Abstract
We investigated the effect of treatment with an aldose reductase inhibitor, insulin or select neurotrophic factors on the generation of oxidative damage in peripheral nerve. Rats were either treated with streptozotocin (STZ) to induce insulin-deficient diabetes or fed with a diet containing 40% D-galactose to promote hexose metabolism by aldose reductase. Initial time-course studies showed that lipid peroxidation and DNA oxidation were significantly elevated in sciatic nerve after 1 week or 2 weeks of STZ-induced diabetes, respectively, and that both remained elevated after 12 weeks of diabetes. The increase in nerve lipid peroxidation was completely prevented or reversed by treatment with the aldose reductase inhibitor, ICI 222155, or by insulin, but not by the neurotrophic factors, prosaptide TX14(A) or neurotrophin-3. The increase in nerve DNA oxidation was significantly prevented by insulin treatment. In contrast, up to 16 weeks of galactose feeding did not alter nerve lipid peroxidation or protein oxidation, despite evidence of ongoing nerve conduction deficits. These observations demonstrate that nerve oxidative damage develops early after the onset of insulin-deficient diabetes and that it is not induced by increased hexose metabolism by aldose reductase per se, but rather is a downstream consequence of flux through this enzyme. Furthermore, the beneficial effect of prosaptide TX14(A) and neurotrophin-3 on nerve function and structure in diabetic rats are not due to amelioration of increased lipid peroxidation.
1. Introduction
Neuropathy is one of the most frequent complications of diabetes mellitus, affecting up to half of those with this disease. Loss of sensation, when combined with microvascular disease and impaired wound healing, results in a pathologic cascade that makes diabetes mellitus the leading cause of lower limb amputation in western societies [1] and a significant economic burden for public health systems. Neurological complications include nerve conduction slowing, resistance to ischemic conduction block and altered sensory perception that often begin early in the disease, while pathologic changes to unmyelinated and myelinated nerve fibers as well as other components of the endoneurial microenvironment become evident later (reviewed in [2]). Although elevated blood glucose levels have been identified as a primary metabolic disturbance underlying diabetic neuropathy (reviewed in [3]), the interrelationships between downstream consequences of hyperglycemia and their role in nerve damage remain unresolved.
The initial downstream metabolic consequences of hyperglycemia include non-enzymatic glycosylation of proteins that ultimately form advanced glycosylation end products, and increased glucose metabolism by hexokinase as well as by aldose reductase, the first enzyme of the polyol pathway. The contribution of exaggerated polyol pathway flux to nerve damage has been emphasized by specific inhibitors of aldose reductase (ARIs) that ameliorate early hyperglycemia-induced functional and structural nerve disorders (reviewed in [4]). Recent work has implicated oxidative stress as a potential downstream consequence of glucose metabolism by aldose reductase, and anti-oxidants prevent or reverse a range of functional and biochemical nerve defects that are also amenable to ARI treatment (reviewed in [5]). However, the precise mechanisms by which increased polyol pathway flux generates oxidative damage remains under investigation. One potential mechanism is depletion of the co-factor NADPH, which is necessary for the activity of both aldose and glutathione reductases, so that too much substrate-driven glucose metabolism by aldose reductase may deplete nerve NADPH levels and impede regeneration of reduced glutathione (GSH), a prominent component of anti-oxidant defense systems in nerve [6]. Reports that nerve GSH levels are reduced in diabetic rats and can be corrected by treatment with an ARI [7] support this contention. However, other consequences of increased flux through the polyol pathway downstream of aldose reductase activity may also prompt nerve GSH depletion and oxidative stress, and a number of mechanisms by which diabetes produces oxidative damage to the nerve have been suggested (reviewed in [8]).
In the present study, we investigated the specific contribution of the first step of the polyol pathway, hexose metabolism by aldose reductase, to the generation of oxidative damage in peripheral nerve. To do this, we adopted two approaches, the pharmacological inhibition of aldose reductase in STZ-diabetic rats, which prevents all flux through the polyol pathway, and galactose intoxication, which promotes excessive galactose metabolism by aldose reductase and accumulation of the osmotically actitive dulcitol, but no further downstream metabolic activity. Findings prompted additional studies designed to evaluate whether neurotrophic factors known to correct nerve dysfunction in diabetic rodents do so by ameliorating oxidative damage.
