Abstract
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause cystic fibrosis (CF). The most common mutation, ΔF508, omits the phenylalanine residue at position 508 in the first nucleotide binding domain (NBD1) of CFTR. The mutant protein is retained in the endoplasmic reticulum and degraded by the ubiquitin-proteasome system. We demonstrate that expression of NBD1 plus the regulatory domain (RD) of ΔF508 CFTR (ΔFRD) restores the biogenesis of mature ΔF508 CFTR protein. In addition, ΔFRD elicited a cAMP-stimulated anion conductance response in primary human bronchial epithelial (HBE) cells isolated from homozygous ΔF508 CF patients. A protein transduction domain (PTD) could efficiently transduce (∼90%) airway epithelial cells. When fused to a PTD, direct addition of the ΔFRD peptide conferred a dose-dependent, cAMP-stimulated anion efflux to ΔF508 HBE cells. Hsp70 and Hsp90 associated equally with WT and ΔF508 CFTR, whereas nearly twice as much of the Hsp90 cochaperone, Aha1, associated with ΔF508 CFTR. Expression of ΔFRD produced a dose-dependent removal of Aha1 from ΔF508 CFTR that correlated with its functional rescue. These findings indicate that disruption of the excessive association of the cochaperone, Aha1, with ΔF508 CFTR is associated with the correction of its maturation, trafficking and regulated anion channel activity in human airway epithelial cells. Thus, PTD-mediated ΔFRD fragment delivery may provide a therapy for CF.—Sun, F., Mi, Z., Condliffe, S. B., Bertrand, C. A., Gong, X., Lu, X., Zhang, R., Latoche, J. D., Pilewski, J. M., Robbins, P. D., Frizzell, R. A. Chaperone displacement from mutant cystic fibrosis transmembrane conductance regulator restores its function in human airway epithelia.
Keywords: CFTR, Aha1, rescue, PTD
Loss of the cystic fibrosis transmembrane conductance regulator (CFTR) anion conductance from the apical membranes of airway epithelia disrupts regulation of the airway surface liquid layer. This leads to impaired mucociliary clearance, airway infection, and inflammation characteristic of cystic fibrosis (CF) (1, 2). The common ΔF508 mutation of CFTR is present on at least one allele in >90% of CF patients, and ∼50% of patients are ΔF508 homozygotes (3, 4). A central issue in CF disease is the inability of this common CFTR variant to achieve the native, folded state that will exit from the endoplasmic reticulum (ER) and traffic to the epithelial cell apical membrane (5).
When acquisition of the native conformation is retarded, CFTR is thought to maintain excessive or prolonged interactions with molecular chaperones (6,7,8), which then target the protein for degradation by mechanisms that police the ER for misfolded or incompletely complexed proteins (9). ER-associated degradation (ERAD) involves ubiquitination of aberrant proteins and their delivery to the proteasome for digestion. If ERAD lags behind the rate of protein synthesis, or during treatment with proteasome inhibitors, aggregates of the mutant protein accumulate. CFTR was the first integral membrane mammalian protein to be identified as a substrate for ubiquitin-proteasome mediated degradation (10, 11), and it has served as a model for the growing list of diseases of protein conformation, which account for a diverse set of pathological etiologies (12).
Essentially all of the ΔF508 CFTR produced by the cell is destroyed by ERAD. Also, due to its complex folding pattern, ∼60–70% of the wild-type (wt) protein may be similarly degraded (13), although this may vary among cell types (14). The proteolytic cleavage patterns of the immature forms of wt and ΔF508 CFTR are similar, whereas the digestion pattern of mature wt CFTR is different (15). This finding supports the concept that at least a portion of the ER-retained mutant CFTR is present in an intermediate conformation that is formed along the normal CFTR folding pathway, as opposed to the formation of a variant protein structure. For ΔF508 CFTR, this intermediate conformation cannot proceed beyond a critical step in the folding process, but this implies that ΔF508 CFTR could be rescued if it were possible to facilitate this step.
A variety of experimental conditions, such as reduced temperature, incubation with chemical chaperones, or pharmacological correctors (16,17,18), can promote the escape of ΔF508 CFTR from the ER, yielding a functional anion channel at the cell surface (19,20). In addition, investigators have reported restoration of ΔF508 CFTR function by coexpression of various partial CFTR constructs or subdomains from wt CFTR (21,22,23). However, a consensus as to which CFTR subdomains are effective in mutant protein rescue is not apparent, and the mechanism of this effect remains obscure. In addition, CFTR fragment-induced rescue has been observed primarily in cells exogenously overexpressing both the CFTR fragment and full-length ΔF508 CFTR.
In the present study, we determined the efficacy of several CFTR fragments in mediating the rescue of ΔF508 CFTR maturation in exogenous expression systems and extended the findings to primary human bronchial epithelial (HBE) cells. The mechanism of rescue was inferred from selective changes in the association of ΔF508 CFTR with a specific chaperone cofactor that is involved in CFTR biogenesis. In addition, we show that linking the effective fragment to a peptide sequence that facilitates protein entry into airway cells promotes CFTR maturation in HBE cells derived from homozygous ΔF508 patients.
MATERIALS AND METHODS
DNA constructs
Various domains of CFTR or ΔF508 CFTR were amplified by polymerase chain reaction (PCR). An EcoRI or EcoRV site with kozak sequence and an initial codon at the NH2 terminus and a stop codon with an XhoI site at the COOH terminus were created for NBD1 (aa 413–586), NBD1-RD (aa 413–838), ΔFNBD1 (aa 413–585), and ΔFRD (aa 413–837). An HA-tag was added to the COOH termini of NBD1, ΔFNBD1, and ΔFRD. These domains were subsequently cloned into the pCDNA3.1 expression vector (confirmed by sequencing). Full-length CFTR and ΔF508 CFTR, derived from pBQ4.7, and pBQ4.7ΔF508, respectively, were subcloned into pcDNA3.1 at NotI and EcoRV sites.
