Abstract
Physiological estrogens, including estrone (E1), estradiol (E2), and estriol (E3), fluctuate with life stage, suggesting specific roles for them in biological and disease processes. We compared their nongenomic signaling and functional actions in GH3/B6/F10 rat pituitary tumor cells. All hormones caused prolactin release at 1 min; the lowest effective concentrations were 10−11 M E2, 10−10 M E1, and 10−7 M E3. All estrogens increased the oscillation frequency of calcium (Ca) spikes, with the same time delay (∼200 s) at all levels (10−15 to 10−9 M). At some concentrations, E1 and E3 provoked more Ca-responding cells than E2. The amplitude and volume of Ca peaks were elevated by all hormones at ≥10−15 M. All hormones caused cell proliferation, with the lowest effective concentrations of E2 (10−15 M) > E1 (10−12 M) > E3 (10−10 M); E2 caused higher maximal cell numbers at most concentrations. All estrogens caused oscillating extracellular-regulated kinase (ERK) activations, with relative potencies of E1 and E2 > E3. All estrogens were ineffective in activation of ERKs or causing proliferation in a subline expressing low levels of membrane estrogen receptor-α. Dose-response patterns were frequently nonmonotonic. Therefore, the hormones E1 and E3, which have been designated “weak” estrogens in genomic actions, are strong estrogens in the nongenomic signaling pathways and functional responses in the pituitary.—Watson, C. S., Jeng, Y.-J., Kochukov, M. Y. Nongenomic actions of estradiol compared with estrone and estriol in pituitary tumor cell signaling and proliferation.
Keywords: calcium, ERK activation, prolactin release, nonmonotonic dose responses, membrane estrogen receptors
Life stage-specific, fluctuating levels of several physiological estrogens suggest a role for them in specific biological and disease processes (reviewed in ref. 1). Many diseases (e.g., Parkinson’s, Alzheimer’s, Tourette’s, attention deficit hyperactivity disorder, osteoporosis, heart disease, and partum-related depression) worsen in women after menopause or pregnancy, or fluctuate in premenopausal females, suggesting a protective effect of estrogens or altered vulnerabilities due to changing levels of estrogens. Reports that only very large doses of estradiol (E2) can improve some conditions thought to be associated with estrogen withdrawal (e.g., mood disorders; ref. 2) suggest that the action of other E2 metabolites could be involved. There are also apparent associations between the levels of some estrogen metabolites and vulnerabilities to certain cancers (3). The possible involvement of different estrogen metabolites in these important biological and disease processes requires that they be fully examined for both genomic and nongenomic actions; the nongenomic actions of most estrogen metabolites are unknown.
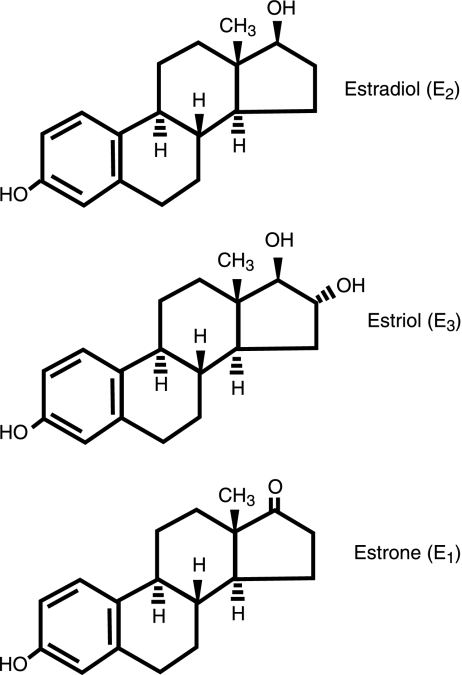
Although E2 (in the high picomolar to nanomolar range) is the physiological estrogen most often associated with female reproductive phase function and with the development of cancers in reproductive tissues, other endogenous estrogenic compounds can be more prevalent during specific life phases. These other estrogens may have significant effects on tissue development, function, and disease states. Estrone (E1) is a significant estrogenic hormone contributor in both reproductive (∼0.5–1 nM) and postmenopausal (150–200 pM) women and in men; estriol (E3) levels are significantly higher in pregnant women (∼10–100 nM) than in nonpregnant women (<7 nM; ref. 4). Decreased E3 levels in pregnancy have been associated with complications of eclampsia (5) and the incidence of Down’s syndrome in offspring (6). Fluctuations of ovarian hormones in perimenopause consist of intermittent high and low E2 levels, followed eventually by chronically lower levels, and the predominance of E1 (7); these changes can be correlated with behavioral and other disturbances, as can pubertal and menstrual cycle-based fluctuations (8). All three of these estrogenic compounds (see Fig. 1) are also produced by aromatases in placenta (especially E3; ref. 9) and other nonreproductive tissues (brain: ref. 10; fat cells: ref. 11), where their effects may extend beyond reproductive functions (12). For example, E3 has protective effects against the development of arthritis in certain experimental models (13), as was known previously for E2. Effects in the brain, bone, the cardiovascular system, and many other tissues could be affected differentially by all three of these endogenous estrogenic compounds during different life stages.
Figure 1.
Structure of the predominant physiological estrogens.
