Abstract
This study investigated the factors responsible for migration and homing of magnetically labeled AC133+ cells at the sites of active angiogenesis in tumor. AC133+ cells labeled with ferumoxide-protamine sulfate were mixed with either rat glioma or human melanoma cells and implanted in flank of nude mice. An MRI of the tumors including surrounding tissues was performed. Tumor sections were stained for Prussian blue (PB), platelet-derived growth factor (PDGF), hypoxia-inducible factor-1α (HIF-1α), stromal cell derived factor-1 (SDF-1), matrix metalloproteinase-2 (MMP-2), vascular endothelial growth factor (VEGF), and endothelial markers. Fresh snap-frozen strips from the central and peripheral parts of the tumor were collected for Western blotting. MRIs demonstrated hypointense regions at the periphery of the tumors where the PB+/AC133+ cells were positive for endothelial cells markers. At the sites of PB+/AC133+ cells, both HIF-1α and SDF-1 were strongly positive and PDGF and MMP-2 showed generalized expression in the tumor and surrounding tissues. There was no significant association of PB+/AC133+ cell localization and VEGF expression in tumor cells. Western blot demonstrated strong expression of the SDF-1, MMP-2, and PDGF at the peripheral parts of the tumors. HIF-1α was expressed at both the periphery and central parts of the tumor. This work demonstrates that magnetically labeled cells can be used as probes for MRI and histological identification of administered cells.—Arbab, A. S., Janic, B., Knight, R. A., Anderson, S. A., Pawelczyk, E., Rad, A. M., Read, E. J., Pandit, S. D., Frank, J. A. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings.
Keywords: MRI, magnetically labeled AC133+ cells, tumors, angiogenesis
Tumor growth and metastasis strongly depend on angiogenesis (1). Angiogenic factors, such as vascular endothelial growth factor (VEGF), that are released by tumor cells stimulate angiogenesis by promoting activation, proliferation, sprouting, and migration of endothelial cells to the tumor tissue and therefore allow for a rapid formation of new blood vessels (2,3,4,5). Endothelial cells that contribute to tumor neovasculatures can originate from sprouting and co-option of neighboring preexisting vessels (6,7,8). However, there is emerging evidence indicating that bone marrow derived endothelial progenitor cells (EPCs) also contribute to the vasculogenesis and growth of certain tumors (9,10,11,12,13). A subpopulation of CD34+ human hematopoietic stem cells identified by the cell-surface molecule CD133 (AC133) have been shown to be more specific for endothelial differentiation and angiogenesis (14,15,16,17). Higher expression of genes regulating angiogenesis was also observed in AC133+ cells (18). Additionally, our previously published results (19) showed migration and incorporation of intravenously administered CD34+/AC133+ cells into the xenoplanted tumors’ neovasculature. EPCs collected from bone marrow or peripheral or cord blood have been used in different animal tumor or ischemia models to determine if these cells have the capacity to become part of the neovasculatures or vasculogenesis in tissues (20,21,22,23). Although the role and the involvement of intravenously administered AC133+ cells in the formation of tumor neovasculature is controversial (12, 24, 25), there have been no studies depicting whether intratumor administration of AC133+ cells participate in tumor neovasculogenesis.
Tumor angiogenesis and vasculogenesis are processes that are strongly influenced by tumor microenvironmental factors such as tumor growth and size, hypoxic state, and expression levels of cytokines and chemokines, as well as the other tissue factors (26,27,28,29). Hypoxia that is pronounced the most within the central region of tumor tissue has been correlated with tumor angiogenesis. However, recent evidence (30, 31) has suggested that the development of neovasculatures at the periphery of the tumor can be induced from the surrounding tissues and tumor interface. Furthermore, AC133+ EPCs have been shown to contribute to vessel formation at the periphery and in the tumor core, both experimentally and clinically (24). Therefore, determining the geographical location of EPCs within the tumor may allow for the identification of extracellular and stromal components that orchestrate incorporation of EPCs and may provide a thorough understanding as to why certain tumors or tumors of a specific size only partially respond to antiangiogenic therapy. In addition, identification of tumor-specific extracellular matrix and a chemo/cytokine profile could provide for therapeutic targets and the ability to tailor the treatment specifically for each malignancy.
The capacity of EPCs or AC133+ stem cells to migrate and incorporate into the tumor neovasculatures is usually demonstrated by histopathology or immunohistochemistry (32, 33). Recently, it was demonstrated that an MRI could also monitor the migration of systemically administered magnetically labeled mammalian cells to different organs and tumors and at sites of active angiogenesis (19, 34,35,36). Ferumoxide is a Food and Drug Administration (FDA) -approved superparamagnetic iron oxide (SPIO) contrast agent that is used for hepatic MRI, and the iron oxide nanoparticles are able to saturate magnetically at low fields resulting in an extremely high T2 relaxation rate in solution. Protamine sulfate, another FDA-approved agent used for the treatment of heparin overdose, can be mixed with ferumoxide and used as an efficient and effective method for labeling any kind of mammalian cells (37). Cells labeled with a ferumoxide-protamine sulfate (FePro) complex can be imaged using T2- and T2*-weighted MRI techniques.
The objectives of this study were to determine whether cellular MRI can detect the migration of magnetically labeled AC133+ cells in relation to tumor growth when coimplanted with tumor cells, and whether the migration of AC133+ cells is related to the expression of angiogenic and other tumor or surrounding tissue microenvironmental factors. We hypothesize that if hypoxia is the sole factor for stimulating angiogenesis, the coimplanted AC133+ cells will remain at the center of the tumor to support neovascularization. Alternatively, if angiogenic factors from within the growing tumor or at the tumor-tissue interface are expressed in sufficient amount, AC133+ cells will migrate toward those areas. Visualizing implanted AC133+ cell differential migration by MRI at different stages of tumor growth or development can provide insight into some of the molecular mechanisms involved in in vivo tumor angiogenesis.
MATERIALS AND METHODS
Preparation of AC133+ cells
CD133+ (AC133+) cells, a subpopulation of human CD34+ hematopoietic stem cells, were obtained from normal volunteers entered into Intramural Review Board (IRB) -approved protocols by leukopheresis techniques. The peripheral blood progenitor cell product was enriched for AC133+ cells by immunomagnetic selection with the CliniMacs System (Miltenyi, Auburn, CA, USA). AC133+ cells were also collected from cord blood with an IRB-approved protocol using MidiMACS system (Miltenyi). Freshly prepared or cryopreserved/thawed AC133+ cells were incubated in either Stemspan medium (StemCell Technologies, Vancouver, BC, Canada) or Stemline II medium (Sigma, St. Louis, MO, USA) containing 40 ng/ml stem cell factor, 40 ng/ml FLT3, and 10 ng/ml thrombopoietin (all from CellGenix, Antioch, IL, USA) and cultured for at least 5 days, with the cell concentration kept at 1 × 106/ml by addition of fresh medium on alternate days. The cells were periodically analyzed by flow cytometry to assess the expression of cell lineage-specific surface markers. On the day of cell labeling, cell concentration in the culture was determined and all cells were centrifuged, with the supernatant retained for future use (see below). Labeled cells were analyzed for labeling efficiency, viability, and phenotypic markers, as described previously (37). Phenotypical markers were analyzed from at least three different cultures of cells, and the following markers were determined: CD34, CD133, CD31, CXCR4, CD19, CD3, CD14, and CD45 (see Supplemental Materials).