2. Material and Methods
2.1. Animals
All studies were performed using adult female Sprague-Dawley rats (Harlan Industries, San Diego CA, USA). Animals were housed 2–3 per cage with free access to food and water and maintained in a vivarium approved by the American Association for the Accreditation of Laboratory Animal Care. These studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Biochemical and physiologic parameters of rats that supplied nerve for some of the studies presented below have already been reported and, where such archival material has been used, the pertinent citation is provided.
2.2. Induction of diabetes
Two different models of experimental diabetes, streptozotocin (STZ)-induced diabetes and galactose feeding, were used. Insulin-deficient diabetes was induced following an overnight fast by a single intra-peritoneal injection of STZ (50 mg/kg; Sigma) dissolved in 0.9% sterile saline. Non-fasting blood sugar levels of 15 mM or above were used to define diabetes. Experimental galactosemia was induced by feeding a diet containing 40% D-galactose by weight and supplemented with 100% of the micronutrients required by rats (Purina, Richmond, IN).
2.3. Drugs
STZ was purchased from Sigma (St. Louis, MO, USA). Prosaptide TX14 (A), a 14 amino acid peptide fragment (TXLIDNNATEEILY, where X=D-alanine) from the neurotrophic region of saposin C, was a gift from Myelos (San Diego, CA, USA) and was dissolved in phosphate-buffered saline. Human recombinant neurotrophin-3 (NT-3) was obtained from Amgen (Thousand Oaks, CA) and the ARI, ICI 22155, was a gift from Dr. D. Mirrlees of Zeneca Pharmaceuticals (Macclesfield, UK).
2.4. Experimental protocols
In initial time course studies, STZ-diabetic rats were maintained for up to 12 weeks, while galactose-fed rats were maintained on the 40% galactose diet for up to 16 weeks before tissue collection. Using a strip operated reflectance meter (OneTouch Ultra, Lifescan, Inc., Milpitas, CA, USA), hyperglycemia was confirmed in a blood sample obtained by tail prick 3 days after STZ injection and also in another sample obtained immediately prior to sacrifice. Efficacy of the galactose-feeding regimen at inducing peripheral nerve disorders was confirmed by measuring motor and sensory conduction velocity (MNCV and SNCV) in the sciatic nerve immediately prior to sacrifice and tissue collection. Nerve conduction was measured under isoflurane anesthesia in the sciatic nerve using single square wave stimuli (10 V, 0.05ms) delivered via stimulating electrodes placed at the sciatic notch and Achilles tendon, and with the resulting M and H waves captured to a digital oscilloscope via recording electrodes placed in the interosseous muscles of the ipsilateral hind paw (see [9] for details).
2.4.1. ARI and insulin
Two different treatment protocols were used to study the effects of aldose reductase inhibition or insulin on nerve lipid peroxidation. In the prevention study, treatment with the ARI, ICI 222155 (20 mg/kg, six days a week by oral gavage), or insulin was initiated after confirmation of hyperglycemia and was continued for 4 weeks. Insulin treatment in diabetic rats was carried out using slow-dissolving pellets that deliver approximately 2–4 units of insulin per day (Linshin, Scarborough, Ontario, Canada). Pellets were implanted under the skin and glucose levels were checked weekly in blood samples obtained by tail prick. Implants were replaced when blood sugar levels increased to above 15 mM, a value widely used to define the presence of hyperglycemia in STZ-injected rats. In the reversal study, the same treatment regimens were maintained but they were initiated after 4 weeks of untreated diabetes. In both studies, rats were killed and sciatic nerves were removed after 4 weeks of treatment and stored as described below. The efficacy of ARI and insulin treatment in reducing flux through the polyol pathway was confirmed by measuring sciatic nerve sugar and polyol content by gas chromatography [11].
