AAV2-CBA-eGFP, AAV2-CBA-MIF, or AAV2-CBA-[C60S]-MIF (all at 1×109 viral gc/well). The amplified PCR products (385 bp; lanes 1–6) were digested by the restriction endonuclease PstI (lanes 5 and 6 only) and then determined by 2.5% agarose gel electrophoresis. L, 100-bp DNA ladder; lane 1, mock transduction (PBS); lane 2, AAV2-CBA-GFP; lanes 3 and 5, AAV2-CBA-MIF; and lanes 4 and 6, AAV2-CBA-[C60S]-MIF. E) Western blot showing the levels of 12-kDa MIF protein in SD rat neuronal cultures 3 days after the following treatments: lane 1, AAV2-CBA-eGFP (1×109 viral gc/well); lane 2, AAV2-CBA-eGFP (5×109 viral gc/well); lane 3, AAV2-CBA-MIF (1×109 viral gc/well); lane 4, AAV2-CBA-MIF (5×109 viral gc/well); and lane 5, mock transduction. Also shown are the levels of a control protein, β-actin, under each treatment condition.
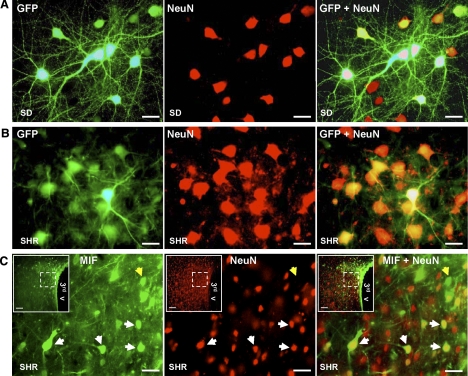
The neuronal specificity of transduction produced by these vectors in vitro was demonstrated by incubation of cerebral cortical neuronal cultures with AAV2-CBA-eGFP (1×109 viral gc/well), which resulted in a high level of neuron-specific GFP expression, on the basis of colabeling with immunoreactive NeuN (Fig. 4A). These AAV2-based vectors were also highly effective in vivo, as use of this vector resulted in increased expression of GFP or MIF within PVN neurons in SHRs and normotensive rats. Bilateral injection of AAV2-CBA-eGFP (0.5 μl; 1×109 gc) into the PVN of SHRs or WKY or SD rats elicited significant neuronal expression of GFP within 7days, and expression persisted at ∼4.0 months after the injection. The fluorescence micrographs in Fig. 4B demonstrate GFP within PVN neurons of an SHRs at 4.0 months postinjection, as indicated by colabeling with the neuron-specific marker NeuN (red). Similar labeling was seen in WKY and SD rats (data not shown). Bilateral injection of AAV2-CBA-MIF (0.5 μl; 1×109 gc) into the PVN of SHRs or WKY or SD rats elicited a similar time-dependent expression of MIF within PVN neurons. The representative fluorescence micrographs presented in Fig. 4C show increased MIF expression in PVN neurons from an SHR at 4 months after AAV2-CBA-MIF injection (white arrows on micrographs). As expected, in the SHRs immunoreactive MIF was also observed in non-neuronal (possibly glial) cells (yellow arrows in micrographs in Fig. 4C). This result is consistent with our above-mentioned findings (Fig. 2) that endogenous MIF is present in astroglia in the PVN of SHRs.
Figure 4.
AAV2-CBA-eGFP- and AAV2-CBA-MIF-mediated neuronal transduction of GFP and MIF. A) Representative fluorescence micrographs from SD rat neuronal cultures showing GFP fluorescence (left), NeuN immunostaining (center), and merged images (GFP+NeuN; right) after incubation with AAV2-CBA-eGFP (1×109 viral gc/well) for 3 days (×40 view). B) Adult male SHRs were microinjected bilaterally into the PVN with AAV2-CBA-eGFP (0.5 μl; 1×109 gc). Four months later, rats were euthanized, and brains were removed and sectioned in preparation for detection of GFP and immunostaining with NeuN. Images are high-power (×40) fluorescence micrographs from the same cell field in the PVN, showing GFP fluorescence (left), NeuN immunostaining (center), and merged images (GFP+NeuN; right). Data are representative of 4 rats. C) Adult male SHRs were microinjected bilaterally into the PVN with AAV2-CBA-MIF (0.5 μl; 1×109 gc). Four months later, rats were euthanized, and brains were removed and sectioned in preparation for detection of MIF and NeuN via immunostaining. Images are high-power (×40) fluorescence micrographs from the field of cells outlined in the inset, showing MIF immunostaining (left), NeuN immunostaining (center), and merged images (MIF+NeuN; right). White arrows indicate MIF-expressing neurons; yellow arrows indicate MIF immunoreactivity within non-neuronal cells, probably glia. Insets: low-power (×10) fluorescence micrographs. 3rd v, third cerebroventricle. Data are representative of 6 rats. Scale bars = 50 μm; 100 μm (insets).
