Abstract
The growth factor, vascular endothelial growth factor (VEGF), induces angiogenesis and promotes endothelial cell (EC) proliferation. Affymetrix gene array analyses show that VEGF stimulates the expression of a cluster of nuclear-encoded mitochondrial genes, suggesting a role for VEGF in the regulation of mitochondrial biogenesis. We show that the serine threonine kinase Akt3 specifically links VEGF to mitochondrial biogenesis. A direct comparison of Akt1 vs. Akt3 gene silencing was performed in ECs and has uncovered a discrete role for Akt3 in the control of mitochondrial biogenesis. Silencing of Akt3, but not Akt1, results in a decrease in mitochondrial gene expression and mtDNA content. Nuclear-encoded mitochondrial gene transcripts are also found to decrease when Akt3 expression is silenced. Concurrent with these changes in mitochondrial gene expression, lower O2 consumption was observed. VEGF stimulation of the major mitochondrial import protein TOM70 is also blocked by Akt3 inhibition. In support of a role for Akt3 in the regulation of mitochondrial biogenesis, Akt3 silencing results in the cytoplasmic accumulation of the master regulator of mitochondrial biogenesis, PGC-1α, and a reduction in known PGC-1α target genes. Finally, a subtle but significant, abnormal mitochondrial phenotype is observed in the brain tissue of AKT3 knockout mice. These results suggest that Akt3 is important in coordinating mitochondrial biogenesis with growth factor-induced increases in cellular energy demands.—Wright, G. L., Maroulakou, I. G., Eldridge, J., Liby, T. L., Sridharan, V., Tsichlis, P. N., Muise-Helmericks, R. C. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase.
Keywords: angiogenesis, VEGF signal transduction, Akt kinase, PGC-1α, mitochondrial protein import
Vascular endothelial growth factor (VEGF) is widely accepted to be the main inducer of angiogenesis (1). Angiogenesis involves a complex series of events including endothelial cell (EC) proliferation, dissolution of basement membranes, migration, and motility. Although it is not generally appreciated, angiogenesis is a profoundly anabolic process that requires the de novo generation of new vascular tissue and deposition of extra cellular matrix. Processes such as angiogenesis, which require remodeling and new tissue production, demand higher levels of ATP synthesis than quiescent tissue. In tissue, the great majority of ATP is produced by mitochondrial oxidative phosphorylation. The amount of cellular volume occupied by mitochondria varies from 4% in fibroblast to 40% in adult cardiomyocytes. The factors that determine the mitochondrial mass within a cell are poorly understood. When cells proliferate or grow larger, mitochondrial mass is maintained within a remarkable narrow range when expressed as a percentage of the cellular volume (2, 3). With the mitochondrial expansion that accompanies exercise, metabolic cues such as AMP, and Ca2+ have been suggested to play a role in signaling increased mitochondrial biogenesis through AMP-activated and calcium/calmodulin-dependent kinases (4, 5). However, much less is known about what controls the mitochondrial biogenesis that occurs in processes such as cellular proliferation. Thus, while it is biologically appropriate that signaling pathways activated by metabolic stress or contractile cues would stimulate mitochondrial reticulum expansion, with such processes such as cellular proliferation, additional mechanisms appear to exist that anticipate, rather than react to, metabolic demands.
Maintenance of mitochondrial mass requires a close coordination between both nuclear and mitochondrial gene expression and recruitment or import of new mitochondrial proteins into preexisting mitochondrial compartments. The coordination between nuclear and mitochondrial gene expression is controlled primarily on the level of transcription by a series of “master” regulators. One such regulator is PGC-1α (peroxisome proliferator-activated receptor γ coactivator-1α), which acts upstream of and in consort with the nuclear respiratory factors NRF-1 and NRF-2. NRF-1 and NRF-2 are responsible for the transcriptional activation of the major mitochondrial DNA (mtDNA) transcriptional regulator, transcription factor A (TFAM) (6), which is responsible for the regulation of mtDNA transcription. These factors then provide, at least in part, the integral mechanism linking and coordinating nuclear and mitochondrial gene expression.
Respiratory protein complexes are composed of both nuclear and mitochondrial encoded subunits that must be recognized, sorted, and imported into the mitochondria and correctly assembled into the subcompartments of the organelle. Import of these mitochondrial preproteins is controlled by translocases located in the outer membrane (TOMs) that recognize signal sequences in the N terminus of the unfolded preproteins and within the inner membrane (TIMs) that control and sort mitochondrial proteins (7,8,9). Assembly of mature respiratory complexes is controlled by a complement of numerous proteases and chaperones. The protein import machinery is yet another level at which mitochondrial biogenesis is closely regulated. Clearly, the control of mitochondrial biogenesis requires complex interacting pathways, many of which are incompletely defined.
Previously, we described a VEGF signaling pathway that leads to the regulation of Akt3 through the sphingosine-1-phosphate S1P3/Edg3 (10). These findings suggested a potential new regulatory pathway, whereby VEGF, through the control of S1P3/Edg3 expression, results in the up-regulation of Akt3 kinase via increased mRNA expression in primary ECs. The VEGF→Akt3 pathway was found to be discrete from that described for the constitutively expressed Akt1, which is regulated via phosphorylation (11, 12). In contrast, Akt3 appears to be primarily regulated by changes in expression (10). Evidence indicating that the Akt isoforms have redundant functions has led to the current view that total Akt activity is the important parameter for determining signaling through this pathway. However, several recent studies have suggested that the different Akt isoforms may have separable downstream signaling functions (13,14,15,16,17,18). The following study has explored the later model and has found a discrete role for Akt3 in the control of mitochondrial biogenesis in response to growth factor stimulation. Our findings suggest that this pathway plays a role in coupling the metabolic requirements of a growing tissue with mitochondrial energy-producing capacity.
MATERIALS AND METHODS
Tissue culture, growth factors, and transfection
Pooled, multiple-donor human umbilical vein ECs (HUVECs) (Lonza, Basal, Switzerland) were maintained at 37°C with 5% CO2 in endothelial basal medium 2 (EBM; Lonza) supplemented with EGM-2 SingleQuots, as described by Cambrex procedures. The SingleQuots include IGF1, EGF, and VEGF. Serum starvation was performed at 80–90% confluence in RPMI 1640 supplemented with 0.1% fetal calf serum (Life Technologies, Gaithersburg, MD, USA) for 24 h followed by stimulation with VEGF165 (20 ng/ml; R&D Systems, Minneapolis, MN, USA).
HUVECs were transfected using the Amaxa nucleofection system (Amaxa, Gaithersburg, MD, USA) in procedures described by the manufacturer. Briefly, 2 × 106 cells were transfected per cuvette in 100 μl of HUVEC Nucleofector solution using the A034 setting on the nucleofector. All transfections were monitored by expression of green fluorescent protein (GFP) using a GFP expression vector pFP-C1 (Clontech, Mountainview, CA, USA) or a GFP-directed RNAi (Amaxa). SMARTpool RNAi directed specifically against the Akt1 or Akt3 isoforms was purchased from Upstate Biotechnology (Lake Placid, NY, USA).
