Abstract
Minimally invasive hepatic resection was first described by Gagner et al. in the early 1990s and since then has become increasingly adopted by hepatobiliary and liver transplant surgeons. Several techniques exist to transect the hepatic parenchyma laparoscopically and include transection with stapler and/or energy devices, such as ultrasonic shears, radiofrequency ablation and bipolar devices. We believe that coagulative techniques allow for superior anatomic resections and ultimately permit for the performance of more complex hepatic resections. In the stapling technique, Glisson's capsule is usually incised with an energy device until the parenchyma is thinned out and multiple firings of the staplers are then used to transect the remaining parenchyma and larger bridging segmental vessels and ducts. Besides the economic constraints of using multiple stapler firings, the remaining staples have the disadvantage of hindering and even preventing additional hemostasis of the raw liver surface with monopolar and bipolar electrocautery. The laparoscopic stapler device is, however, useful for transection of the main portal branches and hepatic veins during minimally invasive major hepatic resections. Techniques to safely perform major hepatic resection with the above techniques will be described with an emphasis on when and how laparoscopic vascular staplers should be used.
Keywords: laparoscopic, hepatic, resection, major, minor, benign, malignant, stapler, stapling technique
Introduction
Because of the successes seen with other types of major laparoscopic surgery, i.e. laparoscopic surgery for colorectal cancer, interest has risen in applying these techniques to the entire abdomen. The hepato-pancreato and biliary (HPB) system has been considered the last bastion of laparoscopic surgery due to a combination of anatomical complexity of this system and the lack of surgeons with experience in both laparoscopy and HPB surgery 1,2,3,4. As opposed to pancreatic resections, which often involve the head of the gland and require multiple anastamoses, interest in minimally invasive techniques for hepatic surgery has risen over the years because anastamoses are rarely indicated. Many authors insist on the existence of laparoscopically accessible hepatic segments in the peripheral segments of the liver (segments II, II, IVb and V) and non-laparoscopic segments that are the high and deep segments in the right side of the liver (segments VIa, VII and VII) 5. Because of this, laparoscopic and hand-assisted resection of lateral and peripheral liver segments has become more common in the management of benign and malignant tumors 6,7,8. Other teams report, however, that all segments of the liver can be approached with totally laparoscopic techniques 3.
In general, surgical resection is preferred to ablative procedures in the treatment of primary and secondary hepatic malignancy 3.Guiding principles of hepatic resection are the need to leave the patient with at least 30% of functional hepatic reserve and at least 1 cm of tumor-free resection margin for malignant tumors 5,9,10.Laparoscopy is particularly useful in cases when resectability is uncertain prior to surgery. According to the Clinical Risk Score advocated by Fong et al. evaluation of five factors can predict the presence of occult intrahepatic or extrahepatic disease that may make patients unresectable 11. These factors include: presence of more than one liver tumor, positive node status of primary tumor, disease-free interval of < 1 year, presence of liver tumor > 5 cm and CEA level > 200 ng/mL. If any patient has > 2 of these factors, occult disease rendering patients unresectable will be found in 42% of cases. Because of this, the routine use of laparoscopy with concomitant laparoscopic ultrasound can save patients from unnecessary laparotomy 11.
We employ the laparoscopic approach because of reports that show benefits in terms of operative time, estimated blood loss (EBL) and length of stay (LOS) after peripherally located hepatic resections performed laparoscopically as compared to traditional techniques 12. Although an increasing number of centers have started using the hand-assisted technique for hepatic resections, we prefer totally laparoscopic techniques because of reports of decreased LOS when compared to lap-assisted or open resections 6,12,13. Because of concerns for massive hemorrhage, risk of gas embolism and port site recurrences and adequacy of resection margins for malignancies via the laparoscopic approach, major hepatectomies are currently being performed in only a few highly specialized centers 3,5,9,10,12,14,15,16,17,18,19,20,21. The great disparity in laparoscopic experience and ability has revealed that aside from anesthetic considerations and contraindications to the pneumoperitoneum itself, the only absolute contraindication to a laparoscopic procedure from a surgical point of view is operator ability and not the patient's pathology; need for complex vascular and biliary reconstruction remain relative contraindications 3.
