Abstract
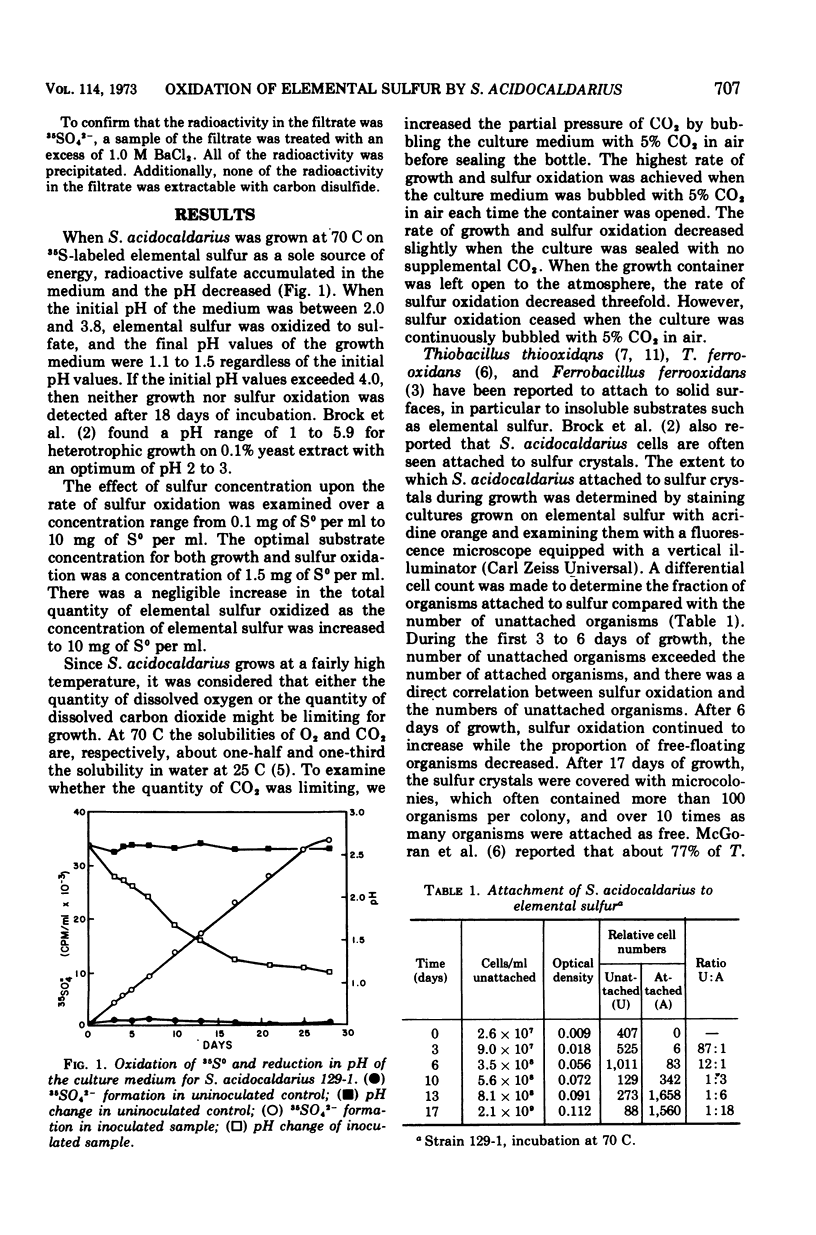
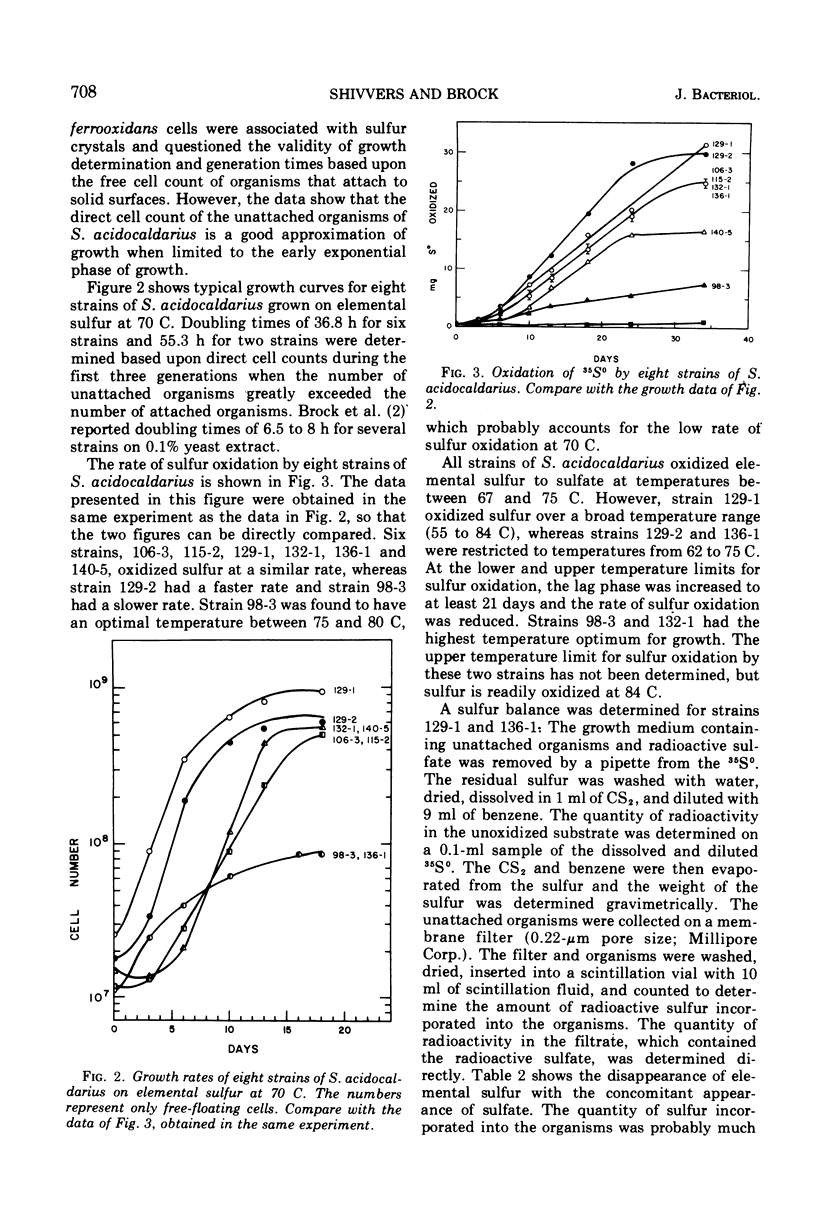
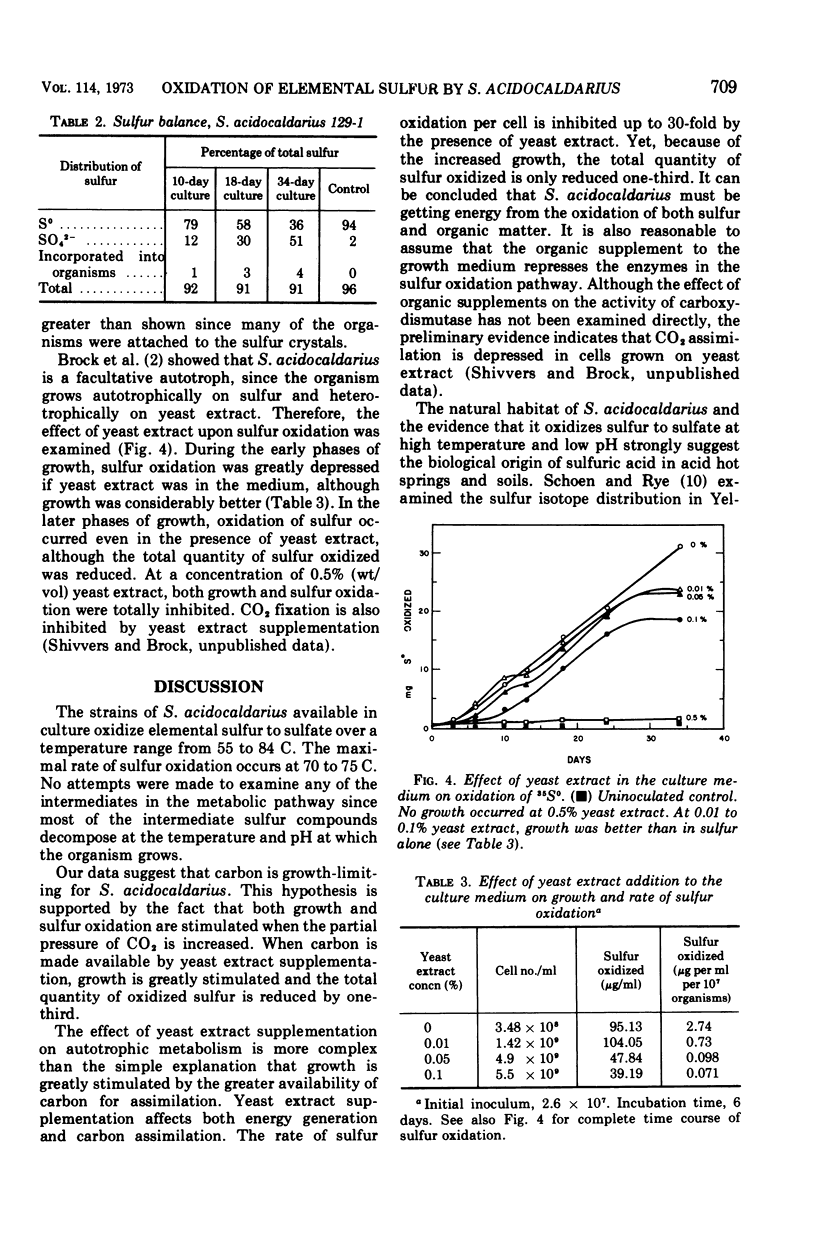
Oxidation of elemental sulfur by Sulfolobus acidocaldarius, an autotroph which grows at high temperatures and low pH, was examined by use of 35S-labeled elemental sulfur. When cultured at pH 3.2 and 70 C, S. acidocaldarius oxidized elemental sulfur essentially quantitatively to sulfuric acid. Oxidation rate paralleled growth rate and decrease in pH of the culture medium. Elemental sulfur was not oxidized under these conditions if the culture was poisoned with formaldehyde. During the growth phase, the proportion of cells attached to the sulfur crystals increased progressively, and in the