Expression, pulse-chase, infection, and immunoprecipitation (IP)
CHO cells were transfected with ΔF508 CFTR or ΔF508 CFTR plus ΔFRD. At 24 h post-transfection, cells were labeled with 35S-Met/Cys (100 μCi/ml) for 30 min and then chased for the indicated times. Cells were lysed in 1× RIPA buffer, and the cell lysates were immunoprecipitated with CFTR antibodies 24-1 and M3A7 overnight at 4°C. After incubation (2–3 h) with protein A and G beads, pelleted beads were washed 3 times with 1× RIPA buffer. 35S-labeled proteins were analyzed by SDS-PAGE and autoradiography. In steady-state assays, precipitated proteins were phosphorylated in vitro with 5 U of PKA catalytic subunit and 10 μCi [γ-32P]ATP, resolved by SDS-PAGE, and detected by autoradiography (24, 25).
For coimmunoprecipitation (co-IP) studies, human embryonic kidney (HEK) 293 cells were transfected with plasmids containing CFTR, a CFTR fragment, or enhanced green florescent protein (EGFP) as control. After 48 h, cells were lysed [150 mM NaCl, 50 mM HEPES (pH 8.0), 1% Nonidet P-40, 1 mM EDTA, 10% glycerol, and protease inhibitors]. Equal amounts of total protein were subjected to IP with anti-CFTR antibody (as above) or anti-V5 (monoclonal antibody to a simian virus protein sequence, Invitrogen) as a control. CFTR-interacting chaperones were then detected by immunoblot (IB).
Transduction with CFTR fragments or EGFP was performed using either recombinant adenoviruses or a peptide transduction domain (PTD) -linked fusion protein. Viruses were generated using the ViraPower Adenoviral Expression System (Invitrogen, Carlsbad, CA, USA). Crude viral stocks were amplified twice, and recombinant adenoviruses were purified and titrated as described previously (26). His-tagged fusions with the peptide transduction domain, PTD5, were expressed in bacteria and purified on a nickel-agarose column, which was described previously (27).
Construction of PTD5 fusion proteins
The construction of the PTD5-EGFP or PTD5-ΔFRD fusion protein was performed by PCR. The 12 amino acids of PTD-5 were inserted at the amino terminus of EGFP or ΔFRD, whereas a 6-histidine amino acid tag was inserted at the carboxy terminus. The fusion proteins were expressed in the pET3b plasmid in Escherichia coli and purified on a nickel column (27).
Subcellular fractionation
The cells were washed with ice-cold phosphate buffered saline (PBS) twice, followed by homogenization with 0.25 M sucrose in 10 mm HEPES (pH 7.5) in a Dounce homogenizer on ice. The homogenate was centrifuged at 600 g for 10 min, 8000 g for 10 min, and 105,000 g for 60 min at 4°C. The resuspended extract from each sediment was further fractionated to provide the organelle fractions of nuclei, mitochondria, and endoplasmic reticulum. The final supernatant of the 105,000 g centrifugation step was used as the cytoplasmic fraction. The Golgi fraction was purified as reported previously (28).
Confocal microscopy
Confocal microscopy was performed as described previously (24, 25, 29). Briefly, after fixation in 2% paraformaldehyde and permeabilization with 2% paraformaldehyde plus 0.1% Triton X-100, cells were washed 3 times with buffer B [0.5% bovine serum albumin and 0.15% glycine (pH 7.4) in PBS]. After blocking with purified goat serum, cells were incubated with primary antibody against the His tag for 1 h, followed by 3 washes in buffer B, and subsequent incubation with Alexa568 (red)-labeled secondary antibodies (Molecular Probes, Eugene, OR, USA) for 1 h. After washing with buffer B, the cell nuclei were stained with cytox green (green label). The coverslips were then mounted for confocal microscopy. Images were analyzed and processed using Metamorph imaging software (Molecular Devices, Downingtown, PA, USA).
Functional assays for plasma membrane CFTR
HBE cells were isolated from the proximal airways dissected from excess pathological lung tissue of ΔF508 homozygous CF patients undergoing lung transplantation under an IRB-approved protocol using established methods (29). CF genotypes were obtained from medical records or from a commercial lab (Genzyme Genetics, Framingham, MA, USA) using cells isolated from the lung tissue. For fluorescence assays, these cells were cultured on glass coverslips for 2 wk, and cAMP-stimulated anion efflux was determined from the intensity response of the halide-sensitive fluorophore, SPQ [6-methoxy-N-(3-sulfopropyl)quinolinium] to cAMP agonists [10 μM forskolin (FSK) plus 100 μM isobutylmethlxanthine (IBMX)]. Cells were loaded with fluorophore by hypoosmotic shock in a NaI-based Ringer’s solution and were allowed to recover for 30 min before sequential perfusion on the microscope stage with solutions containing NaI, NaNO3, NaNO3 plus agonists, and again to NaI, as described previously (31). Procedures were performed at 37°C. The time course of fluorescence intensity, relative to that at time 0, F1/F0, provided normalization for dye loading. The data (mean±se) were obtained from ∼20 cells on each of two coverslips from each patient; each experiment was repeated at least twice.
For whole-cell patch-clamp recordings (32), HBE cells on coverslips were transferred to a chamber on an inverted microscope and perfused at 37°C with a phosphate-buffered bath solution (mM): 145 NaCl, 0.4 KH2PO4, 1.6 K2HPO4, 1.0 MgCl2, 1.5 CaCl2, and 5 glucose. Patch pipettes were fabricated from borosilicate capillaries (Warner Instrument Corporation, Hamden, CT, USA) on a micropipette puller (P-97, Sutter Instrument Co. Novato, CA, USA). After fire polishing, pipettes had resistances of 1.5–3 MΩ when filled with the pipette solution (mM): 125 KCl, 1.2 NaH2PO4, 4.8 Na2HPO4, 1 MgCl2, 5 glucose, 0.5 EGTA, 2 ATP, 0.05 GTP. After establishing the whole-cell configuration, membrane voltage was clamped at –30 mV using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA, USA) interfaced with a PC running pClamp 8.1 software. Whole-cell currents were recorded in response to voltage steps from –100 to +60 mV before and after bath addition of 20 μM forskolin. Whole-cell conductance (Gm) was calculated as the slope of the current-voltage (I-V) relation between –60 and +40 mV and normalized to cell size by the membrane capacitance estimate obtained at the outset of each experiment.