Increased MIF expression in the PVN decreases baseline blood pressure in SHRs but not in WKY rats
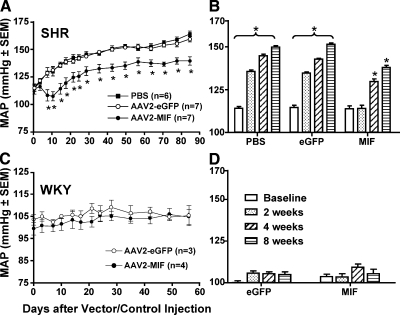
Based on the above expression data, we examined whether increased expression of MIF in the PVN of SHRs or WKY rats would alter basal blood pressure. Eight-week-old SHRs were fitted with telemetry pressure transducers, followed 10–14 days later by microinjection of either AAV2-CBA-MIF (1×109 gc in 0.5 μl), AAV2-CBA-eGFP (1×109 gc in 0.5 μl), or PBS (0.5 μl) bilaterally into the PVN. MAP and HR were monitored via telemetry before vector injections into the PVN, and for 12 wk after vector injections. Before the PVN injections, baseline MAP values in the AAV2-CBA-eGFP- and PBS-treated SHRs were 114.6 ± 3.2 mmHg (n=7 rats) and 114.1 ± 2.6 mmHg (n=6 rats), respectively. As expected, MAP increased steadily over the next 12 wk, reaching levels of 158.6 ± 2.6 mmHg (n=7 rats) and 163.3 ± 2.5 (n=6) in the GFP-transduced and PBS-treated SHRs, respectively (Fig. 5A). In both control groups there was a significant increase in baseline MAP by 2 wk after GFP or PBS treatment (Fig. 5B). In contrast, in the MIF-transduced SHRs, there was an initial dip in MAP, and the increase in baseline MAP over time was significantly less (Fig. 5A). Before the PVN injection of AAV2-CBA-MIF, baseline MAP was 113.8 ± 4.6 mmHg (Fig. 5A), and 12 wk after MIF transduction MAP had increased to only 139 ± 3.7 mmHg (n=7 rats). In the MIF-transduced rats, the increase in MAP with time did not become significant until 4 wk after MIF treatment (Fig. 5B). MIF transduction into the PVN did not produce a significant change in HR compared with that in GFP-transduced and PBS-treated rats (Table 1).
Figure 5.
Reduction of baseline MAP in SHRs but not in WKY rats after increased MIF expression in PVN neurons. A, B) Eight-week-old male SHRs were fitted with telemetry pressure transducers, followed 10–14 days later by microinjection of either AAV2-CBA-MIF (1×109 gc in 0.5 μl), AAV2-CBA-eGFP (1×109 gc in 0.5 μl), or PBS (0.5 μl) bilaterally into the PVN. MAP and HR were monitored via telemetry for the next 12 wk. Shown in A is baseline MAP plotted against time after vector/control injections. Baseline MAP before viral vector injections is plotted on the y axis. *P < 0.05 vs. corresponding time point in the AAV2-CBA-eGFP group. Shown in B are the changes in MAP in each group of rats at 2, 4, and 8 wk after vector/control injections, compared with baseline MAP before viral vector injections. Data are means ± se from 7 rats (both AAV2-CBA-MIF and AAV2-CBA-eGFP groups) and 6 rats (PBS group); *P < 0.05 vs. baseline. C, D) Eight-week-old male WKY rats were fitted with telemetry pressure transducers, followed 10–14 days later by microinjection of either AAV2-CBA-MIF (1×109 gc in 0.5 μl) or AAV2-CBA-eGFP (1×109 gc in 0.5 μl) bilaterally into the PVN. Shown in C is baseline MAP plotted against time after vector injections. Baseline MAP before viral vector injections is plotted on the y axis. Shown in D are the changes in MAP in each group of rats at 2, 4, and 8 wk after vector/control injections, compared with baseline MAP before viral vector injections. Data are means ± se from 3 rats (AAV2-CBA-eGFP group) and 4 rats (AAV2-CBA-MIF group).
