Abstract
Background. Patients noted to have an inadequate future liver remnant on pre operative volumetric assessment are considered to be candidates for portal vein embolization (PVE). A subset of patients undergo laparoscopic intervention prior to PVE for staging purposes or to address the primary in Stage IV colon cancer. These patients usually undergo PVE as a subsequent additional procedure by the transhepatic route. The aim of this study was to assess the feasibility of portal vein ligation by the laparoscopic approach in suitable patients. Materials and methods. A retrospective review of a prospectively maintained database was performed to identify patients that underwent laparoscopic portal vein ligation (LPVL). The demographic, clinical, radiographic, operative and volumetric details were collected to determine the feasibility of portal vein ligation. Results. A total of nine patients underwent LPVL as part of a two stage procedure in preparation for subsequent major hepatectomy. With a median age of 67 yrs, the diagnoses included: colorectal metastasis (five patients), cholangiocarcinoma (three patients) and hepatocellular carcinoma (one patient). The ligation involved the right portal vein in all and was performed with silk ligature (seven patients) and clips (two patients). Volumetric data was available in six patients which showed a mean increase from 209.1 cc±97.76 to 495.83 cc±310.91 (increase by 181.5%) In two patients, inadequate hypertrophy mandated later embolization by percutaneous technique. Five patients underwent subsequent major hepatic resection as planned. The remaining four patients were noted to have progression of disease that precluded the planned procedure. There were no complications associated with LPVL. Conclusions. LPVL is feasible and can be safely performed. In a select group of patients, it may be considered as an alternative to subsequent embolization and thereby potentially absolve the need for an additional procedure with its attendant complications.
Keywords: laparoscopy, portal vein ligation, future liver remnant
Introduction
Portal vein occlusion (embolization/ligation) is undertaken in patients with an inadequate future liver remnant (FLR) prior to planned subsequent major hepatectomy 1,2,3. In the majority of patients the decision to proceed with portal vein occlusion is made on pre operative volumetric assessment and the patients then undergo portal vein embolization (PVE). A subset of patients are determined to be candidates for portal vein occlusion during staging laparoscopy when unexpected bilobar involvement is detected 4,5,6,7. In addition, patients with colorectal cancer and synchronous hepatic involvement that are taken to the operating room for laparoscopic resection of the primary could be potential candidates for portal vein occlusion. These patients are usually subjected to PVE as a separate procedure at a subsequent stage. The ability to ligate the portal vein at the same time as laparoscopy in eligible patients could potentially avoid another procedure in the future with its attendant complications. The aim of this study was to assess the feasibility of laparoscopic portal vein ligation (LPVL) in suitable patients at the same time as the initial laparoscopy.
Materials and methods
All patients who underwent LPVL for hepatobiliary malignancies were identified from a prospectively maintained database (11/2005 to 06/2007) Data relating to patient demographics, primary diagnosis and extent of hepatic involvement on CT scan was collected. LPVL was performed as described.
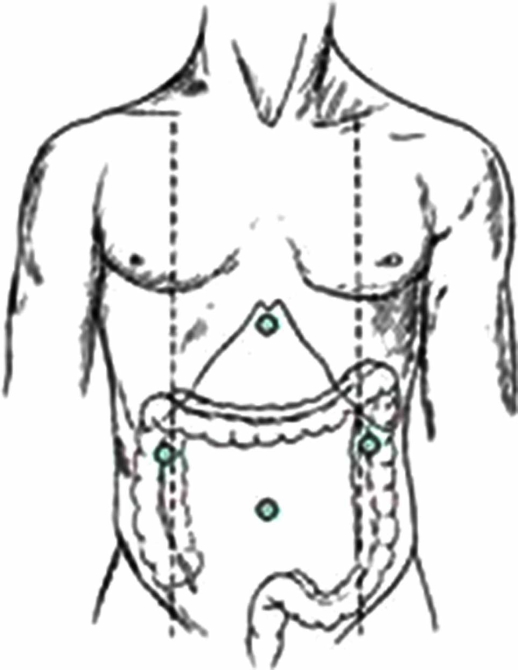
The patient is placed supine on the operating table with the positioning of trocars as shown in Figure 1. Access to the abdomen was gained by the Hasson technique and the pneumoperitoneum was maintained at 12 mmHg. After performing diagnostic laparoscopy, the need for portal vein ligation was determined in each patient based on the combination of pre operative radiological and intra op findings. An intra op ultra sound examination of the liver was performed in all patients.