Preparation of tumor cells
Rat glioma cells (C-6, CLL-107; American Type Culture Collection, Manassas, VA, USA) and human amelanotic melanoma cells (A375; American Type Culture Collection) were used to generate tumors in nude mice. Both tumor cell lines were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. On the day of implantation, tumor cells were washed and resuspended at 1 × 106 cells (for rat glioma) and 3 × 106 cells (for human melanoma) per 50 μl and mixed with either iron oxide-labeled or -unlabeled 1 × 105 to 1 × 106 (for glioma) and 3 × 106 (for melanoma) AC133+ cells per 50 μl. Higher numbers of labeled AC133+ cells were used in the melanoma compared with the glioma model because of the slower growth of melanoma.
Animal model
All animal experiments were performed according to the protocol approved by our animal care and use committee at the National Institutes of Health and Henry Ford Health System. We have used two different tumor cell lines (C-6: rat glioma; and A375: human melanoma) to subcutaneously implant the tumor into the flanks of 6- to 8-wk-old female BALB/c nude mice (Charles River Laboratories, Wilmington, MA, USA). Two cell lines were used to determine the reproducibility of our findings in two different tumor conditions. For rat glioma, 1 × 106 cells in 50 μl of medium were implanted subcutaneously into the flanks. For melanoma, 3 × 106 cells in 50 μl were used. There were 48 mice included in this study. Of the 48, 14 mice were inoculated with glioma and live magnetically labeled AC133+ cells; 9 mice received glioma and live unlabeled AC133+ cells; 16 mice received melanoma and live magnetically labeled AC133+ cells; and 9 mice received melanoma and dead magnetically labeled AC133+ cells. For every condition, there were at least 3 animals in each group (but there were at least 4 mice for magnetically labeled live cells) and based on MRI availability, at least 2–3 animals from each group underwent in vivo MRI at different stages of tumor growth (0.5, 1.0, and 1.5 cm in size). Most of the implanted glioma exhibited fast and steady growth, reaching the size of 0.5 cm by 7–10 days, 1 cm by 14–17 days, and 1.5 cm by 21–24 days. Melanoma was slow in growth. Most of the implanted melanoma grew to 1 cm by 21 days after implantation. Instead of specific time points, we scanned the animals at specific stages of tumor growth, which was based on our previous research (19), in which we noted more migration of stem cells toward the sites of active angiogenesis when tumors grew to 1 cm in size. Tumors were measured on two dimensions every alternate day by a veterinary technician, and when the tumors attained the desirable size in any dimension, animals underwent the MRI. Soon after the MRI, animals were perfused and the tumors were harvested for further study or ex vivo MRI.
Preparation of FePro complex and AC133+ cell labeling
Commercially available ferumoxide suspension (Feridex IV, Berlex Laboratories, Wayne, NJ, USA) was used. Preservative-free protamine sulfate (10 mg/ml; American Pharmaceuticals Partner Inc., Schaumburg, IL, USA) was prepared as a fresh stock solution of 1 mg/ml in distilled water at the time of use. FePro preparation and cell labeling were performed as we described previously (37). The final concentration of ferumoxide and protamine sulfate was 50 and 2.25 μg/ml, respectively. After FePro labeling, cells were labeled with fluorescent dye DiI (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s protocol, washed, and resuspended with tumor cells to achieve a concentration of 1 × 105 to 3 × 106 AC133+ cells in 50 μl. For the C-6 glioma tumor model, the ratio of tumor cells to AC133+ cells ranged from 10:1 to 1:1, and for the melanoma model the ratio was kept at 1:1. To determine whether dead AC133+ cells would migrate along with tumor cells, labeled AC133+ cells were incubated with sterile 3% paraformaldehyde for 10 min and washed repeatedly with sterile PBS and resuspended with tumor cells (i.e., 3×106 dead AC133+ cells mixed with 3×106 melanoma cells). Unlabeled AC133+ cells were also mixed with tumor cells and implanted to determine whether unlabeled cells would contribute to any changes in signal intensity on T2*-weighted images on cellular MRI.
In vivo MRI protocol
Mice were imaged with a 7-T MR microimaging system (Bruker, Billerica, MA, USA) with 2.0- to 3.9-mT/cm gradients, using either a Bruker birdcage volume or surface coil located over the tumor. Mice were anesthetized with 1.5–2% isofluorane or halothane by nosecone and were physiologically monitored. A 5-mm water phantom was placed under the tumor-bearing leg, and axial images over the flank were acquired with a 2.6-cm2 field of view (FOV) and 0.5-mm slice thickness. An MRI was performed using a 2-dimensional, T2*-weighted gradient echo pulse sequence [TR/TE=500/4.3 ms, number of excitations (NEX)=8; 384×384 matrix zero-filled to 512×512; 70×70×500 μm resolution] or a 3-dimensional T2*-weighted gradient echo sequence (TR/TE=30/10 ms; 128×128×128 matrix; 20×20×20 mm3 FOV; NEX=4).
Ex vivo MRI protocol
Mice were euthanized at selected time points for ex vivo MRI followed by histological analysis of the tumors. The mice were deeply sedated using CO2 inhalation and were euthanized by cervical dislocation. Soon after cervical dislocation, the chest was opened quickly; a hole was made in the right auricle and perfused through the left ventricle with PBS followed by 4% paraformaldehyde (PFA). Immediately after, tumors with surrounding tissues were removed and placed into 4% PFA and 3% sucrose. Tumors and surrounding tissue were imaged in perfluoroether (Fomblin, Ausimont USA, Thorofare, NJ, USA) using a 7-T MR microimaging system, (Bruker). T2*-weighted, 3-dimensional gradient echo sequence (TR/TE=280/5.3 ms) or fast gradient echo sequence (TR/TE=14–18/3.5 ms), at 60- to 90-μm isotropic resolution were performed. FOV ranged from 2.0 to 2.7 cm in plane and 3.2 to 3.7 cm in the read dimension for multiple tumors imaged simultaneously. The minimum NEX was 16. The size of the matrix varied based on the total tumor volume to adjust isotropic resolution.
Implanted AC133+ cells in tumor neovasculatures
To determine whether locally administered AC133+ cells mixed with tumor cells would incorporate into tumor vasculogenesis, frozen tumor sections (at 10–20 μm) were prepared, washed with PBS, and incubated overnight with fluorescein isothiocyanate (FITC) -labeled tomato lectin (1:100 dilution, Vector Laboratory, Burlingame, CA, USA). Slides were washed, counterstained with DAPI, and analyzed under a fluorescent microscope (BX40; Olympus, Tokyo, Japan). Although fluorescently labeled lectin was used intravenously in our previous study (19) to delineate the endothelial lining of functioning neovasculatures, intravenously administered lectin would not delineate endothelial cells that had not formed functioning neovasculatures. Our ex vivo technique delineates endothelial lining in both functioning and nonfunctioning neovasculatures. Consecutive sections were stained with Perl’s reagent for iron-positive cells at the corresponding sites of detected DiI-positive cells. Frozen sections were also stained for CD31 with FITC-conjugated secondary antibody.