TABLE 1.
Baseline HR values in SHR and WKY rats before and after AAV2-CBA-eGFP, AAV2-CBA-MIF, or PBS injections into the PVN
| Time | SHR
|
WKY
|
|||
|---|---|---|---|---|---|
| PBS (n=6) | eGFP (n=7) | MIF (n=7) | eGFP (n=3) | MIF (n=4) | |
| Baseline | 315 ± 13 | 315 ± 13 | 343 ± 5 | 301 ± 6 | 306 ± 11 |
| 2 wk | 318 ± 9 | 330 ± 12 | 333 ± 6 | 295 ± 7 | 321 ± 13 |
| 4 wk | 329 ± 14 | 344 ± 9 | 317 ± 11 | 323 ± 8 | 311 ± 6 |
| 8 wk | 340 ± 16 | 353 ± 9 | 307 ± 11 | 317 ± 5 | 318 ± 7 |
Values are mean ± se beats/min; nrats/group. There are no significant differences between any of the HR values.
Eight-week-old WKY rats were fitted with telemetry pressure transducers, and microinjected with either AAV2-CBA-MIF or AAV2-CBA-eGFP bilaterally into the PVN, exactly as described above for the SHRs. Before the injection of AAV2-CBA-MIF or AAV2-CBA-eGFP into these rats, baseline MAP values were 99.3 ± 2.8 mmHg (n=4 rats) and 103.5 ± 2.5 mmHg (n=3 rats), respectively. Telemetry recordings over the next 8 wk revealed that increased MIF expression in the PVN of WKY rats did not significantly alter baseline MAP or HR (Fig. 5C, D; Table 1). For example, at 8 wk after PVN transduction of MIF or GFP, the baseline MAP values in WKY rats were 104.7 ± 3.3 mmHg (n=4 rats) and 105.3 ± 4.5 mmHg (n=3 rats), respectively.
Increased [C60S]-MIF expression in the PVN does not alter baseline blood pressure in SHRs
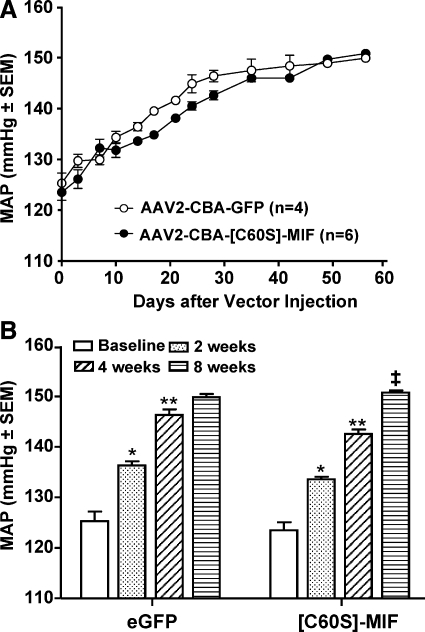
To test the role of the TPOR activity of MIF in the MAP-lowering action of this molecule, we determined the effects of overexpression of the mutant protein [C60S]-MIF within the PVN on the baseline blood pressure of SHRs. [C60S]-MIF contains the same number of amino acids as MIF but is devoid of TPOR activity because of a single C → S mutation at position 60 (25). Eight-week-old SHRs were fitted with telemetry pressure transducers, followed 10–14 days later by microinjection of either AAV2-CBA-[C60S]-MIF (1×109 gc in 0.5 μl) or AAV2-CBA-eGFP (1×109 gc in 0.5 μl) bilaterally into the PVN. MAP and HR were monitored via telemetry for the next 8 wk. Before the PVN injections, baseline MAP values in the AAV2-CBA-[C60S]-MIF- and AAV2-CBA-eGFP -treated SHRs were 123.5 ± 3.9 mmHg (n=6 rats) and 125.3 ± 3.8 mmHg (n=4 rats), respectively. From the time course data shown in Fig. 6, it is clear that injection of AAV2-CBA-[C60S]-MIF bilaterally into the PVN did not reduce the progressive increase in MAP in SHRs. For example, at 8 wk after AAV2-CBA-[C60S]-MIF and AAV2-CBA-eGFP injection, baseline MAP values were 150.8 ± 1.1 mmHg (n=6 rats) and 149.9 ± 1.3 mmHg (n=4 rats), respectively. Likewise, HR was not altered in these animals after AAV2-CBA-[C60S]-MIF injections. For example, before the PVN injections, baseline HR values in the AAV2-CBA-[C60S]-MIF- and AAV2-CBA-eGFP-treated SHRs were 317.8 ± 4.2 mmHg (n=6 rats) and 320.4 ± 4.1 mmHg (n=4 rats), respectively. At 2, 4, and 8 wk after AAV2-CBA-[C60S]-MIF treatment, HR values were 316.4 ± 9.6, 333.8 ± 7.4, and 337.5 ± 12.1 beats/min, respectively (n=6 rats). These values are no different from those measured in the GFP-transduced rats at the same time points (317.4±9.1, 335.9±7.0, and 338.8±6.0 beats/min, respectively, at 2, 4, and 8 wk after AAV2-CBA-eGFP injection, n=4 rats).