Gene array analyses
For Affymetrix gene chip analysis, Affymetrix (Santa Clara, CA, USA) human genome U133A GeneChips, which contain representations of ∼22,000 genes were used. RNA was isolated from ECs either untreated or treated with VEGF for 16 h, in triplicate, using a Qiagen RNA isolation microspin column purification (Qiagen, Valencia, CA, USA). For each GeneChip analysis, total RNA (10 μg) was converted into double-stranded cDNA using a T7-(dT)24 primer (Genset, San Diego, CA, USA) and the Custom SuperScript cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Biotin-labeled cRNA was synthesized from cDNA by in vitro transcription (Enzo BioArray HighYield RNA Transcript Labeling Kit; Enzo Life Sciences, Farmingdale, NY, USA) and then fragmented. For each sample, 15 μg of fragmented cRNA was hybridized to the GeneChip Array as described in the Affymetrix GeneChip expression Analysis Technical Manual. GeneChip arrays were scanned using an Affymetrix GeneArray Scanner 2500, and the probe intensities were quantified using Affymetrix MAS5.0 software with average chip intensities scaled to 150 U. Affymetrix hybridization and analyses were performed by the Medical University of South Carolina DNA Microarray Facility (directed by S. Argraves and J. Barth). Fold changes were tabulated for each gene and are shown in Table 1.
TABLE 1.
Primer sequences used in RT-PCR
| Primer | Sequence |
|---|---|
| S26 forward (F) | CTCCGGTCCGTGCCTCCAAG |
| S26 reverse (R) | CAGAGAATAGCCTGTCTTCAG |
| Akt1 F | ATGAGCGACGTGGCTATTGTGAAG |
| Akt1 R | GAGGCCGTCAGCCACAGTCTGGAT |
| Akt3 F | ATGAGCGATGTTACCATTGT |
| Akt3 R | CAGTCTGTCTGCTACAGCCTG |
| CoxI F | GGCCTGACTGGCATTGTATT |
| CoxI R | TGGCGTAGGTTTGGTCTAGG |
| CoxII F | TCCATGATCACGCCCTCATA |
| CoxII R | TAAAGGATGCGTAGGGATGG |
| NADII F | AAGCAACCGCATCCATAATC |
| NADII R | TCAGAAGTGAAAGGGGGCTA |
| MCAD F | TTGAGTTCACCGAACAGCAG |
| MCAD R | TCCAAGTCCAAGACCTCCAC |
| β Pydehydrogenase F | TCCCTGGAATTCAGAGGATG |
| β Pydehydrogenase R | AGCACCAGTGACACGAACAG |
| NRF1 F | GTGGCAGGACTTTCTGC |
| NRF1 R | AAGGATTCCTGGGAAGGAGA |
| ATP synthase (F0) F | TGCTGCAACAGTAGGAGTGG |
| ATP synthase (F0) R | AAGGCTGCCAAAGACTGTTC |
| Rprotein L3 F | ATCTTTGGACGTGGCAGAAC |
| Rprotein L3 R | ACTCTGGCTCATCGAAGCTC |
| Cox8 F | TACCTCCTGCTTCGTGACCT |
| Cox8 R | AGGTCTCCAGGTGTGACAGG |
| Lysyl tRNA synthetase F | TCGGGGAGACATAATTGGAG |
| Lysyl tRNAsynthetase R | TCAGCTCACCCTTCTTGGTT |
| Tomm70 F | GGGAGTGGACCAGACAAAGA |
| Tomm70 R | GCTGGAGTGCAGTGGCTATT |
| Tim23 F | GCTGGCCTTCTTTACGATTG |
| Tim23 R | CTGCACCTCGTGTTTTCTCA |
Primers were picked using Primer 3 software provided by the Whitehead Institute for Biomedical Research.
To assess Akt1 vs. Akt3 specific target genes, Atlas nylon-based gene arrays (Clonetech, Inc., Palo Alto, CA, USA) were used for microarray analyses in procedures described by the manufacturer. Briefly, total RNA isolated from ECs transfected with RNAi directed against Akt1 or Akt3 was used to create 32P-radiolabeled probes that were used to hybridize to the Atlas Select Human Oncogene array. This array contained ∼600 genes that were differentially expressed between MCF7 breast carcinoma cells and MCF7-ErbB2, a breast carcinoma overexpressing the tyrosine kinase receptor, ErbB2. Akt1 vs. Akt3-specific changes in gene expression were assessed by autoradiography followed by analysis using AtlasImage software in procedures provided by the manufacturer (Clonetech).
Southern blot analysis
Cells were lysed overnight at 37°C in 1× RIPA Lysis Buffer (50 mM Tris, pH 7.5; 1% Triton X-100; 150 mM NaCl; 0.1% SDS; 1% sodium deoxycholate; and 40 mM NaF) supplemented with Proteinase K (Sigma), and subjected to phenol:chloroform extraction. Total genomic DNA was isolated by ethanol precipitation. Equal amounts of total DNA, as assessed by both optical density and visualization of ethidium bromide-stained agarose gels, were digested to completion using EcoRI, resolved on 0.7% agarose gels, and transferred to nylon membranes (Stratagene, La Jolla, CA, USA). Southern blots were probed using a mitochondrial specific CoxII probe created by reverse transcriptase-polymerase chain reaction (RT-PCR) using the primers shown in Table 1.
RT-PCR
For semiquantitative RT-PCR, cDNA was synthesized from total RNA (2–5 μg) with a Superscript First-Strand Synthesis Kit purchased from Invitrogen, using Oligo(dT) following the manufacturer’s instructions. PCR reactions contained equal amounts of cDNA and 1.25 μM of the appropriate primer pair (Sigma-Proligo, St. Louis, MO, USA). All primer sequences used in these analyses are shown in Table 1. Cycling conditions were 94°C for 5 min; 20–35 cycles of 94°C for 1 min, 50–65°C (based on primer Tm) for 1 min, 72°C for 1 min 45 s + 2 s/cycle; 72°C for 7 min and cooled to 4°C. Cycle number was empirically determined to be within the linear range of the assay for each primer pair used. All semiquantitative RT-PCR was performed in tandem with S26 primers as an internal control. Products were run on 1–1.5% (based on product size) agarose gels and visualized on a Bio-Rad Molecular Imaging System.
Real-time PCR was performed using a Brilliant CYBR Green QPCR kit in combination with an Mx3000P real-time PCR system, both purchased from Stratagene. Real-time PCR was performed at least in triplicate at least two independent times. For real-time PCR of genomic DNA, internal control primers that detect 18S rRNA (Ambion, Austin, TX, USA) or primers that detect S26 were used. Real-time PCR was performed at least three independent times and at least in triplicate. Error bars indicate sd.
Western blot analysis
Cells were washed 1× in PBS, and total protein was isolated by lysis in 1× RIPA lysis buffer supplemented with complete protease inhibitors without EDTA (Roche, Palo Alto, CA, USA) for 10 min on ice followed by 15 min centrifugation at 15,000 g. The BCA protein assay (Pierce, Rockford, IL, USA) was used to determine protein concentrations using instructions provided by the manufacturer. Equal amounts of protein were resolved on SDS-10% polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica MA, USA). Blots were blocked in Blotto [5% nonfat dry milk in TS (150 mM NaCl and 10 mM Tris, pH 7.4) plus 0.1% Tween-20] for 30 min and then probed with the antibodies indicated overnight at 4°C. The antibodies used for Western blot analysis were anti-Akt3 and anti-p85 subunit of PI3 kinase (Millipore-Upstate) and anti-Akt1 (Santa Cruz, Santa Cruz, CA, USA). Appropriate horseradish peroxidase-conjugated secondary antibodies were purchased from Invitrogen-Caltag. Proteins were visualized using Luminol Reagent 50 (Stratagene, San Diego, CA, USA).