Immunohistochemistry and Prussian blue stain
After ex vivo MRI, tumors and surrounding tissues were divided in half for the preparation of the frozen sections and paraformaldehyde fixation with embedding in paraffin. Sections (6–10 μm) were cut from representative tumors (at least 2) from each experimental setting. Consecutive slides from the frozen or paraffin block sections were evaluated by immunohistochemical staining for the expression of endothelial cell markers using anti-human CD31, von Willebrand Factor (vWF; all from Novocastra, Newcastle-upon-Tyne, UK), and VE-cadherin (Chemicon International, Temecula, CA, USA). Tumor sections were also stained for different angiogenic factors using either anti-human or anti-rat vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), matrix metalloproteinase-2 (MMP-2), hypoxia-inducible factor-1α (HIF-1α), and stromal cell derived factor-1 (SDF-1). All antibodies were bought from Lab Vision Corporation (Fremont, CA, USA) except SDF-1, which was obtained from R&D Systems (Minneapolis, MN, USA). Either the same or consecutive sections were stained with Perl’s reagent for iron detection (Prussian blue staining), according to our previously described method (37). From a single paraffin block, 10–20 consecutive sections were obtained. More than 30 sections were obtained from the frozen block (whole) of the tumor. Ten to twelve sections were obtained at 200 μm intervals. Randomly selected consecutive slides were stained for different factors. To determine whether the migrated (to the peripheral part of tumors) iron-positive cells were indeed of human origin or whether host macrophages had phagocytosed some of the iron-positive cells or released SPIO nanoparticles from human labeled cells, resulting in a low signal intensity on MRI, consecutive sections were stained with a human specific anti-mitochondrial antibody (Chemicon) and anti-mouse F4/80 antibody (specific for mouse macrophages; Serotec, Raleigh, NC, USA). Consecutive sections were also used as negative control in which the primary antibody was omitted, but all other procedures were performed in an identical manner. Immunohistochemistry was performed with standard procedures using horseradish peroxidase (HRP) -tagged secondary antibodies. After being stained, all sections were imaged with a microscope at ×50, and 20–30 randomly selected areas were captured where the most abundant positive cells were observed. Then, all the slides were submerged in xylene to remove the cover slips, and the sections were restained for iron by using standard Prussian blue staining procedures and cover slipped. The sections were scanned under a microscope to determine the exact locations of human mitochondrial and F4/80-positive cells and photomicrographed to show the colocalization of double-positive cells.
Western blotting
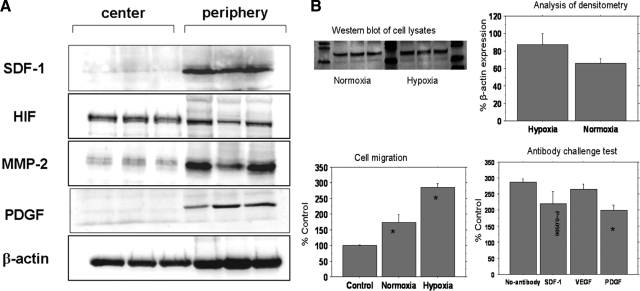
To confirm the immunohistochemistry findings and to mimic core biopsy techniques, separate sets (3 animals at each time point) of animals bearing subcutaneous gliomas were euthanized at tumor sizes of 1 and 1.5 cm. Tumors were snap-frozen, and 2-mm midsections were selected for further cutting into 5 small pieces (1 central and 4 corner; Fig. 1). Collected tissues were mechanically pulverized over dry ice, and total protein (30–80 mg of the frozen tissue powder per sample) was extracted using ProteoExtract Complete Mammalian Proteome Extraction Kit (Calbiochem, San Diego, CA, USA), according to the manufacturer instructions. Protein concentration in recovered protein extracts was determined with Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Hercules, CA, USA), using BSA as a standard. Forty micrograms of total protein was resolved by electrophoresis using 8–12% SDS-polyacrylamide gels, transferred to the nitrocellulose membrane (Bio-Rad), and probed with specific primary antibodies: mouse monoclonal IgGs against HIF-1α, SDF-1, β-actin (all from Chemicon), MMP-2, and rabbit polyclonal IgG against PDGF (from Lab Vision). Primary antibody binding was detected by incubating the membrane in the presence of HRP-conjugated secondary polyclonal goat antibody, and the signal was detected by enhanced chemiluminescence using the ECL Detection Kit (Amersham Biosciences, Piscataway, NJ, USA).
Figure 1.
Schematic representation of tumor sections used to extract total protein for Western blot analysis of expression of different angiogenic and chemoattractant factors in the tumor and the surrounding tissues. At first, 2-mm-thick sections from the center of the tumors (1.5 cm in size) were excised (left), and these sections were further cut into 5 separate pieces, as depicted at right. After a 2-mm portion of the tumor tissues was cut from all sides (marked as peripheral part), the leftover tumor tissues were considered the center part of the tumor. C, central; P, peripheral.
In vitro hypoxia and migration studies
To determine the effect of hypoxia, SDF-1, VEGF, and PDGF on the migration of AC133+ cells, we created an in vitro model to produce hypoxic-state glioma cells. C-6 cells (1×106) were plated in tissue culture flasks (T25 cm2) and allowed to grow to 80–90% confluence inside the normoxic (∼20 oxygen) incubator. To generate hypoxic conditions, cells were washed twice, replenished with 5 ml of serum-free growth medium, and incubated inside the hypoxic incubator (<2% oxygen) for 7 h, after which supernatant and cells were collected separately. Similarly, control cells were incubated in the normoxic incubator, and both cells and supernatant were collected separately. Whole-cell lysates were generated from the collected cells, and Western blot analysis was performed to determine the expression of HIF-1α. Briefly, cell pellets were lysed with CelLytic-M (Sigma), and protein concentration was measured using the BCA Protein Assay (Pierce, Rockford, IL, USA). Seventy micrograms of total protein was resolved on 10% SDS-PAGE gel, transferred to nitrocellulose membrane, and detected using mouse anti-HIF-1α (1:100; Serotec) and rabbit-anti- hβ-actin (1:1000; Sigma) primary antibodies, followed with HRP-conjugated (1:2000) secondary antibodies. Bands were visualized by chemiluminescence (Amerscham Biosciences), quantified by spot densitometry using Kodak Image Station 1000 (Eastman Kodak Company, Rochester, NY, USA), and normalized to β-actin.
Supernatants from both normoxic and hypoxic states were used to determine their effect on stem cells migration with or without the presence of neutralizing anti SDF-1, VEGF, and PDGF antibodies (1 μg/ml concentration mixed with supernatant). Cell migration studies were performed using QCM Chemotaxis 3-μm 96-well Cell Migration Assay Kit (Chemicon) according to the supplier’s experimental protocol. Each sample included 1 × 105 cells, and migration was detected as fluorescence that was read using a 520 filter set. The studies were repeated to determine reproducibility.
Statistical analysis
All data are expressed as mean ± se. Analysis for significant differences was performed with 1-way ANOVA. A value of P < 0.05 was considered significant.
RESULTS
Viability, proliferation, and cell surface marker expression of FePro-labeled AC133+ cells
After culture for at least 5 days, flow cytometric analysis of the AC133+ cells showed preservation of CD34, CD133 markers. Cells were not remarkably positive for other lineages (lin−). There were no changes in the phenotypical expression after the labeling of cell with FePro. FePro-labeled cells showed similar proliferation compared with that of the control on MTT assay (see Supplemental Materials).