Liver biopsies, wedge resections and segmentectomies are usually been done with the laparoscopic bipolar device and ultrasoninc energy shears. Smaller lesions in the very lateral aspects of segments 2 and 3 can be resected with the laparoscopic GIA stapler device. We routinely use laparoscopic stapler devices for left lateral segmentectomies and major resections. The laparoscopic vascular stapler is particularly useful when transection of hepatic veins is necessary although then can also be used for transection of portal structures. Although some centers advocate transection of the hepatic parenchyma with laparoscopic staplers, we routinely use the laparoscopic bipolar device in addition to either ultrasonic energy (SonoSurg, Olympus Surgical America/Harmonic Scalpel, Johnson & Johnson, New Brunswick, NJ, USA) for normal hepatic tissue or bipolar thermal energy (Ligasure, Covidien, Norwalk, CT, USA) for patients with cirrhotic livers.
Preoperative work-up
Aside from routine laboratory examination and medical and anesthesiology clearance, all patients should get a chest X-ray to rule out additional pulmonary disease. Radiologic examination of the liver should begin with a transabdominal ultrasound to confirm that tumors will be visible with intra-operative ultrasound. All patients that are candidates for surgical resection should undergo preoperative helical CT scan and all lesions that are located next to large vascular or biliary structures should also get an MRI with three-dimensional reconstruction PET scans are considered on a case-by-case basis. Intra-operative laparoscopic sonography should also be performed to rule out additional lesions and confirm resectability.
The set-up in the operating room
All patients should receive preoperative deep venous thrombotic prophylaxis. All patients without isolated lesions in the deep posterior segments (segments IVa, VII and VIII) are placed supine on the operating table 22. After general enodotracheal intubation, a foley catheter and an orogastric tube are placed. A central venous line is placed in patients with a history of congestive heart failure, or with poor peripheral access, or if a major resection is planned. The patient's arms are tucked along the sides of the patient to allow for unencumbered access to the patient. The patient is then placed in the low lithotomy position (a.k.a “French position”) with the legs bent at the knees and spread apart to allow for the operator to stand in between the legs without limitation of the laparoscopic instruments. Patients with lesions of the right side of the liver have their right upper abdomen elevated with padding placed under their back to enhance exposure of this area. Patients requiring major resections of the left liver do not require any additional pad placement 15. For patients with lesions in the deep and posterior segments they are put in a modified left lateral position with their right arm supported above their head, their lower body is still placed with the legs spread 22. The patient's chest is then strapped in place to prevent slipping during the procedure. An autostatic self-retaining table-mounted liver retractor is then placed on the right side of the operating table as high up as possible, this device is particularly useful when doing major right hepatectomy, on the left side the falciform ligament is usually adequate to hold the liver in place. Ideally, a robotically controlled camera holder is placed on the left side of the patient, if not a surgical assistant to hold the camera 23.
Specific surgical equipment that will be necessary include a bipolar cautery forceps (Medtronic France S.A.S., Boulogne-Billancourt, France) and ultrasonic shears that are particularly useful for the dissection around the portal triad and portal vein and for division through the hepatic parenchyma. In patients with cirrhotic livers, the Ligasure device seems to obtain superior hemostasis during this portion of the procedure (Covidien, Norwalk, CT, USA). Multiple laparoscopic linear staplers will be required for transection of larger vessels and should also be available to transect the hepatic parenchyma. As mentioned, a flexible laparoscopic ultrasound probe with color-flow Doppler is required to confirm resectability of tumors, and to identify other lesions. A hand port and full laparotomy tray is kept in every room should urgent conversion be needed. In the early port of surgeons experience with these techniques we recommend placing the hand port routinely in case rapid control of hemorrhage is required. The port can be placed in the upper midline and used for extraction of the specimen. If laparoscopic instruments need to be placed through the hand port they can be passed directly through the port as necessary.