The vast majority of studies on the mechanisms of estrogen (and other steroid) actions over the past 40 years were focused on nuclear transcription (genomic) effects (reviewed in ref. 14). Although nongenomic estrogen actions were first suggested early in the investigation of steroid mechanisms (15), most studies of them are relatively recent. Membrane-initiated signaling pathways include complex webs of interacting signals that can converge to ramp up or down a particular function and can have immediate mechanistic consequences (such as rapid release of peptide hormones from preloaded secretory vesicles; ref. 16). These signals can also lead to later downstream consequences after the accumulation of signaling cascade products or the phosphorylation of transcription factors (reviewed in ref. 17). Many types of known membrane-initiated signaling cascade mechanisms can be involved in nongenomic estrogenic signaling (reviewed in refs. 18, 19). Although we are beginning to understand more about the various ways in which E2 acts via membrane receptor-initiated pathways, we currently know little about the nongenomic responses to the other prominent physiological estrogens (E1 and E3). Other physiological estrogens besides E2 have always been considered to be “weak” estrogens because of their actions in the genomic response pathways (20). The possible potency of these diverse estrogen metabolites in signaling pathways initiated in membrane estrogen receptors thus needs to be explored.
In the pituitary, estrogens facilitate both synthesis and regulated secretion of prolactin (PRL; ref. 21). In addition, limited estrogen-induced cell proliferation is part of the normal response of the pituitary but can also produce pituitary tumors (22). PRL is believed to be a growth factor for many tissues, including breast and prostate (23, 24), so its overproduction or excessive release by estrogens could lead to pathologies or tumors of these target tissues (25). We have studied membrane-initiated, E2-induced mechanistic and functional actions in a rat pituitary tumor cell subline (GH3/B6) for several years, and we (26, 27) recently demonstrated that representatives of another class of so-called weak estrogens (as defined via the genomic mechanism; environmental estrogens) can cause potent nongenomic signaling effects in these cells whenever the membrane estrogen receptor is expressed at high levels.
Steroids and their mimetics can have very different effects at different concentrations, especially in nongenomic signaling pathways. We (28, 29) have shown previously that estrogens operating via these pathways can cause the activation of extracellular-regulated kinases (ERKs), a subclass of mitogen-activated protein kinases (MAPKs), in pituitary tumor and breast cancer cells. These responses to E2 and some environmental estrogens, plus PRL release in pituitary cells, all have nonclassical, bimodal dose-response patterns (with for example picomolar and nanomolar peaks of activity and inactive concentrations in between; refs. 26, 28,29,30). The scientific literature contains many conflicting conclusions about responses to diverse estrogen classes (physiological, dietary, environmental, and pharmaceutical) and individual compounds within them, but this may be due to the study of single or few doses, which could yield different results when nonclassical dose responses are involved. Only by doing careful, wide-range dose-response studies at the mechanistic level can such discrepancies be resolved.
MATERIALS AND METHODS
Materials and Cells
We purchased phenol red-free Dulbecco’s modified Eagle medium (DMEM) from Mediatech (Herndon, VA, USA); horse serum from Gibco-BRL (Grand Island, NY, USA); and defined supplemented calf sera and fetal bovine sera from Hyclone (Logan, UT, USA). Paraformaldehyde and glutaraldehyde were purchased from Fisher Scientific (Pittsburgh, PA, USA). We purchased Fura-2/AM from Molecular Probes (Eugene, OR, USA). Antibodies used in the ERK phosphorylation studies were purchased from Cell Signaling (Danvers, MA, USA). All other materials were purchased from Sigma (St. Louis, MO, USA).
GH3/B6 cells were originally subcloned as rapidly E2-responsive cells (31) in electrophysiological assays. We further subcloned these cells for high expression levels of mERα (16) creating the GH3/B6/F10 (F10) subclone used in all of these studies (except Figs. 5C and 6C, where a low-expressing subclone D9, was used). For the present experiments, F10 cells were subsequently reselected by immunopanning for highly enriched expression of mERα and then used between passages 10 and 20. We have published a series of papers (16, 26, 29, 30, 32,33,34,35,36) describing the involvement of mERα in various nongenomic signaling and functional tests and also demonstrating the specificity of the response (i.e., not elicited by 17αE2; blocked by ICI 187 870). We routinely culture these cells in DMEM containing 12.5% horse serum, 2.5% defined supplemented calf serum, and 1.5% fetal calf serum. They are then switched to various hormone-free media before our experimental designs (see below).
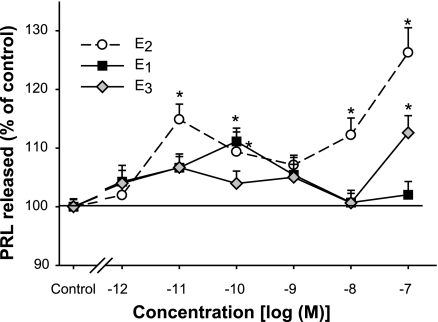
Figure 2.
Rapid PRL release caused by different concentrations of E2 compared with E1 and E3. PRL released into the medium at 1 min was measured by RIA (n=18 samples over 3 experiments). Value for percentage of control represents the percentage of the basal PRL secretion (compared with vehicle-treated cells) at each estrogen concentration, normalized to the cell number in each well. Error bars around the controls are quite small and sometimes obscured by the symbol. *P < 0.05 vs. vehicle treatment.
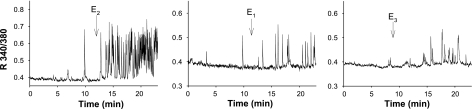
Figure 3.
E1 and E3, like E2, can produce rapid [Ca2+]i increases in GH3/B6/F10 cells. Hormone-induced time-dependent [Ca2+]i changes from a single representative cell are shown for each estrogen. All hormones were applied at a concentration of 10 pM. The x axes of all graphs show the time elapsed (min). The y axes show the ratio of fluorescence signals collected at 510 nm emission using 340 or 380 nm excitation wavelengths. Arrows indicate the time of estrogen application.