MRI of tumors
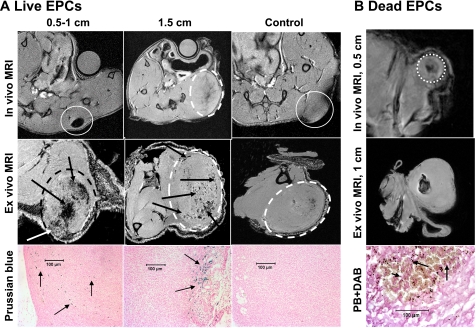
Both in vivo and ex vivo MRI showed hypointensity areas due to T2* shortening of the FePro-labeled cells in the central as well as peripheral part of the tumors (Fig. 2). Hypointense regions were observed mostly at the periphery of the implanted glioma and in the melanoma tumors that were >1 cm in size. Tumor cells mixed with unlabeled cells did not show any hypointense regions within the small tumors (0.5 or 1 cm). However, small focal hypointense regions were observed in larger tumors that could be due to areas of hemorrhage or slow blood flow (Fig. 2A, control, ex vivo). The distributions of these focal hypointense regions were not similar to the ones seen in similar sized tumors that were transplanted with viable FePro-labeled AC133+ cells (see Fig. 2A, tumor at 0.5 and 1.5 cm). These localized low signal intensity areas were seen in both tumors types at larger tumor sizes. When tumors were mixed with dead FePro-labeled AC133+ cells, hypointense regions within the center of the tumor were observed. There were no hypointense regions observed at the periphery of the tumor on in vivo and ex vivo MRI (Fig. 2B). Figure 3 shows MRI and Prussian blue staining images of a 1-cm melanoma that received FePro-labeled live AC133+ cells at the time of tumor inoculation. Prussian blue positive human cells were detected at the tumor periphery and in the deeper parts of the tumor in the animals that received viable labeled AC133+ cells. The Prussian blue positive cells exhibited endothelial cell morphology (i.e., elongated shape) and were incorporated into ongoing vasculogenesis within the tumor (Fig. 3). In animals with tumors that were coimplanted with dead FePro-labeled AC133+ cells, the MRI demonstrated a focal central area of hypointensity that corresponded to the areas of Prussian blue positive cells that were round and smaller in size as compared with the cells observed in tumors coimplanted with viable labeled AC133+ cells (Fig. 2).
Figure 2.
In vivo and ex vivo MRI images of implanted tumors mixed with either live FePro-labeled and unlabeled (control) AC133+ cells or labeled dead AC133+ cells. A) In vivo and ex vivo MRI images of implanted rat glioma mixed with live FePro-labeled and unlabeled (control) AC133+ cells. Note the loss of central low signal intensity (due to large number of labeled cells) in tumor measuring 0.5–1 cm compared with that measuring 1.5 cm (arrows on ex vivo images). These low signal intensities are different from the low signal intensity seen in control tumor (ex vivo). Corresponding DAB-enhanced Prussian blue (PB) staining shows iron-positive cells both in the central and peripheral part in the small tumor but in the peripheral portion only in the 1.5-cm tumor, with no iron-positive cells in the tumor injected with unlabeled cells. White circles indicate tumors on in vivo and ex vivo MRI. B) In vivo and ex vivo MRI images of implanted human melanoma mixed with magnetically labeled dead AC133+ cells at a tumor size of 0.5 and 1 cm. Note the localized low signal intensity in both tumors at sizes of 0.5 and 1 cm. Corresponding DAB-enhanced Prussian blue staining shows iron particles scattered in the central part of the tumor. Note the morphology of dead iron-positive cells (round, arrows), which is different from that of live cells (elongated; see Fig. 3). White circle indicates tumor on in vivo MRI.
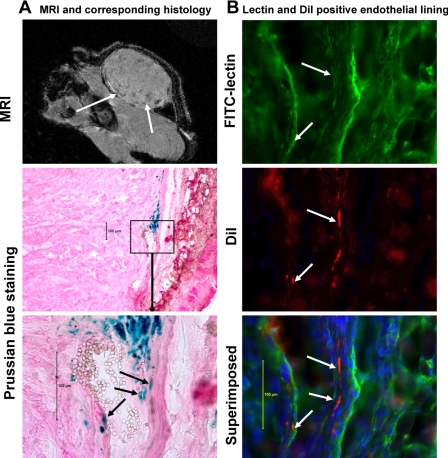
Figure 3.
Migration and incorporation of locally administered magnetically labeled AC133+ cells in human melanoma tumor in mouse. A) Gradient echo MRI shows low signal intensity areas mostly at the periphery of the tumors (white arrows). Representative histology sections show incorporation of Prussian blue-positive cells (staining performed after fluorescent microscopic images were obtained; see below) along the margin of a blood vessel (note the red blood cells within the lumen). B) To prove whether implanted live FePro-labeled AC133+ cells incorporated in the formation of tumor angiogenesis (vasculature), tumor vasculature (endothelial lining) was delineated by FITC-labeled tomato lectin, and fluorescent microscopic images were obtained both at FITC (lectin) and rhodamine (DiI-labeled cells) channels. The nucleus of the cells was delineated by DAPI staining. After fluorescent microscopy, the same section was stained for Prussian blue (for iron-positive cells) and bright-field microscopic images were obtained to match the area of fluorescent positive cells. Fluorescent microscopic images show incorporation of locally implanted DiI-labeled AC133+ cells (white arrows) into the tumor vasculatures. Arrows indicate lectin-positive endothelial lining and DiI-positive AC133+ cells. Note the corresponding Prussian blue-stained image indicating the presence of iron and DiI dye in the same cells.
Labeled AC133+ cells and formation of blood vessels
To determine whether locally implanted AC133+ cells mixed with tumor cells incorporated into the tumor neovasculatures, AC133+ cells were double labeled with FePro and fluorescent dye DiI, mixed with melanoma cells and implanted into the flanks of mice. Tumors at 1 cm in size were collected, and frozen sections were made to identify the endothelial lining of blood vessels using FITC-labeled tomato lectin. Figure 3 shows the incorporation of DiI-positive cells into the endothelial lining of blood vessels within the tumor. Prussian blue staining of consecutive sections also demonstrated the incorporation of iron-positive cells into the endothelial lining of the tumor blood vessels. To determine whether these DiI-positive cells incorporated into endothelial lining expressed endothelial cell markers, frozen sections were stained for the presence of CD31 molecules. DiI-positive cells that incorporated into the endothelial lining also exhibited CD31 expression (Fig. 4A). Prussian blue staining of the consecutive section showed the presence of multiple iron-positive cells (Fig. 4A).
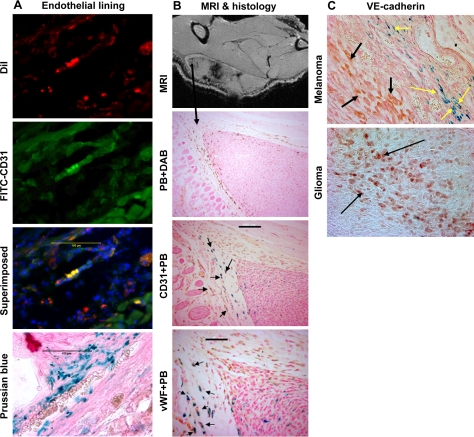
Figure 4.