Confocal microscopy
Transfected cells were seeded onto poly-l-lysine (Sigma) -coated cover slips and allowed to attach overnight. All incubations were performed at room temperature. Cells were fixed in 3.7% formaldehyde in 1× PBS (pH 7.4) for 20 min, washed in PBS, and permeabilized in 0.1% Triton X-100 for 20 min. After washing in PBS, fixed cells were blocked with 5% BSA in 1× PBS for 30 min with gentle agitation. Cells were incubated with the primary antibody (concentrations of which were empirically determined) for 1 h, washed in PBS, and incubated with the appropriate secondary antibody for 1 h. Washed cover slips were mounted in Gel/Mount (Biomeda Corp. Foster City, CA, USA) for visualization using confocal microscopy. For rhodamine phalloidin staining (Molecular Probes, Eugene, OR, USA), fixed and permeabilized cells were incubated in a 1:40 dilution in PBS for 20 min at room temperature prior to mounting. Nuclei were counterstained using TO-PRO-3 iodide (1:1000; Molecular Probes). Confocal microscopy was performed using the Olympus IX70 microscope equipped with the Fluoview confocal system (Olympus, Tokyo, Japan). Z stacks were performed with a step size of 0.4 μm and an ×60 oil immersion objective. The PGC1-GFP expression construct (19), and the PGC1-myc expression construct (19) were previously described and obtained from Addgene (Cambridge, MA, USA). The primary antibody used to detect PGC-1-myc expression was obtained from (Cell Signaling, Boston MA, USA), and the secondary Alexa Fluor 488 goat anti-mouse antibody was purchased from Molecular Probes.
Analysis of electron micrographs
Akt3-null mice were previously described (20). Six-week-old mice were anesthetized and perfused with 10 ml of heparin containing saline followed by 150 ml of 2.5% electron microscopy (EM) -grade gluteraldehyde in 0.1 M sodium cacodylate (pH 7.4) for 30 min. After perfusion, the brains were removed, and portions of the cerebral cortex were embedded in LR white resin. Electron microscopy and micrography were performed at the Emory University EM Core Facility. Mitochondrial structures were manually traced, and their area (measured in pixels) and density were measured using Adobe Photoshop software (Adobe Systems, San Jose, CA, USA). The total remaining nonmitochondrial area was also noted, allowing mitochondrial volume to be expressed as a percentage of total. Analyses was performed on 10 sections from each of 8 brains (4 wild-type and 4 Akt3−/− knockout brains), and significance was determined with Student’s t test (P<0.05). The morphometric analysis of electron micrographs was performed in a masked procedure, with generation and analysis of data performed by different individuals.
QO2 measurements
Cells (2×106) were detached from the dishes using trypsin and a rubber policeman and suspended in the culture medium. Oxygen consumption (QO2) was measured polarographically with a Clarke-type electrode in a water-jacketed airtight chamber, as described previously (20).
RESULTS
VEGF stimulates a cellular program for mitochondrial biogenesis
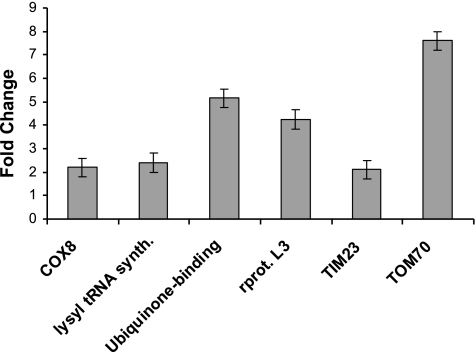
Expression profiling in yeast has defined sets of genes, especially the ribosomal proteins, which cycle coordinately with growth or increased metabolic rates. Increased expression of these genes has been used in yeast to correlate growth stages to mitochondrial function (21). Analysis of a series of Affymetrix gene arrays assessing changes in gene expression in primary ECs treated with VEGF revealed that at least 50 different nuclear encoded mitochondrial genes were regulated in a VEGF-dependent manner (Table 2). Many of these genes showed changes <2-fold; however, changes in gene expression using the Affymetrix gene arrays were measured after 16 h of VEGF stimulation, perhaps reducing the overall intensity of the response to VEGF stimulation. Six genes identified as up-regulated at 16 h of VEGF stimulation were selected for further analysis on the basis of their close association with the mitochondrial biogenesis program. An earlier VEGF effect (6 h) on the expression of a ribosomal protein subunit, a cytochrome c oxidase subunit (COX), a tRNA synthetase, a mitochondrial ubiquinone-binding protein, and the two major mitochondrial import proteins of the inner and outer mitochondrial membranes (Tim23 and Tom70, respectively) was tested by real-time PCR. As shown in Fig. 1, VEGF causes an induction of the expression of all six nuclear encoded genes that varies from >2-fold (Cox8, tRNA synthetase) to 8-fold (Tom70). These findings suggest that VEGF stimulates the expression of several nuclear encoded mitochondrial genes within 6 h of stimulation, potentially linking VEGF to mitochondrial biogenesis.
TABLE 2.
Nuclear encoded mitochondrial genes found to be regulated by VEGF in EC by Affymetrix gene array analysis
| Accession no. | Nuclear encoded mitochondrial gene | Fold change |
|---|---|---|
| NM_013410.1 | Adenylate kinase 3 (AK3) | −1.59 |
| BC003375.1a | Mitochondrial ribosomal protein L3 | +1.3 |
| NM_000016.1 | Acyl-coenzyme A dehydrogenase | +1.28 |
| NM_030579.1 | Cytochrome b5 outer mitochondrial membrane precursor (CYB5-M) | +1.53 |
| NM_001172.2 | Arginase, type II (ARG2) | −2.29 |
| NM_006327.1a | Translocase of inner mitochondrial membrane 23 (yeast) homolog (TIM23) | +1.2 |
| AB019219.1 | Similar to yeast pre-mRNA splicing factors, Prp1Zer1 and Prp6 | +1.94 |
| NM_020243.1 | Mitochondrial import receptor Tom22 (LOC56993) | +1.24 |
| NM_003321.1 | Tu translation elongation factor, mitochondrial (TUFM) | +1.26 |
| BC005957.1 | Solute carrier family 25 (mitochondrial carrier; peroxisomal membrane protein | +1.34 |
| NM_021107.1 | Mitochondrial ribosomal protein S12 (MRPS12) | +1.1 |
| NM_000688.1 | Aminolevulinate, delta-, synthase 1 (ALAS1) | +1.2 |
| NM_014765.1 | Translocase of outer mitochondrial membrane 20 (yeast) homolog | +1.2 |
| NM_001862.1 | Cytochrome c oxidase subunit Vb (COX5B) | +1.3 |
| NM_001863.2 | Cytochrome c oxidase subunit VIb (COX6B) | +1.24 |
| NM_000714.2 | Benzodiazapine receptor (peripheral) (BZRP) | +1.2 |
| Z93241 | Contains three novel genes, a mitochondrial ATP synthase G chain | +2.15 |
| NM_001190.1 | Branched chain aminotransferase 2, mitochondrial (BCAT2) | +1.2 |
| NM_001995.1 | Fatty-acid-coenzyme A ligase, long-chain 1 (FACL1) | +1.34 |
| W46388 | Superoxide dismutase 2, mitochondrial | −1.3 |
| AI832239a | Mitochondrial ribosomal protein L23 | +1.2 |
| D13119.1 | P2 mRNA for ATP synthase subunit c | +1.28 |