RESULTS
Biochemical evidence of fragment-induced ΔF508 CFTR maturation
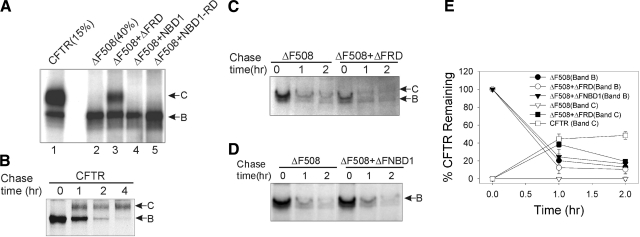
CFTR progression beyond the ER was monitored from the conversion of core-glycosylated, nascent CFTR (band B) to the complex glycosylated mature form (band C). We tested several CFTR fragments for mutant protein rescue by coexpression with full-length ΔF508 CFTR in CHO cells and assessed the appearance of band C in the steady state. The CFTR subdomains evaluated included the wt and ΔF508 versions of NBD1 or NBD1 plus the R domain (see Materials and Methods). Among these, expression of a construct comprising NBD1 plus the R domain from ΔF508 CFTR, termed ΔFRD, was most active in facilitating the appearance of mature ΔF508 CFTR (Fig. 1A. lane 3). Mature protein was not detected in CHO cells expressing ΔF508 CFTR alone (lane 2), or in cells coexpressing ΔF508 CFTR with wt or ΔF508 NBD1 (lane 4). Coexpression of ΔF508 CFTR with wt NBD1-RD yielded a small amount of the mature form (lane 5), noted with a more prolonged exposure than that shown. However, ΔFRD was clearly the most effective of the CFTR fragments examined. At steady state, its coexpression yielded ∼10% of the level of mature protein formed in cells expressing full-length wt CFTR under similar conditions, and this occurred without an increase in the expression of the immature form of ΔF508 CFTR, suggesting that this fragment was not simply increasing mature protein by augmenting mutant CFTR expression.
Figure 1.
ΔFRD expression promotes ΔF508 CFTR maturation. A) CHO cells were transfected with wt CFTR; ΔF508 CFTR; or ΔF508 CFTR plus ΔFRD, NBD1, or NBD1-RD. Equal amounts of total proteins (∼500 μg) were immunoprecipitated with anti-CFTR and phosphorylated using [γ-32P]ATP and PKA as described in Materials and Methods. The phosphorylated proteins, except as indicated, were fractionated on SDS-PAGE and detected by autoradiography. Arrows indicate mature CFTR band C or immature CFTR band B. B) Pulse-chase experiments in CHO cells transfected with wt CFTR and the kinetics of nascent CFTR degradation and conversion to mature CFTR were assayed as described in Materials and Methods. C) Identical to panel B, except cells were transfected with ΔF508 CFTR (ΔF508) or ΔF508 plus ΔFRD. D) Same as in panel C, except cells were transfected with ΔF508 CFTR or ΔF508 plus ΔFNBD1. E) Quantitation of the intensities of CFTR bands B and C at the indicated times from all experiments of the type shown in panels B–D. Band intensities at later times are expressed as a percentage of the CFTR band B density at t = 0 (100%). Data presented as mean ± se (n=3).
To determine whether ΔFRD influenced early steps in CFTR biogenesis, we performed pulse-chase experiments in CHO cells expressing wt or ΔF508 CFTR. The conversion of nascent wt CFTR to the mature form followed previously described kinetics (13), and this is illustrated in Fig. 1B. The mature protein was not formed in cells expressing ΔF508 CFTR alone; however, in cells expressing ΔF508 CFTR plus ΔFRD, a significant amount of the mature form of mutant CFTR was generated after a 1 h chase (Fig. 1C). Restoration of ΔF508 CFTR maturation was not observed in cells coexpressing ΔF508 NBD1 (termed ΔFNBD1 in Fig. 1D). These findings indicate that expression of the ΔFRD fragment permits a fraction of ΔF508 CFTR to escape the ER quality control system and to traffic to the plasma membrane.
Quantitation of the degradation and maturation kinetics of ΔF508 CFTR from several pulse-chase experiments is illustrated in Fig. 1E. The rate of immature ΔF508 CFTR degradation was independent of ΔFRD expression with a half-life, t1/2, of ∼40 min. Thus, the maturation of ΔF508 CFTR facilitated by expression of the ΔFRD fragment was not due to interference with its degradation. This finding, together with the increase in C-to-B band ratio observed at steady state, indicates that ΔFRD promoted folding of the mutant, to elicit the post-ER processing of about one-third (38±5.3%) of the initially labeled ΔF508 CFTR after a 1 h chase. This maturation efficiency was not significantly different from that of wt CFTR (44±6.5%) within the same timeframe. However, the rescued ΔF508 CFTR appeared less stable than wt CFTR. One-fifth (20±5.5%) of initially labeled ΔF508 CFTR remained after 2 h chase, whereas mature wt CFTR was relatively resistant to degradation (48±4.2% remained at same chase period). This finding is consistent with data indicating that ΔF508 CFTR that progresses beyond the ER when cells are cultured at low temperature or exposed to chemical chaperones remains unstable (33).