Figure 1. .
Position of trocars.
The portal triad was dissected from the right side with the help of harmonic scalpel. The bile duct was dissected and elevated to expose the main portal vein. Further dissection was performed in the cranial direction to identify the portal bifurcation. The right portal vein was now dissected and encircled with a vessel loop. The right portal vein was now occluding with either clips or silk ligature. The details of additional procedures, performed at the same time as LPVL, mortality, morbidity and post operative course was obtained.
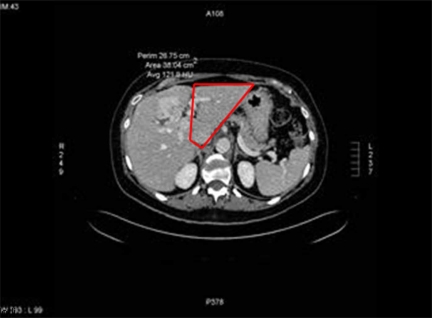
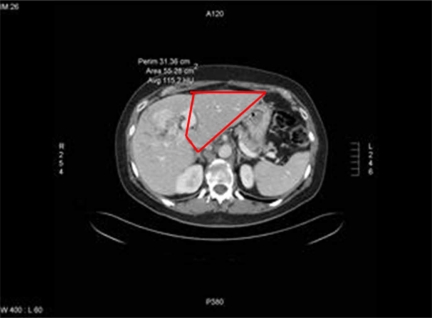
The extent of hepatic involvement was assessed on CT scan prior to LPVL. The majority of patients underwent CT scan 3–6 weeks after LPVL with some undergoing CT scan 7–8 weeks afterwards. The degree of hypertrophy (DOH) was assessed by performing volumetric assessments on the pre and post LPVL scans. (Figures 2 and 3). Volumetric measurements were performed by a radiologist by comparing the volume of the FLR before and after LPVL. The volume of the FLR was calculated by the following formula: remnant liver volume×100÷(total liver volume minus tumor volume). The percent change in size or DOH of the FLR was calculated by the following formula: DOH=(FLR volume: post LPVL) minus (FLR volume: pre LPVL)÷(FLR volume: pre PVL)×100.
Figure 2. .
CT Scan prior to laproscopic portal vein ligation.
Figure 3. .
CT Scan post laproscopic portal vein ligation.
Results
A total of nine patients underwent LPVL for various hepatobiliary malignancies. The patient demographics and clinical details are shown in Table I Colorectal metastasis accounted for 55% of the patients included in the study. One patient with colorectal metastasis received chemotherapy prior to LPVL.
Table I. Demographics and clinical details of nine patients included in the study.
| Pt # | Age | Sex | Diagnosis | Extent of hepatic involvement | Other hepatic procedure | Extra hepatic procedure |
|---|---|---|---|---|---|---|
| 1 | 49 | M | CLM | III, IV, VI, VII, VIII | Wedge excision seg III + biopsy PLN | None |
| 2 | 59 | M | CCA | I, IV (single lesion, size = 6.5 cm | None | None |
| 3 | 72 | F | CCA | I, VI, VII | Biopsy PLN | None |
| 4 | 62 | M | CLM | IV, V, VI, VII, VIII (single lesion measuring 12 cm) | Cholecystectomy | None |
| 5 | 77 | M | HCC | Extensive involvement of right lobe | Cholecystectomy | None |
| 6 | 67 | M | CLM | Multiple lesions in entire right liver and lesion in II, III | Wedge excision x 2-left lobe | Laparoscopic LAR |
| 7 | 73 | M | CLM | III, IV, V, VI, VII | Wedge excision seg III | Laparoscopic left Hemicolectomy |
| 8 | 49 | M | CLM | Multiple lesions in entire right liver | None | Laparoscopic LAR + TME + ileostomy + portacath |
| 9 | 75 | M | CCA | I, IV, VII, VIII | Cholecystectomy | None |
CLM = colorectal liver metastasis; CCA = cholangiocarcinoma; HCC = hepatocellular carcinoma; PLN = portal lymph nodes; LAR = low anterior resection; TME = total mesorectal excision.