Immunohistochemistry showing endothelial markers in administered cells. A) Immunohistochemistry of randomly selected melanoma frozen sections show expression of human CD31 in the implanted tumor. DiI-positive cells (AC133+ cells, red), human CD31+ cells stained with FITC conjugated secondary antibody (green), and combined superimposed image of the section indicating DiI-positive cells transform into endothelial cells (yellow). Prussian blue staining of the consecutive section shows multiple iron-positive cells at the corresponding sites. Note the red blood cells within the lumen on Prussian blue section. Scale bars = 100 μm. B) Immunohistochemistry of randomly selected rat glioma paraffin sections shows expression of both human CD31 and vWF in the implanted tumor. Ex vivo MRI shows low signal intensity areas both in the center and at the periphery of a 1-cm tumor. DAB-enhanced Prussian blue staining shows migration of labeled AC133+ cells at the periphery of the tumor. Double staining, CD31 (brown) followed by Prussian blue (blue) of the consecutive section shows double-positive cells (black arrows). Double staining, vWF (brown) followed by Prussian blue (blue) of the consecutive section shows double-positive cells (black arrows). Expression of both vWF and CD31 in iron-positive cells indicates the maturation of AC133+ cells into endothelial cells. DAB-enhanced Prussian blue image is ×10. Scale bars = 50 μm (vWF+ PB); 100 μm (CD31+ PB). C) Immunohistochemistry of randomly selected human melanoma and rat glioma paraffin sections shows expression of VE-cadherin in the tumor cells as well as in Prussian blue-positive cells. Double staining, VE-cadherin (brown) followed by Prussian blue of a section from implanted human melanoma mixed with FePro-labeled AC133+ cells. Yellow arrows show VE-cadherin- and Prussian blue-positive cells that are placed along the lining of vessels. Black arrows show VE-cadherin-positive melanoma cells. VE-cadherin staining of glioma shows numerous positive tumor cells (dark brown, black arrows).
Endothelial cell marker expression
To determine whether implanted AC133+ cells express endothelial markers, glioma sections were subjected to immunohistochemistry for the presence of CD31, VE-cadherin and vWF. In glioma, implanted AC133+ cells showed positive for CD31 and vWF markers, indicating AC133+ cell transformation into the functional and mature endothelial cells (Fig. 4B). Double staining with Prussian blue also showed both iron and endothelial marker positive cells at the sites of low signal intensity areas seen on the MRI (Fig. 4B). Multiple VE-cadherin and Prussian blue positive cells were seen at the corresponding sites of implanted AC133+ cell migration and incorporation (Fig. 4C). Both malignant melanoma and glioma cells also showed positive for VE-cadherin expression (Fig. 4C).
Expression of angiogenic factors and migration of labeled AC133+ cells
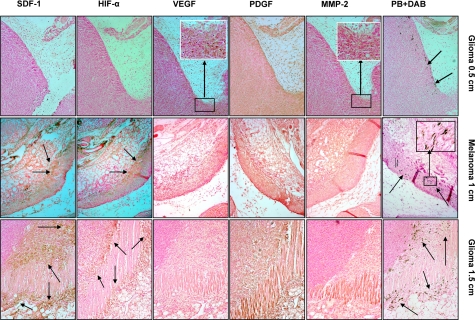
To identify whether migration of locally implanted labeled AC133+ cells is related to the expression of angiogenic or other factors within the tumor or tumor surrounding tissue, consecutive slides of implanted rat glioma tumor were subjected to immunohistochemical and Prussian blue staining (Fig. 5). Tumors at all stages of growth demonstrated generalized PDGF expression within the tumor and adjacent surrounding tissues. Figure 5 (left column) shows the expression of different factors and Prussian blue staining in rat glioma at 0.5 cm in size. There was no remarkable expression of HIF-1α and SDF-1 observed at the sites of labeled AC133+ cell migration (arrows in Prussian blue positive DAB), although the expression of these two factors was observed in other parts of the tumor (data not shown). A few tumor cells (glioma) expressed MMP-2 (inset, brown cells) and VEGF (inset, brown cells) at the corresponding sites of labeled AC133+ cells. Migration of labeled AC133+ cells was related to the expression of HIF-1α and SDF-1 in large (>1.0 cm) tumors (melanoma, middle column; glioma, right column). Localized areas of expression of HIF-1α and SDF-1 were observed at the sites of tumor invasion into the surrounding muscles or tissues, where migration of labeled AC133+ cells was prominent (Prussian blue positive DAB, arrows). Neither MMP-2 nor VEGF colocalized with migrated labeled EPC. PDGF expression was also not localized at the sites of AC133+ cell migration.
Figure 5.
Expression of different angiogenic and chemoattractant factors at the sites of migrated labeled AC133+ cells in tumors. Top row: expression of different angiogenic and chemoattractant factors at the sites of migrated labeled AC133+ cells in rat glioma at a tumor size of 0.5 cm (consecutive sections). Prussian blue staining shows migration of cells at the periphery of the tumor (arrows). MMP-2 and VEGF staining show expression of these factors at the corresponding sites of cell migration (inset, brown cells). PDGF staining shows generalized expression both in the tumor and adjacent surrounding tissues. HIF-1α and SDF-1 staining do not show expression of the factors at the corresponding sites of migrated cells. Middle row: expression of different angiogenic and chemoattractant factors at the sites of migrated labeled AC133+ cells in human melanoma at a tumor size of 1 cm (consecutive sections). Prussian blue staining shows migration of cells at the periphery of the tumor and at the sites of invasion into surrounding muscles and tissues (arrows). MMP-2 staining shows mild expression of MMP-2 throughout the tumor and surrounding tissues. PDGF staining also shows generalized expression both in the tumor and adjacent surrounding tissues. VEGF staining shows no localized expression of these factors at the corresponding sites of cell migration. HIF-1α and SDF-1 staining show very strong localized expression of the factors at the corresponding sites of migrated cells (arrows). Inset: multiple DAB enhanced Prussian blue positive cells. Bottom row: Expression of different angiogenic and chemoattractant factors at the sites of migrated labeled AC133+ cells in rat glioma at a tumor size of 1.5 cm (consecutive sections). Prussian blue staining shows migration of cells at the periphery of the tumor and at the sites of invasion into surrounding muscles and tissues (arrows). MMP-2 staining shows mild expression of MMP-2 throughout the tumor and surrounding tissues. PDGF staining shows generalized expression both in the tumor and adjacent surrounding tissues. VEGF staining shows no localized expression of these factors at the corresponding sites of cell migration. HIF-1α and SDF-1 staining show very strong localized expression of the factors at the corresponding sites of migrated cells (arrows). All images ×10.
Identification of human cells and host macrophages
To identify whether the iron-positive cells were indeed the administered human cells, sections were stained with human specific anti-mitochondrial antibody followed by Prussian blue staining. Consecutive sections were also stained for mouse specific macrophage marker (anti-mouse F4/80 antigens) followed by Prussian blue to identify whether host macrophages contained iron. Figure 6 shows multiple human mitochondria-positive cells (brown) around blood vessels at the peripheral part of the tumor (2 randomly selected regions), and these cells also show iron on Prussian blue staining. Figure 7 shows sections stained with anti F4/80 antibodies and negative control of the same area from consecutive sections. There were multiple F4/80-positive cells observed throughout the tumors, and localized accumulated F4/80-positive cells are shown here (Fig. 7A). None of the cells show iron on the Prussian blue-stained section (Fig. 7B). Negative control images (Fig. 7C, D) from two randomly selected areas show no definite brown cells indicative of nonspecific staining.