2.4.2. Neurotrophic factors
Treatment with TX14(A) (1 mg/kg in 250 µl of phosphate-buffered saline thrice weekly by subcutaneous injection) was initiated after confirmation of STZ-induced hyperglycemia and continued for 16 weeks. Treatment with human recombinant NT-3 (1 mg/kg in 250 µl of phosphate-buffered saline thrice weekly by subcutaneous injection) was initiated 2 months after confirmation of STZ-induced diabetes and continued for an additional month. Efficacy of these treatment regimes against a range of nerve disorders in STZ-diabetic rats has already been reported [9, 12] and the material used in the present study was derived from the same animals after archiving at −70°C.
2.5. Tissue extraction and homogenization
At the end of each study, rats were anesthetized and killed by decapitation. Sciatic nerves were then quickly removed, immediately frozen in liquid nitrogen and stored at −70°C until used. For analysis, sciatic nerve segments (~ 2 cm) were weighed and homogenized in 400 µl of PBS buffer (pH 7.4), containing 5 mM butylated hydroxytoluene (Sigma-Aldrich) and 0.4% protease inhibitor cocktail (Sigma-Aldrich). The samples were centrifuged at 10,000g for 15 minutes at 4°C and the supernatants were collected for assay of protein concentration (BCA™ Protein Assay Kit; Pierce, Rockford, IL, USA), lipid peroxidation and protein oxidation (see below).
2.6. Lipid peroxidation: malondialdehyde (MDA) plus 4-hydroxyalkenal (HAE) assay
Measurement of total MDA+HAE levels was performed using a commercial kit (LPO-586 assay, Oxis International Inc., CA, USA). The method is based on the reaction of the chromogenic reagent, N-methyl-2-phenylindole, with MDA and HAE at 45°C [13]. Two hundred microliters of homogenate were used for measurements of total MDA+HAE, according to the manufacturer’s instructions. The absorbance of chromogenic product was measured at 586 nm with a Uvikon 930 spectrophotometer (Kontron Instruments, USA) and compared to the absorbance in corresponding MDA standards. The total MDA+HAE concentration was expressed as µmoles per mg protein.
2.7. Protein oxidation: 2, 4 dinitrophenylhydrazone (DNP)-derivatized protein assay
The levels of oxidatively modified proteins were measured by immunoblotting, using an assay kit (Chemicon International Inc., CA, USA; OxyBlot™ Protein Oxidation Detection assay). The kit provides reagents for sensitive immunodetection of the carbonyl groups introduced into protein side chains by oxidative stress. Briefly, sciatic nerve homogenates were pre-incubated with 2,4 dinitrophenylhydrazine (DNPH) or derivatization-control solution. The carbonyl groups in the protein side chains were derivatized to DNP by reaction with DNPH. Derivatized-protein samples were directly dot-blotted onto a membrane that was incubated with specific primary antibody to the DNP moiety of the proteins (1:150) for 1 hour at room temperature, followed by incubation with peroxidase-linked goat anti-rabbit IgG antibody (1:300) for 45 min at room temperature. Chemiluminescence was developed with extended-duration substrate (Lumi-Light Western Blotting, Roche, Indianapolis, IN, USA) and detected using a Hyperfilm-ECL (Amersham Biosciences, Piscataway, NJ, USA). Intensities of dots were determined using Quantity One® software developed by Bio-Rad. Data were expressed as the ratio of DNP-derivatized protein intensity to actin intensity. Actin intensity was quantified after pre-incubation of membranes with Restore™ Western Blot Stripping Buffer (Pierce, Rockford, IL, USA) for 15 minutes at room temperature. Following this step, membranes were incubated with mouse monoclonal anti-actin antibody (1:2000; Sigma-Aldrich) for 1 hour and then with a peroxidase-linked goat anti-mouse IgG antibody (1:10000) for 45 minutes. Chemiluminescence and dot intensity were determined as described above.
