Abstract
The clinical experience using a novel technique of liver resection with vascular staplers for dissection of hepatic parenchyma, was documented most recently in a prospective manner. These data have clearly demonstrated for the first time that stapler hepatectomy is a safe and fast dissection technique in major liver surgery (e.g. hepatectomy) which is feasible in a routine clinical setting.
Keywords: liver resection, parenchymal transection, stapler hepatectomy, liver resection technique, endo vascular stapler
Introduction
Improvements in surgery very often depend on technical novelties. The introduction of stapling devices has been one of the most important improvements in surgery of the last decades and had a great impact on many different aspects. Today, staplers have become widely accepted for many types of open and laparoscopic procedures 1,2,3,4,5,6. They provide fast and reliable sutures, thus saving time, decreasing blood-loss and increasing security. Resection of liver parenchyma is one of the most common procedures in hepatobiliary surgery 7,8. One of the most important steps of this type of operation is the transection of the liver parenchyma. Since intraoperative hemorrhage is one of the main contributors to morbidity and mortality, the Pringle maneuver (PM) is still used quite frequently 9. While liver resection should be radical enough to safely resect tumors the procedure should be performed in a tissue preserving manner. The additional demands of modern hepatic surgery are to omit PM or vascular control in general 10,11 and thus to decrease warm ischemia/reperfusion injury of the remnant liver 11,12,13,14,15,16. New techniques for liver resection aim to not only being radical enough to offer curative treatment to underlying diseases while adequate functional tissue is left, but also to prevent any injury to remnant liver while there is minimal blood loss 11,14,15,16,17. Therefore, optimal techniques aim to minimize bleeding and prevent ischemia/reperfusion injury to the liver by avoidance of methods of vascular control 13,18,19,20. In about 80% of stapler hepatectomies, methods of vascular control are not needed, as reported elsewhere 21. Only in few cases PM or other methods of blood inflow control are applied based on the surgeon's personal judgement.
For resection of liver parenchyma, various methods have been established over the past few years. Ultrasound dissection, microwave tissue coagulation and transection using water jets are among the improvements for liver resection that surgeons can choose from 7,22,23,24,25,26,27. Further, the development of modern methods of coagulation, e.g. argon beam and electrocoagulation, offer fast and reliable techniques for prevention of seepage and have revolutionized hepatic surgery 28.
Today, vascular staplers have become an accepted tool in liver surgery. They are routinely used for dissection of hepatic vessels 29,30,31. More than fifteen years ago, hepatic wedge resection with various types of stapling devices has been tried, but publications on this are limited 32. In the 1990s, an operative procedure was developed that allows transection of liver parenchyma with staplers 33. This has been introduced to the head of our institution by Leslie H. Blumgardt, Sloan Kettering Memorial Cancer Center, New York, NY, USA. Since October 2001 it has become the standard technique for liver resection in our department. Depending on the surgeons choice, more than 70% of all major hepatectomies have been performed as stapler hepatectomy over the last six years in our department 21. Until now, we have performed more than 550 staplerhepatectomies with great success. The first 300 stapler hepatectomies underwent a critical prospective analysis 21,34. Here we review our first experiences with endo-GIA vascular staplers for parenchymal transection during liver resection 21.
Stapler hepatectomy
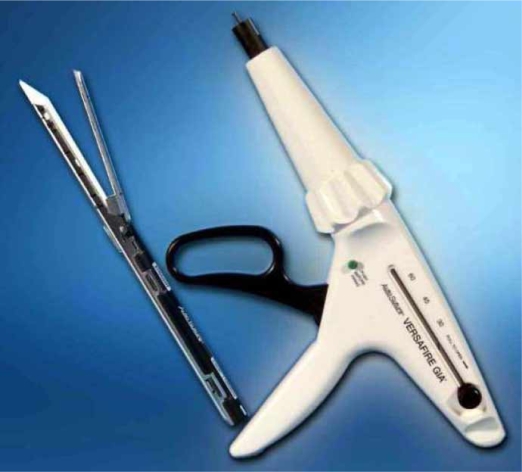
The stapler can be assembled quite easily. After the staple cartridge is attached to a lengthy shaft the stapler is ready to use. It can be rotated freely, thus allowing easy handling and exact placement of the device (Figure 1). When the device is fired, two triple suture lines are performed simultaneously. Division of tissue is performed between these triple lines and vessels are securely closed.