Figure 6.
Proof that human cells contain SPIO nanoparticles. To determine whether the migrated (to the peripheral part of tumors) iron-positive cells are indeed of human origin, sections were stained with human specific anti mitochondrial antibody. After staining, all sections were imaged with a microscope at ×50, and 20–30 randomly selected areas were captured where most abundant positive cells were observed. All slides were then submerged in xylene to remove the cover slips, the sections were restained for iron by using standard Prussian blue staining procedures, and cover slips were emplaced. The sections were scanned under a microscope to determine the exact locations of human mitochondrial positive cells and photomicrographed to show the colocalization of double-positive cells. Randomly selected areas show abundant cells carrying human mitochondria (brown cells and arrows; A, C). The same areas show multiple iron-positive cells (B, D). Note the exact match of iron- and human mitochondria-positive cells (arrows; A–D). A few representative cells are indicated by the arrows. Scale bars = 50 μm.
Figure 7.
Host macrophages and iron-positive cells. To determine whether host macrophages phagocytose some iron-positive cells or released SPIO nanoparticles from labeled human cells, consecutive sections were stained with anti-mouse F4/80 antibody (specific for mouse macrophages), photomicrographed, and stained for iron. Consecutive sections were also used as negative control where primary antibody was omitted, but all other procedures were identical. Multiple localized F4/80-positive cells (brown cells) are seen (A), which show no iron on Prussian blue staining (B). Images from negative control section show no brown cells (C, D). Scale bars = 50 μm.
Western blot analysis of the expression of angiogenic factors
To confirm the findings of immunohistochemistry analysis and to mimic core biopsy techniques, whole protein extracted from large tumors (>1.0 cm) was analyzed by Western blotting (Fig. 8A). Specific antibodies against SDF-1, PDGF, and MMP-2 detected high expression levels in the peripheral parts of the tumor. Antibodies against HIF-1α detected greater level of expression in the center compared with the periphery of the tumor. The housekeeping protein β-actin was used as a control for loading.
Figure 8.
Western blot analysis of hypoxic cell lysate and cell migration studies. A) Western blot analysis of expression of different angiogenic and chemoattractant factors in whole-tissue lysates of implanted rat glioma tumors (samples from 3 different tumors). Forty micrograms of total tumor tissue protein was resolved by electrophoresis using 8–12% SDS-polyacrylamide gels. On transfer to the nitrocellulose membrane, proteins were detected by immunobloting, using specific antibodies against proteins of interest. Immunoblotting using specific antibodies against SDF-1, PDGF, and MMP-2, respectively, reveals high expression levels in the periphery of the tumor. Antibodies against HIF-1α reveal HIF-1α expression in the center and the periphery of the tumor, with higher levels of expression within the center of the tumor. Housekeeping protein β-actin was used as a control for loading. B) Effects of hypoxic condition on stem cell migration and HIF-1α expression. Top left: Western blots of whole-cell lysates show the expression of HIF-1α in tumor cells cultured under normoxic and hypoxic conditions (triplicate samples). Top right: analysis of densitometry of HIF-1α immunoblots, expressed as percentage of β-actin expression, shows higher HIF-1α expression in the hypoxic condition. Bottom left: cell migration graph compares migration of AC133+ cells toward the supernatants collected from normoxic and hypoxic tumor cells compared with control condition (*P<0.05). Cell migration to the hypoxic supernatant is higher than that to the normoxic supernatant (P<0.01). Bottom right: antibody challenge test compares migration of AC133+ cells toward the supernatants collected from normoxic and hypoxic tumor cells with and without 1 μg/ml of neutralizing anti-SDF-1, anti-VEGF, and anti-PDGF antibodies. A significant decrease in migration of cells is observed in wells treated with anti-PDGF (*P<0.01), and anti-SDF-1 antibody also inhibits cell migration.
In vitro hypoxic and migration studies
Tumor cells cultured under the hypoxic conditions expressed higher levels of HIF-1α than the cells grown in normoxic conditions (Fig. 8B). Supernatants from both normoxic and hypoxic cells induced directional migration of stem cells. However, cell migration in response to the hypoxic supernatant was significantly higher than that observed in response to control stem cell medium and normoxic supernatant (Fig. 8B). When antibodies against SDF-1, VEGF, and PDGF were added separately, there was a significant decrease in the migration of cells in chambers that contained anti-PDGF antibody. There was also a borderline significant decrease in chambers that contained anti-SDF-1 antibody (P=0.0506). Addition of anti-VEGF antibodies did not alter the migration of cells. These findings indicate that PDGF and SDF-1 play an important role as chemoattractants within the tumor microenvironment.
DISCUSSION
This study demonstrates that migration of locally implanted magnetically labeled AC133+ cells to the periphery of the tumor at the site of active angiogenesis can be monitored and tracked by cellular MRI. Previously, cellular MRI showed the incorporation of intravenously administered SPIO-labeled Sca1+ and AC133+ cells into ongoing tumor vasculogenesis in implanted intracranial glioma or flank tumors (19, 36). Current results are consistent with our earlier study (19) that used confocal microscopy and immunohistochemistry demonstrating the contribution of endothelial precursor cells in tumor neovasculature. Interestingly, the present experiments show that the coimplantation of AC133+ cells mixed with tumor cells resulted in the presence of AC133+ cells at the periphery of the tumor, as demonstrated by MRI and histology. If the two populations of cells (AC133+ cells and tumor) are completely mixed when implanted in the flank of the animal, one would expect a homogeneous distribution of the human AC133+ cells throughout the tumor mass, assuming that all cancer cells secrete cytokines equally. During the tumor growth in melanoma and glioma cells, the migration of FePro-labeled AC133+ cells from the center to the periphery of the tumor was detected by MRI. However, not all of the AC133+ cells migrated to the tumor periphery, possibly due to cell death or other factors that were not detected by immunohistochemistry analysis, such as an increase in tumor interstitial pressure. It is important to note that not all iron-positive cells that migrated to the peripheral part of the tumors incorporated into the ongoing neovascularization. However, the cells that were positive for iron by Prussian blue stain were also positive for human specific marker (anti-mitochondria). Similar to our previously reported results (19), the host macrophages (F4/80-positive cells) accumulated at the periphery of the flank tumors and these cells were not positive for iron by Prussian blue staining. These findings indicate that the Prussian blue positive cells that migrated to the tumor periphery were viable cells and did not release iron oxide nanoparticles that could be phagocytosed by the macrophages. Moreover, the low signal intensity areas at the periphery of the tumors seen on MRI are due to migrated and viable cells incorporating into the neovasculature.
Although some groups reported correlation between tumor hypoxia and angiogenesis, recent studies have shown that ongoing development of the neovasculature in the periphery of the tumor, usually at the tumor-tissue interfaces, is driven by hypoxia as well as the other factors (30, 31). We hypothesized that both tumor hypoxia and other microenvironmental tissue factors released from tumors and surrounding tissues may be important for differential migration and homing of AC133+ cells to the tumor periphery. To address this issue, we have implanted tumor cells mixed with either live or dead double-labeled (FePro+DiI) AC133+ cells in the flanks of nude mice. As tumor cells proliferated to form a coherent mass, initial co-opting of local vessels followed by local centralized tumor hypoxia and release of angiogenic factor to stimulate the grow of the neovasculature was expected. Cellular MRI demonstrated the migration of live AC133+ cells toward periphery of the tumor, indicating that the AC133+ cell trafficking to the periphery of the tumors was influenced by the cytokines produced by tumor or surrounding tissue.