2.8. DNA oxidation: 8-OH-dG EIA assay
The amount of 8-OH-dG in sciatic nerves from control and STZ-diabetic rats was determined using the Bioxytech 8-OHdG assay kit, a competitive enzyme-linked immunosorbent assay (OXIS Health Products Portland, OR, USA). Sciatic nerve DNA was isolated using a DNeasy® Tissue kit (Quiagen, MD, USA), according to the manufacturer’s instructions. After measuring the concentration of DNA in the samples by their absorbance at 260 nm, 100 µl of extracted DNA samples was resuspended in 135 µL of ddH2O, 15 µL of 200 mM ammonium acetate (pH 5.3), 15 µL of 5 mM of ZnSO4 and 6 units of nuclease P1. Samples were mixed, incubated for 30 min at 37 °C with an argon overlay and then 15 µL of 1M of TRIS- HCl (pH 8.5), 15 µL of 5 mM EDTA and 2 units of alkaline phosphatase were added. After mixing, samples were first incubated for 30 min at 37 °C with an argon overlay and then purified using Millipore Ultra-free MC PLGC Centrifugal Filter Units. Finally, DNA-digested samples were added to the microtiter plate pre-coated with 8-OHdG, and the assay was performed according to the manufacturer’s instructions. Results were expressed as ng 8-OH-dG per µg total DNA.
2.9. Statistical analysis
The two-tailed, unpaired t-test was used in studies involving two groups. Differences between three or more groups were tested using one-way ANOVA, after which multiple comparisons were made using the Dunnett’s or Bonferroni’s post-hoc tests. Data are reported as group mean ± SEM.
3. Results
3.1. Lipid peroxidation and DNA oxidation, but not protein oxidation, were increased in nerves from STZ-diabetic rats
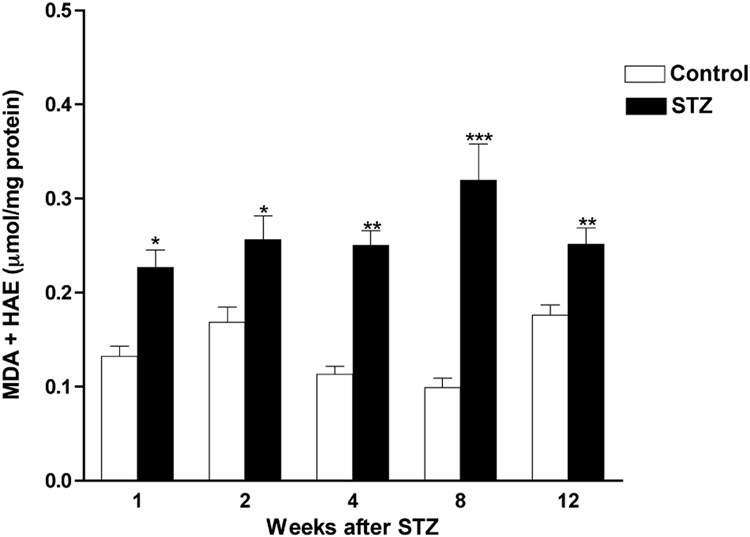
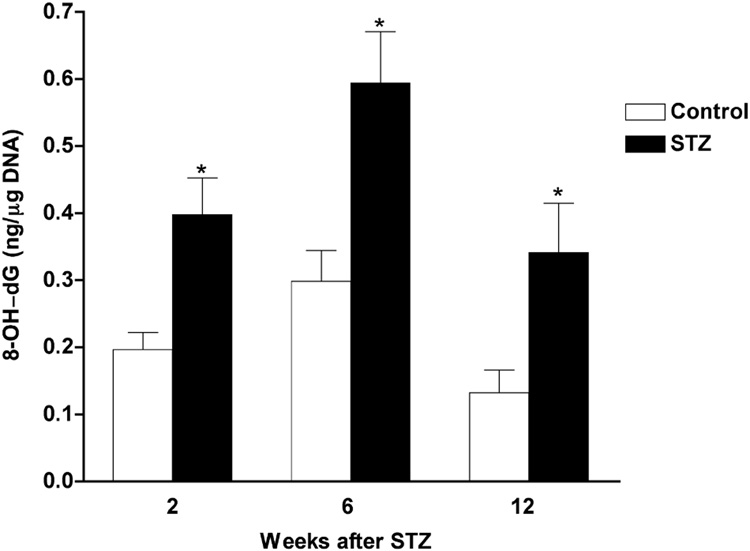
All STZ-diabetic rats had blood sugar levels above the 15 mM lower limit and most were above the upper detection limit (33.3 mM) for the glucose analyzer, whereas all control rats had blood sugar levels less than 15 mM (data not shown). After 12 weeks of STZ-induced diabetes, sciatic nerve had significantly higher MDA+HAE (Figure 1) and 8-OH-dG (Figure 2) levels compared to nerve from control rats, indicating increased nerve lipid peroxidation and DNA oxidation, respectively. However, the ratio of DNP-derivatized protein per unit of actin in sciatic nerve was not significantly different between age-matched control and STZ-diabetic rats (control: 0.667 ± 0.088, N = 11; diabetic: 0.550 ± 0.095; mean ± SEM, N = 9 per group), suggesting that nerve protein oxidation is not increased after this duration of diabetes.