| NM_004074.1a | Cytochrome c oxidase subunit VIII (COX8) | +1.23 |
| NM_001152.1 | Solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 5 | +1.2 |
| AF285758.1 | Lysyl-tRNA synthetase | +1.2 |
| NM_004541.2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex | +1.64 |
| BC000147.1 | Similar to malic enzyme 2, NAD(+)-dependent | +1.2 |
| NM_001689.1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c (subunit 9) | +1.33 |
| NM_001697.1 | ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit | +1.2 |
| NM_004889.1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit f, isoform 2 | +1.2 |
| NM_021129.1 | Pyrophosphatase (inorganic) (PP) | +1.22 |
| NM_000690.1 | Aldehyde dehydrogenase 2, mitochondrial (ALDH2) | +1.1 |
| AI961224 | Solute carrier family 25 (mitochondrial carrier; adenine nucleotide translocator), member 6 | +1.2 |
| BC001917.1 | Malate dehydrogenase 2, NAD (mitochondrial) | +1.2 |
| M26700.1a | Ubiquinone-binding protein mRNA | +1.83 |
| NM_005138.1 | SCO (cytochrome oxidase deficient, yeast) homolog 2 | −2.62 |
| NM_000188.1 | Hexokinase 1 (HK1) | +1.4 |
| AF086790.1 | Aconitase precursor (ACON) | −1.47 |
| NM_003002.1 | Succinate dehydrogenase complex, subunit D | +1.3 |
| M27093.1 | Branched chain alpha-keto acid dehydrogenase transacylase subunit (E2b) | −2.7 |
| AF276920.1 | Thioredoxin 2 (TRX2) | +1.3 |
| NM_014820.1a | Translocase of outer mitochondrial membrane 70 (yeast) homolog A (TOMM70A) | +1.1 |
| NM_005412.1 | Serine hydroxymethyltransferase 2 (mitochondrial) (SHMT2) | +1.3 |
| NM_003000.1 | Succinate dehydrogenase complex, subunit B, iron sulfur (Ip) (SDHB) | +1.1 |
| NM_000126.1 | Electron-transfer-flavoprotein, alpha polypeptide (glutaric aciduria II) (ETFA) | +1.23 |
| BG165094 | Translocase of outer mitochondrial membrane 20 (yeast) homolog | +1.2 |
| NM_001359.1 | 2,4-Dienoyl CoA reductase 1, mitochondrial (DECR1) | +1.2 |
| NM_012458.1 | Translocase of inner mitochondrial membrane 13 (yeast) homolog B (TIMM13B) | +1.2 |
| NM_002453.1 | Mitochondrial translational initiation factor 2 (MTIF2) | +1.4 |
| NM_001689.1 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit c (subunit 9) isoform 3 | +1.33 |
| BC003633.1 | Translocase of outer mitochondrial membrane 70 (yeast) homolog | +1.2 |
| NM_005011.1a | Nuclear respiratory factor | +1.76 |
Gene was confirmed at 6 h of VEGF treatment by real-time PCR.
Figure 1.
VEGF stimulates a cellular program for mitochondrial biogenesis. Real-time RT-PCR analysis of gene expression from total RNA isolated from ECs either serum starved or treated with VEGF (20 ng/ml) for 6 h using primers listed in Table 1. Fold changes in gene expression of six nuclear encoded mitochondrial genes as compared to 18S rRNA expression as an internal control are shown. All real-time PCR was performed in triplicate.
The serine threonine kinase Akt3 is linked to mitochondrial gene expression
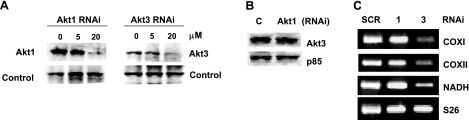
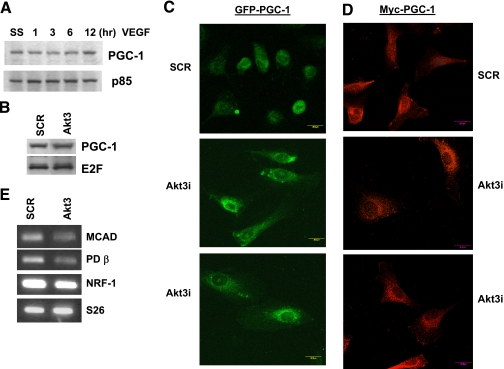
Our published findings show that VEGF stimulates the expression of Akt3 via a pathway requiring the Gi protein-coupled receptor, S1P3 (Edg3) and that this pathway is separable from that described for Akt1 (10). The hypothesis that Akt3 plays a nonredundant role downstream of VEGF is supported by the findings that Akt3 is regulated in a markedly different manner than Akt1. To determine whether there was a role for Akt3 independent of that for Akt1, RNAi was used to specifically block Akt1 or Akt3 expression in ECs. Western blot analysis of ECs transfected with RNAi directed against Akt1 or Akt3 showed the RNAis to be effective in selectively blocking the expression of each kinase (Fig. 2A) with no effect on the other in transfected ECs, indicating that each RNAi is isoform specific (Fig. 2B).
Figure 2.
Silencing of Akt3 inhibits mitochondrial gene expression. A) Western blot analysis of Akt1 and Akt3 expression, resulting from transfection of two different amounts of isoform-specific RNAi. B) Western blot analysis of total Akt3 protein levels in cells transfected with scrambled control (C) or Akt1 RNAi. The p85 PI3 kinase subunit is shown as a loading control. C) RT-PCR of total RNA isolated from ECs transfected with RNAi against scrambled control (SCR), Akt1 (1), or Akt3 (3), using primers directed against the mitochondrial encoded genes, COXI, COXII, and NADH dehydrogenase. The ribosomal subunit RNA S26 is shown as an internal control. All analyses were performed at least 3 independent times.
To determine whether there was a role for Akt3 discrete from that of Akt1, a comparison between probes generated from ECs transfected with RNAi directed against Akt1 vs. Akt3 was used to probe an array for 578 genes differentially expressed in MCF7 breast carcinoma cells as compared to MCF7 cells overexpressing ErbB2. These arrays were chosen because of the important role of Akt1 as a downstream effector of Her2/neu (17, 22,23,24,25). The most marked change in expression between cells transfected with Akt1 RNAi vs. those transfected with Akt3 RNAi was decreased mtDNA in ECs where Akt3 was silenced (data not shown). These results were confirmed and extended by comparing the expression levels of three genes encoded by mtDNA: COXI, COXII, and NADH dehydrogenase in Akt1, Akt3, and scrambled control transfected cells (Fig. 2C). These results indicate that mtDNA expression is specifically reduced in Akt3 knockdown cells.
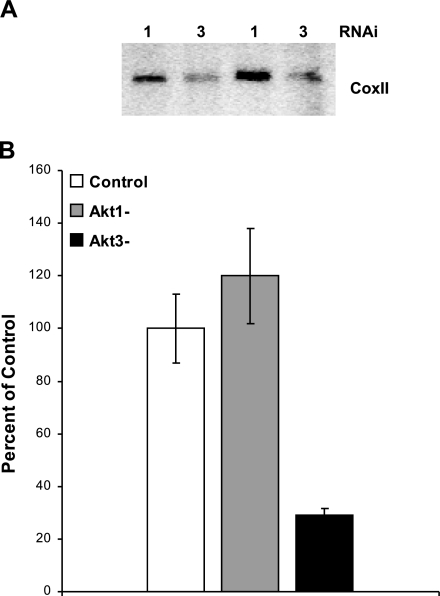
Because total mitochondrial gene expression can be influenced by mtDNA copy number, we assessed whether Akt3 silencing affected the amount of total mtDNA by Southern blot analysis using a probe for the mitochondrial gene, COXII (Fig. 3A). Total DNA was isolated from ECs transfected with either Akt1 or Akt3 RNAi and digested with EcoRI, which yields a single 16-kb mitochondrial fragment. Equal amounts of digested DNA were resolved by gel electrophoresis and subjected to Southern blot analysis. There is a marked decrease in mtDNA content in Akt3 knockdown cells vs. Akt1 knockdown cells. To quantitate the changes in mtDNA content, real-time PCR was performed on genomic DNA isolated from ECs transfected with scrambled control, Akt1, or Akt3 RNAi using primers directed against COX II (26). As shown in Fig. 3B, silencing of Akt3 resulted in an ∼60% reduction in mtDNA content as compared to control. These results also indicate that knockdown of Akt1 has no effect on overall mtDNA content in ECs and suggest that mtDNA copy number is specifically linked to Akt3.