Trochar placement
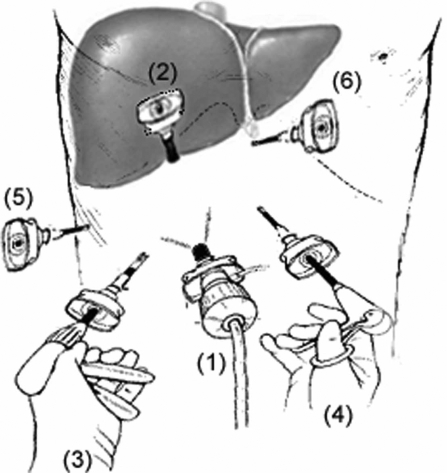
After pneumoperitoneum is obtained and maintained at a pressure of 10–15 torr, a 10 mm camera port is placed approximately 1 hand-breadth below the right costal margin along a line in between the mid-clavicular line and midline (Figure 1). All the peritoneal surface and contents of all four quadrants are visually inspected with a 30° laparoscope to rule out additional undiagnosed pathology. If the absence of metastatic disease is confirmed, the next port is placed just below the costal margin along the nipple line under direct visualization. This second port is a 12 mm trocar to permit for the introduction of the laparoscopic ultrasound and a complete staging ultrasound examination of the liver is performed. If respectability is confirmed, the remaining ports are placed under direct visualization. Two working 5 mm ports are placed to the left and right of the camera port. Another 5 mm port is placed in the subxiphoid region for the surgical assistant and the last 5 mm port is placed along the anterior axillary line and is used for the autostatic liver retractor device when needed.
Figure 1. .
Port placement. The camera port (12 mm) is placed approximately 7 cm below the right costal margin along a line in-between the mid-clavicular line and midline. A second port (12 mm) is placed just below the costal margin along the mid-axillary line. Two working ports (5 mm) are placed to the left and the right of the camera port. A fifth port (5 mm) is placed along the right anterior axillary line for liver retraction, and the final port (5 mm) is placed in the sub-xiphoid region.
Mobilization of the liver
Once the decision is made to proceed with major hepatic resection, the patient is placed in reverse-Trendelenberg and the round ligament is retracted anteriorly to enhance exposure of the hepatoduodenal ligament. For right hepatectomies the falciform ligament is preserved, but the right triangular ligament is incised and followed until it joins the right aspects of the coronary and retrocaval ligaments. The increased mobility of the liver sometimes enables isolation of the right hepatic vein (RHV) at this stage of the procedure if the anterior aspect of the Vena Cava can be dissected free. For left lateral lobectomies, the left triangular ligament is transected, but the falciform ligament is maintained to maintain upward retraction of the liver. For left hepatectomies the falciform ligament must be taken down, which can be done at the end of the procedure if the upward retraction of this structure is helpful 15. The left triangular ligament should be followed to the ligamentum venosum and the left aspect of the coronary ligament and the superior aspects of the left side of the retrocaval ligaments are identified and incised. As with the RHV the increased mobility of the liver that results can enable isolation of the left hepatic vein (LHV) retrohepatically at this stage of the procedure.
Isolation and transection of the hepatic inflow
Prior to dissecting the portal triad, an umbilical or vascular tape should be placed around it by incisong the lesser ometum and passing the tape through the Foramen of Winslow. This will allow for the laparoscopic performance of the Pringle maneuver if necessary. The structures of the portal triad may need to be dissected out individually for selective ligation during major resections, however, when possible transection of more than one structure with a laparoscopic stapling device is a valid option. This is particularly useful for left hepatectomies because of the fact that the pars transversus of the left branch of the portal vein is particularly long before it trifurcates. For left lateral lobectomies, the structures of the portal triad supplying segments 2 and 3 are found directly in the umbilical fossa. When major resctions are planned, the hepatoduodenal ligament is dissected cranially starting from the confluence of the cystic and common bile duct (CBD). For left hepatectomies lateral retraction of the CBD will expose the left hepatic artery as it comes off the bifurcation. This structure is doubly clipped and transected if not transected with a laparoscopic vascular stapler. The CBD is retracted medially for right hepatectomies to expose the right hepatic artery, which is then similarly clipped and cut if it has not been incorporated into a transection with the stapler.