Figure 1. Time course of increased lipid peroxidation in nerves from STZ-diabetic rats.
Levels of MDA+HAE were measured in sciatic nerves from age-matched control and diabetic rats after varying durations of diabetes. Data are presented as mean ± SEM (N = 5–9 per group) and were analyzed by two-tailed, unpaired-t test. * = p<0.05, ** = p<0.01 and *** = p<0.001 versus appropriate control.
Figure 2. Time course of increased DNA oxidation in nerves from STZ-diabetic rats.
Levels of 8-OH-dG were evaluated in sciatic nerves from age-matched control and diabetic rats after varying durations of diabetes. Data are presented as mean ± SEM (N = 5–7 per group) and were analyzed by two-tailed, unpaired-t test. * = p<0.05 versus appropriate control.
To determine the stability of tissue stored at −70°C, lipid peroxidation levels were measured in sciatic nerves from non-diabetic rats stored for 2 weeks and 10 years. MDA+HAE levels in these nerves were virtually identical (2 weeks: 0.223 ± 0.027 µmoles/mg protein, mean ± SEM, N = 11; 10 years: 0.218 ± 0.022, N = 8), indicating long-term stability under our storage conditions.
3.2. Galactose feeding did not increase nerve lipid peroxidation or protein oxidation
After 8 or 16 weeks on a diet containing 40% D-galactose, body weight of galactose-fed rats was significantly reduced compared to that of control rats (8 weeks: 279 ± 6 g versus 251 ± 5 g, control versus galactose-fed, respectively, mean ± SEM, N = 8–11 per group, p<0.05, unpaired t-test; see [9] for 16 week data). Eight or 16 weeks of galactose feeding impaired nerve function, as indicated by significantly reduced MNCV (8 weeks: 67.1 ± 3.6 m/s versus 55.4 ± 2.3 m/s, control versus galactose-fed, respectively, p<0.05; see [9] for 16 week data) and SNCV (8 week: 55.3 ± 2.1 m/s versus 45.8 ± 1.1 m/s, control versus galactose-fed, respectively, p<0.05; see [9] for 16 week data). Sciatic nerve levels of MDA+HAE in galactose-fed rats were not significantly different from those in age-matched controls at either time point (Table 1). DNP-derivatized proteins from sciatic nerve of galactose-fed rats were also not significantly different from those in control rats after 8 or 16 weeks of galactose feeding (Table 1). These data suggest that galactose feeding, unlike STZ-induced diabetes, does not induce either lipid peroxidation or protein oxidation.
Table 1.
Lipid peroxidation and protein oxidation in sciatic nerves from control and galactose-fed rats.
| MDA + HAE (µmoles/ mg protein) | DNP-derivatized protein/actin ratio | |||
|---|---|---|---|---|
| 8 weeks | 16 weeks | 8 weeks | 16 weeks | |
| Control | 0.155 ± 0.017 | 0.169 ± 0.015 | 0.395 ± 0.023 | 0.604 ± 0.082 |
| Galactose | 0.195 ± 0.019 | 0.183 ± 0.019 | 0.349 ± 0.028 | 0.658 ± 0.174 |
Data are mean ± SEM (N = 7–11 per group) and were analyzed with unpaired, two-tailed t-tests. Differences are not significant.
3.3. Increased lipid peroxidation and DNA oxidation in nerve was apparent after as little as 1 or 2 weeks of diabetes, respectively
After observing increased nerve lipid peroxidation and DNA oxidation resulting from 12 weeks of diabetes, we evaluated the levels of MDA+HAE and 8-OH-dG in sciatic nerves from rats with diabetes of different durations and with blood sugar concentration > 15 mM at onset and sacrifice. Levels of MDA+HAE were already significantly elevated in sciatic nerve after 1 week of STZ-induced diabetes and remained elevated at all subsequent time points evaluated (Figure 1). Levels of 8-OH-dG were also significantly elevated in sciatic nerve after 2 weeks and remained elevated after 6 or 12 weeks of STZ-induced diabetes (Figure 2).