Evidence of functional ΔF508 CFTR at the cell surface
To determine whether the biochemical evidence of ΔFRD-evoked ΔF508 CFTR rescue translates to the formation of anion channels at the cell surface, we initially measured cAMP-stimulated anion efflux using the halide-sensitive fluorophore SPQ in primary cultures of HBE cells isolated from homozygous ΔF508 CF patients (ΔF/ΔF HBE). ΔF/ΔF HBE cells were infected with recombinant adenovirus carrying full-length wt CFTR (AdCFTR), ΔFRD (AdΔFRD), or EGFP (AdEGFP). EGFP monitored the transduction efficiency, which showed that >90% of cells exhibited GFP fluorescence 2 days postinfection. As shown in Fig. 2A, ΔF/ΔF HBE cells infected with AdCFTR exhibited a rapid dequenching of SPQ fluorescence on cAMP-stimulation, whereas cells expressing EGFP did not respond significantly. However, ΔF/ΔF HBE cells transduced with AdΔFRD showed a significant fluorescence response on the stimulation. As a control, HeLa cells infected with AdΔFRD did not exhibit an anion conductance response (Fig. 2B) so that the airway cell response was not due to a conductance property of ΔFRD itself. These data confirm the biochemical results and indicate that expression of ΔFRD in ΔF/ΔF HBE cells produces a cAMP-regulated anion transport response.
Figure 2.
ΔFRD transduction increases cAMP-stimulated anion permeability and conductance in ΔF/ΔF human airway cells. A) Time course of relative SPQ fluorescence in response to cAMP agonists (10 μM FSK plus 100 μM IBMX) in ΔF/ΔF HBE cells infected with AdCFTR, AdΔFRD, or AdEGFP; multiplicity of infection (MOI) = 200. Cells were placed on microscope stage and perfused with NaI media at t = 0; indicated solution changes were made at 3 min intervals. Data are from multiple coverslips using cells from one patient; representative of data from three patients. B) SPQ assays in Hela cells infected with AdΔFRD or AdCFTR, performed as in panel A; MOI = 50. C) I-V relations obtained during whole-cell patch-clamp recordings from ΔF/ΔF HBE cells infected with AdCFTR (circles) or AdΔFRD (squares); currents obtained under basal (closed symbols) and cAMP-stimulated (open symbols) conditions. Holding potential = –50 mV. D) Identical to panel C, except the cells were infected with AdEGFP. E) A typical trace of the whole-cell currents obtained at –50 mV. F) Summary of peak cAMP-induced whole-cell conductance (ΔGm), normalized for apparent cell surface area (nS/pF) in ΔF/ΔF HBE cells infected with AdCFTR (n=7), AdΔFRD (n=6), or AdGFP (n=7). Statistical probabilities are indicated.
Next, we measured whole-cell currents in ΔF/ΔF HBE by patch clamp. The I-V relations illustrated in Fig. 2C show that ΔF/ΔF HBE cells transduced with AdCFTR exhibited a low basal Cl− conductance that increased >20-fold in response to cAMP stimulation with forskolin. Moreover, ΔF/ΔF HBE cells infected with AdΔFRD showed a similar cAMP-dependent increase in whole-cell Cl− current. The activated conductance, which was blocked by 100 μM glibenclamide (data not shown), exhibited a linear I-V relation characteristic of CFTR. The low basal conductance of cells transduced with AdEGFP was not augmented by forskolin (Fig. 2D). The cAMP-stimulated Cl− currents of AdCFTR and AdΔFRD transduced cells were reversed to baseline levels on agonist washout (Fig. 2E). On average, ΔF/ΔF HBE cells transduced with AdΔFRD resulted in a cAMP-dependent membrane conductance change (ΔGm) that was 86% of that observed in cells transduced with AdCFTR (Fig. 2F). A comparison of data of Figs. 1 and 2 shows an apparent disparity between the ΔGm response of HBE cells and the biochemical expression of mature CFTR (∼10% of wt levels) at steady state in CHO cells. While we do not know the levels of mature CFTR protein obtained in ΔF/ΔF HBE cells, a disproportionate relation between functional responses and CFTR protein expression level has been observed previously (34). The results from SPQ and patch-clamp assays demonstrate that ΔFRD expression promotes the delivery of functional ΔF508 CFTR channels to the surface of CF patient-derived airway cells.
Chaperone interactions with ΔF508 CFTR
Next, we examined the effect of ΔFRD on the expression levels of several molecular chaperones implicated in CFTR biogenesis, including Hsp70, Hsp90, Hdj-2, calnexin, and cysteine string protein (Csp), in CHO and Calu-3 cells. As determined by IB (Supplemental Fig. 1), steady-state chaperone expression levels were not affected by ΔFRD expression and, therefore, would not account for its action on mutant CFTR maturation. Next, we determined whether introduction of ΔFRD altered the interaction of wt or ΔF508 CFTR with a subset of chaperones by co-IP. Hsp70, Hdj-2, and Hsp90 were found to interact with the immature forms of both wt and ΔF508 CFTR (6,7,8). As shown in Fig. 3A, Hsp70 and Hsp90 associated with both wt and ΔF508 CFTR to a similar degree; that is, their binding did not distinguish between the immature forms of wt and ΔF508 CFTR (top panel lane 2 vs. 4). Moreover, the coexpression of ΔFRD did not alter these CFTR-chaperon interactions (top lanes 3 and 5) and, as control, the presence of ΔFRD did not alter our ability to immunoprecipitate the full-length CFTRs (bottom panel). Using quantitative IPs, we found ∼0.5% of endogenous Hsp70 and ∼0.01% of endogenous Hsp90 associated with wt or mutant CFTR band B at steady state.
Figure 3.
Preferential interaction of Aha1 with ΔF508 CFTR is reduced by ΔFRD. A) HEK293 cells were transfected as follows: wt CFTR plus EGFP (lane 2); wt CFTR plus ΔFRD (lane 3); ΔF508 CFTR plus EGFP (lane 4); ΔF508 CFTR plus ΔFRD (lanes 5 and 6). Equal amounts of total protein (∼50 μg) were subjected to IP with anti-CFTR (lanes 2–5) or anti-V5 (lane 6) monoclonal antibodies. Of the total protein used in the IP of lane 2, 5% was loaded in lane 1 as input. Coprecipitated Hsp70 (top panel), Hsp90 (middle panel), or precipitated CFTR (bottom panel) were detected by IB. HC, antibody heavy chain. B) HEK293 cells were transfected with ΔF508 CFTR plus EGFP (lanes 1, 7); ΔF508 CFTR plus ΔFRD (lanes 2–4); wt CFTR plus EGFP (lane 5); wt CFTR plus ΔFRD (lanes 6). Wt or ΔF508 CFTR:ΔFRD plasmid DNA ratios are indicated above the panels. Following co-IP (protocol identical to A), IBs were performed with anti-Aha1 or anti-CFTR. Relative Aha1 density (given below panel) gives the average of two independent experiments. Total proteins (30 μg) were loaded for detection of ΔFRD or Aha1 expression (bottom panels).