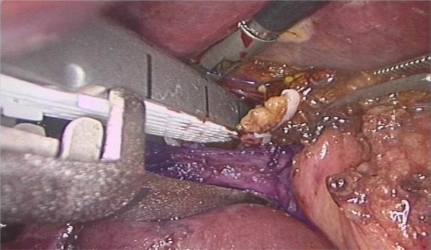
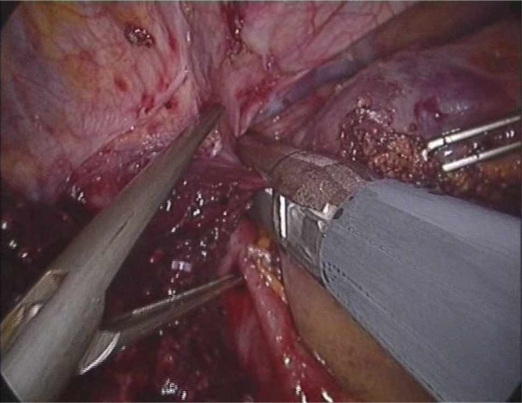
The laparoscopic stapler device is less useful for transection of the portal inflow into the right liver because the structures often bifurcate early into an anterior and posterior branch. Also, because of the angle of these structures, it is often necessary to place the distal ends of the stapler into the underlying hepatic parenchyma (Figure 2). This can cause unnecessary bleeding and increases the risk of damaging deeper structures such as the inferior vena cava (IVC). For an extended right hepatectomy it is also necessary to take the right branch of the left hepatic artery as it supplies segment IV and this usually cannot be incorporated into a stapler and needs to be done separately. This is also the case when this structure needs to be taken for a central hepatectomy.
Figure 2. .
Stapler placed on posterior branch of right portal vein (blue).
The left branch of the portal vein can be found in the umbilical fossa as it trifurcates into branches supplying segments II, III and IV. As with the arterial supply, the right branch of the portal vein can be found in the hilar plate. Once these structures are skeletonized for approximately 1 cm, they can be transected with a stapler or clipped and cut as necessary. We have started reinforcing clips on the portal vein stumps with a suture ligature due to delayed massive hemorrhage in one patient. In some cirrhotic patients with large portal vein branches the laparoscopic vascular stapler may be particularly useful due to the size of these structures, two firings of the vascular stapler may sometimes be required.
The final structure of the portal triad that needs to be located and isolated for major right and extended hepatectomy if not taken as a whole triad in the laparoscopic stapler device is the biliary tree. It can sometimes be difficult to locate the hepatic ducts, as a result, when the duct in question is believed to be isolated, it is transected with the laparoscopic shears until bile is seen for confirmation. The bile duct is then oversewn with absorbable suture. As mentioned, it is usually only necessary to isolate the right hepatic duct/s because of the diffculty in placing the stapler device in this region. We believe that post-operative bile leaks in major right hepatectomy can be reduced by identifying the biliary system individually. As mentioned, because of the length of the left portal triad, the ease of use of the stapler device obviates the need to isolate the biliary system in routine cases.
Isolation of the hepatic outflow
When possible it is advantageous to approach the hepatic veins retrohepatically. As opposed to the open approach, the laparoscopic retrohepatic approach can often times reveal a relatively easy isolation of these structures. Although it may be tempting to complete the dissection of these structures during the mobilization of the liver, a more aggressive retrohepatic dissection of the hepatic veins should be performed after the hepatic inflow is controlled. When this is not possible, the hepatic veins must be approached as with open surgery via an anterior approach (Figure 3). The retrohepatic dissection begins along the anterior surface of the IVC and proceeds in a cranial fashion. Most perforators can be controlled with the bipolar forceps; however, larger venous branches should be clipped and cut prior to transection. For right hepatectomies, a window is created between the right and middle hepatic veins (MHV) and the RHV is dissected for approximately 1 cm to allow for the placement of the laparoscopic vascular stapler after the hepatic inflow and parenchyma are transected. For left hepatectomies RHV is similarly dissected until a window between it and the MHV is created. For extended left hepatectomies this window is not necessary, as the MHV will also be taken before it comes off of the LVH. For central hepatectomies, the MHV can only be approached anteriorly. For extended left hepatectomies, both the LVH should be approached laterally and dissected superiorly until the branch of the MHV can be approached retrohepatically.
