Abstract
Background. The aim of the present study was to evaluate the feasibility and efficacy of the LigaSure vessel sealing system on a large scale when used for liver resection. Methods. We retrospectively analyzed the short-term outcomes of 277 patients undergoing hepatectomies with the use of the LigaSureR system. Results. There were two hospital deaths (0.7%), and the morbidity rate was 25.3%. Mean blood loss during liver transection was 352±422 ml, and the liver transection speed was 1.9±0.86 cm2/min. The number of ties required during liver transection was 13.2±13. The morbidity and mortality rate was similar when comparing patients with injured livers (chronic hepatitis or cirrhosis) and those with normal livers, but liver transection speed was faster in those with normal livers when compared with those with injured livers (2.00±0.88 vs. 1.57±0.63 cm2/min, p=0.001). Conclusions. The LigaSure system can be applied safely in patients undergoing liver resection, regardless of whether cirrhosis is present or not.
Keywords: liver resection, blood loss, LigaSure
Introduction
Surgical techniques for liver resection have improved dramatically over the past two decades 1,2,3. Various studies have demonstrated that the postoperative morbidity and mortality for patients undergoing liver resection is closely related to the degree of intraoperative blood loss, the majority of which occurs during transection of the liver parenchyma 4,5,6. Therefore, the particulars of the technique employed for liver transection are critical for optimal outcomes.
Several techniques have been employed to reduce intraoperative blood loss during liver resection. The Pringle maneuver is the most widely accepted technique to achieve bloodless hepatectomy 4,7. Recently, randomized controlled trial showed that intraoperative blood salvage was effective in reducing intraoperative blood loss 8. The cavitational ultrasonic surgical aspirator (CUSA) has also been used to facilitate bloodless hepatectomy, but a randomized clinical trial comparing ultrasonic and manual clamp transection of the liver showed no difference in blood loss and operation time 9. The crush clamping method is another simple and widely adopted technique used during liver transection, and Lesurtel and colleagues reported that the crush clamping technique was superior in terms of resection time, blood loss, and blood transfusion frequency when compared with the CUSA, Hydrojet, and dissecting sealers 10.
The LigaSure Vessel Sealing System (Valleylab, Boulder, CO, USA) is a novel hemostatic device that can seal blood vessels up to 7 mm in diameter by denaturing collagen and elastin within the vessel wall and in the surrounding connective tissue. In a randomized clinical trial, the use of LigaSure system reduced operation time and blood loss in patients undergoing hemorrhoidectomy and gastrectomy 11,12,13. We previously reported that the use of LigaSure plus crush clamping during liver resection allowed rapid and safe division of the liver parenchyma 8.
The goal of the present study was to analyze the safety and efficacy of the LigaSure sealing system combined with crush clamping in 277 patients undergoing hepatectomies.
Methods
Patients
Between May 2005 and October 2007, 315 consecutive patients underwent liver resection in Cancer Institute Hospital (Tokyo, Japan). The Patients who underwent liver resection with concomitant lymph node dissection or bile duct reconstruction and those who underwent laparoscopic liver resection were excluded from this study. Thus, data from a total of 277 patients (87.9%) who underwent liver resection using the LigaSure system were analyzed for this study.
Analysis
Prospectively collected data were retrospectively analyzed. Intraoperative data, including operative time (min), liver transection time (min), liver transection area (cm2), portal triad clamp time (Pringle time; min), estimated blood loss during liver transection and operation (ml), central venous pressure (cm H2O) and total number of ligations during liver transection, were recorded for subsequent analysis. The estimated blood loss during liver transection was calculated as total blood loss minus the estimated blood loss before the liver transection. The transection speed (cm2/min) was calculated as transection area divided by transection time. The maximum tumor size, tumor number, type of hepatectomy, concomitant resection, and preoperative liver status (normal or injured) were recorded. Minor hepatectomy was defined as limited resection of two or less Couinaud segments, and major hepatectomy as resection of three or more Couinaud segments. Injured liver was defined as chronic hepatitis or cirrhosis in pathological findings of the resected specimen. Postoperative variables were analyzed and included complications, length of hospital stay (days), and mortality. Operative mortality was defined as any death resulting from a complication during operation.