Figure 6.
Increased expression of [C60S]-MIF in PVN neurons does not alter baseline MAP in SHRs. Eight-week-old male SHRs were fitted with telemetry pressure transducers, followed 10–14 days later by microinjection of either AAV2-CBA-[C60S]-MIF (1×109 gc in 0.5 μl) or AAV2-CBA-eGFP (1×109 gc in 0.5 μl) bilaterally into the PVN. A) Baseline MAP plotted against time after vector injections. Baseline MAP before viral vector injections is plotted on the y axis. B) Changes in MAP in each group of rats at 2, 4, and 8 wk after vector injections, compared with baseline MAP before viral vector injections. Data are means ± se from 6 rats (AAV2-CBA-[C60S]-MIF group) and 4 rats (AAV2-CBA-eGFP group). *P < 0.001 vs. baseline; **P < 0.001 vs. 2 wk; ‡P < 0.001 vs. 4 wk.
Increased MIF expression in the PVN: effect on cardiac hypertrophy in SHRs
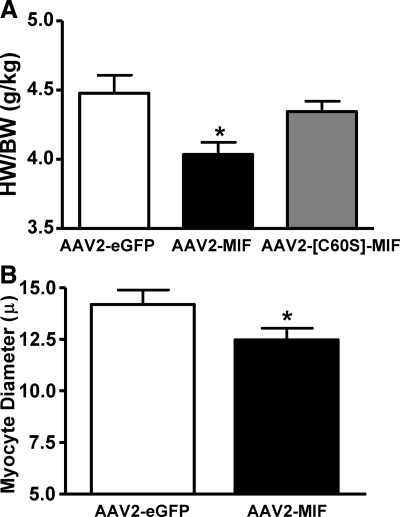
After the above experiments using SHRs, rats were euthanized and hearts were removed for determination of the effects of MIF transduction into the PVN on cardiac hypertrophy. The data presented in Fig. 7A are the heart weight/body weight (HW/BW) ratios of SHRs after AAV2-CBA-MIF, AAV2-CBA-eGFP, or AAV2-CBA-[C60S]-MIF treatments as above. These data indicate that the MIF-transduced rats display significant decreases in HW/BW ratio compared with the AAV2-CBA-eGFP-treated rats. However, AAV2-CBA-[C60S]-MIF did not significantly alter the HW/BW ratio, compared with that in AAV2-CBA-eGFP-treated rats (P=0.09). In further analyses, the hearts from the same AAV2-CBA-MIF- and AAV2-CBA-eGFP-treated rats were processed for assessment of cardiac myocyte diameter. The data indicate that the AAV2-CBA-MIF-treated rats have a significant decrease in cardiac myocyte diameter (Fig. 7B), consistent with the decrease in HW/BW ratio.
Figure 7.
Reduction in cardiac hypertrophy after increased MIF expression in PVN neurons of SHRs. A) HW/BW ratios in the AAV2-CBA-eGFP-, AAV2-CBA-MIF- and AAV2-CBA-[C60S]-MIF-injected (all at 1×109 gc in 0.5 μl) SHRs (from Figs. 5 and 6) were determined at the end of the experiments, after the cardiovascular recordings. Data are means ± se from 6 to 9 rats. *P < 0.05 vs. AAV2-CBA-eGFP- and AAV2-CBA-[C60S]-MIF-treated rats. B) Cardiac myocyte diameters in the AAV2-CBA-MIF- and AAV2-CBA-eGFP-treated rats. Data are means ± se from 6 rats in each group. *P < 0.05 vs. AAV2-CBA-eGFP-treated rats.