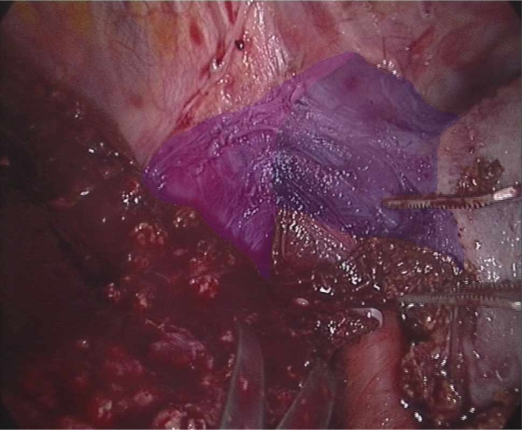
Figure 3. .
Dissection of left hepatic vein in preparation for the completion of a totally laparoscopic left hepatectomy. The middle hepatic vein has been highlighted in purple and the left hepatic vein has been highlighted in blue.
Transection of the hepatic parenchyma
The fundamental rule of this portion of the procedure is to lower the central venous pressure (CVP) as much as possible, because this will decrease blood loss from the divided parenchyma. Unfortunately, due to the effect of pneumoperitoneum on the central line transducer, CVP readings are not reliable during pneumoperitoneum. Due to this, visual examination of the IVC is the best way to assess true filling pressures, even though CVP readings prior to insufflation can give a useful estimate of filling pressures. Optimal CVP is done when the IVC looks half-empty and fluctuates with the movements of the heart and ventilator. To reduce the risk of CO2 gas embolism, the intra-abdominal pressure is reduced to 10 mmHg, although this is dependent on visibility and maintenance of domain.
For normal hepatic parenchyma, hepatic parenchymal transection is performed laparoscopically with the harmonic scalpel in the dominant hand and with the laparoscopic bipolar forceps in the other. Although most branches can be controlled with the bipolar cautery forceps, when larger segmental vessels are encountered they are clipped or suture ligated. The harmonic scalpel is particularly useful during the dissection of larger vessels because on its lowest setting it works like the Cavitron ultrasonic dissector (CUSA™, Radionics, Burlington, MA, USA) without the added disadvantage of the constant oozing of the irrigation fluid and deflation of the pneumoperitoneum of the actual cavitron. Once the major vessels of the liver are dissected with the ultrasonic scalpel, the laparoscopic shears are used to complete the isolation of these structures. Alternatively, the laparoscopic GIA staplers can be used, however, we have noted increased blood loss when this technique is used and the vessels aren't first isolated and the stapler device is placed blindly into the parenchyma. The LigaSure vessel sealing system (Covidien, Norwalk, CT, USA) provides the best hemostasis to either of the above-mentioned techniques for hepatotomy in cirrhotic patients.
The line of transection for major hepatectomies follows the lines of demarcation caused by transection of the arterial and portal flow of the respective segments. Prior to division of the hepatic parenchyma, Glisson's capsule is scored and any large segmental branches are clipped prior to division with the ultrasonic scalpel. Because of the amount of smoke created during this part of the procedure, a smoke evacuator or filtering device is recommended to maintain visibility.
Transection of the hepatic outflow
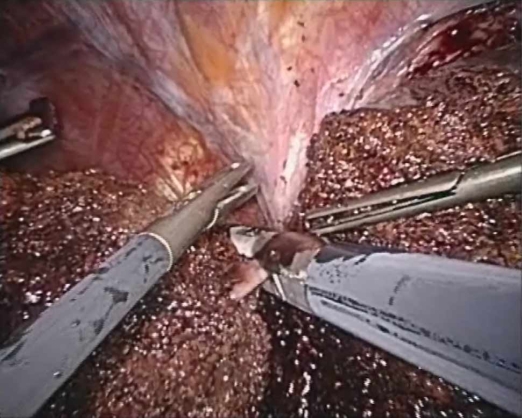
If not done prior, the complete mobilization of the respective attachments to the side of the liver to be resected are taken down. The transection of the hepatic veins is performed with a laparoscopic GIA vascular stapler (Figures 4 and 5). The proper identification and isolation of the hepatic veins may be the most difficult aspect of these procedures. To facilitate this part of the procedure, the upper aspect of the corresponding retrocaval ligament should be transected to maximize the visualization and control of these vessels. When difficulty arises, a hand port can be placed in the subcostal region to permit manual palpation of this region and ensure adequate dissection. Prior to transection with the endoscopic stapler, a laparoscopic vascular clamp should be passed into the abdomen in case urgent clamping is necessary. As mentioned, if it is not possible to identify the hepatic veins retrohepatically they can be isolated anteriorly after transection of the hepatic parenchyma.