Figure 1. .
Endo-GIA vascular stapler. Hepatectomies are performed in our department with endo-GIA vascular staplers. Picture depicts the compouds of the stapler.
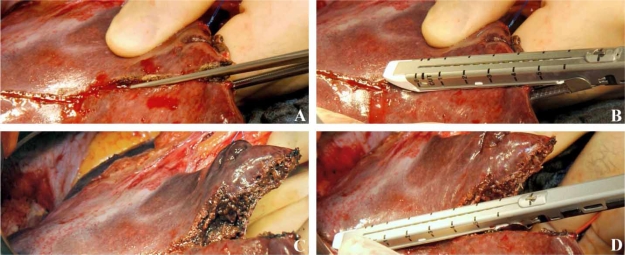
Hepatectomies are performed in our department with the above described stapler, which has been approved for this indication in the USA (FDA 510(k) number K061095), based on a highly standardized protocol in patients with both benign and malign liver disease as described elsewhere 21. Briefly, after opening the abdominal wall using a reversed L-shaped incision and exploration of the abdominal cavity, the liver is mobilized. Short hepatic veins and caudate veins from the inferior vena cava are clipped. If a hemihepatectomy is performed, the corresponding pedicle with portal vein, artery and bile duct and the corresponding hepatic vein are separated using linear vascular staplers. The appropriate hepatic artery is clipped additionally. After demarcation of the transectional line, a straight clamp is used for fracturing of hepatic parenchyma. Subsequently, this portion of liver is transected with a vascular stapler (Figure 2). The clamp and the stapler are used in an alternating manner until complete resection of the liver is achieved. During the liver resection a central venous pressure higher than 5 cm H2O seems to be associated with an increased blood loss. Therefore, the central venous pressure should be as low as possible to reduce bleeding 35. Besides mono and bipolar electrocoagulation, argon beam coagulation is used at the end of resection to achieve complete haemostasis.
Figure 2. .
Stepwise transection of liver tissue for resection. (A) A straight clamp is used for fracturing of hepatic parenchyma. (B) Subsequently, this portion of liver is transected with a vascular stapler. (C) Vessels of the transectional plane are securely closed. (D) The clamp and the stapler are used in an alternating manner until complete resection of the liver is achieved.
Costs
For some authors, it is doubtful whether staplers, being expensive surgical tools, can be used cost effectively 36. Undeniably, linear vascular staplers increase the total material costs significantly. Nonetheless, since stapler hepatectomy has been introduced to our department, it has become the technique of our choice and both, the total operative time and need for transfusions decreased, being main contributors to costs of liver resection 21,34. Thus, mean costs for a hepatectomy decreased by 2.400, – [euro] per case. For our analysis of costeffectiveness, material costs, costs for anaesthesia and surgery itself and costs of hospital and ICU stay were considered relevant 21,34.