DISCUSSION
The major findings of this study are 1) that SHRs exhibit a lack of MIF expression within PVN neurons, 2) that restoration of MIF within PVN neurons of SHRs significantly attenuates the time-dependent increases in blood pressure and cardiac hypertrophy that are normally observed in these animals, and 3) that these beneficial actions of MIF appear to be mediated via the intrinsic TPOR activity of the MIF molecule. As such, the data presented here are the first demonstration that MIF can lower high blood pressure in SHRs and that this protein may constitute a novel therapeutic target in hypertension.
The rationale for the present study stems from a number of our previous investigations. First, we had established that Ang II increases MIF expression in hypothalamic neurons from normotensive rats in vitro and that CNS-applied Ang II increases MIF levels in WKY and SD rat PVN in vivo (17, 20). Second, we demonstrated that MIF acts intracellularly to inhibit the chronotropic actions of Ang II in normotensive rat PVN neurons in vitro, an effect mediated via the TPOR activity of the MIF molecule (18, 19). Importantly, we had also determined that transient increases in expression of MIF within PVN neurons of normotensive rats attenuate the pressor effect of CNS-injected Ang II, while not altering baseline blood pressure (20). These data established elevated levels of MIF in the PVN as an intracellular negative regulator of the CNS-mediated cardiovascular actions of Ang II in normotensive rats. The picture in SHRs is different. Whereas our in vitro experiments indicated that exogenously applied MIF inhibited the enhanced chronotropic action of Ang II in SHR hypothalamic neurons (20), it was also clear that Ang II failed to increase expression of endogenous MIF in these cells (20). This result suggests that the negative regulatory actions of MIF are absent in SHRs. Considering that the PVN-mediated actions of Ang II are enhanced in SHRs and contribute to the development of hypertension observed in these animals (12,13,14,15), we developed the idea that the lack of a MIF regulatory mechanism in PVN neurons of SHRs might contribute to the increased sensitivity of these neurons to Ang II (12) and ultimately to hypertension in these animals. These ideas are reinforced by the results from the present study, which indicate that Ang II fails to increase MIF expression in the PVN of SHRs, that MIF appears to be reduced in or absent from PVN neurons in SHRs, and that restoration of MIF in PVN neurons of SHRs via viral-mediated gene delivery lowers blood pressure in SHRs.
The data presented in this study also raise a number of questions that will be the subject of our further studies. One immediate question concerns the intracellular mechanism of action of MIF within the PVN in achieving a reduction in basal blood pressure in SHRs. The results of our experiments involving transduction of [C60S]-MIF into the PVN of SHRs clearly indicate a role for the TPOR moiety of MIF, consistent with our in vitro data (18, 19) and with current opinions on the mechanism of action of MIF in various systems (26,27,28,29). Based on these findings, one possibility is that MIF acts via its TPOR activity to scavenge reactive oxygen species (ROS) and in doing so interrupts Ang II signaling (which is ROS-dependent) and the enhanced Ang II-induced chronotropic actions at the PVN (17, 20, 25). Our in vitro data, which indicate that regulatory actions of MIF on neuronal K+ current involve a TPOR/ROS scavenging mechanism, certainly support this notion (19). However, although the bulk of our data suggest that MIF exerts its regulatory actions via an intracellular mechanism (17,18,19), we cannot exclude the possibility that the transduced MIF is also secreted from the neurons and exerts extracellular actions. For example, the interactions of MIF with pro- and antiinflammatory cytokines in the PVN must be investigated as other studies have indicated that a balance between these mechanisms in the hypothalamus controls the autonomic output that ultimately leads to heart failure (30). This information is especially important because MIF is well known to increase secretion of proinflammatory cytokines from non-neuronal cells (26, 28).