The right portal vein was ligated in all patients. Metallic clips were used in two patients and silk ligature (2–0 silk) was used in the subsequent seven patients to occlude the right portal vein. Seven patients underwent other hepatic procedures such as wedge excision of lesions in the left lobe, biopsy of portal lymph nodes and cholecystectomy at the same time as LPVL (Table I). In three patients, simultaneous resection of the colorectal primary was performed. The mortality rate was zero and there were no morbidities related to LPVL. The mean length of stay was 6.7 days (±1.4). After excluding the patients who underwent a simultaneous colorectal procedure, the mean length of stay was 3.5 days (±1.4).
The extent of hepatic involvement prior to LPVL is shown in Table I All the patients had extensive involvement of the right lobe of the liver. The caudate lobe and the left lateral segment were involved in three patients. In patients with involvement of the left lateral segment, wedge excision was performed simultaneously with LPVL.
Volumetric data was available in six patients of whom three patients did not undergo the planned subsequent major hepatic rescection. In these three patients volumetric data was performed beyond the customary four week period. (Table II) The pre operative mean volume for all six patients was 209.1 + / − 97.76. Post LPVL volumes increased to a mean of 495.83 + / − 310.91. The mean difference in volume between pre and post LPVL was 286.83 + / − 313.94. The difference in volume ranged from 22 ml to 877 ml. The mean DOH was 181.5%. The maximal DOH was noted in patient # 9 (423%) with a diagnosis of cholangiocarcinoma.
Table II. Volumetric data.
| Volumetric data |
|||||
|---|---|---|---|---|---|
| Patient # | Diagnosis | Pre op (ml) | Post op (ml) | Difference in volume (ml) | DOH (%) |
| 1 | CLM | 218.6 | 1095 | 877 | 401 |
| 2 | CCA | 349 | 510 | 161 | 46 |
| 3 | CCA | 223 | 245 | 22 | 10 |
| 6 | CLM | 106 | 292 | 186 | 175 |
| 7 | CLM | 267 | 357 | 90 | 34 |
| 9 | CCA | 91 | 476 | 385 | 423 |
| Mean (+/ − SD) | 209.1 + / − 97.76 | 495.83 + / − 310.91 | 286.83 + / − 313.94 | 181.5 | |
CLM = colorectal liver metastasis; CCA = cholangiocarcinoma; DOH = degree of hypertrophy.
Five patients (55%) underwent a subsequent major hepatectomy as planned. Three patients underwent a right hepatectomy and two patients required an extended right hepatectomy to obtain a R0 resection. The caudate lobe was resected in two patients. In patient #1 there was evidence of new lesions (<1cm) on the non-embolized side that required wedge resections. The remaining four patients were noted to have progression of disease that precluded the planned major hepatectomy.
Discussion
Portal vein occlusion has become an integral component in the treatment algorithm of patients with inadequate FLR 1,2,3. Portal vein occlusion redirects blood to the FLR and has been shown to reduce the risk of complications due to peri operative liver failure 1,2,3. This is usually accomplished by the technique of PVE which was initially described by Makucchi et al. in 1984 8 and substantiated in a later report in 1990 9 for patients with cholangiocarcinoma. Kinoshita et al. described the similar technique in patients with hepatocellular carcinoma in 1986 10. The clinical benefit of PVE have been documented in patients with colorectal hepatic metastasis 11,12,13 and hepatocellular carcinoma 14,15,16. Although PVE is the preferred choice by many, a role for PVL has also been demonstrated by some authors 17,18,19.