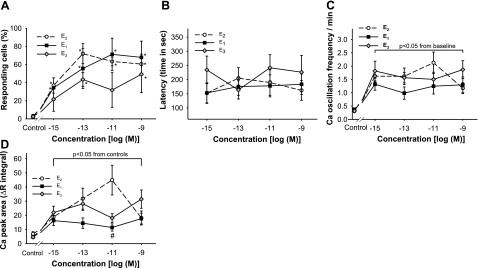
Figure 4.
Ca2+ volume measurements, percentage of responding cells, Ca2+ oscillation frequency, and response initiation timing of Ca2+ influx due to three physiological estrogens at different concentrations. A) Number of responding cells per dish was recorded as a percentage of the total number of cells recorded simultaneously (n=3–5 dishes; 31–83 individual cells for each data point). *P < 0.05 vs. vehicle treatment. B) Delay time of E1-, E2-, and E3-induced changes in Ca2+ oscillation frequency. C) Increases in Ca2+ oscillation frequency compared with basal frequency (set to 0 on the plot), in response to E1, E2, and E3. D) Ca2+ influx values (R340/380) show the ratio of FURA-2 fluorescence signals collected at 500 nm emission using 340 or 380 nm excitation wavelengths. Values were assessed over a 10 min time period. The integral of the Ca2+ response was estimated by the total area under the Ca2+ peaks elicited by E1, E2, and E3 treatment. #P < 0.05 vs. E2 treatment at the same concentration.
Figure 5.
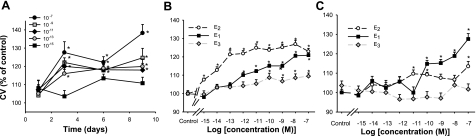
Proliferative effects of estrogens on F10 (mER-enriched) vs. D9 (mER-depleted) subtypes of GH3/B6 cells, measured by the crystal violet assay. A) F10 cells grown in the presence of different concentrations of E2 over a 9-day time course. Proliferation at day 3 was selected as the analysis time point for subsequent experiments (see B, C). B) Influence of different concentrations of E2 compared with E1 and E3 (n=24 samples; 3 experiments) on F10 cells. C) Influence of different concentrations of E2 compared with E1 and E3 (n=24 samples; 3 experiments) on D9 cells. *P < 0.05 vs. vehicle treatment.
Figure 6.
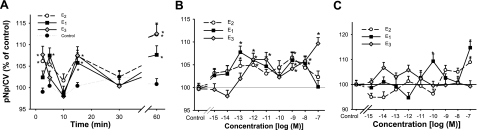
Effects of physiological estrogens (E2, E1, and E3) on ERK activation (phosphorylation) in F10 (mER-enriched) vs. D9 (mER-depleted) subtypes of GH3/B6 cells, measured by our quantitative plate assay for phosphorylated ERKs. Values are reported as the amount of paranitrophenol (pNp) generated as normalized to the crystal violet (CV) value for cell number for each well. Error bars around the controls are quite small and are sometimes obscured by the symbol. Data are presented as a percentage of vehicle-treated controls, which were set to 100 (n=24 samples; 3 experiments/graph). A) Time-dependent changes in the phosphorylation status of ERKs due to estrogen treatments at 1 nM. B) Concentration-dependent changes in the phosphorylation status of ERKs in mERα-enriched F10 cells after 5 min estrogen treatments. C) Concentration-dependent changes in the phosphorylation status of ERKs in low-mERα-expressing D9 cells in response to estrogens at 5 min. *P < 0.05 vs. vehicle treatment.
PRL release measured via RIA
Cells (0.5–0.7×106) were plated in poly-d-lysine-coated 6-well plates. After serum deprivation in ITS-defined medium (5 μg/ml insulin, 5 μg/ml transferin, 5 ng/ml sodium selenite, and 0.1% BSA) for 48 h, this medium was removed and DMEM/0.1% BSA with or without the appropriate estrogen or vehicle control (ethanol) was added. The cells were incubated for 1 min and centrifuged at 4°C (350 g for 5 min). The supernatant was then collected and stored at −20°C until RIA. Concentrations of PRL were determined using components of the rat PRL RIA kit from the National Institute of Diabetes and Digestive and Kidney Disease and the National Hormone and Pituitary Program (Baltimore, MD, USA). Briefly, RIA buffer (80% PBS, 20% DMEM, and 2% normal rabbit serum), 100 μl cold standard (rat PRL-RP-3) or unknown sample rPRL-s-9 antiserum (final dilution of 1:437,500 in RIA buffer), and [125I]-rat-PRL (PerkinElmer, Waltham, MA, USA; using 15,000 counts per tube diluted in RIA buffer) were combined and incubated with shaking overnight at 4°C. Anti-rabbit IgG (R-0881; Sigma) was added to a final dilution of 1:9, and the samples were incubated with shaking at room temperature (RT) for 2 h. One milliliter of polyethylene glycol solution [1.2 M polyethylene glycol (P-6667; Sigma) and 50 mM Tris, pH 8.6] was then added, and the samples were incubated with shaking at RT for 15 min. The samples were then centrifuged at 4000 g for 10 min at 4°C, the supernatant was decanted, and the pellet was counted in a Wizard 1470 Gamma Counter (PerkinElmer). The PRL concentration was then calculated and normalized to the crystal violet values (see below) representing cell number. These measurements were done in three different experiments (on different days).