Surgical technique
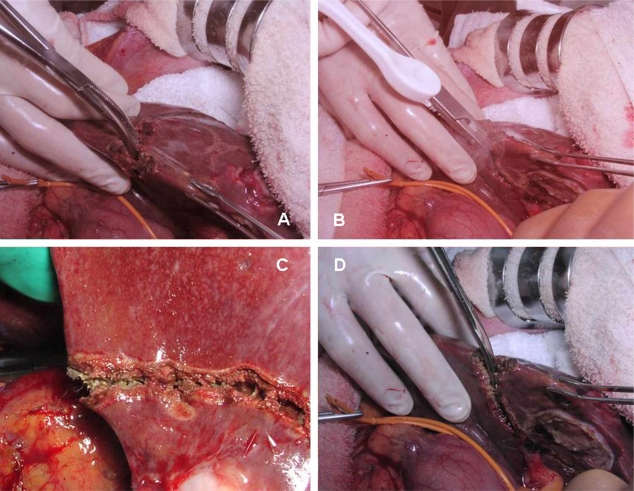
All operations in this study were performed or supervised by two senior surgeons (Yamamoto J and Saiura A). After laparotomy by J incision, the whole liver was examined by intraoperative ultrasonography. Liver transection was performed using a crush clamping technique and an intermittent Pringle maneuver with periods of 15 minutes of clamping and 5 minutes of unclamping (Figure 1). The liver transection was accomplished in three steps. First, the liver parenchyma was crushed using Kelly forceps (Figure 1A) and then aspirated, revealing the residual vessels or Glissonian sheaths. Second, the residual tissue was sealed using the LigaSure Standard handset (Figure 1B,C) at a power level of 2. Glissonian sheaths or hepatic veins of diameter >3 mm were ligated with 4–0 Vicryl (Ethicon, CO, USA) (Figure 2). Finally, the center of the sealed zone was divided with scissors (Figure 1D), and electric cautery was used to seal the remaining tissue. No clips were used. The bile leak test was performed routinely after liver transection.
Figure 1. .
Crush clamping method with the LigaSure system. (A) Crushing of the liver parenchyma using Kelly forceps. (B,C) Sealing the residual tissue using the LigaSure system. (D) Dividing the center of the sealed zone.
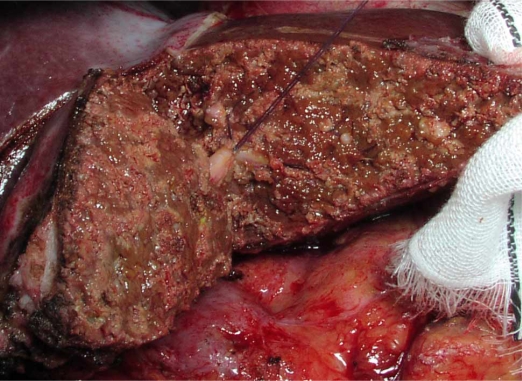
Figure 2. .
The raw surface was shown. The Glissonian sheath of segment 3 was ligated.
Statistical analysis
Quantitative variables were compared using student t-test. Comparisons between groups were performed using the Chi-square test. Statistical analyses were conducted using a statistical analysis package (SPSS 9.0, SPSS Inc. Chicago, IL). A p–value <0.05 was considered to represent statistical significance.
Results
Clinical data from 277 patients were analyzed. The mean age of the patients was 63.4±10.6 (years±SD). One hundred seventy-seven males and 100 females were included. Cause of disease was metastatic liver tumor (n=188), primary liver carcinoma (n=72), and various benign conditions (n=17). Histological examination of resected liver showed normal liver in 233 patients (84%) and chronic liver disease or cirrhosis in 44 patients (16%). Major hepatectomy was performed in 71 patients (25%), and minor hepatectomy was performed in 206 patients (75%). In patients with synchronous metastases arising from gastrointestinal malignancy, liver resection was performed synchronously after resection of the primary tumor. Combined gastrointestinal resection was performed synchronously in 41 patients (14%; colorectal, n=24; stomach, n=17) prior to liver resection. Eight patients (2.8%) required hepatic vein reconstruction due to metastatic invasion from a primary colorectal cancer. The maximum diameter of resected tumor was 4.0±2.9 (mean ±SD) cm, with solitary tumors in 160 patients (57%) and multiple tumors in 117 patients (43%).