Figure 3.
Akt3 is required for maintenance of mtDNA copy number. A) Southern blot analysis of total genomic DNA isolated from ECs transfected with either Akt1 or Akt3 RNAi and probed using COXII, a gene encoded by the mitochondria. Two independent transfections are shown. B) Real-time PCR of total genomic DNA isolated from ECs transfected using either control scrambled RNAi or RNAi directed against Akt1 (Akt1-) or Akt3 (Akt3-) using primers directed against COXII and the ribosomal subunit RNA S26 as an internal control. The graph represents the change in DNA copy number as a percentage of control. All real-time analyses were performed in triplicate.
Akt3 silencing results in a reduction in O2 consumption and nuclear mitochondrial gene expression and in perinuclear mitochondrial localization
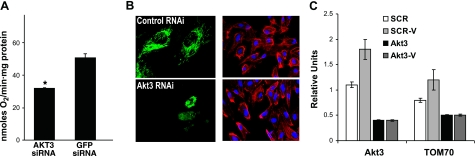
To determine whether the reduction in mtDNA correlated with changes in mitochondrial respiration, QO2 was measured in cells transfected with either control or Akt3 RNAi. As shown in Fig. 4A, blockade of Akt3 expression resulted in a significant decrease in cellular respiration as compared to control cells, correlating with the decreased mtDNA expression and copy number.
Figure 4.
Silencing of Akt3 results in a decreased QO2 and nuclear mitochondrial gene expression. A) Respiration of mitochondria in living cells was measured using a Clark-type O2 electrode in an oxygen consumption chamber. The graph represents nanomoles of O2 consumed per minute relative to total cell number, as assessed by quantitation of total protein. *P > 0.05. B) Left panels: fluorescent images of ECs cotransfected with control RNAi or Akt3 RNAi plus a GFP chimera containing a mitochondrial import sequence to specifically label mitochondria. Right panels: images of cells transfected with control or Akt3 RNAi followed by staining with rhodamine phallodin and Topro to visualize actin filaments and nuclei, respectively. C) RT-PCR analysis of Akt3 and TOM70 expression in cells transfected with scrambled control (SCR) or Akt3 RNAi and either serum starved or treated with VEGF (-V) (20 ng/ml) for 6 h.
Changes in mitochondrial morphology and localization were directly visualized in Akt3-depleted ECs using a GFP fused to the mitochondrial signal sequence that directs GFP to the mitochondria (27), in cells transfected either with Akt3 or control RNAi. As shown by the fluorescent images in Fig. 4B, silencing of Akt3 resulted in a more perinuclear distribution of mitochondria. The mitochondria also appear more condensed, and there are potentially fewer mitochondria. Perinuclear mitochondrial distributions are commonly seen during times of mitochondrial stress, such as during oxidative stress, necrotic cell death, or during drug-induced mitochondrial depletion (28,29,30). However, Akt3 blockade alone does not result in increased apoptosis (6) or in marked changes in cell morphology (Fig. 4B, right panel). Taken together, these results indicate a role for Akt3 in controlling mitochondrial biogenesis and basal activity and stability.
Akt3 links VEGF to mitochondrial biogenesis
Our published work shows that VEGF stimulation results in an increase in the expression of Akt3. To position Akt3 downstream of VEGF in the regulation of mitochondrial gene expression, we assessed whether blockade of Akt3 would affect the VEGF induction of nuclear mitochondrial gene expression. Whereas the mitochondrial genome only encodes 13 of >1300 mitochondrial proteins (31), mitochondrial biogenesis relies heavily on the activity of the mitochondrial protein import apparatus. Accordingly, the expression levels of the major import protein, TOM70, were measured. The induction of TOM70 expression by VEGF was diminished in cells transfected with an RNAi directed against Akt3, indicating that TOM70 expression is Akt3 dependent (Fig. 4C). These findings indicate that stimulation of ECs with VEGF, through a pathway dependent on Akt3, results in an increase in mitochondrial biogenesis and that this pathway modulates both nuclear and mitochondrial gene expression.
Akt3 controls PGC-1α nuclear localization
PGC-1α has emerged as the “master regulator” of mitochondrial biogenesis (32,33,34). This transcriptional coactivator stimulates mitochondrial biogenesis by increasing the expression of nuclear respiratory factors (NRF1 and 2) and other transcription factors that regulate mitochondrial biogenesis. Indeed, overexpression of PGC-1α is sufficient to direct a marked increased mitochondrial mass (35, 36). PGC-1α activity can be regulated by changes in its expression via nitric oxide (37, 38), calmodulin-dependent protein kinase (CaMK) (39) and oxidative stress (40). p38 MAPK can also affect PGC-1α activity by direct phosphorylation (41). To test whether the VEGF→Akt3 pathway was affecting the expression of PGC-1α, Western blot analyses were performed under conditions of VEGF stimulation. As shown in Fig. 5A, VEGF stimulation does not markedly effect the expression of PGC-1α, although there may be a slight increase in expression after 12 h of treatment. Blockade of Akt3 similarly has no effect on the expression levels of PGC-1α (Fig. 5B).
Figure 5.
Blockade of Akt3 expression results in exclusion of PGC-1α from the nucleus. A) Western blot analysis of total protein from cells serum starved (SS) then stimulated with VEGF for the times indicated and probed for PGC-1α expression. PI3K p85 subunit is shown as a loading control. B) Western blot analysis of PGC-1α expression in cells transfected with scrambled (SCR) or Akt3 RNAi. E2F is shown as a loading control. C) Confocal images of cells cotransfected with a GFP-PGC-1α expression construct and SCR or Akt3 RNAi. D) Confocal immunofluorescent images of cells cotransfected with a myc-PGC-1α expression construct and SCR or Akt3 RNAi. E) RT-PCR of total RNA isolated from ECs transfected with RNAi against either SCR or Akt3, using primers directed against the nuclear encoded mitochondrial genes NRF-1, MCAD, and PDβ. The ribosomal subunit RNA S26 is shown as an internal control.
It has been previously demonstrated that at least in the liver, two Akt-dependent mechanisms exist to control PGC-1α-dependent gene expression. The first involves a direct phosphorylation of FoxO1, resulting in its cytoplasmic localization and blockade of its interaction with PGC-1α-dependent target genes (42). The other involves the direct phosphorylation of PGC-1α, which results in its exclusion from the nucleus (43). Although these functions are apparently Akt2 specific in the liver, we tested whether Akt3 blockade could influence the subcellular localization of PGC-1α. To this end, we expressed GFP-tagged PGC-1α or an Myc-tagged PGC-1α in ECs transfected either with control or Akt3 RNAi. As shown in Fig. 5C, blockade of Akt3 results in a redistribution of GFP-PGC-1α from a nuclear/perinuclear localization to a more cytoplasmic localization. To confirm this redistribution, we performed a similar experiment using an myc-tagged PGC-1α expression construct. As shown in Fig. 5D, similar results were obtained. Cytoplasmic localization of PGC-1α correlates with a reduction in the expression of the PGC-1α-dependent target genes (44,45,46): NRF-1, medium chain acyl-CoA dehydrogenase (MCAD), and pyruvate dehydrogenase β (PDβ) (Fig. 5E). These results suggest that Akt3 controls the subcellular trafficking of PGC-1α in ECs, blockade of Akt3 inhibiting PGC-1α activity, and thus nuclear encoded mitochondrial gene expression.