Preferential binding of Aha1 to ΔF508 CFTR is reduced by ΔFRD
In contrast to Hsp70 and Hsp90, the interaction of Aha1, an Hsp90 cochaperone, with wt and ΔF508 CFTR differed significantly. On average, 1.7 times more Aha1 was associated with ΔF508 CFTR relative to wt CFTR (Fig. 3B, lanes 1 vs. 5). Importantly, the interaction between Aha1 and ΔF508 CFTR was attenuated (lanes 2, 3) or abolished (lane 4) by coexpression of ΔFRD in a dose-dependent manner. The relatively weaker interaction of Aha1 with wt CFTR was also markedly reduced by ΔFRD expression (lanes 5 vs. 6). Again, the presence of ΔFRD did not alter CFTR IP efficiency. Thus, the expression of ΔFRD resulted in a dose-dependent maturation of ΔF508 CFTR that correlated with a reduced interaction with Aha1. These findings suggest that the displacement of excess ΔFRD from ΔF508 CFTR results in an energetically more favorable folding environment for the mutant, allowing a fraction of ΔF508 CFTR to exit the ER (35). The lower efficacy of ΔFRD-mediated rescue in HEK293 compared with CHO cells likely reflects differences in these cell lines, which were observed previously in Aha1 knockdown experiments (35). Together, these results demonstrate that Aha1 differentiates ΔF508 CFTR from wt CFTR and that ΔFRD disrupts the preferential association of Aha1 with the mutant protein.
PTD5 transduces human airway epithelial cells
PTDs are small positively charged peptides identified in diverse proteins such as HIV Tat and Drosophila antennapedia (Antp). They can facilitate the efficient delivery of relatively large proteins across cell membranes in an energy and receptor-independent manner (36). We have identified and characterized a class of positively charged peptides that can facilitate protein transduction in a variety of cell types, including airway epithelial cells (27).
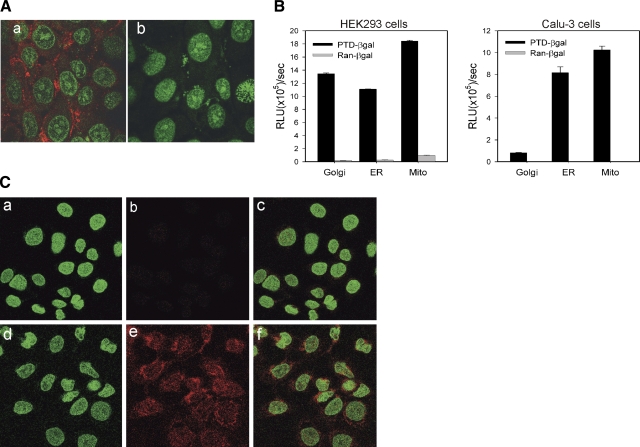
To determine whether the transduction peptide PTD5 can facilitate protein uptake by human airway cells, a PTD5-biotin-streptavidin-Cy3 complex was incubated with Calu-3 cells in culture medium. Three hours postadministration, the cells were analyzed for transduction by fluorescence confocal microscopy. A significant Cy3 signal was observed in the PTD5-treated Calu-3 cells (Fig. 4Aa), but not in control peptide treated cells (Fig. 4Ab). Airway cell uptake of a PTD5-EGFP fusion protein was also observed (Supplemental Fig. 2). Next, we examined the distribution of a PTD5-β-gal fusion protein following transduction of HEK293 cells and Calu-3 cells. The ability of PTD5 to facilitate delivery of the β-gal protein to several intracellular organelles following internalization was examined by analysis of β-gal activity in fractionated organellar compartments, as described in Materials and Methods. Figure 4B shows that PTD5 was able to deliver β-gal to ER, Golgi, and mitochondria. Finally, we tested whether PTD-ΔFRD polypeptide efficiently transduces human airway epithelial cells. No detectable PTD-ΔFRD was observed at time 0 when the polypeptide was added to ΔF508 CFTR expressing CFBE41o- cells at a concentration of 0.4 mg/ml (Fig. 4Ca–c), whereas robust signals were detected 150 min following incubation with PTD-ΔFRD (Fig. 4Cd–f). Approximately 90% of the cells stained positive for the epitope carried by the fusion protein. Taken together, these results suggest that PTD5 can facilitate the efficient transduction of protein into airway cells and, therefore, in principle, this approach could be employed for the luminal delivery of therapeutic proteins for treatment of pulmonary diseases such as CF.
Figure 4.
PTD-5 facilitates delivery of marker proteins and ΔFRD into airway epithelial cells. A) PTD-5 streptavidin-Cy3 (a) was added to Calu-3 culture medium at 37°C; a random peptide streptavidin-Cy3 was used as control (b). Three hours after addition of the fusion peptide complexes, the intracellular localization of the Cy 3 fluorescence signal (red) was visualized by confocal microscopy. Nuclei were counter stained with cytox green (green). B) PTD5-β-Gal fusion protein, or a random control peptide (Ran-β-gal) (both 2 μg/ml), was added to the culture media bathing HEK293 or Calu-3 cells at 37°C. Three hours postaddition, the cells were washed extensively, and organelles were isolated by differential centrifugation. β-Gal activity of the purified organellar fractions was analyzed using a Galacto-light kit (Applied Biosystems, Foster City, CA, USA) after normalization of the compartment volumes. C) PTD-ΔFRD fusion protein (0.4 mg/ml) was added to the medium of CFBE41o-ΔF cells at room temperature. At t = 0 (a–c) and 150 min post-transduction (d–f), the cells were extensively washed and then fixed and permeablized. Immunostaining was performed using His antibody against the fusion protein tag (red). Nuclei were counterstained with cytox green (green).