Cytosolic free [Ca2+]i imaging
Cells were harvested with 0.25% trypsin-0.02% EDTA, plated on poly-d-lysine-treated 35/22 mm glass-bottom dishes (Willco Wells, Amsterdam, Netherlands) at a density of 100,000 cells/mm3, and incubated at 37°C for 48–72 h. At 12 h before an experiment, cells were incubated in serum-free DMEM. On the day of the experiment, the cells were loaded with 2.5 μM of the Ca2+-sensitive fluorescent dye Fura-2AM for 1 h at RT, washed 3 times, and then incubated at RT for 1–4 h before live Ca2+ imaging experiments. The physiological solution used for Fura-2 loading and live-cell imaging contained 150 mM NaCl, 5.5 mM KCl, 1 mM MgCl2, 4 mM CaCl2, 7 mM glucose, and 10 mM HEPES, pH 7.4.
The cell imaging setup included a Nikon 200E microscope with ×40 SuperFluo lens and computer-controlled illumination system (Sutter Instruments, Novato, CA, USA) equipped with a digital monochrome-cooled charge-coupled Roper Coolsnap HQ camera (Roper Scientific, Tucson, AZ, USA). Fluorescent emission at 510 nm from regions of interest (corresponding to a single cell) was acquired online with the MetaFluor software (Universal Imaging, Downington, PA, USA). The signal was obtained in dual 340 and 380 nm excitation mode, and the average intensity of fluorescence in each region was used to estimate 340:380 ratios (R340/380), reflecting [Ca2+].
MetaMorph (Universal Imaging) and SigmaPlot (Systat Software, San Jose, CA, USA) scientific software were used for conversion and analysis of acquired data. For quantitative characterization of Ca2+ oscillation amplitude, area, and frequency in live cells, the AutoFit function of the PeakFit software (Systat Software) with manual adjustments was used. The peak threshold was chosen empirically as ΔR ≥ 0.05. Individual cells were considered responsive to estrogen treatment when they demonstrated an increase in Ca2+ oscillation frequency compared with basal and showed at least 0.25 Ca2+ spikes per minute as estimated over a 10 min time interval, with a delay no more than 10 min from the addition of an estrogen in the bath. Subsequent comparative analysis of cell responses was performed on these defined responsive cells only.
Cell number measurements: the crystal violet assay
Subconfluent cells growing in serum-containing medium were seeded into a 96-well plate (1000 cells/well), allowed to attach overnight, and treated with estrogens in DMEM medium containing 1% 4× charcoal-stripped FBS (feeding every 2–3 days) for 9 days. Cell numbers were assessed by the crystal violet assay after 1, 3, 6, and 9 days, and it was determined that day 3 was the appropriate day to compare the proliferative effects of estrogens at different concentrations. Crystal violet stains multiple cellular constituents that can be assessed by extracting the dye and reading at 560–590 nm, the values being proportional to cell number measured with other techniques (37). On the day of cell number analysis, cells from which the medium had been removed were fixed for 20 min in 2% paraformaldehyde and 0.1% glutaraldehyde in PBS, stained for 30 min with a 0.1% solution of crystal violet, and destained in deionized water. The dye was released by 10% acetic acid at RT for 30 min, and the A590 signal of the extract was then read in a microplate reader. We used 8 samples (one column of wells) for each treatment group, and experiments were repeated 3 times using different passages of cells.
ERK assays
We developed this assay to assess activated ERK 1 and 2 levels in fixed cells (29). Cells were plated at a density of 10,000 cells/well in 96-well plates. The day after plating, growth media were replaced with DMEM containing 1% charcoal-stripped (4×) serum (to deprive cells of steroids) for 48 h. Cells were then washed with DMEM once before the estrogens (or 0.0001% ethanol vehicle) were added for different times (0–60 min). The cells were then fixed with 2% paraformaldehyde/0.2% picric acid at 4°C for 48 h. After fixation, the cells were permeabilized with PBS containing 2% BSA and 0.1% Triton X-100 for 1 h at RT. Then the cells were washed 3 times with PBS, and primary antibody (Ab) against pERK (1:400 in PBS/1% BSA/0.1% Triton X-100) was added. After overnight incubation at 4°C, the cells were washed 3 times with PBS, and the biotin-conjugated secondary Ab (Vector Labs, Burlingame CA, 1:300) in PBS/1% BSA was added for 1 h at RT. The cells were again washed with PBS, incubated with Vectastain ABC-AP solution (Vector Labs) for 1 h at RT, and again washed 3 times with PBS, followed by addition of Vectastain alkaline phosphatase substrate plus levamasole (an endogenous phosphatase inhibitor). Plates were incubated in the dark for 30 min at 37°C, and the signal for the phosphatase product para-nitrophenol was read at A405 in a model 1420 Wallace microplate reader (PerkinElmer). The number of cells in each well was determined by the crystal violet assay, as described above. We routinely used cholesterol as a negative control and phorbol 12-myristate 13-acetate (TPA) as a positive control to ascertain expected cell behavior in these assays. All experiments were repeated at least 3 times using different passages of cells on different days.
Statistics
Data from the measurements of PRL release, cell number, and ERK activity were analyzed by 1-way ANOVA followed by multiple comparisons vs. the control group (Holm-Sidak method). Paired t tests were used to compare Ca2+ signaling characteristics before and after estrogen treatments, while an ANOVA with the Dunn’s method for multiple comparisons was used for E1, E2, and E3 treatment series. The Sigma Stat 3 program (Systat Software, Chicago, IL, USA) was used for all statistical analyses. Significance was accepted at P < 0.05.