Figure 4. .
Transection of right hepatic vein with laparoscopic GIA stapler device, note laparoscopic vascular clamp in right side of the field. This device should always be in the abdomen prior and during to transection of any major vascular structure with the laparoscopic vascular stapler in case torrential hemorrhage should occur.
Figure 5. .
Transection of the left hepatic vein with laparoscopic GIA stapler device, note laparoscopic vascular clamp in left side of the field.
Discussion
No prospective randomized controlled trials have been published comparing open to laparoscopic hepatic resections, however, one case-controlled study exists comparing laparoscopic left lateral segmentectomy to open historical controls. In this study 18 patients that underwent laparoscopic bisegmentectomies of segments II and III were identified. The study found longer operative and portal clamping times for the laparoscopic approach, but noted significantly less intra-operative blood loss. Neither group had any mortalities, and the complication rate was 11% in the laparoscopic group compared to 15% in the open group. Complications relating specifically to the surgery were only noted in the open group and consisted of hemorrhage, sub-phrenic abscess and biliary leak 7.
In a study 89 laparoscopic liver resections over a 10-year period were reported. The majority of cases were performed for malignant disease (73%). Major hepatectomy was performed in 43%, and conversion to open was necessary in 13% of all cases. Mortality was reported in one patient (1.1%) secondary to a bile leak; and complications occurred in 16% of patients that underwent minor hepatectomies and increased to 29% after major hepatectomy. The authors concluded that totally laparoscopic hepatectomy was feasible and safe for even major hepatic resections with similar long-term survival, but acknowledged the considerable learning curve associated with these procedures 3. In an American study of minimally invasive liver resections, the authors reported an operative complication rate of 9.3%, however, overall complication rates were not reported 24.
The results from three different HPB centers with a large experience in open resection of hepatocellular cancer were analyzed to ascertain rates of morbidity and mortality (Table 1) so that a comparison with the laparoscopic experience in the literature could be evaluated (Table 2) 25,26,27. Similarly three recently published reports from HPB centers with experience in open resection of hepatic metastases were analyzed 28,29,30. Neuroendocrine metastases to the liver were excluded from this analysis because of the fact that they do not have a similar natural history to other secondary tumors to the liver. As a group, 31% of open hepatectomies for primary liver cancer suffered a post-operative complication and 21% in the group that underwent hepatic resection for secondary lesions. As can be seen these results are comparable to the laparoscopic experience reported in the literature 3.Table 2
Table 1. Comparison of morbidity in the published literature from three large centers who perform open resections for secondary liver tumors (not including neuroendocrine metastases).
| Laurent (2001) 28 | 3Zacharias (2004) 30 | Jaeck (2004) 29 | Total (mean) | |
|---|---|---|---|---|
| Overall complications(%) | 8 | 41 | 15.1 | 21 |
| Pulmonary | 0 | 21 | 0 | 7 |
| Ascites (%) | NR | 5.3 | NR | 5.3 |
| Hepatic insufficiency (%) | 2.5 | 1.7 | 0 | 1.4 |
| Hemorrhage (%) | 0 | 0 | 0 | 0 |
| Bile leak (%) | 0 | 1.7 | 0 | 0.6 |
| Intra-abdominal collection (%) | 0 | 5.3 | 0 | 1.8 |
| Obstruction (%) | 0 | 0 | 0 | 0 |
| Wound | ||||
| Infection | 0 | 1.7 | 3 | 1.6 |
| Evisceration | 0 | 1.7 | 0 | 0.6 |
| Other | 5 | 25 | 12.1 | 14 |
Table 2. Comparison of morbidity in the published literature from three European centers who perform open resections for primary liver cancer.