It will also be important to discern which neuronal population(s) in the PVN is/are important for the regulatory actions of MIF on blood pressure in the SHRs. In the present studies we used a CBA promoter that produces MIF transduction primarily in neurons in the CNS (31). However, it does not discriminate between different neuronal populations within the PVN, and so expression of MIF after AAV2-CBA-MIF will proceed in all neuronal types at the site of injection. PVN neurons that influence arterial pressure regulation include multiple neuronal phenotypes with several projection sites (32). For example, one major output of the parvocellular PVN comprises neurons expressing corticotropin-releasing hormone, with some coexpression of vasopressin, that project to the median eminence (33). The corticotropin-releasing hormone and vasopressin then act in the pituitary to stimulate secretion of adrenocorticotropic hormone, which in turn stimulates the synthesis and secretion of glucocorticoids from the adrenal cortex. Elevated glucocorticoids lead to hypertension and other cardiovascular disease risk factors (34). Another identified output of the PVN consists of neurons that project to the spinal cord and/or the rostral ventral lateral medulla to control sympathetic nerve activity (33). These neurons are not fully characterized, but glutamate, vasopressin, corticotropin-releasing hormone, and oxytocin have been implicated as neurotransmitters (33, 35). Other PVN neurons that may influence blood pressure regulation project to the locus coeruleus or the nucleus tractus solitarius. Approximately 30% of neurons projecting to the locus coeruleus express corticotropin-releasing hormone (36), whereas neurons that project to the nucleus tractus solitarius express corticotropin-releasing hormone, oxytocin, vasopressin, or other neurotransmitters (37). Although the complex and overlapping roles of various neurotransmitters used by PVN projection neurons make it a difficult task, it will be essential in future work to develop techniques to express MIF selectively in specific neuronal populations within the PVN and determine the effects on blood pressure, plasma glucocorticoids, and sympathetic nerve activity.
From a more global perspective, it has become apparent that MIF is an important regulator in cardiovascular disease, exerting both deleterious and protective actions (38, 39). The present studies suggest a further protective action of MIF and continue its identification as a factor that has a key role in the CNS-mediated regulation of blood pressure. The data provided here indicate that a MIF-mediated negative regulatory mechanism that is present in the PVN of normotensive rats is absent from SHRs and that increased expression of MIF in neurons at this site in SHRs decreases the progression of hypertension in these animals. Although our studies suggest that MIF may constitute a novel therapeutic target for hypertension, it will be important to determine whether MIF has beneficial actions in other forms of experimental hypertension and whether MIF levels are similarly deficient in neurons from brains of human hypertensive patients before proceeding.
Acknowledgments
This work was supported by National Institutes of Health grant HL-076803 (C.S.). We thank Arseniy Shabashvili for technical assistance.
References
- Grassi G, Mancia G. Neurogenic hypertension: is the enigma of its origin near the solution? Hypertension. 2004;43:154–155. doi: 10.1161/01.HYP.0000109870.99110.7e. [DOI] [PubMed] [Google Scholar]
- Schlaich M P, Lambert E, Kaye D M, Krozowski Z, Campbell D J, Lambert G, Hastings J, Aggarwal A, Esler M D. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Osborn J W, Fink G D, Sved A F, Toney G M, Raizada M K. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Allen A M. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved A F. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- Stocker S D, Cunningham J T, Toney G M. Water deprivation increases fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Akine A, Montanaro M, Allen A M. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci. 2003;108:17–21. doi: 10.1016/j.autneu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Chen Q H, Toney G M. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol. 2001;281:R1844–R1153. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- Ferguson A V, Washburn D L, Latchford K J. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226:85–96. doi: 10.1177/153537020122600205. [DOI] [PubMed] [Google Scholar]
- McKinley M J, Allen A M, May C N, McAllen R M, Oldfield B J, Sly D, Mendelsohn F A. Neural pathways from the lamina terminalis influencing cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol. 2001;28:990–992. doi: 10.1046/j.1440-1681.2001.03592.x. [DOI] [PubMed] [Google Scholar]
- Li Y F, Wang W, Mayhan W G, Patel K P. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol. 2006;290:R1035–R1043. doi: 10.1152/ajpregu.00338.2004. [DOI] [PubMed] [Google Scholar]