later phases of growth over 10 times more cells were attached to sulfur than were free. Doubling times for eight strains growing on elemental sulfur varied from 37 to 55 h. The organism grows much more rapidly on yeast extract than on sulfur. In a medium containing both sulfur and yeast extract, sulfur oxidation was partially inhibited, although growth was excellent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Fliermans C. B., Brock T. D. Ecology of sulfur-oxidizing bacteria in hot acid soils. J Bacteriol. 1972 Aug;111(2):343–350. doi: 10.1128/jb.111.2.343-350.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGoran C. J., Duncan D. W., Walden C. C. Growth of Thiobacillus ferrooxidans on various substrates. Can J Microbiol. 1969 Jan;15(1):135–138. doi: 10.1139/m69-024. [DOI] [PubMed] [Google Scholar]
- Mosser J. L., Mosser A. G., Brock T. D. Bacterial origin of sulfuric Acid in geothermal habitats. Science. 1973 Mar 30;179(4080):1323–1324. doi: 10.1126/science.179.4080.1323. [DOI] [PubMed] [Google Scholar]
- SCHAEFFER W. I., HOLBERT P. E., UMBREIT W. W. Attachment of Thiobacillus thiooxidans to sulfur crystals. J Bacteriol. 1963 Jan;85:137–140. doi: 10.1128/jb.85.1.137-140.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen R., Rye R. O. Sulfur isotope distribution in solfataras, yellowstone national park. Science. 1970 Dec 4;170(3962):1082–1084. doi: 10.1126/science.170.3962.1082. [DOI] [PubMed] [Google Scholar]