Akt3-knockout animals have an abnormal mitochondrial phenotype
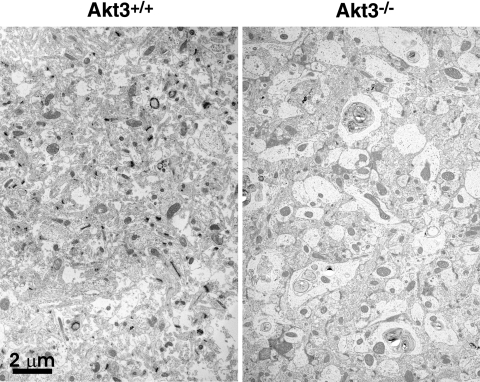
Given the evidence for a selective role for Akt3 in mitochondrial biogenesis in our culture model, it was of interest to explore the importance of Akt3 on mitochondria in vivo. The Akt3-knockout (Akt3-KO) animal has a moderate phenotype, with the most prominent change being decreased brain size (47, 48). Using a series of electron micrographs (Fig. 6) of brain sections from wild-type and Akt3-KO animals, we performed a morphometric analysis. Results of these analyses indicate that the mitochondrial volume expressed as a percentage of total cellular area is not significantly different between the Akt3-KO and control animals. However, the Akt3-KO animals do display significantly fewer and larger mitochondrial structures (Table 3 and Fig. 6). Consistent with swelling, the mitochondria of the Akt3-KO animals are measurably less electron dense than those of the controls. These data suggest that although the mitochondrial phenotype in the brains of the Akt3-KO animals is subtle, it does establish a significant and indispensable role for Akt3 in mitochondrial biogenesis.
Figure 6.
Akt3-KO animals display fewer and larger mitochondria. Representative electron micrographs of brain sections in wild-type and Akt3-KO animals showing enlarged and fewer mitochondria.
TABLE 3.
Morphometric analysis of mitochondrial content of brain tissue of Akt3-KO mice
| Genotype |
 mitochondrial size (pixels) mitochondrial size (pixels) |
Mitochondrial volume (% of total) | Mitochondria per unit area |
 mitochondrial density (AU) mitochondrial density (AU) |
|---|---|---|---|---|
| Akt3+/+ | 12197 ± 751 | 6.2 ± 0.3% | 5.4 ± 0.4 | 103 ± 1.8 |
| Akt3−/− | 13910 ± 638* | 5.9 ± 0.4% | 4.4 ± 0.3* | 96 ± 2.0* |
Brain tissue of Akt3-KO mice displays fewer but larger, less-dense mitochondria as compared to control mice. AU, arbitrary units.
P < 0.05 vs. control.
DISCUSSION
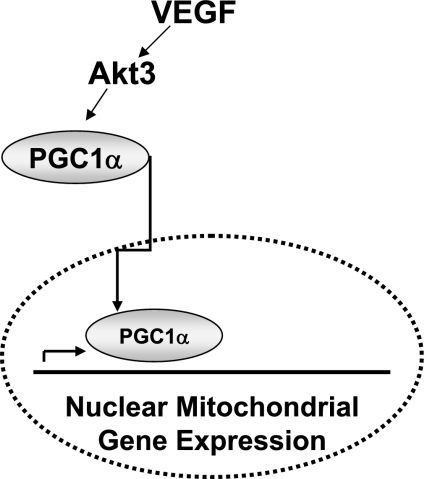
An increasing amount of literature suggests that individual Akts have specific roles in different cell types. Our findings support the model shown in Fig. 7, where Akt3 in primary ECs functions within a VEGF signaling pathway, leading to the regulation of a cellular program of mitochondrial biogenesis, at least in part, through the control of PGC-1α nuclear trafficking. Specific inhibition of Akt3, but not of Akt1, results in a reduction in mtDNA copy number and gene expression, indicating that Akt3, in particular, affects mitochondrial biogenesis and potentially the stability or turnover of the mitochondria. These molecular changes are manifested at the functional level, as we show that inhibition of Akt3 results in decreased cellular respiration and changes in mitochondrial distribution and stability. Mitochondrial biogenesis is regulated by the coordination between nuclear and mitochondrial gene expression. VEGF stimulation results in a marked increase in nuclear encoded mitochondrial gene expression, which is controlled by Akt3. In addition, we show a subtle but statistically significant mitochondrial phenotype in Akt3 null animals, i.e., fewer and larger per unit area. Taken together, our findings suggest that Akt3 plays a role in stimulating mitochondrial biogenesis in some conditions.
Figure 7.
Model of VEGF stimulation of mitochondrial biogenesis. VEGF stimulation results in the activation of Akt3, which controls the nuclear localization of the master regulator of mitochondrial biogenesis, PGC-1α, resulting in the increased expression of nuclear encoded mitochondrial gene expression.
The Akt family of kinases comprises three highly homologous members; Akt1, Akt2, and Akt3 (49, 50). Overexpression of constitutively activated forms of individual family members results in similar phenotypes in epithelial cells and in lymphoid cells. Transgenic animals expressing myristylated forms of Akt1, Akt2, and Akt3 from the lck promoter results in lymphoma (51). Oncogenesis induced by expression of these constitutively activated kinases is identical, indicating that these kinases share substrate specificity in the generation of the oncogenic phenotype. This, in combination with the mild nature of the Akt1 and Akt2 knockouts, suggests redundancy in downstream function (52, 53). These findings have been used to argue that the Akt family members have overlapping functions and to suggest that the observed phenotypes are due to a reduced level of total Akt activity of functionally redundant isoforms (13). However, there is growing evidence that these three kinases have distinct functions in distinct cell types: 1) insulin-induced glucose uptake is preferentially controlled by Akt2, not by Akt1 (15, 54); 2) Akt3, not Akt1, modulates G2/M phase transition in ovarian carcinoma (55); 3) individual family members can be differentially activated by EGF stimulation (18); and 4) in breast cancer, Akt1 controls tumor cell motility and invasion (16, 17).
Our results show that Akt3 is required for stimulation of mitochondrial biogenesis downstream of VEGF stimulation in primary ECs. Because Akt2 is not expressed in ECs, this cell type provides an opportunity to compare and contrast Akt1 vs. Akt3 function in the absence of Akt2. Using a direct comparison between Akt1 and Akt3 silencing, we have consistently shown that Akt3 gene silencing results in a reduction in mitochondrial DNA content, oxygen consumption, and in both nuclear and mitochondrial specific gene expression. Indeed, at least in ECs, our findings suggest that Akt3 may play a compensatory role for Akt2. Others have shown a role for Akt2 in the regulation of insulin-dependent PGC-1α transcriptional control in the liver (42, 43). We show that Akt3 may function similarly in ECs, with blockade of Akt3 resulting in the reduction of PGC-1α-dependent gene expression and a shuttling of PGC-1α out of the nucleus. Interestingly, however, we have not been able to demonstrate a direct interaction between Akt3 and PGC-1α (data not shown) or whether Akt3 acts directly or indirectly with PGC-1α in primary ECs remains to be determined.