3.4. ARI or insulin treatment prevented and/or reversed diabetes-induced increases of lipid peroxidation and DNA oxidation in sciatic nerves
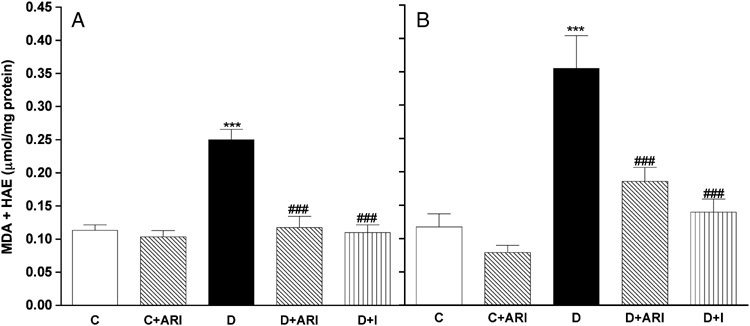
To further study the impact of flux through aldose reductase and hyperglycemia on the induction and maintenance of diabetes-induced increases in nerve oxidative stress, two different experiments were conducted. In the prevention study, treatment with the ARI, ICI 222155, or insulin were initiated after confirmation of hyperglycemia and continued for 4 weeks. In the reversal study, the same doses and regimen were used but treatments were initiated after 4 weeks of untreated diabetes and continued for a further 4 weeks. In both studies, ARI treatment prevented or reversed accumulation of the polyol-pathway products, sorbitol and fructose, without affecting nerve glucose levels (see Table 2 for the reversal study and [14] for the prevention study), while insulin treatment prevented or reversed accumulation of glucose, sorbitol and fructose in the sciatic nerve of diabetic rats. MDA+HAE levels in sciatic nerves from control rats treated with ICI 222155 did not differ from levels found in untreated control rats (Figure 3A and B). MDA+HAE levels in sciatic nerves from STZ-diabetic rats were significantly higher than those obtained from control rats when sciatic nerves were removed after 4 (Figure 3A) or 8 weeks (Figure 3B) of diabetes. Treatment with ICI 222155 or insulin significantly prevented (Figure 3A) and reversed (Figure 3B) the diabetes-induced increase of nerve lipid peroxidation. The levels of MDA+HAE in nerves from diabetic rats treated with ICI 222155 or with insulin were not different from those obtained from control rats (Figure 3A and B).
Table 2.
Body weight, blood glucose and nerve sugar levels in control rats, diabetic rats and diabetic rats treated with the aldose reductase inhibitor, ICI 222155, or insulin for the last 4 weeks of an 8-week period of diabetes.
| Nerve | ||||||
|---|---|---|---|---|---|---|
| Body weight | Blood glucose | glucose | sorbitol | fructose | myo-inositol | |
| (g) | (mM) | (nmol/mg nerve dry weight) | ||||
| Control | 273±7* | All < 15 | 4.1±0.6* | 0.6±0.4* | 0.6±0.3* | 12.7±1.5* |
| Diabetic | 236±7 | All > 15 | 42.7±3.9 | 3.6±0.5 | 20.0±1.6 | 9.6±0.5 |
| Diabetic +ARI | 243±8 | All > 15 | 56.3±3.9 | 0.1±0.1* | 3.2±0.4* | 11.9±0.7* |
| Diabetic +insulin | 283±7* | All < 15 | 1.3±0.4* | 0.3±0.3* | 0.1±0.1* | 11.4±1.1* |
Data are presented as mean ± SEM (N = 8–16 per group) and were analyzed by one-way ANOVA, after which multiple comparisions were made with Dunnett’s post-hoc test.
p<0.05 vs untreated diabetic group.
Figure 3. Treatment with the ARI, ICI 222155, or insulin completely prevented and reversed the diabetes-induced increase in nerve lipid peroxidation.