Blood loss and the need for vascular control
In surgery, hemorrhage can be one of the most important intraoperative problems and also is considered to be an important determinant factor for postoperative outcome. Therefore, one of the modern surgery's aims is to prevent blood loss and to reduce patient morbidity 37. With refinement of surgical techniques the overall need for PM and other methods of vascular inflow control in liver resections has decreased. Especially stapler hepatectomy offers some great advantages. Both the median intraoperative blood loss (major resection: 800 ml; minor resection 500 ml) and the median operative time (major resection: 240 min; minor resection: 155 min) with a median of only 7 minutes for the parenchymal phase are decreased compared with conventional techniques, while methods for vascular control (e.g. PM, total vascular exclusion (TVE) or intermittend TVE) are not needed in most cases, thus preventing damage to the remnant liver tissue due to ischemia/reperfusion injury 10,11. One should also keep in mind that in many cases of hepatectomy steatotic or otherwise affected organs are being operated. These organs may have a shorter ischemia tolerance and therefore are more susceptible to ischemia/reperfusion injury 37. Recent data suggests that vascular control promotes growth of colorectal metastases 9,38, which are one of the main indications to liver parenchyma resection (approximately 37% in our institution). With modern techniques like stapler hepatectomy PM, TVE and intermittent TVE can no longer be considered to be state of the art and can no longer be employed with clean conscience as a routine 11.
Complications
Overall morbidity and mortality are at low levels comparable to other high-volume centers performing hepatectomies with conventional techniques 21,34,39. In a recent article, we have published the risk and outcome analysis of the first 300 cases of stapler hepatectomy in our department 21. Overall morbidity and mortality were 4.3 and 33%, respectively. Blood loss higher than 1.200 ml and total operative time greater than 180 min could be identified as main predictors to postoperative complications (p<0.01). Concomitant extrahepatic resection or age did not correlate to increased morbidity.
Conclusion
Based on our recent publication, stapler hepatectomy can be used as a technique for both minor and major resection of liver parenchyma for malign and benign liver disease 21,29. To our experience, the use of vascular linear staplers for the parenchymal phase of liver resection is a fast, safe and cost-effective surgical procedure. While overall morbidity and mortality rates are comparable to those of other high-volume centers using conventional standard resection techniques, vascular control is not needed as a standard, thus reducing potentially ischemia/reperfusion injury after liver resection.
Acknowledgements and disclosures
The authors would like to thank Jörg Rodrian for intraoperative photos.
References
- 1.Delaitre B, Maignien B. Laparoscopic splenectomy—technical aspects. Surg Endosc. 1992;6:305–8. doi: 10.1007/BF02498866. [DOI] [PubMed] [Google Scholar]
- 2.Chowbey PK, Sharma A, Khullar R, Mann V, Baijal M, Vashistha A. Laparoscopic subtotal cholecystectomy: a review of 56 procedures. J Laparoendosc Adv Surg Tech A. 2000;10:31–4. doi: 10.1089/lap.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Mentzer SJ. Improved technique for hilar vascular stapling. Ann Thorac Surg. 1992;53:165–6. doi: 10.1016/0003-4975(92)90784-2. [DOI] [PubMed] [Google Scholar]
- 4.Klaiber C, Wagner M, Metzger A. Various stapling techniques in laparoscopic appendectomy: 40 consecutive cases. Surg Laparosc Endosc. 1994;4:205–9. [PubMed] [Google Scholar]