Tumor angiogenesis is a very complex process, and different factors, such as HIF-1α, VEGF, MMPs, PDGF, bovine fibroblast growth factor, and others, are considered to be important for tumor neoangiogenesis. Hypoxia-inducible transcription factors trigger a coordinated angiogenic and arteriogenic response by inducing expression of VEGF, VEGF receptor 1, VEGF receptor 2, PDGF-BB, angiopoietin 2, nitric oxide synthase, TGF-β1, and others (38, 39). In patients with tumors and stroke, the level of serum VEGF was increased and the number of circulating EPCs correlated with the level of circulating VEGF (40, 41). VEGF is important in initiating the mobilization of bone marrow-derived EPCs by acting on the VEGFR2. However, the role of VEGF in the migration and homing of EPCs in tumor angiogenesis is not clearly understood. In the current study, an MRI was performed at different time points of tumor growth, and histology sections were obtained to determine the expression of the markers known to be responsible for angiogenesis (such as HIF-1α, VEGF, MMPs, PDGF, and SDF-1) in the tumor and surrounding tissues. Independent of tumor type, serial histological sections were stained for PDGF, HIF-1α, SDF-1, MMP-2, and VEGF, in comparison with adjacent tissue sections stained with Prussian blue. These sections exhibited strong expression of SDF-1 and HIF-1α at the sites where labeled AC133+ cells were present. Expression of PDGF and MMP-2 was also observed; however, VEGF expression was not prominent. The role of VEGF in the migration and homing of AC133+ cells toward the tumor periphery at sites of ongoing angiogenesis is unclear. Although weak expression of VEGF was observed in small tumors (i.e., 0.5 cm) in areas where the implanted AC133+ cells were detected, other parts of the tumor where labeled AC133+ cells were not observed by Prussian blue staining demonstrated similar or higher levels of VEGF expression. Expression of PDGF by glioma cells is well documented (42, 43); however, our immunohistochemistry data did not reveal any increase in PDGF expression within the areas in which the AC 133+ cells had migrated and were detected in the tumors. PDGF could be a contributing factor in tumor angiogenesis, but its influence on the migration and homing of human CD34+/AC133+ cells has not been documented yet. Our in vitro cell migration studies using supernatants from cells grown under hypoxic conditions indicated that PDGF may play a role as a chemoattractants in the migration of AC133+ cells. MMP-2 did not appear to contribute to the migration and homing of AC133+ cells to sites of vasculogenesis.
SDF-1 has been shown to be a potent chemoattractant for bone marrow-derived hematopoietic (CD34+) stem cells. AC133+ cells, a subpopulation of CD34+ cells, were used in the current study (44,45,46). Both CD34+ and AC133+ cells exhibited abundant expression of CXCR4, a receptor for SDF-1, and in vitro studies (47,48,49) have also shown an SDF-1 dose-dependent migration of CD34+/AC133+ cells. There have also been reports (50, 51) showing HIF-1α-dependent up-regulation and expression of SDF-1 in tumors. In this current study, we observed high expression of SDF-1 at the sites that corresponded to the sites of HIF-α expression in 1.5-cm tumors, by immunohistochemistry and Western blot analysis. Migration of ferumoxide-labeled AC133+ cells was also prominent at the sites of higher SDF-1 expression that indicated that homing of AC133+ cells was related to the expression of SDF-1. HIF-1α dependent SDF-1 expression might be the factor for AC133+ cells homing to these angiogenic sites. SDF-1 expression has been shown to be upregulated in response to HIF-1α (50,51,52). In the current study, the Western blot results indicated that in large flank tumors SDF-1 was highly expressed in the periphery, corresponding to areas where the AC133+ cells were observed. In addition, immunoblotting showed that HIF-1α was also expressed within the same area as SDF-1 and that PDGF and MMP-2 were present in the periphery of tumor. However, analysis of central tumor regions revealed no PDGF expression, while MMP-2 was expressed at lower levels in comparison to the tumor periphery. Based on our in vitro cell migration studies, PDGF might be responsible for the migration and homing of administered AC133+ at the sites of tumor. Contrary to the immunoblotting results, immunohistochemistry analysis of both the 1 and 1.5 cm tumors revealed that PDGF and MMP2 were expressed throughout the center and the periphery of the tumor. Since in the current study immunohistochemistry showed a time-dependent expression pattern of different factors that accompanied tumor growth, it is possible that the separate set of tumors used for Western blotting analysis exhibited slightly different time course of expression of the analyzed factors.
Limitation
It was not part of this study to determine the number of administered cells that migrated to the periphery of the tumor and how many of these migrated cells incorporated into neovascularization. Our previous study (53) indicated that determination of exact number of migrated and accumulated magnetically labeled cells is not possible, based on the current imaging and analytic parameters. Moreover, quantification of the number iron-positive cells on Prussian blue stain (which is a daunting task) may not reflect only the administered human cells but uptake by other cells (54). Our study design did not include coregistration of histology sections with MRI images. It was not the original intent of the study due to absence of anatomical landmark in and around the tumors.
CONCLUSIONS
In conclusion, using magnetic labeling and cellular MRI, migration and homing of locally administered AC133+ cells can be tracked in vivo. Cellular MRI can be used for cell migration studies where different transgenic tumor or cell models can be used to identify the factors related to the migration. Magnetic labeling of AC133+ cells may be complementary to fluorescent or luminescence-based technique to follow the migration and homing of EPCs or stem cells in tumor or in ischemic tissues in response to different angiogenic or chemotaxic factors.
Acknowledgments
We thank Gene T. Yocum for help during the labeling of AC133+ cells at the National Institutes of Health. We thank Dr. A. S. M. Iskander for help in sectioning of collected tumor samples and collection of AC133+ cells from cord blood, and Dr. Hanh M. Khuu for supervision during collection of AC133+ from volunteers. This work was supported in part by the Intramural Research Program of the Clinical Center at the National Institutes of Health and an extramural funding grant (5R01CA122031).