| Laurent (2005) 25 | Dupont (2005) 26 | Baton (2007) 27 | Total (mean) | |
|---|---|---|---|---|
| Overall complications (%) | 23 | 28 | 42 | 31 |
| Ascites (%) | 0 | 2.4 | 5 | 2.5 |
| Hepatic insufficiency (%) | 4.6 | 3.6 | 8 | 5.4 |
| Hemorrhage (%) | 0 | 3.6 | 2 | 1.9 |
| Bile leak (%) | 3.7 | 3.6 | 5 | 4.1 |
| Angiocholitis (%) | 0 | 2.4 | 2 | 1.5 |
| Intra-abdominal collection (%) | 4.6 | 4.8 | 15 | 8.1 |
| Wound | 3.6 | |||
| Infection | 2.7 | |||
| Evisceration | 0.9 | 0 | 0 | 1.2 |
| Peritonitis | 0.9 | 0 | 0 | 0.3 |
| Other | 5.6 | 2.4 | 8 | 5.3 |
As experience has grown world wide, other centers have noted increased short-term benefits for patients undergoing laparoscopic minor hepatic resections of decreased analgesic requirements and shorter hospital stays when compared to historical open controls: average hospital stay of 3.5 days and one day of analgesic use 31. Furthermore, indications to perform laparoscopic resection of liver tumors have also been found to be safe in patients with hepatocellular carcinoma and Child's A cirrhosis 20. Some Authors have appropriately concluded that laparoscopic resections of simpler hepatic segments such as a bisegmentectomy of segments II and III, should probably be considered the standard of care 32.
Conclusions
Since the first report of a laparoscopic liver resection in 1992, laparoscopic resection of peripheral hepatic segments has become increasingly more common in the surgical treatment of both benign and malignant tumors. The minimally invasive approach to resections of the entire liver, however, is still only being performed in highly specialized centers do to lingering concerns about feasibility and efficacy. Minimally invasive techniques for hepatic resections of the entire liver are feasible and safe, and high-volume centers that specialize in these procedures can have results similar to historical open series. The laparoscopic stapler device can be a useful adjunct to laparoscopic hepatic resection, however, a full armamentarium should be at the disposable of the minimally invasive surgeon to ensure the safe performance of these procedures.
References
- 1.Gumbs AA, Gayet B. The laparoscopic duodenopancreatectomy: the posterior approach. Surg Endosc. 2008;22(2):539–540. doi: 10.1007/s00464-007-9635-8. [DOI] [PubMed] [Google Scholar]
- 2.Gumbs AA, Gres P, Madureira FA, Gayet B. Laparoscopic vs. open resection of noninvasive intraductal pancreatic mucinous neoplasms. J Gastrointest Surg. 2008;12:707–12. doi: 10.1007/s11605-007-0311-z. [DOI] [PubMed] [Google Scholar]
- 3.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93(1):67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 4.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, et al. Laparoscopic versus open hepatic resections for benign and malignant neoplasms – a meta-analysis. Surgery. 2007;141(2):203–11. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterik M, Marino M, et al. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236(1):90–7. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH. Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg. 2000;135(7):854–9. doi: 10.1001/archsurg.135.7.854. [DOI] [PubMed] [Google Scholar]
- 7.Lesurtel M, Cherqui D, Laurent A, Tayar C, Fagniez PL. Laparoscopic versus open left lateral hepatic lobectomy: a case-control study. J Am Coll Surg. 2003;196(2):236–42. doi: 10.1016/S1072-7515(02)01622-8. [DOI] [PubMed] [Google Scholar]
- 8.Mouiel J, Katkhouda N, Gugenheim J, Fabiani P. Possibilities of laparoscopic liver resection. J Hepatobiliary Pancreat Surg. 2000;7(1):1–8. doi: 10.1007/s005340050146. [DOI] [PubMed] [Google Scholar]
- 9.Masutani S, Sasaki Y, Imaoka S, Iwamoto S, Ohashi I, Kameyama M, et al. The prognostic significance of surgical margin in liver resection of patients with hepatocellular carcinoma. Arch Surg. 1994;129(10):1025–30. doi: 10.1001/archsurg.1994.01420340039007. [DOI] [PubMed] [Google Scholar]
- 10.Shirabe K, Takenaka K, Gion T, Fujiwa Y, Shimada M, Yanaga K, Maeda T, Kajiyama K, Sugimachi K. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg. 1997;84(8):1077–80. [PubMed] [Google Scholar]