- 5.Lewis RJ. The role of video-assisted thoracic surgery for carcinoma of the lung: wedge resection to lobectomy by simultaneous individual stapling. Ann Thorac Surg. 1993;56:762–8. doi: 10.1016/0003-4975(93)90975-n. [DOI] [PubMed] [Google Scholar]
- 6.Fowler DL, White SA. Laparoscopy-assisted sigmoid resection. Surg Laparosc Endosc. 1991;1:183–8. [PubMed] [Google Scholar]
- 7.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–80. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schemmer P, Friess H, Büchler MW. Recent advances in surgical therapy for primary and metastatic liver cancer. Ann Surg Hepatol. 2002;7:124–33. [Google Scholar]
- 9.van der Bilt JDW, Livestro DP, Borren A, van Hillegersberg R, Borel Rinkes IHM. European survey on the application of vascular clamping in liver surgery. Dig Surg. 2007;24:423–35. doi: 10.1159/000108325. [DOI] [PubMed] [Google Scholar]
- 10.Schemmer P, Büchler MW. Invited Commentary on van der Bilt JDW, Livestro DP, Borren A, van Hillegersberg R, Borel Rinkes IHM. European survey on the application of vascular clamping in liver surgery. Dig Surg. 2007;24(6):423–35. doi: 10.1159/000108325. [DOI] [PubMed] [Google Scholar]
- 11.Rahbari NN, Wente MN, Schemmer P, Diener MK, Hoffmann K, Motschall E, Schmidt J, Weitz J, Büchler MW. Impact of portal triad clamping on outcome after hepatic resection – a systematic review and meta-analysis. Br J Surg. 2008;95(4):424–32. doi: 10.1002/bjs.6141. [DOI] [PubMed] [Google Scholar]
- 12.Heaton N. Advances and methods in liver surgery: haemostasis. Eur J Gastroenterol Hepatol. 2005;17(Suppl 1):S3–S12. doi: 10.1097/00042737-200504001-00002. [DOI] [PubMed] [Google Scholar]
- 13.Nuzzo G, Giuliante F, Giovannini I, Vellone M, De Cosmo G, Capelli G. Liver resections with or without pedicle clamping. Am J Surg. 2001;181:238–46. doi: 10.1016/s0002-9610(01)00555-4. [DOI] [PubMed] [Google Scholar]
- 14.Schemmer P, Bunzendahl H, Thurman R, et al. Glycin verlängert das Überleben nach warmer Ischämie und Leberteilresektion im Tiermodell. Deutsche Gesellschaft für Chirurgie/Chirurgisches Forum. 2000;29:331–4. [Google Scholar]
- 15.Nickkholgh A, Barro-Bejarano M, Liang R, Zorn M, Mehrabi A, Gebhard MM, Büchler MW, Gutt CN, Schemmer P. Signs of reperfusion injury following CO2 pneumoperitoneum: an in vivo microscopy study. Surg Endoscop. 2008;22(1):122–8. doi: 10.1007/s00464-007-9386-6. [DOI] [PubMed] [Google Scholar]
- 16.Kincius M, Liang R, Nickkholgh A, Hoffmann K, Flechtenmacher C, Ryschich E, Gutt CN, Gebhard MM, Schmidt J, Büchler MW, Schemmer P. Taurine protects from liver injury after warm ischemia in rats: the role of Kupffer cells. Eur Surg Res. 2007;39:275–83. doi: 10.1159/000102982. [DOI] [PubMed] [Google Scholar]
- 17.Hofland J, Henny CP. Bloodless (liver) surgery? The anesthetist's view. Dig Surg. 2007;24:265–73. doi: 10.1159/000103657. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie S, Dixon E, Bathe O, Sutherland F. Intermittent hepatic vein—total vascular exclusion during liver resection: anatomic and clinical studies. J Gastrointest Surg. 2005;9:658–66. doi: 10.1016/j.gassur.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Emond J, Wachs ME, Renz JF, Kelley S, Harris H, Roberts JP, Ascher NL, Lim RC, Jr. Total vascular exclusion for major hepatectomy in patients with abnormal liver parenchyma, Arch Surg. 1995;130:824–30; discussion 830–1. [DOI] [PubMed] [Google Scholar]
- 20.Dixon E, Vollmer CM, Jr, Bathe O, Sutherland F. Vascular occlusion to decrease blood loss during hepatic resection. Am J Surg. 2005;190:75–86. doi: 10.1016/j.amjsurg.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Schemmer P, Friess H, Hinz U, Mehrabi A, Kraus TW, Z'graggen K, Schmidt J, Uhl W, Büchler MW. Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg. 2006;30:419–30. doi: 10.1007/s00268-005-0192-9. [DOI] [PubMed] [Google Scholar]