The decision to occlude the portal vein is usually based on pre operative volumetric assessment of the FLR. These patients are subjected to PVE through the ipsilateral or contralateral liver lobe by the transhepatic approach. A subset of patients who are candidates for staged hepatectomy undergo laparoscopic intervention prior to PVE. This includes patients with Stage IV colon cancer who are taken to the operating room for laparoscopic resection of the primary. In addition, some patients are determined to be candidates for staged hepatic resection at the time of staging laparoscopy 4,5,6,7. These patients undergo PVE as an additional procedure at a later stage. The ability to ligate the portal vein at the time of initial laparoscopy could potentially avoid this additional procedure. The aim of this study was to assess feasibility of LPVL in patients with various hepatobiliary malignancies.
The results of our study demonstrate that LPVL is feasible in a select group of patients. There were no complications in relation to LPVL. In patients requiring laparoscopic resection of the colorectal primary, simultaneous LPVL did not lead to increased morbidity. Laparoscopy also enabled us to perform wedge resections of tumors in the FLR at the time of LPVL. LPVL was associated with acceptable DOH (increase in volume of FLR by 181.5%). Five patients (55%) underwent a subsequent major hepatectomy as planned. The evidence of progression of disease precluded a major hepatectomy in the remaining four patients.
There are some potential advantages to the approach of LPVL. Portal vein ligation at the time of laparoscopy avoids a subsequent second procedure. LPVL can also avoid the morbidity that is associated with PVE. PVE is known to be associated with several technical and liver related complications 20,21. The rate of complications associated with PVE has been reported to be in the range of 12.8–15% 20,21. The complications associated with PVE include, haemobilia, haemoperitoneum, arterial puncture, puncture site haematomas, subcapsular haematomas, pseudoaneurysm, pneuomothorax, migration of embolic material to FLR, occlusion of main portal vein, arteriovenous and arterioportal fistulas.
Some authors have suggested that PVE in the presence of disease in the FLR can lead to disproportionate hypertrophy of the tumors in relation to normal liver 22,23. Elias et al. 22 noted that in patients with functionally intact liver parenchyma, the growth rate of metastasis was more rapid than that of the normal liver. The ability to resect minimal disease in the FLR at the same time as LPVL could potentially decrease the risk of disease progression. Another advantage of laparoscopic PVL is that it does not preclude subsequent PVE if required. Failure to undergo hypertrophy following ligation can be seen in patients with variations in right portal vein anatomy which has been noted to be present in 17% of patients. Two patients in our study did not demonstrate adequate DOH of hypertrophy following PVL. These patients underwent subsequent PVE by the transhepatic route and were noted to have adequate DOH.
The results of our study demonstrate the LPVL is feasible, safe and is associated with acceptable DOH. This approach may be suitable in a select group of patients that need to go to the operating room initially for addressing the primary lesion or staging purposes. LPVL helps to avoid a subsequent procedure with its attendant complications. The ability to address minimal disease in the FLR at the same time could also potentially reduce the risk of disease progression in the FLR. Therefore, in a select group of patients, LPVL can be considered as a viable alternative to PVE.