- Harding J W, Felix D. Angiotensin-sensitive neurons in the rat paraventricular nucleus: relative potencies of angiotensin II and angiotensin III. Brain Res. 1987;410:130–134. doi: 10.1016/s0006-8993(87)80033-1. [DOI] [PubMed] [Google Scholar]
- Gutkind J S, Kurihara M, Saavedra J M. Increased angiotensin II receptors in brain nuclei of DOCA-salt hypertensive rats. Am J Physiol. 1988;255:H646–H650. doi: 10.1152/ajpheart.1988.255.3.H646. [DOI] [PubMed] [Google Scholar]
- Meyer J M, Felten D L, Weyhenmeyer J A. Measurement of immunoreactive angiotensin II levels in microdissected brain nuclei from developing spontaneously hypertensive and Wistar Kyoto rats. Exp Neurol. 1990;107:164–169. doi: 10.1016/0014-4886(90)90154-k. [DOI] [PubMed] [Google Scholar]
- Reja V, Goodchild A K, Phillips J K, Pilowsky P M. Upregulation of angiotensin AT1 receptor and intracellular kinase gene expression in hypertensive rats. Clin Exp Pharmacol Physiol. 2006;33:690–695. doi: 10.1111/j.1440-1681.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- Perez D M, Karnik S S. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–161. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- Sun C, Li H, Leng L, Raizada M K, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci. 2004;24:9944–9952. doi: 10.1523/JNEUROSCI.2856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li H, Gao Y, Matsuura T, Upchurch P A, Raizada M K, Sumners C. Lack of macrophage migration inhibitory factor regulation is linked to the increased chronotropic action of angiotensin II in SHR neurons. Hypertension. 2007;49:528–534. doi: 10.1161/01.HYP.0000257877.11495.cb. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sun C, Leng L, Kapurniotu A, Bernhagen J, Bucala R, Martynyuk A E, Sumners C. Macrophage migration inhibitory factor increases neuronal delayed rectifier K+ current. J Neurophysiol. 2006;95:1042–1048. doi: 10.1152/jn.00499.2005. [DOI] [PubMed] [Google Scholar]
- Li H, Gao Y, Freire C D, Raizada M K, Toney G M, Sumners C. Macrophage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J. 2006;20:1748–1750. doi: 10.1096/fj.06-5836fje. [DOI] [PubMed] [Google Scholar]
- Barik S. Site-directed mutagenesis by double polymerase chain reaction (megaprimer method) White E A, editor. Totowa, NJ, USA: Humana Press; Methods in Molecular Biology, Vol. 15, PCR ProtocolsCurrent Methods and Applications. 1993:277–287. doi: 10.1385/0-89603-244-2:277. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Hildinger M, O'Connor E, Gao G P, Wilson J M. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- Rohr U P, Wulf M A, Stahn S, Steidl U, Haas R, Kronenwett R. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J Virol Methods. 2002;106:81–88. doi: 10.1016/s0166-0934(02)00138-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. San Diego, CA, USA: Academic Press; The Rat Brain in Stereotaxic Coordinates. 1998 [Google Scholar]
- Kleemann R, Kapurniotu A, Frank R W, Gessner A, Mischke R, Flieger O, Juttner S, Brunner H, Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele M, Bernhagen J. Link between macrophage migration inhibitory factor and cellular redox regulation. Antioxid Redox Signal. 2005;7:1234–1248. doi: 10.1089/ars.2005.7.1234. [DOI] [PubMed] [Google Scholar]
- Kudrin A, Ray D. Cunning factor: macrophage migration inhibitory factor as a redox-regulated target. Immunol Cell Biol. 2008;86:232–238. doi: 10.1038/sj.icb.7100133. [DOI] [PubMed] [Google Scholar]
- Zimmerman M C, Lazartigues E, Lang J A, Sinnayah P, Ahmad I M, Spitz D R, Davisson R L. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhang Z H, Wei S G, Chu Y, Weiss R M, Heistad D D, Felder R B. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101:304–312. doi: 10.1161/CIRCRESAHA.107.148940. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chen S, Sonntag C F, Sumners C, Klein R L, King M A, Hughes J A, Meyer E M. Recombinant adeno-associated virus serotype 2 effectively transduces primary rat brain astrocytes and microglia. Brain Res Brain Res Protoc. 2004;14:18–24. doi: 10.1016/j.brainresprot.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Swanson L W, Sawchenko P E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Benarroch E E. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Walker B R. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]
- Stocker S D, Simmons J R, Stornetta R L, Toney G M, Guyenet P G. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventral lateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes B A S, Valentino R J, Xu G, Van Bockstaele E J. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko P E. Evidence for differential regulation of corticotropin-releasing factor and vasopressin immunoreactivities in parvocellular neurosecretory and autonomic-related projections of the paraventricular nucleus. Brain Res. 1987;437:253–263. doi: 10.1016/0006-8993(87)91641-6. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;2008 117:1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]
- Miller E J, Li J, Leng L, McDonald C, Atsumi T, Bucala R, Young L H. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]