RESULTS
Because our F10 cell line (enriched for the expression of the membrane form of ERα) was previously shown to respond rapidly to E2 by releasing stored PRL (16) and because other “weak” xenoestrogens have been shown to be potent in nongenomic responses (38,39,40), including PRL release (30), we wondered whether the other prominent physiological estrogen metabolites, E1 and E3, could also cause this response. Figure 2 shows that 0.1 nM E1 could release PRL, which is a quite potent action and far more potent than that shown for genomic E1 responses. E3 could only cause PRL release at 100 nM concentrations, but these levels are achieved physiologically during the later stages of human pregnancy.
Our previous studies (35) showed that E2 at low concentrations evoked robust Ca2+ mobilization responses dependent on L-type voltage-gated Ca2+ channels in these mERα-enriched cells. In Fig. 3, we demonstrate that both E1 and E3 (in comparison to E2) could cause relatively rapid Ca2+ ingress into cells at the quite low concentration of 10 pM, which is in the lowest ranges of physiological concentrations in both females at multiple life stages and males. There was no constant pattern in this behavior for vehicle-treated cells. A small percentage of vehicle-treated cells (as well as untreated cells) occasionally showed increases in Ca2+ oscillation frequency or decreases in the spontaneous firing rate. Both spontaneous and estrogen-induced increases in the Ca2+ firing rates, reflecting increase in membrane excitability, looked similar overall.
In Fig. 4, we examined other measurable aspects of the Ca2+ response that could give us more detail about the mechanisms. Latency time of the response can give some indication of direct vs. indirect effects of the ER on the channel, oscillation frequency reflects membrane firing, and the amplitude of Ca2+ spikes or the magnitude of the response (measured as a ΔR340/380 integral) depend on the density of calcium channels, as well as their activation state. Because these cells do not all express mERα continuously (for instance varying between different phases of the cell cycle; ref. 41), it is typical to find between 20 and 60% of cells expressing mERα. We also found that cells that were completely quiescent before treatment had very low response rates (5–20% for E2, 0–40% for E3, and a very variable 5–60% for E1); therefore, we included only cells that showed some pretreatment activity.
All three estrogens caused an increasing number of Ca2+-responding cells across these physiological doses (Fig. 4A). Overall, E2 and E1 caused the most robust responses and were active at 10−15 M. E3 was effective at as low as 0.1 pM but was not effective at all tested concentrations. All three estrogens (Fig. 4B) showed similar delays between hormone application and the beginning of the Ca2+ influx, which probably indicates ion channel opening after a cascade of signaling events, rather than direct opening of the channel by mER interaction. The oscillation frequency of channel openings for all three estrogens had similar phasing (Fig. 4C). The peak amplitudes of the intracellular Ca2+ concentration spikes caused by the three estrogens (data not shown) were similar but not identical; significant increases were observed after as little as 100 fM E1 (P<0.01; n=10) and 10 pM E2 (P<0.05; n=10) treatments. When we measured the areas under the Ca2+ peaks (the volume of the Ca2+ entry) over a period of 10 min, we saw some separation in potency between these hormones (Fig. 4D). At a concentration of 10 pM, E2 clearly caused a more robust response, but at other physiological concentrations all three hormones caused a similar response. These findings suggest that these hormones use similar receptors and probably draw on the same pool of signaling pathways, although they may not use them all identically.
The F10 cell proliferation response to E2 was essentially maximal by 3 days at all proliferation-inducing concentrations of E2 (Fig. 5A), so we chose this time point to determine the concentration dependence of this response for all hormones. The only concentration tested in this time course that did not cause significant increases in cell number was the 10−15M concentration. In Fig. 5B, the comparison of the different estrogens shows that E2 was the most potent in this response, significant responses being evident at as low as fM. However, both E1 (effective at pM and above) and E3 (effective at 0.1 nM and above) were also significantly active at low, physiologically relevant concentrations. To determine whether the mERα-expressing status of the cells could effect this response, we compared the proliferation response to that in another clonal line of these cells (the D9 subclone) previously isolated for its very low levels of mERα expression (16, 42). Figure 5C shows that although these cells did respond at the higher E1 and E2 concentrations, they were clearly less sensitive, in keeping with their lower mERα levels.
We then tested activation by phosphorylation of ERKs (subtypes 1 and 2) in mERα-enriched F10 cells by E2, compared with E1 and E3. We first determined the time-dependent changes shown in Fig. 6A. As we have observed previously, ERK activation by phosphorylation was an oscillating response, with waves of phosphorylation (29) separated by probable action of phosphatases (28), as we have seen previously for E2. Although the phasing of the first activation was slightly different between these estrogenic hormones (E3 was faster, E1 slower, and E2 fast but longer lasting), all three hormones basically gave a similarly rapid response. E1 was not quite as effective as E2 and E3 for the later sustained peak of activation (at 60 min). Dose-dependent changes in ERK activation caused by E2, E1, and E3 in F10 cells are shown in Fig. 6B. These responses were evident at quite low doses for all three hormones (100 fM for E1 and E2; 1 pM for E3). Again the dose responses for all three hormones can be described as nonmonotonic, as is typical of other nongenomic estrogenic responses that we have measured both in this study and in many of our past studies (26, 29, 30, 43). In Fig. 6C, the ERK responses to these hormones are shown for the mERα-depleted subline D9. Responses were evoked only at much higher estrogen concentrations in these cells. For E2, a 100 nM concentration was necessary to achieve a significant response, although E1 could elicit responses at 0.1 and 100 nM concentrations. E3 was unable to elicit a response at any tested concentration over this wide range. We also routinely used positive and negative controls to assess the response range of a given batch of cells after 5 min of treatment. For F10 cells, the phosphorylated ERK level as a result of treatment with 1 nM cholesterol (negative ligand control) was 101.8 ± 1.2% of vehicle controls and with 20 nM TPA (positive response control) was 111.3 ± 2.2%. For D9 cells treated with 1 nM cholesterol, the response was 101.4 ± 2.1%, and with 20 nM TPA was 112.3 ± 1.9%.