Functional rescue by a PTD-ΔFRD fusion protein
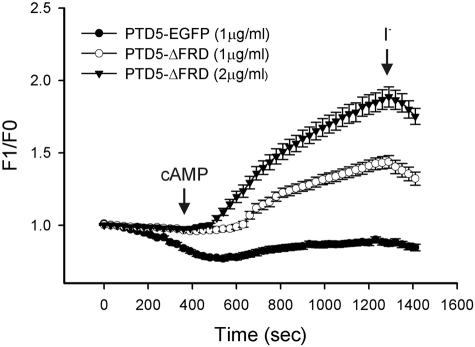
To determine whether PTD-mediated delivery of ΔFRD can rescue ΔF508 CFTR, a PTD5-ΔFRD fusion protein was constructed and purified from a bacterial expression system (Supplemental Fig. 3). The control PTD5-EGFP fusion protein effectively transduced polarized Calu-3 cells. More than 90% of cells exhibited green fluorescence after a 1 h incubation, whereas a control peptide-EGFP fusion was an ineffective transducer of EGFP expression (Supplemental Fig. 3). We then asked whether a PTD5-ΔFRD fusion protein could rescue functional ΔF508 CFTR maturation in ΔF/ΔF HBE cells. We observed a dose-dependent increase in cAMP-stimulated anion efflux from ΔF/ΔF airway cells by SPQ fluorescence (Fig. 5) after 2 h of treatment with PTD5-ΔFRD. The relative magnitude of this response was similar to that observed after viral transduction of HBE cells with ΔFRD or wt CFTR (Fig. 2). These data demonstrate that the PTD5-ΔFRD peptide effectively transduces airway cells to rescue cAMP-dependent ΔF508 CFTR function.
Figure 5.
PTD5-ΔFRD fusion peptide restores cAMP-stimulated anion permeability to ΔF/ΔF HBE cells. ΔF/ΔF HBE cells were incubated with the indicated concentrations of PTD5-ΔFRD or PTD5-EGFP for 2 h, followed by SPQ fluorescence assay of cAMP-stimulated halide efflux as described in Materials and Methods and Fig. 2.
DISCUSSION
CFTR biogenesis has served as a model for so-called conformational diseases, in which alterations in ER protein processing or quality control underlie a diverse set of pathologies (9, 12). Changes in ER protein processing may lead to stable misfolded protein conformations that produce dominant-negative effects, aggregation-prone conformations that produce cellular stress and toxicity (e.g., neurodegenerative diseases), or unstable misfolded protein conformations that lead to functional deficiencies. The loss of anion channel function that results from deficient ΔF508 CFTR biogenesis conforms to the latter category of conformational disease.
The results of this study demonstrate that a fragment of ΔF508 CFTR comprising NBD1 plus the R domain (ΔFRD), when introduced into cells expressing full-length ΔF508 CFTR, partially corrects the maturation and function of the mutant protein. This phenomenon was observed in cells transiently expressing both ΔF508 CFTR and ΔFRD, as well as in primary airway epithelial cells endogenously expressing ΔF508 CFTR, when ΔFRD was expressed from recombinant adenovirus or the peptide was added directly, fused to a protein transduction domain.
In attempting to resolve the mechanism, we examined ΔFRD-induced alterations in chaperone interactions with full-length ΔF508 CFTR that may explain this phenomenon. First, in contrast with Hsp70 and Hsp90, the Hsp90 cochaperone Aha1 associated preferentially with ΔF508 CFTR. Preferential binding of Aha1 to ΔF508 CFTR has not been reported previously; however, the silencing of Aha1 resulted in ΔF508 CFTR maturation (35). Second, expression of ΔFRD reduced the preferential association of Aha1 with ΔF508 CFTR. Thus, our results suggest that ΔFRD, as at least one consequence of its action, promotes the biochemical and functional maturation of ΔF508 CFTR by reducing its association with the cochaperone, Aha1. Data presented here are consistent with the prevailing theory that prolonged and/or excessive interactions of unfolded proteins with molecular chaperones target them for degradation when the native conformation is not achieved (37), and they suggest that the interaction of CFTR with Aha1 is an important factor in this respect. It is likely, however, that changes in other components of interacting chaperone complexes contributing to ΔFRD-mediated ΔF508 CFTR correction remain to be identified.
Molecular chaperones play essential roles both in protein biogenesis, where they facilitate folding and suppress aggregation, and in protein degradation, where they contribute to quality control by promoting the recognition and proteolysis of misfolded proteins. Two major molecular chaperone families that are associated with CFTR during its biogenesis are Hsp40-Hsp70 (8) and Hsp90 and its regulators (7, 35). Aha1 is a cochaperone of Hsp90 that stimulates its ATPase activity and thereby modulates the dynamics of chaperone-client protein interactions (35). The silencing of Aha1 resulted in ΔF508 CFTR maturation, indicating that it is possible to promote a favorable folding environment for ΔF508 CFTR by perturbing its interactions with chaperones. Similar in principle to the silencing of Aha1, ΔFRD fragment delivery efficiently displaced Aha1 from its association with full-length ΔF508 CFTR, and this resulted in maturation of the mutant protein.