Fifty-three repeat hepatectomies (19%) were included. The mean amount of blood loss during operation and during liver transection was 630±652 ml and 352±422 ml, respectively. The mean liver transection speed and transection area was 1.93±0.86 cm2/min and 74.0±50.2 cm2, respectively. The mean number of ties required during liver transection was 13.2±13. The mean central venous pressure was 5.3±2.7 cm H2O. The mortality and morbidity rate was 0.7 and 25.2%, respectively. Two patients died due to massive hemorrhage (first postoperative day) and liver failure (59th postoperative day), respectively. The mean postoperative hospital stay was 16.7±11.3 days. The incidence of bile leak was 5.7% (n=16), but all bile leaks resolved spontaneously. Other complications included pleural or ascites (n=21, 7.5%), wound infection (n=11, 3.9%), postoperative hemorrhage (n=3, 1.0%), sepsis (n=3, 1.0%), ileus (n=2, 0.7%), liver failure (n=2, 0.7%) and various others (n=10, 3.6%).
Preoperative status included 233 patients with normal livers and 44 patients with injured livers (Table I). There were two hospital deaths in the normal liver group. The morbidity rate was similar in both groups (23% vs. 31%, p=0.252). The amount of blood loss during liver transection and the transection area was similar in both groups. However, the liver transection speed was significantly greater in the normal liver group than in the injured liver group (2.00 cm2/min vs. 1.57 cm2/min, p=0.001). The total number of ties required during liver transection was similar when comparing the two groups.
Table I. Outcome of liver transection in patients with normal and injured livers.
| Normal liver n=233 | Injured liver n=44 | p | |
|---|---|---|---|
| Postoperative hospital stay | 16.6±11.6 | 17.4±10.2 | |
| Mortality | 2 | 0 | 0.707 |
| Morbidity | 54 | 14 | 0.252 |
| Blood loss during transection (mL) | 333±379 | 444±587 | 0.283 |
| Transection area (cm2) | 75±52 | 68±38 | 0.353 |
| Transection speed (cm2/min) | 2.00±0.88 | 1.57±0.63 | 0.001 |
| Central Venous Pressure (cm H2O) | 5.34±2.6 | 5.32±3.0 | 0.982 |
| Number of ligations during liver transection | 13.1±13 | 13.7±8.9 | 0.783 |
Discussion
The present study investigated the short-term outcomes for patients undergoing liver resection with the use of the LigaSure system and the crush clamping method. A previous randomized controlled study of 60 patients demonstrated that a faster and equally safe hepatectomy was achieved with the LigaSure system when compared with the crush clamping method without the use of the LigaSure system. The present study confirmed these findings in a larger patient population, with the exception that there were two hospital deaths. One patient died on the first postoperative day due to massive intraoperative hemorrhage secondary to tearing of middle hepatic vein in the context of a metastatic lesion located at the root of the middle hepatic vein. This patient had undergone hepatectomy on three prior occasions secondary to recurrent colorectal metastases. The second patient died on the 59th postoperative day due to postoperative liver failure. This patient had undergone hepatectomy once before secondary to colorectal liver metastases. In both the cases, the LigaSure system did not seem directly related to the mechanism of death.
The LigaSure system has been used in many surgical procedures. A reduction in intraoperative blood loss and a shortening of operation duration were determined in various operation. Previous studies have reported that the LigaSure system may present some technical difficulties when sealing the bile duct 14. In this study, bile ducts in the portal tract of less than <3 mm in diameter were successfully sealed with the LigaSure system, and the incidence of bile leak was 5.7%, which is consistent with previous studies and which suggests that even thin bile ducts in the minor portal tract can be sealed safely by this device 15,16. However, our experience suggests that it may be difficult to seal very small vessels with the LigaSure system. Thus, tiny Glissonian sheaths directly originated from main trunk such as Glissonian sheeths of the caudate lobe should be ligated with conventional techniques.