DISCUSSION
It is evident from our data that physiological estrogens that were previously categorized as weak can potently activate multiple signaling and functional responses in pituitary cells. There were some, but few, differences between E2 and the other metabolites in the parameters of Ca2+ signaling, and the differences in potency for the PRL release and ERK activation differed by no more than one order of magnitude at low physiological concentrations. The most notable discrepancy was in cell proliferation, where E2 effects were evident at femtomolar levels, whereas E1 and E3 were effective at 1 pM and 0.1 nM and above, respectively; however, all hormones caused substantial cell number changes well within physiological concentration ranges. Therefore, changing levels of all of these prominent estrogen metabolites could be the basis for some life stage-specific disease biases in women. It is useful to remember that earlier work from the Clark laboratory (20) maintained that such estrogens were not really weak; when their concentrations were maintained continuously by implants, as opposed to single injections, they could actually elicit tissue growth of the uterus. Perhaps chronic maintenance of these membrane-initiated signaling events contributes eventually to most of the major tissue growth responses. As shown by our earlier work, the rapid release of preformed PRL from pituitary tumor cells (within minutes) is a consequence of E2 and environmental estrogen action via the nongenomic pathway (e.g., refs. 16, 32, 34, 36); here we show that this response is also evoked by these endogenous estrogenic metabolites. We also corroborated our previous observations that the level of mERα expressed by different cell sublines can be correlated with the sensitivity of these responses to estrogenic regulation (16); where these responses still occur at the higher concentrations, they may be attributed to remaining mERα in these cells or other ERs.
The nongenomic signaling and functional response sensitivities to these different estrogens did not match and probably should not be expected to necessarily. Unlike transcriptional responses (relatively similar mechanistically), nongenomic responses are quite diverse in mechanism and types of participating proteins. The functional responses initiating from the membrane (PRL secretion, ERK activation, perhaps part of cell proliferation) undoubtedly involve multiple diverse signaling pathways as our past work showed (selective pathway inhibitors only partially inhibit the responses; different estrogenic ligands elicit these separate pathways to different extents.) For example, multiple types of signaling pathways drive the secretory response. Although Ca2+ is necessary to trigger the response, a Ca2+ signal is not sufficient to accomplish everything that is part of a fully elicited secretory response. There are also steps such as docking secretory vesicles to membranes and aligning and stabilizing the machinery of membrane fusion. We have shown in the past that just raising the levels of Ca2+ in these cells (to the highest degree by KCl depolarization) is inadequate to cause a maximal secretory response compared with that induced by E2 (35). These different estrogen metabolites appear to elicit other parts of this response besides Ca2+ channel opening differently or with different degrees of efficiency. Cell proliferation also involves many different signaling systems (e.g., all cellular constituents must be doubled; the processes of mitosis and cytokinesis are each very complex processes involving multiple and different kinds of signaling pathways.) So again, we might expect different compounds acting at the membrane to elicit these processes with different efficacies or potencies.
The ability of estrogens to facilitate cancer cell growth is well known but still incompletely understood mechanistically. Stimulating cells to proliferate involves making new cellular constituents (the genomic actions most often studied) but also involves evoking rapid signals that can alter the phosphorylation status of cell cycle proteins and their regulators (44,45,46) or cause secretion of growth factors or cytokines (47,48,49). All three physiological estrogens studied here caused time-dependent patterns of ERK phosphorylation, with apparent periods of dephosphorylation between activation periods, an oscillating pattern often observed by others in diverse tissues. In addition, all three estrogens were potent in their phosphorylation of ERK, although with some unique concentration-dependent patterns. ERK activations, and especially the later (sustained) ERK activations, have often been linked to the ability of cells to proliferate (50) and so may be associated with that functional response here. Whether these signaling cascades initiated by estrogen metabolites also lead to key cell cycle protein phosphorylations remains to be determined.
Regardless of whether cell proliferation in our mER-enriched F10 cell line represents normal vs. tumor cell regulation or direct regulation by estrogens vs. indirect regulation via the PRL released by the actions of these estrogens, there may be consequences for health. Released PRL acting as a growth factor in an autocrine, paracrine, or endocrine fashion can also affect a number of other tissues (25). Other possible functional consequences of changes in PRL activity include coordination or miscoordination of the female hormonal cycle with preparation of tissues for reproduction (e.g., mammary gland) and changes in the control of reproductive behavior. Hyperprolactinemia is a recognized cause of infertility, as well as behavioral illnesses. Therefore, these estrogenic metabolites could be involved in a variety of functions and malfunctions.
Although the changes we observed in multiple responses are small, they are significant, and it is likely that endogenous regulatory biology is more controlled by such small but significant changes (as opposed to the very large changes often observed in experimental systems studied using overexpressed proteins). The potency with which these estrogens act in our assays (compared with those published elsewhere for well-known highly estrogen-dependent cells) may have been affected by our use of a completely defined medium (with just the addition of insulin, transferin, BSA, and selenium) for the PRL and Ca2+ assays, and our use of 4× charcoal stripped serum in our proliferation and ERK assays (vs. the usual singly charcoal-stripped serum often used in media for proliferation assays). We feel that better definition of the assay system (rigorous elimination of the steroids present in serum-containing media) takes precedence over robustness of the response, as long as the system still yields significant results.