In addition, these findings demonstrate a novel downstream function for VEGF, stimulation of mitochondrial biogenesis via the regulation of Akt3. Our previously published findings suggest that Akt3 is regulated by a VEGF-driven pathway dependent on the sphingosine-1-phosphate receptor, S1P3/Edg3. A distinction between the regulation of Akt1/2 and Akt3 exists; activation of S1P3 results in an up-regulation of Akt3 expression in primary ECs, which is either concurrent with or precedes its activation by phosphorylation. Whether the VEGF induction of the mitochondrial biogenesis program is also dependent on S1P3 is presently under investigation but would provide an additional level at which to regulate cellular energetics in ECs.
Although mitochondria are the primary source of cellular energy production through oxidative phosphorylation, they are increasingly viewed as important for other diverse cellular processes, such as calcium homeostasis and apoptosis. Defects in mitochondrial function contribute to many human diseases, such as cancer and cardiovascular disease (56, 57). Mechanisms exist to increase mitochondrial oxidative capacity in response to cellular energy demands. It is important to note that Akt3 expression, compared to other family members, has a more restricted pattern; Akt3 is most highly expressed in the brain and heart (47, 48), which are shown to have a higher mitochondrial content than other organs of the body (58, 59). It is probably this reason then, we were able to find a mild, but significant, mitochondrial phenotype in brain tissue per se in the Akt3-KO animals, having consistently fewer mitochondria per square area than wild-type controls. In many knockout animal models, the chronic absence of a signal is often compensated by other mechanisms. Therefore, while the evidence of mitochondrial dysfunction is subtle in the Akt3-KO brains, these findings suggest an important role for Akt3, which is incompletely compensated for, at least in brain tissue. The Akt3-null animals do not have a phenotype related to heart development, even though this tissue does contain a large concentration of mitochondria. Given the complexities and the tissue-specific control of mitochondrial biogenesis, it is unclear if the heart should display similar phenotype as brain. However, a change in cardiac function in the Akt3-null animals has not yet been addressed. Whether Akt3-null animals display a more severe reduction in vascular-specific mitochondrial content is presently under investigation.
The major phenotype of Akt3-null animals is a postnatal reduction in brain size that is attributed to fewer and smaller cells. Whether this reduction is due to changes in cell proliferation or survival is not presently known. Concerning discussion of functional consequences of the larger but less numerous mitochondria that exist within the brains of Akt3-null mice, it is likely that additional compensatory mechanisms (either during development or specific to brain tissue) exist. Clearly, the phenotype in vivo was more subtle than that observed in cell culture, suggesting some degree of compensation. We can speculate that an energetic deficit during the development may underlie the smaller brain. However, this remains speculative, as we have no direct evidence linking brain size with the mitochondrial abnormalities.
Interestingly, the PGC1α-null animal also exhibits a neurological phenotype, both behavioral and degenerative (60, 61) and has increased sensitivity (increased neurodegenerative effects) to inducers of reactive oxygen species (ROS) (62). Mitochondrial metabolism is linked to the production of ROS; unpaired electrons react with oxygen-generating superoxide, which plays an important role in neurodegeneration, as well as in cardiac disease and in aging. Whether Akt3-null animals have an increased sensitivity to ROS remains to be determined. However, this could be a contributing factor to the overall reduction in cell number in the brains of Akt3-null animals. Decreased mitochondrial function has been correlated with neurodegeneration, and Akt3 could also play a role in this context as well.
Mitochondrial biogenesis is largely thought to be regulated at the level of transcription. NRF-1 and NRF-2 are responsible for the transcriptional activation of the major mtDNA transcriptional regulator, TFAM (6), which is responsible for the regulation of mtDNA transcription. mtDNA encodes 13 genes, whose proteins are essential components of the respiratory complexes I, III, IV, and V (63, 64). All remaining mitochondrial proteins are encoded within the nucleus. Nuclear-encoded mitochondrial proteins are synthesized in the cytosol and must be imported and processed by complex import machinery prior to their functional incorporation within the mitochondrial proteome. The relation between protein import and mitochondrial reticulum expansion has received little attention; however, because the vast majority of mitochondrial proteins are imported, the two processes are likely to be closely linked. Thus, we consider it highly significant that the major import protein, Tom70, is regulated by Akt3. These findings also suggest that Tom70 is controlled in a PGC-1α-dependent fashion.
Angiogenesis is thought to be responsible for the majority of new blood vessel formation in the adult (65). Processes, such as angiogenesis, that require remodeling and new tissue production demand higher levels of ATP synthesis than quiescent tissue. We provide evidence that VEGF, functioning at least in part through the regulation of Akt3, stimulates a cellular program controlling mitochondrial biogenesis. Signaling pathways that coordinate cell growth and cellular remodeling with metabolic capacity (i.e., mitochondrial biogenesis) have been inferred to exist, but very little is known about the nature of this regulation. Delineation of this novel pathway and its regulation of mitochondrial biogenesis may lay the groundwork for the design of new angiogenic therapies for the treatment of a broad range of human diseases linked to mitochondrial function (66, 67) and may provide insight into the fundamental processes that regulate mitochondrial mass.
Acknowledgments
This work was supported in part by grants from National Institute of Neurological Disorders and Stroke (P20 RR16434) and National Institute of Heart, Lung, and Blood (HL084565) to R.M.H. We thank R. Visconti for help with photography and L. Obeid, Y. Hannun, C. Beeson, and R. Schnellman for their helpful suggestions.
References
- Yancopoulos G D, Davis S, Gale N W, Rudge J S, Wiegand S J, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Posakony J W, England J M, Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol. 1977;74:468–491. doi: 10.1083/jcb.74.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes G W, Mahler H R, Perlman R S. Nuclear gene dosage effects on mitochondrial mass and DNA. J Cell Biol. 1974;61:565–574. doi: 10.1083/jcb.61.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D A, Irrcher I, Ljubicic V, Joseph A M. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209:2265–2275. doi: 10.1242/jeb.02182. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren J M, Young L H, Pypaert M, Mu J, Birnbaum M J, Shulman G I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virbasius J V, Scarpulla R C. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M T, Hoogenraad N J. Mitochondrial-nuclear communications. Annu Rev Biochem. 2007;76:701–722. doi: 10.1146/annurev.biochem.76.052305.091720. [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann J M. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- MacKenzie J A, Payne R M. Mitochondrial protein import and human health and disease. Biochim Biophys Acta. 2007;1772:509–523. doi: 10.1016/j.bbadis.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber C B, Eldridge J, Taha T A, Obeid L M, Muise-Helmericks R C. Modulation of total Akt kinase by increased expression of a single isoform: Requirement of the sphingosine-1-phosphate receptor, Edg3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp Cell Res. 2006;312:1164–1173. doi: 10.1016/j.yexcr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Leung S, Stark G R. Activation of phosphatidylinositol 3-kinase in response to interleukin- 1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X D, Xu P Z, Chen M L, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen W S, Crawford S E, Coleman K G, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17:1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulukos K E, Pognonec P, Sariban E, Bailly M, Lagrou C, Ghysdael J. Rapid and transient expression of Ets2 in mature macrophages following stimulation with cMGF, LPS, and PKC activators. Genes Dev. 1990;4:401–409. doi: 10.1101/gad.4.3.401. [DOI] [PubMed] [Google Scholar]