Our search for CFTR subdomains capable of ΔF508 CFTR rescue was not exhaustive due to the efficacy identified in ΔFRD. Other investigators have reported ΔF508 CFTR rescue with partial CFTR constructs that contain wt NBD1, a process that has been termed complementation (21,22,23). In the present work, however, it was clear that a wt NBD1-RD fragment was only marginally active compared to its ΔF508 counterpart. Presumably, the maturation of ΔF508 CFTR induced by expression of subdomains from wt CFTR is encouraged by the presence of a native partial structure. In polytopic proteins, including CFTR, domain folding is thought to occur cotranslationally, whereas the interfaces between domains assemble more slowly following translation of the full-length protein (38, 39). The issue of domain-domain interactions appears critical in mutant CFTR assembly, as inferred from several findings. First, marked structural differences between wt and ΔF508 NBD1 were not apparent from their crystal structures (40), with the caveat that the solved ΔF508 NBD1 contained suppressor mutations to promote solubility. Second, the F508 residue is surface exposed in the NBD1 crystal structure (41), and modeling studies of CFTR in relation to intact ABC transporters whose structures are solved imply that this surface interacts with cytoplasmic loops of the transmembrane domains (42). Third, studies of protease sensitivity, as a measure of folded protein conformation, suggest that the ternary structure of CFTR is obtained post-translationally and that domain interactions are critical in conferring stability to the full-length protein (43, 44). These findings suggest that wt CFTR fragments may promote the biogenesis of full-length ΔF508 CFTR by providing a structural scaffold that, at least transiently, circumvents inadequate domain interactions that underlie ΔF508 CFTR instability. If this is true, then the mechanism of ΔFRD rescue is likely to be mechanistically different from that produced by wt CFTR fragments. Rather, our data suggests that the efficacy of ΔFRD is based on its ability to alter the chaperone-mediated folding environment of ΔF508 CFTR.
Finally, protein transduction domains can be used to deliver full-length proteins as well as small peptides into cells. The advantage to protein transduction appears to be the efficiency of delivery. For example, intraperitoneal injection of a PTD-β-gal complex resulted in the transduction of a large percentage of cells within different tissues including liver, muscle, lung, and even brain (36). Given that the therapeutic proteins to be delivered are identical to endogenous proteins, the immune response to the injected proteins appears to be minimal, depending on the size and type of transduction domain used. Thus PTDs may be useful in the treatment of acute diseases as well as chronic diseases, both genetic and acquired. Delivery of functional, therapeutic proteins using PTDs has been demonstrated in several models of disease. Using a Tat PTD-Bcl-XL fusion protein, several groups have demonstrated the ability to protect neuronal cells from apoptosis following brain ischemia (45). In addition, the delivery of peptides derived from p16, a cell cycle regulatory protein, and p53, a proapoptotic tumor suppressor, have been shown to limit tumor growth (46, 47). For CF, it is likely that therapies targeting the functions of molecular chaperones per se will result in nonspecific or toxic effects on cells and may evoke an unfolded protein response that leads to apoptosis (48). In contrast, protein-specific targeting, as utilized in the present study, may provide a selective therapeutic approach that does not generally perturb the ER quality control machinery. Therefore, this approach may also have implications for other protein folding diseases.
Acknowledgments
We thank Yuee Wang for technical assistance, Dr. John R. Riordan (University of North Carolina, Chapel Hill, NC, USA) for M3A7 antibody, and Dr. William E. Balch (Scripps Research Institute, La Jolla, CA, USA) for polyclonal Aha1 antibody. Funding was provided by the U.S. National Institutes of Health (DK68196, DK72506, and DK725076) and the Cystic Fibrosis Foundation.
References
- Pilewski J M, Frizzell R A. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- Boucher R C. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Kerem B, Rommens J M, Buchanan J A, Markiewicz D, Cox T K, Chakravarti A, Buchwald M, Tsui L C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Davis P B. Cystic fibrosis. Pediatr Rev. 2001;22:257–264. doi: 10.1542/pir.22-8-257. [DOI] [PubMed] [Google Scholar]
- Cheng S H, Gregory R J, Marshall J, Paul S, Souza D W, White G A, O'Riordan C R, Smith A E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Janich S, Cohn J A, Wilson J M. The common variant of cystic fibrosis transmembrane conductance regulator is recognized by hsp70 and degraded in a pre-Golgi nonlysosomal compartment. Proc Natl Acad Sci U S A. 1993;90:9480–9484. doi: 10.1073/pnas.90.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo M A, Jensen T J, Cui L, Hou Y, Chang X B, Riordan J R. Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J. 1998;17:6879–6887. doi: 10.1093/emboj/17.23.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham G C, Lu Z, King S, Sorscher E, Tousson A, Cyr D M. The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J. 1999;18:1492–1505. doi: 10.1093/emboj/18.6.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J L. Chaperoning the maturation of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Lung Cell Mol Physiol. 2001;281:L39–L42. doi: 10.1152/ajplung.2001.281.1.L39. [DOI] [PubMed] [Google Scholar]
- Ward C L, Omura S, Kopito R R. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- Aridor M, Balch W E. Integration of endoplasmic reticulum signaling in health and disease. Nat Med. 1999;5:745–751. doi: 10.1038/10466. [DOI] [PubMed] [Google Scholar]
- Ward C L, Kopito R R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J Biol Chem. 1994;269:25710–25718. [PubMed] [Google Scholar]
- Varga K, Jurkuvenaite A, Wakefield J, Hong J S, Guimbellot J S, Venglarik C J, Niraj A, Mazur M, Sorscher E J, Collawn J F, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Kartner N, Lukacs G L. Limited proteolysis as a probe for arrested conformational maturation of delta F508 CFTR. Nat Struct Biol. 1998;5:180–183. doi: 10.1038/nsb0398-180. [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Lukacs G L, Du K, Caci E, Zegarra-Moran O, Galietta L J, Verkman A S. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Straley K S, Cao D, Gonzalez J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings L R, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack J H, Tung R, Grootenhuis P D, Negulescu P. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Loo T W, Bartlett M C, Clarke D M. Correctors promote maturation of cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by binding to the protein. J Biol Chem. 2007;282:33247–33251. doi: 10.1074/jbc.C700175200. [DOI] [PubMed] [Google Scholar]