References
- 1.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without pre operative portal vein embolization. Arch Surg. 2002;137:675–81. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 2.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–94. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 3.Selzner N, Pestalozzi BC, Kadry Z, Selzner M, Wildermuth S, Clavien PA. Downstaging colorectal metastasis by concomitant unilateral portal vein ligation and selective intra-arterial chemotherapy. Br J Surg. 2006;93:587–92. doi: 10.1002/bjs.5281. [DOI] [PubMed] [Google Scholar]
- 4.Grobmyer SR, Fong Y, D'Angelica MA, DeMatteo RP, Blumgart LH, Jarnagin WR. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastasis. Arch Surg. 2004;139:1326–30. doi: 10.1001/archsurg.139.12.1326. [DOI] [PubMed] [Google Scholar]
- 5.Thaler K, Kanneganti S, Khajanchee Y, Wilson C, Swanstrom L, Hansen PD. The evolving role of staging laparoscopy in the treatment of colorectal hepatic mestastasis. Arch Surg. 2005;140:727–34. doi: 10.1001/archsurg.140.8.727. [DOI] [PubMed] [Google Scholar]
- 6.Mann CD, Neal CP, Metcalfe MS, Pattenden CJ, Dennison AR, Berry DP. Clinical risk score predicts yeild of staging laparoscopy in patients with colorectal liver metastasis. Br J Surg. 2007;94:855–9. doi: 10.1002/bjs.5730. [DOI] [PubMed] [Google Scholar]
- 7.D'Angelica M, Fong Y, Weber S, Gonen M, DeMatteo RP, Conlon K, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Onc. 2003;10:183–9. doi: 10.1245/aso.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 8.Makuuchi M, Takayasu K, Takuma T, et al. Pre operative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to bile duct carcinoma. J Jon Soc Clin Surg 1984;45:14–20 (Japanese). [Google Scholar]
- 9.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Pre operative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–7. [PubMed] [Google Scholar]
- 10.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Pre operative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–8. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki S, Makuuchi M, Kakazu T, Miyagawa S, Takayama T, Kosuge T, et al. Resection for multiple metastatic liver tumors after portal embolization. Surgery. 1994;115:674–7. [PubMed] [Google Scholar]
- 12.Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996;24:1386–91. doi: 10.1053/jhep.1996.v24.pm0008938166. [DOI] [PubMed] [Google Scholar]
- 13.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–6. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109–15. doi: 10.1007/BF01655721. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura T, Nakajima Y, Une Y, Namieno T, Ogasawara K, Yamashita K, et al. Efficacay and safety of pre operative precutaneous transhepatic portal vein embolization with absolute ethanol: a clinical study. Surgery. 1997;83:135–41. doi: 10.1016/s0039-6060(97)90282-8. [DOI] [PubMed] [Google Scholar]
- 16.Yamakodo K, Takeda K, Matsumura K, Nakatsuka A, Hirano T, Kato N, et al. Regeneration of the unembolized liver parenchyma following portal vein embolization. J Hepatol. 1997;27:871–80. doi: 10.1016/s0168-8278(97)80325-x. [DOI] [PubMed] [Google Scholar]
- 17.Kinmanesh R, Farges O, Adballa EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all surgical approach for complete resection of primary gastrointestinal stromal tumours with multiple bilateral liver metastasis. JACS. 2003;197:164–70. doi: 10.1016/S1072-7515(03)00334-X. [DOI] [PubMed] [Google Scholar]
- 18.Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoridis J, et al. Two-stage liver surgery for advanced liver metastasis synchronous with colorectal tumor. Hepatogastroenterology. 2004;51:413–8. [PubMed] [Google Scholar]
- 19.Aussilhou B, Lesurtel M, Sauvanet A, Farges O, Dokmak S, Goasquen N, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of left liver remnant. J Gastrointest Surg. 2008;12:297–303. doi: 10.1007/s11605-007-0410-x. [DOI] [PubMed] [Google Scholar]
- 20.Di Stefano DR, de Baere T, Denys A, Hakime A, Gorin G, Gillet M, et al. Pre operative percutaneous portal vein embolization: evaluation of adverse events in 188 patients. Radiology. 2005;234:625–30. doi: 10.1148/radiol.2342031996. [DOI] [PubMed] [Google Scholar]
- 21.Kodoma Y, Shimizu T, Endo H, Miyamato M, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13:1223–27. doi: 10.1016/s1051-0443(07)61970-8. [DOI] [PubMed] [Google Scholar]
- 22.Elias D, DeBaere T, Roche A, Ducreux M, Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastasis is more rapid than that of liver parenchyma. Br J Surg. 1999;86:784–8. doi: 10.1046/j.1365-2168.1999.01154.x. [DOI] [PubMed] [Google Scholar]
- 23.Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastasis after preoperative hemihepatic portal vein embolization. Hepatology. 2001;34:267–72. doi: 10.1053/jhep.2001.26513. [DOI] [PubMed] [Google Scholar]