We have once again demonstrated that dose responses to estrogens via the nongenomic pathway are nonclassical. These nonmonotonic dose responses were observed for PRL release, some Ca2+ responses, and ERK activation. This is similar to the unusual dose-response patterns we have observed in the past with both physiological and nonphysiological estrogens (26, 30, 43). Others have also observed these characteristics of nongenomic responses (e.g., ref. 39). In addition, nongenomic responses are often observed to exhibit distinct bimodal time activations, similar to the phasing of responses we observed here. Although these phenomena are still unexplained, possibilities include different subpopulations of receptors responding differently because of subcellular location (such as apical vs. basolateral membranes, or nonraft vs. raft or caveolar membranes) or the participation of multiple signaling pathways that are independently activated at different concentrations of estrogens and with different temporal patterns. Oscillating ERK activities can apparently be caused in part by activation of compensatory phosphatases (28). We also note that compounds within a class of steroids acting on nongenomic responses have generally been shown to have different hierarchy of potency, which might be related to their altered binding affinities in the membrane, compared with their nuclear counterparts (reviewed in ref. 19). Whatever the cause for these distinctly separate dose and time segments in the responses with some variations between these estrogen metabolites, it is very clear that these observations demand more detailed dose-response characterizations to understand at what concentrations various estrogens (physiological estrogen metabolites, dietary estrogens, environmental estrogens, or pharmaceutical) exert their actions, either beneficial or harmful.
Elucidating the underlying cellular mechanisms and receptors that regulate responses to various physiological estrogens will allow future examination of how hormone replacements, analogs, and antagonists could replace or alleviate their stage-specific and tissue-specific effects that sometimes are exaggerated to the point of causing severe disorders. Understanding nongenomic estrogenic effects will allow entirely new approaches to these maladies and may offer supporting explanations for changes in medical practice such as the preservation of ovaries in women undergoing hysterectomies. Our studies justify why other physiological estrogens should be considered in the diagnosis and treatment of diseases.
Acknowledgments
We gratefully acknowledge funding from the National Institutes of Environmental Health Sciences and the American Institute for Cancer Research that supported these studies. We also thank Dr. David Konkel for expert editing of our article.
References
- Williams C L. Estrogen effects on cognition across the lifespan. Horm Behav. 1998;34:80–84. doi: 10.1006/hbeh.1998.1480. [DOI] [PubMed] [Google Scholar]
- Richardson T A, Robinson R D. Menopause and depression: a review of psychologic function and sex steroid neurobiology during the menopause. Prim Care Update Ob Gyns. 2000;7:215–223. doi: 10.1016/s1068-607x(00)00049-4. [DOI] [PubMed] [Google Scholar]
- Greenlee H, Chen Y, Kabat G C, Wang Q, Kibriya M G, Gurvich I, Sepkovic D W, Bradlow H L, Senie R T, Santella R M, Ahsan H. Variants in estrogen metabolism and biosynthesis genes and urinary estrogen metabolites in women with a family history of breast cancer. Breast Cancer Res Treat. 2007;102:111–117. doi: 10.1007/s10549-006-9308-7. [DOI] [PubMed] [Google Scholar]
- Greenspan F S, Gardner D G. New York, NY, USA: Lange Medical Books, McGraw Hill; Basic and Clinical Endocrinology, (7th ed.) 2004:925–926. [Google Scholar]
- Shenhav S, Gemer O, Volodarsky M, Zohav E, Segal S. Midtrimester triple test levels in women with severe preeclampsia and HELLP syndrome. Acta Obstet Gynecol Scand. 2003;82:912–915. doi: 10.1034/j.1600-0412.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- Chard T, Macintosh M C. Screening for Down’s syndrome. J Perinat Med. 1995;23:421–436. doi: 10.1515/jpme.1995.23.6.421. [DOI] [PubMed] [Google Scholar]
- Prior J C. Ovarian aging and the perimenopausal transition: the paradox of endogenous ovarian hyperstimulation. Endocrine. 2005;26:297–300. doi: 10.1385/ENDO:26:3:297. [DOI] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Newby D, Aitken D A, Howatson A G, Connor J M. Placental synthesis of oestriol in Down’s syndrome pregnancies. Placenta. 2000;21:263–267. doi: 10.1053/plac.1999.0469. [DOI] [PubMed] [Google Scholar]
- Vanson A, Arnold A P, Schlinger B A. 3 beta-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Deslypere J P, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab. 1985;61:564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- Meinhardt U, Mullis P E. The essential role of the aromatase/p450arom. Semin Reprod Med. 2002;20:277–284. doi: 10.1055/s-2002-35374. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Enhancement of collagen-induced arthritis in female mice by estrogen receptor blockage. Arthritis Rheum. 2001;44:2168–2175. doi: 10.1002/1529-0131(200109)44:9<2168::aid-art370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tsai M J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Pietras R J, Szego C M. Endometrial cell calcium and estrogen action. Nature. 1975;253:357–360. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- Pappas T C, Gametchu B, Yannariello-Brown J, Collins T J, Watson C S. Membrane estrogen receptors in GH3/B6 cells are associated with rapid estrogen-induced release of prolactin. Endocrine. 1994;2:813–822. [Google Scholar]