- Denning G M, Anderson M P, Amara J F, Marshall J, Smith A E, Welsh M J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Sato S, Ward C L, Krouse M E, Wine J J, Kopito R R. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J Biol Chem. 1996;271:635–638. doi: 10.1074/jbc.271.2.635. [DOI] [PubMed] [Google Scholar]
- Owsianik G, Cao L, Nilius B. Rescue of functional DeltaF508-CFTR channels by co-expression with truncated CFTR constructs in COS-1 cells. FEBS Lett. 2003;554:173–178. doi: 10.1016/s0014-5793(03)01162-1. [DOI] [PubMed] [Google Scholar]
- Clarke L L, Gawenis L R, Hwang T C, Walker N M, Gruis D B, Price E M. A domain mimic increases DeltaF508 CFTR trafficking and restores cAMP-stimulated anion secretion in cystic fibrosis epithelia. Am J Physiol Cell Physiol. 2004;287:C192–199. doi: 10.1152/ajpcell.00337.2003. [DOI] [PubMed] [Google Scholar]
- Cormet-Boyaka E, Jablonsky M, Naren A P, Jackson P L, Muccio D D, Kirk K L. Rescuing cystic fibrosis transmembrane conductance regulator (CFTR)-processing mutants by transcomplementation. Proc Natl Acad Sci U S A. 2004;101:8221–8226. doi: 10.1073/pnas.0400459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Hug M J, Bradbury N A, Frizzell R A. Protein kinase A associates with cystic fibrosis transmembrane conductance regulator via an interaction with ezrin. J Biol Chem. 2000;275:14360–14366. doi: 10.1074/jbc.275.19.14360. [DOI] [PubMed] [Google Scholar]
- Sun F, Hug M J, Lewarchik C M, Yun C H, Bradbury N A, Frizzell R A. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- Van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- Mi Z, Mai J, Lu X, Robbins P D. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol Ther. 2000;2:339–347. doi: 10.1006/mthe.2000.0137. [DOI] [PubMed] [Google Scholar]
- Hamilton R L, Moorehouse A, Havel R J. Isolation and properties of nascent lipoproteins from highly purified rat hepatocytic Golgi fractions. J Lipid Res. 1991;32:529–543. [PubMed] [Google Scholar]
- Sun F, Zhang R, Gong X, Geng X, Drain P F, Frizzell R A. Derlin-1 promotes the efficient degradation of the cystic fibrosis transmembrane conductance regulator (CFTR) and CFTR folding mutants. J Biol Chem. 2006;281:36856–36863. doi: 10.1074/jbc.M607085200. [DOI] [PubMed] [Google Scholar]
- Devor D C, Bridges R J, Pilewski J M. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279:C461–C479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- Yang Y, Devor D C, Engelhardt J F, Ernst S A, Strong T V, Collins F S, Cohn J A, Frizzell R A, Wilson J M. Molecular basis of defective anion transport in L cells expressing recombinant forms of CFTR. Hum Mol Genet. 1993;2:1253–1261. doi: 10.1093/hmg/2.8.1253. [DOI] [PubMed] [Google Scholar]
- Schultz B D, Frizzell R A, Bridges R J. Rescue of dysfunctional deltaF508-CFTR chloride channel activity by IBMX. J Membr Biol. 1999;170:51–66. doi: 10.1007/s002329900537. [DOI] [PubMed] [Google Scholar]
- Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache K G, Papsin B, Zerangue N, Stenmark H, Lukacs G L. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol. 2004;164:923–933. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L G, Pickles R J, Boyles S E, Morris J C, Ye H, Zhou Z, Olsen J C, Boucher R C. In vitro assessment of variables affecting the efficiency and efficacy of adenovirus-mediated gene transfer to cystic fibrosis airway epithelia. Hum Gene Ther. 1996;7:51–59. doi: 10.1089/hum.1996.7.1-51. [DOI] [PubMed] [Google Scholar]
- Wang X, Venable J, LaPointe P, Hutt D M, Koulov A V, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, Riordan J R, Kelly J W, Yates J R, 3rd, Balch W E. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Agashe V R, Guha S, Chang H C, Genevaux P, Hayer-Hartl M, Stemp M, Georgopoulos C, Hartl F U, Barral J M. Function of trigger factor and DnaK in multidomain protein folding: increase in yield at the expense of folding speed. Cell. 2004;117:199–209. doi: 10.1016/s0092-8674(04)00299-5. [DOI] [PubMed] [Google Scholar]
- Jansens A, van Duijn E, Braakman I. Coordinated nonvectorial folding in a newly synthesized multidomain protein. Science. 2002;298:2401–2403. doi: 10.1126/science.1078376. [DOI] [PubMed] [Google Scholar]
- Kleizen B, van Vlijmen T, de Jonge H R, Braakman I. Folding of CFTR is predominantly cotranslational. Mol Cell. 2005;20:277–287. doi: 10.1016/j.molcel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Lewis H A, Zhao X, Wang C, Sauder J M, Rooney I, Noland B W, Lorimer D, Kearins M C, Conners K, Condon B, Maloney P C, Guggino W B, Hunt J F, Emtage S. Impact of the deltaF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- Lewis H A, Buchanan S G, Burley S K, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino W B, Hendrickson W A, Hunt J F, Kearins M C, Lorimer D, Maloney P C, Post K W, Rajashankar K R, Rutter M E, Sauder J M, Shriver S, Thibodeau P H, Thomas P J, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J L, Thomas P J. Building an understanding of cystic fibrosis on the foundation of ABC transporter structures. J Bioenerg Biomembr. 2007;39:499–505. doi: 10.1007/s10863-007-9117-7. [DOI] [PubMed] [Google Scholar]
- Du K, Sharma M, Lukacs G L. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- Cui L, Aleksandrov L, Chang X B, Hou Y X, He L, Hegedus T, Gentzsch M, Aleksandrov A, Balch W E, Riordan J R. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp F R, Lu A, Ran R, Graham S H, Chen J. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D R, Ezhevsky S A, Becker-Hapak M, Nagahara H, Wei M C, Dowdy S F. Transduced p16INK4a peptides inhibit hypophosphorylation of the retinoblastoma protein and cell cycle progression prior to activation of Cdk2 complexes in late G1. Cancer Res. 1999;59:2577–2580. [PubMed] [Google Scholar]
- Wang W, El-Deiry W S. Targeting p53 by PTD-mediated transduction. Trends Biotechnol. 2004;22:431–434. doi: 10.1016/j.tibtech.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rapp U K, Kaufmann S H. Glucose-regulated stress proteins and antibacterial immunity. Trends Microbiol. 2003;11:519–526. doi: 10.1016/j.tim.2003.09.001. [DOI] [PubMed] [Google Scholar]