Romano and colleagues reported that the LigaSure system was unable to achieve hemostasis in patients with cirrhotic liver disease undergoing liver transection. In our study, blood loss during liver transection was similar when comparing patients with normal livers and those with injured livers. However, Romano's study did not use routine inflow occlusion, and the present study utilized the Pringle maneuver routinely. Further, the liver parenchyma was crushed by means of the LigaSure forceps in Romano's study rather than the Kelly forceps employed in the present study. Thus, the techniques used in the present study appear to be superior in terms of achieving hemostasis in patients with cirrhosis. However, the slower transection speed for patients with injured livers in the present study may reflect a more meticulous liver transection that could have promoted the high rate of successful hemostasis.
LigaSure can be applied safely in any type of liver and hepatectomy when used with the crush clamping method. Therefore, we think that any hepatic surgeon skilled in the crush clamping method can use the LigaSure system to achieve faster liver transection. However, the sharp tip of the LigaSure forceps can damage small vessels and lead to a hemorrhage. The shape of the LigaSure forceps needs to be improved.
The LigaSure disposable hand piece (LigaSure Standard) used in Japan costs approximately $150 USD per unit. However, the LigaSure system reduced the length of time required for hepatectomy by 11 minutes and reduced the length of time to hemostasis after hepatectomy, thereby reducing total operative time by 30 minutes. These properties likely result in a favorable costbenefit analysis for this system.
In conclusion, the LigaSure system can be applied safely in patients undergoing liver resection, regardless of whether cirrhosis is present or not.
Acknowledgements and disclosures
This study was supported by a Grant-in-Aid for basic research from the Ministry of Education, Culture, Sports, Science and Technology.
References
- 1.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–99. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan ST, Lai EC, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117–20. doi: 10.1002/bjs.1800830138. [DOI] [PubMed] [Google Scholar]
- 5.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406; discussion 406–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, et al. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–10. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 7.Belghiti J, Noun R, Malafosse R, Jagot P, Sauvanet A, Pierangeli F, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369–75. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, et al. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg. 2006;192:41–5. doi: 10.1016/j.amjsurg.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 9.Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, et al. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922–8. doi: 10.1001/archsurg.136.8.922. [DOI] [PubMed] [Google Scholar]
- 10.Lesurtel M, Selzner M, Petrowsky H, McCormack L, Clavien PA. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg 2005;242:814–22, discussion 822–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palazzo FF, Francis DL, Clifton MA. Randomized clinical trial of Ligasure versus open haemorrhoidectomy. Br J Surg. 2002;89:154–7. doi: 10.1046/j.0007-1323.2001.01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee WJ, Chen TC, Lai IR, Wang W, Huang MT. Randomized clinical trial of Ligasure versus conventional surgery for extended gastric cancer resection. Br J Surg. 2003;90:1493–6. doi: 10.1002/bjs.4362. [DOI] [PubMed] [Google Scholar]
- 13.Jayne DG, Botterill I, Ambrose NS, Brennan TG, Guillou PJ, O'Riordain DS. Randomized clinical trial of Ligasure versus conventional diathermy for day-case haemorrhoidectomy. Br J Surg. 2002;89:428–32. doi: 10.1046/j.0007-1323.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- 14.Matthews BD, Pratt BL, Backus CL, Kercher KW, Mostafa G, Lentzner A, et al. Effectiveness of the ultrasonic coagulating shears, LigaSure vessel sealer, and surgical clip application in biliary surgery: a comparative analysis. Am Surg. 2001;67:901–6. [PubMed] [Google Scholar]
- 15.Ijichi M, Takayama T, Toyoda H, Sano K, Kubota K, Makuuchi M. Randomized trial of the usefulness of a bile leakage test during hepatic resection. Arch Surg. 2000;135:1395–400. doi: 10.1001/archsurg.135.12.1395. [DOI] [PubMed] [Google Scholar]
- 16.Man K, Fan ST, Ng IO, Lo CM, Liu CL,Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704–11; discussion 711–703. [DOI] [PMC free article] [PubMed] [Google Scholar]