References
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu M H, Wijelath E S, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage L R, Moore M A, Storb R F, Hammond W P. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Samejima N, Yamazaki K. A study on the vascular proliferation in tissues around the tumor in breast cancer. Jpn J Surg. 1988;18:235–242. doi: 10.1007/BF02471439. [DOI] [PubMed] [Google Scholar]
- Ellis L M, Liu W, Ahmad S A, Fan F, Jung Y D, Shaheen R M, Reinmuth N. Overview of angiogenesis: Biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28:94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Zhang Z G, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- Tomanek R J, Schatteman G C. Angiogenesis: new insights and therapeutic potential. Anat Rec. 2000;261:126–135. doi: 10.1002/1097-0185(20000615)261:3<126::AID-AR7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hotfilder M, Nowak-Gottl U, Wolff J E. Tumorangiogenesis: a network of cytokines. Klin Padiatr. 1997;209:265–270. doi: 10.1055/s-2008-1043960. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner J M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker P H, Verfaillie C M. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–346. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett N R, Crystal R G, Moore M A, Hajjar K A, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar B N, Reinhardt R L, Schwartz R E, Keene C D, Ortiz-Gonzalez X R, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low W C, Largaespada D A, Verfaillie C M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Gill M, Dias S, Hattori K, Rivera M L, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- Gehling U M, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld D K, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- Fan C L, Li Y, Gao P J, Liu J J, Zhang X J, Zhu D L. Differentiation of endothelial progenitor cells from human umbilical cord blood CD 34+ cells in vitro. Acta Pharmacol Sin. 2003;24:212–218. [PubMed] [Google Scholar]
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Pomyje J, Zivny J, Sefc L, Plasilova M, Pytlik R, Necas E. Expression of genes regulating angiogenesis in human circulating hematopoietic cord blood CD34+/CD133+ cells. Eur J Haematol. 2003;70:143–150. doi: 10.1034/j.1600-0609.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- Arbab A S, Pandit S D, Anderson S A, Yocum G T, Bur M, Frenkel V, Khuu H M, Read E J, Frank J A. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24:671–678. doi: 10.1634/stemcells.2005-0017. [DOI] [PubMed] [Google Scholar]
- Murohara T. Therapeutic vasculogenesis using human cord blood-derived endothelial progenitors. Trends Cardiovas Med. 2001;11:303–307. doi: 10.1016/s1050-1738(01)00128-1. [DOI] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–C579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler C M. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Path. 2004;57:965–969. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D A. Umbilical cord blood cells and brain stroke injury: bringing in fresh blood to address an old problem. J Clin Invest. 2004;114:312–314. doi: 10.1172/JCI22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura H, Dhar D K, Kohno H, Kubota H, Fujii T, Ueda S, Kinugasa S, Tachibana M, Nagasue N. Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 2004;10:8554–8560. doi: 10.1158/1078-0432.CCR-0946-03. [DOI] [PubMed] [Google Scholar]
- Detwiller K Y, Fernando N T, Segal N H, Ryeom S W, D'Amore P A, Yoon S S. Analysis of hypoxia-related gene expression in sarcomas and effect of hypoxia on RNA interference of vascular endothelial cell growth factor A. Cancer Res. 2005;65:5881–5889. doi: 10.1158/0008-5472.CAN-04-4078. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A, Hockel M. Tumor hypoxia and malignant progression. Meth Enzymol. 2004;381:335–354. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- Ferrari N, Glod J, Lee J, Kobiler D, Fine H A. Bone marrow-derived, endothelial progenitor-like cells as angiogenesis-selective gene-targeting vectors. Gene Ther. 2003;10:647–656. doi: 10.1038/sj.gt.3301883. [DOI] [PubMed] [Google Scholar]
- Agbulut O, Vandervelde S, Al Attar N, Larghero J, Ghostine S, Leobon B, Robidel E, Borsani P, Le Lorc'h M, Bissery A, Chomienne C, Bruneval P, Marolleau J P, Vilquin J T, Hagege A, Samuel J L, Menasche P. Comparison of human skeletal myoblasts and bone marrow-derived CD133+ progenitors for the repair of infarcted myocardium. J Am Coll Cardiol. 2004;44:458–463. doi: 10.1016/j.jacc.2004.03.083. [DOI] [PubMed] [Google Scholar]
- Moore X L, Lu J, Sun L, Zhu C J, Tan P, Wong M C. Endothelial progenitor cells’ “homing” specificity to brain tumors. Gene Ther. 2004;11:811–818. doi: 10.1038/sj.gt.3302151. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner J M, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Arbab A S, Jordan E K, Wilson L B, Yocum G T, Lewis B K, Frank J A. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351–360. doi: 10.1089/104303404322959506. [DOI] [PubMed] [Google Scholar]
- Anderson S A, Shukaliak-Quandt J, Jordan E K, Arbab A S, Martin R, McFarland H, Frank J A. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann Neurol. 2004;55:654–659. doi: 10.1002/ana.20066. [DOI] [PubMed] [Google Scholar]
- Anderson S A, Glod J, Arbab A S, Noel M, Ashari P, Fine H A, Frank J A. Noninvasive MR imaging of magnetically labeled stem cells to directly identify neovasculature in a glioma model. Blood. 2005;105:420–425. doi: 10.1182/blood-2004-06-2222. [DOI] [PubMed] [Google Scholar]
- Arbab A S, Yocum G T, Kalish H, Jordan E K, Anderson S A, Khakoo A Y, Read E J, Frank J A. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104:1217–1223. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- Yoshida D, Kim K, Noha M, Teramoto A. Hypoxia inducible factor 1-alpha regulates of platelet derived growth factor-B in human glioblastoma cells. J Neurooncol. 2006;76:13–21. doi: 10.1007/s11060-005-3279-0. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Fomchenko E I, Holland E C. Platelet-derived growth factor-mediated gliomagenesis and brain tumor recruitment. Neurosurg Clin N Am. 2007;18:39–58. doi: 10.1016/j.nec.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Westermark B, Heldin C H, Nister M. Platelet-derived growth factor in human glioma. Glia. 1995;15:257–263. doi: 10.1002/glia.440150307. [DOI] [PubMed] [Google Scholar]
- Rigolin G M, Mauro E, Ciccone M, Fraulini C, Sofritti O, Castoldi G, Cuneo A. Neoplastic circulating endothelial-like cells in patients with acute myeloid leukaemia. Eur J Haematol. 2007;78:365–373. doi: 10.1111/j.1600-0609.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- Rustemeyer P, Wittkowski W, Greve B, Stehling M. Flow-cytometric identification, enumeration, purification, and expansion of CD133+ and VEGF-R2+ endothelial progenitor cells from peripheral blood. J Immunoassay Immunochem. 2007;28:13–23. doi: 10.1080/15321810601025549. [DOI] [PubMed] [Google Scholar]
- Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–66. [PubMed] [Google Scholar]
- Mohle R, Bautz F, Rafii S, Moore M A, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- Jo D Y, Rafii S, Hamada T, Moore M A. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbab A S, Yocum G T, Rad A M, Khakoo A Y, Fellowes V, Read E J, Frank J A. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- Jin D K, Shido K, Kopp H G, Petit I, Shmelkov S V, Young L M, Hooper A T, Amano H, Avecilla S T, Heissig B, Hattori K, Zhang F, Hicklin D J, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett N R, Crystal R G, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4(+) hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceradini D J, Kulkarni A R, Callaghan M J, Tepper O M, Bastidas N, Kleinman M E, Capla J M, Galiano R D, Levine J P, Gurtner G C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Arbab A S, Iskander A S M, Rad A M, Anderson S A, Pandit S D, Read E J, Frank J A. Migration of endothelial progenitor cells is related to HIF-1a induced SDF-1 expression. Mol Imaging. 2006;5:233. [Google Scholar]
- Rad A M, Arbab A S, Iskander A S, Jiang Q, Soltanian-Zadeh H. Quantification of superparamagnetic iron oxide (SPIO)-labeled cells using MRI. J Magn Reson Imaging. 2007;26:366–374. doi: 10.1002/jmri.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelczyk E, Arbab A S, Chaudhry A, Balakumaran A, Robey P G, Frank J A. In vitro model of brdu or iron oxide nanoparticle uptake by activated macrophages from labeled stem cells: implications for cellular therapy. Stem Cells. 2008;26:1366–1375. doi: 10.1634/stemcells.2007-0707. [DOI] [PubMed] [Google Scholar]