- 22.Arru M, Pulitanò C, Aldrighetti L, Catena M, Finazzi R, Ferla G. A prospective evaluation of ultrasonic dissector plus harmonic scalpel in liver resection. Am Surg. 2007;73:256–60. [PubMed] [Google Scholar]
- 23.Yao P, Gunasegaram A, Ladd LA, Chu F, Morris DL. Inline radiofrequency ablation-assisted laparoscopic liver resection: first experiment with stapling device. ANZ J Surg. 2007;77:480–4. doi: 10.1111/j.1445-2197.2007.04099.x. [DOI] [PubMed] [Google Scholar]
- 24.Lai PBS, Lee KF, Wong J, Li AKC. Techniques for liver resection: a review. Surgeon. 2007;5:166–74. doi: 10.1016/s1479-666x(07)80044-8. [DOI] [PubMed] [Google Scholar]
- 25.Corvera CU, Dada SA, Kirkland JG, Garrett RD, Way LW, Stewart L. Bipolar pulse coagulation for resection of the cirrhotic liver. J Surg Res. 2006;136:182–6. doi: 10.1016/j.jss.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Satoi S, Kamiyama Y, Matsui Y, Kitade H, Kaibori M, Yamamoto H, Yanagimoto H, Takai S, Kwon AH. Clinical outcome of 214 liver resections using microwave tissue coagulation. Hepatogastroenterology. 2005;52:1180–5. [PubMed] [Google Scholar]
- 27.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–3. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckley ML, Ghafourpour KL, Indresano AT. The use of argon beam coagulation to control hemorrhage: a case report and review of the technology. J Oral Maxillofac Surg. 2004;62:615–8. doi: 10.1016/j.joms.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Fan S. Use of the endo-GIA vascular stapler for hepatic resection. Asian J Surg. 2003;26:193–6. doi: 10.1016/S1015-9584(09)60301-8. [DOI] [PubMed] [Google Scholar]
- 30.Ramacciato G, Aurello P, D'Angelo F, Caramitti A, Barillari P, Fornasari V. Effective vascular endostapler techniques in hepatic resection. Int Surg. 1998;83:317–23. [PubMed] [Google Scholar]
- 31.Ramacciato G, Balesh AM, Fornasari V. Vascular endostapler as aid to hepatic vein control during hepatic resections. Am J Surg. 1996;172:358–62. doi: 10.1016/S0002-9610(96)00199-7. [DOI] [PubMed] [Google Scholar]
- 32.Lefor AT, Flowers JL. Laparoscopic wedge biopsy of the liver. J Am Coll Surg. 1994;178:307–8. [PubMed] [Google Scholar]
- 33.Blumgart LH. Resection of the liver. J Am Coll Surg. 2005;201:492–4. doi: 10.1016/j.jamcollsurg.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Schemmer P, Friess H, Dervenis C, Schmidt J, Weitz J, Uhl W, Büchler MW. The use of endo-GIA vascular staplers in liver surgery and their potential benefit: a review. Dig Surg. 2007;24:300–5. doi: 10.1159/000103662. [DOI] [PubMed] [Google Scholar]
- 35.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–60. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 36.Izbicki JR, Gawad KA, Quirrenbach S, Hosch SB, Breid V, Knoefel WT, Küpper HU, Broelsch CE. Is the stapled suture in visceral surgery still justified? A prospective controlled, randomized study of cost effectiveness of manual and stapler suture. Chirurg. 1998;69:725–34. doi: 10.1007/s001040050481. [DOI] [PubMed] [Google Scholar]
- 37.Schemmer P, Schoonhoven R, Swenberg JA, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG. Gentle organ manipulation during harvest as a key determinant of survival of fatty livers after transplantation in the rat. Transpl Int. 1999;12:351–9. doi: 10.1007/s001470050239. [DOI] [PubMed] [Google Scholar]
- 38.Nicoud IB, Jones CM, Pierce JM, Earl TM, Matrisian LM, Chari RS, Gorden DL. Warm hepatic ischemia–reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res. 2007;67:2720–8. doi: 10.1158/0008-5472.CAN-06-3923. [DOI] [PubMed] [Google Scholar]
- 39.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406; discussion 406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]