- Bae S S, Cho H, Mu J, Birnbaum M J. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- Irie H Y, Pearline R V, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge J S. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroulakou I G, Oemler W, Naber S P, Tsichlis P N. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Okano J, Gaslightwala I, Birnbaum M J, Rustgi A K, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem. 2000;275:30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- Ichida M, Nemoto S, Finkel T. Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor gamma Coactivator-1 alpha (PGC-1alpha) J Biol Chem. 2002;277:50991–50995. doi: 10.1074/jbc.M210262200. [DOI] [PubMed] [Google Scholar]
- Schnellmann R G, Monks T J, Mandel L J, Lau S S. 2-Bromohydroquinone-induced toxicity to rabbit renal proximal tubules: the role of biotransformation, glutathione, and covalent binding. Toxicol Appl Pharmacol. 1989;99:19–27. doi: 10.1016/0041-008x(89)90107-5. [DOI] [PubMed] [Google Scholar]
- Tu B P, Kudlicki A, Rowicka M, McKnight S L. Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science. 2005;310:1152–1158. doi: 10.1126/science.1120499. [DOI] [PubMed] [Google Scholar]
- Pianetti S, Arsura M, Romieu-Mourez R, Coffey R J, Sonenshein G E. Her-2/neu overexpression induces NF-κB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IκB-α that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- Schmitz K J, Otterbach F, Callies R, Levkau B, Holscher M, Hoffmann O, Grabellus F, Kimmig R, Schmid K W, Baba H A. Prognostic relevance of activated Akt kinase in node-negative breast cancer: a clinicopathological study of 99 cases. Mod Pathol. 2004;17:15–21. doi: 10.1038/modpathol.3800002. [DOI] [PubMed] [Google Scholar]
- Tokunaga E, Kimura Y, Oki E, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Baba H, Maehara Y. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. Int J Cancer. 2006;118:284–289. doi: 10.1002/ijc.21358. [DOI] [PubMed] [Google Scholar]
- Marmor M D, Skaria K B, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Mambo E, Chatterjee A, Xing M, Tallini G, Haugen B R, Yeung S C, Sukumar S, Sidransky D. Tumor-specific changes in mtDNA content in human cancer. Int J Cancer. 2005;116:920–924. doi: 10.1002/ijc.21110. [DOI] [PubMed] [Google Scholar]
- Yano M, Kanazawa M, Terada K, Namchai C, Yamaizumi M, Hanson B, Hoogenraad N, Mori M. Visualization of mitochondrial protein import in cultured mammalian cells with green fluorescent protein and effects of overexpression of the human import receptor Tom20. J Biol Chem. 1997;272:8459–8465. doi: 10.1074/jbc.272.13.8459. [DOI] [PubMed] [Google Scholar]
- De Gannes F M, Leducq N, Diolez P, Belloc F, Merle M, Canioni P, Voisin P J. Mitochondrial impairment and recovery after heat shock treatment in a human microglial cell line. Neurochem Int. 2000;36:233–241. doi: 10.1016/s0197-0186(99)00118-7. [DOI] [PubMed] [Google Scholar]
- Pletjushkina O Y, Lyamzaev K G, Popova E N, Nepryakhina O K, Ivanova O Y, Domnina L V, Chernyak B V, Skulachev V P. Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim Biophys Acta. 2006;1757:518–524. doi: 10.1016/j.bbabio.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Hallmann A, Milczarek R, Lipinski M, Kossowska E, Spodnik J H, Wozniak M, Wakabayashi T, Klimek J. Fast perinuclear clustering of mitochondria in oxidatively stressed human choriocarcinoma cells. Folia Morphol (Warsz) 2004;63:407–412. [PubMed] [Google Scholar]
- Asin-Cayuela J, Gustafsson C M. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem Sci. 2007;32:111–117. doi: 10.1016/j.tibs.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12:360–365. doi: 10.1016/s1043-2760(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman B M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Scarpulla R C. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Lehman J J, Barger P M, Kovacs A, Saffitz J E, Medeiros D M, Kelly D P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L K, Mansfield C M, Lehman J J, Kovacs A, Courtois M, Saffitz J E, Medeiros D M, Valencik M L, McDonald J A, Kelly D P. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba M O. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba M O, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci U S A. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr P T, Spiegelman B M. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1α expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach K A, Schnellmann R G. Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem. 2007;282:2355–2362. doi: 10.1074/jbc.M608009200. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon J C, Zhang C Y, Krauss S, Mootha V K, Lowell B B, Spiegelman B M. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARγ coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum M J. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1α transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey C J, Yoon J C, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman B M. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla R C, Spiegelman B M. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Schreiber S N, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRα) J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Kurukulasuriya R, Link J T, Madar D J, Pei Z, Richards S J, Rohde J J, Souers A J, Szczepankiewicz B G. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr Med Chem. 2003;10:123–153. doi: 10.2174/0929867033368556. [DOI] [PubMed] [Google Scholar]
- Tschopp O, Yang Z Z, Brodbeck D, Dummler B A, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings B A. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Easton R M, Cho H, Roovers K, Shineman D W, Mizrahi M, Forman M S, Lee V M, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum M J. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol Cell Biol. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T O, Rittenhouse S E, Tsichlis P N. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Mende I, Malstrom S, Tsichlis P N, Vogt P K, Aoki M. Oncogenic transformation induced by membrane-targeted Akt2 and Akt3. Oncogene. 2001;20:4419–4423. doi: 10.1038/sj.onc.1204486. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen J L, Chu Q, Feng F, Birnbaum M J. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Chen W S, Xu P Z, Gottlob K, Chen M L, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzakri K, Karlsson H K, Vestergaard H, Madsbad S, Christiansen E, Zierath J R. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes. 2006;55:785–791. doi: 10.2337/diabetes.55.03.06.db05-0796. [DOI] [PubMed] [Google Scholar]
- Cristiano B E, Chan J C, Hannan K M, Lundie N A, Marmy-Conus N J, Campbell I G, Phillips W A, Robbie M, Hannan R D, Pearson R B. A specific role for AKT3 in the genesis of ovarian cancer through modulation of G(2)-M phase transition. Cancer Res. 2006;66:11718–11725. doi: 10.1158/0008-5472.CAN-06-1968. [DOI] [PubMed] [Google Scholar]
- Singh K K. Mitochondrial dysfunction is a common phenotype in aging and cancer. Ann N Y Acad Sci. 2004;1019:260–264. doi: 10.1196/annals.1297.043. [DOI] [PubMed] [Google Scholar]
- Goffart S, von Kleist-Retzow J C, Wiesner R J. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Gwosdow G, Collipp P J, Maddaiah V T. Effect of growth hormone on weight and mitochondrial protein content of rat heart. Res Commun Chem Pathol Pharmacol. 1976;14:763–766. [PubMed] [Google Scholar]
- Oldendorf W H, Cornford M E, Brown W J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu P H, Tarr P T, Lindenberg K S, St-Pierre J, Zhang C Y, Mootha V K, Jager S, Vianna C R, Reznick R M, Cui L, Manieri M, Donovan M X, Wu Z, Cooper M P, Fan M C, Rohas L M, Zavacki A M, Cinti S, Shulman G I, Lowell B B, Krainc D, Spiegelman B M. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α-null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Leone T C, Lehman J J, Finck B N, Schaeffer P J, Wende A R, Boudina S, Courtois M, Wozniak D F, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy J O, Medeiros D M, Schmidt R E, Saffitz J E, Abel E D, Semenkovich C F, Kelly D P. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi J M, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon D K, Bachoo R, Spiegelman B M. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Rantanen A, Larsson N G. Regulation of mitochondrial DNA copy number during spermatogenesis. Hum Reprod. 2000;15:86–91. doi: 10.1093/humrep/15.suppl_2.86. [DOI] [PubMed] [Google Scholar]
- Lightowlers R N, Chinnery P F, Turnbull D M, Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet. 1997;13:450–455. doi: 10.1016/s0168-9525(97)01266-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain R K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Isner J M, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]