- Watson C S. Signaling themes shared between peptide and steroid hormones at the plasma membrane. STKE. 1999;1999:PE1. doi: 10.1126/stke.1999.12.pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C S, Gametchu B. Proteins of multiple classes participate in nongenomic steroid actions. Exp Biol Med. 2003;228:1272–1281. doi: 10.1177/153537020322801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C S, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc Soc Exp Biol Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- Anderson J N, Peck E J, Jr, Clark J H. Estrogen-induced uterine responses and growth: relationship to receptor estrogen binding by uterine nuclei. Endocrinology. 1975;96:160–167. doi: 10.1210/endo-96-1-160. [DOI] [PubMed] [Google Scholar]
- Dannies P S. Control of prolactin production by estrogen. Litwack G, editor. Orlando, FL, USA: Academic Press; Biochemical Actions of Hormones. 1985:289–310. [Google Scholar]
- Gorski J, Wendell D, Gregg D, Chun T Y. Estrogens and the genetic control of tumor growth. Progress Clin Biol Res. 1997;396:233–243. [PubMed] [Google Scholar]
- Adams A B. Human breast cancer: concerted role of diet, prolactin and adrenal C19-delta 5 steroids in tumorigenesis. Int J Cancer. 1992;50:854–858. doi: 10.1002/ijc.2910500603. [DOI] [PubMed] [Google Scholar]
- Nevalainen M T, Valve E M, Ingleton P M, Nurmi M, Martikainen P M, Harkonen P L. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–627. doi: 10.1172/JCI119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C V, Furth P A, Hankinson S E, Schuler L A. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulayeva N N, Watson C S. Xenoestrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Env Health Perspect. 2004;112:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A L, Bulayeva N N, Watson C S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-α-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Env Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinovic D, Watson C S. Membrane estrogen receptor-alpha levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2005;7:R130–R144. doi: 10.1186/bcr959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulayeva N N, Gametchu B, Watson C S. Quantitative measurement of estrogen-induced ERK 1 and 2 activation via multiple membrane-initiated signaling pathways. Steroids. 2004;69:181–192. doi: 10.1016/j.steroids.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A L, Bulayeva N N, Watson C S. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufy B, Vincent J-D, Fleury H, Pasquier P D, Gourdji D, Vidal A T. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979;204:509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Norfleet A M, Clarke C, Gametchu B, Watson C S. Antibodies to the estrogen receptor-α modulate prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. FASEB J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C H, Watson C S. TX, USA: Texas A&M University, College Station; Cell cycle regulation of membrane estrogen receptor (mER) expression in the rat pituitary tumor cell line GH3/B6. Category 4 (Poster 26), 37, Society of Toxicology-Gulf Coast Chapter, Annual Meeting. 1997 [Google Scholar]
- Norfleet A M, Thomas M L, Gametchu B, Watson C S. Estrogen receptor-α detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- Bulayeva N N, Wozniak A, Lash L L, Watson C S. Mechanisms of membrane estrogen receptor-α-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am J Physiol Endocrinol Metab. 2005;288:E388–E397. doi: 10.1152/ajpendo.00349.2004. [DOI] [PubMed] [Google Scholar]
- Pappas T C, Gametchu B, Watson C S. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Zivadinovic D, Gametchu B, Watson C S. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res. 2005;7:R101–R112. doi: 10.1186/bcr958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero A B, Fuentes E, Ripoll C, Soria B, Nadal A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsarnovszky A, Le H H, Wang H S, Belcher S M. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]
- Watson C S, Bulayeva N N, Wozniak A L, Alyea R A. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C S, Campbell C H, Gametchu B. The dynamic and elusive membrane estrogen receptor-alpha. Steroids. 2002;67:429–437. doi: 10.1016/s0039-128x(01)00172-6. [DOI] [PubMed] [Google Scholar]
- Campbell C H, Watson C S. A comparison of membrane vs. intracellular estrogen receptor- in GH3/B6 pituitary tumor cells using a quantitative plate immunoassay. Steroids. 2001;66:727–736. doi: 10.1016/s0039-128x(01)00106-4. [DOI] [PubMed] [Google Scholar]
- Watson C S, Norfleet A M, Pappas T C, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitiary tumor cells via a plasma membrane version of estrogen receptor. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- Ahamed S, Foster J S, Bukovsky A, Wimalasena J. Signal transduction through the Ras/Erk pathway is essential for the mycoestrogen zearalenone-induced cell-cycle progression in MCF-7 cells. Mol Carcinog. 2001;30:88–98. doi: 10.1002/1098-2744(200102)30:2<88::aid-mc1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Obaya A J, Sedivy J M. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59:126–142. doi: 10.1007/s00018-002-8410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M, Sirbasku D A. A new serum-free method of measuring growth factor activities for human breast cancer cells in culture. In Vitro Cell Dev Biol. 1988;24:911–920. doi: 10.1007/BF02623902. [DOI] [PubMed] [Google Scholar]
- Danielpour D, Ikeda T, Kunkel M W, Sirbasku D A. Identification of an autostimulatory growth factor in the extracts and conditioned medium of GH3/C14 rat pituitary cells in culture. Endocrinology. 1984;115:1221–1223. doi: 10.1210/endo-115-3-1221. [DOI] [PubMed] [Google Scholar]
- Rucci N, Ricevuto E, Ficorella C, Longo M, Perez M, Di G C, Funari A, Teti A, Migliaccio S. In vivo bone metastases, osteoclastogenic ability, and phenotypic characterization of human breast cancer cells. Bone. 34:697–709. doi: 10.1016/j.bone.2003.07.012. [DOI] [PubMed] [Google Scholar]
- English J, Pearson G, Wilsbacher J, Swantek J, Karandikar M, Xu S, Cobb M H. New insights into the control of MAP kinase pathways. Exp Cell Res. 1999;253:255–270. doi: 10.1006/excr.1999.4687. [DOI] [PubMed] [Google Scholar]