- 11.Jarnagin WR, Conlon K, Bodniewicz J, Dougherty E, DeMatteo RP, Blumgart LH, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer. 2001;91(6):1121–8. doi: 10.1002/1097-0142(20010315)91:6<1121::aid-cncr1108>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Schmandra TC, Mierdl S, Hollander D, Hanisch E, Gutt C. Risk of gas embolism in hand-assisted versus total laparoscopic hepatic resection. Surg Technol Int. 2004;12:137–43. [PubMed] [Google Scholar]
- 13.O'Rourke N, Fielding G. Laparoscopic right hepatectomy: surgical technique. J Gastrointest Surg. 2004;8(2):213–6. doi: 10.1016/j.gassur.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Gumbs AA, Gayet B.Totally Laparoscopic Central Hepatectomy. J Gastrointest Surg 2007. Oct. 20 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Gumbs AA, Gayet B. Totally laparoscopic left hepatectomy. Surg Endosc. 2007;21(7):1221. doi: 10.1007/s00464-007-9319-4. [DOI] [PubMed] [Google Scholar]
- 16.Cherqui D. Benign liver tumors. J Chir (Paris) 2001;138(1):19–26. [PubMed] [Google Scholar]
- 17.Cherqui D. Laparoscopic hepatic resection. Useful or futile? Ann Chir. 2002;127(3):171–4. doi: 10.1016/s0003-3944(02)00735-6. [DOI] [PubMed] [Google Scholar]
- 18.Cherqui D. Laparoscopic liver resection. Br J Surg. 2003;90(6):644–6. doi: 10.1002/bjs.4197. [DOI] [PubMed] [Google Scholar]
- 19.Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, et al. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232(6):753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, et al. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243(4):499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359(9304):392–6. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 22.Gumbs AA, Gayet B.Video: the lateral laparoscopic approach to lesions in the posterior segments. J Gastrointest Surg 2008. Jan 11. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23.Gumbs AA, Crovari F, Vidal C, Henri P, Gayet B. Modified robotic lightweight endoscope (ViKY) validation in vivo in a porcine model. Surg Innov. 2007;14(4):261–4. doi: 10.1177/1553350607310281. [DOI] [PubMed] [Google Scholar]
- 24.Koffron AJ, Auffenberg G, Kung R, Abecassis M.Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246(3):385–92; discussion 392–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent C, Blanc JF, Nobili S, Sacunha A, Le Bail B, Bioulac-Sage P, et al. Prognostic factors and longterm survival after hepatic resection for hepatocellular carcinoma originating from noncirrhotic liver. J Am Coll Surg. 2005;201(5):656–62. doi: 10.1016/j.jamcollsurg.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Dupont-Bierre E, Compagnon P, Raoul JL, Fayet G, de Lajarte-Thirouard AS, Boudjema K. Resection of hepatocellular carcinoma in noncirrhotic liver: analysis of risk factors for survival. J Am Coll Surg. 2005;201(5):663–70. doi: 10.1016/j.jamcollsurg.2005.06.265. [DOI] [PubMed] [Google Scholar]
- 27.Baton O, Azoulay D, Adam DV, Castaing D. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg. 2007;204(2):250–60. doi: 10.1016/j.jamcollsurg.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Laurent C, Rullier E, Feyler A, Masson B, Saric J. Resection of noncolorectal and nonneuroendocrine liver metastases: late metastases are the only chance of cure. World J Surg. 2001;25(12):1532–6. doi: 10.1007/s00268-001-0164-7. [DOI] [PubMed] [Google Scholar]
- 29.Jaeck D , Oussoultzoglou E , Rosso E , Greget M , Weber JC , Bachellier P . A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg 2004;240(6):1037–49; discussion 1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zacharias T, Jaeck D, Oussoultzoglou E, Bachellier P, Weber JC. First and repeat resection of colorectal liver metastases in elderly patients. Ann Surg. 2004;240(5):858–65. doi: 10.1097/01.sla.0000143272.52505.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mala T, Edwin B, Rosseland AR, Gladhaug I, Fosse E, Mathisen O. Laparoscopic liver resection: experience of 53 procedures at a single center. J Hepatobiliary Pancreat Surg. 2005;12(4):298–303. doi: 10.1007/s00534-005-0974-3. [DOI] [PubMed] [Google Scholar]
- 32.Chang S, Laurent A, Tayar C, Karoui M, Cherqui D. Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg. 2007;94(1):58–63. doi: 10.1002/bjs.5562. [DOI] [PubMed] [Google Scholar]