Levels of MDA+HAE were measured in sciatic nerves from age-matched control rats (C); ICI 222155-treated control rats (C+ARI; 20 mg/kg), STZ-diabetic rats (D); ICI 222155-treated STZ-diabetic rats (D+ARI; 20 mg/kg) and in insulin-treated STZ-diabetic rats (D+I). Treatments with ICI 222155 or insulin were initiated at the onset of hyperglycemia and continued for 4 weeks (A) or were initiated after 4 weeks of untreated diabetes and continued for 4 weeks (B). Data are presented as mean ± SEM (N = 7–10 per group) and were analyzed by one-way ANOVA, after which multiple comparisons were made with the Bonferroni test. *** = p<0.001 versus untreated control rats and ### = p<0.001 versus untreated diabetic rats.
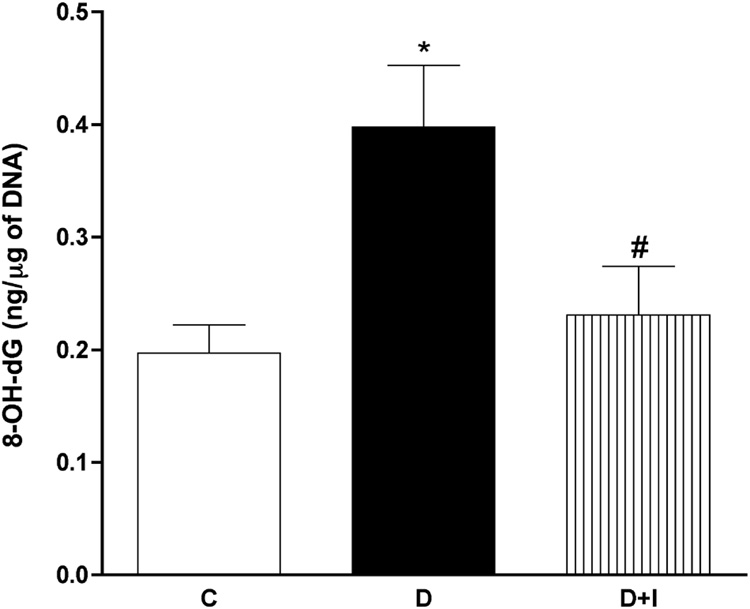
With regard to DNA oxidation, treatment with insulin (initiated after confirmation of hyperglycemia and continued for 4 weeks) significantly prevented the diabetes-induced increase of sciatic nerve 8-OH-dG levels (Figure 4), suggesting that nerve DNA oxidation was caused by the diabetic state rather than a direct toxic effect of STZ.
Figure 4. Treatment with insulin significantly prevented the diabetes-induced increase in nerve DNA oxidation.
Levels of 8-OH-dG were evaluated in sciatic nerves from age-matched control rats (C); STZ-diabetic rats (D) and in insulin-treated STZ-diabetic rats (D+I). Treatments with insulin were initiated at the onset of hyperglycemia and continued for 4 weeks. Data are presented as mean ± SEM (N = 5 per group) and were analyzed by one-way ANOVA, after which multiple comparisons were made with the Bonferroni test. * = p<0.05 versus untreated control rats and # = p<0.05 versus untreated diabetic rats.
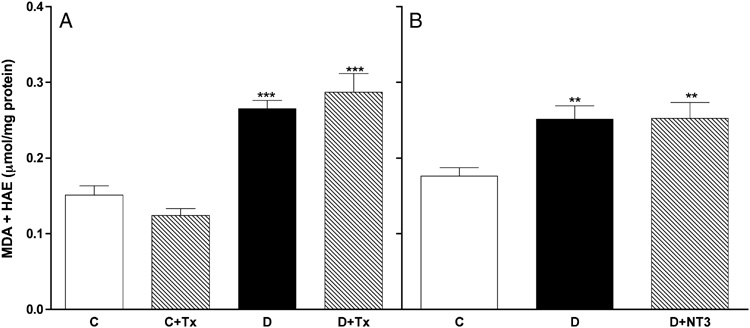
3.5. Treatment with select neurotrophic factors failed to prevent or reverse the diabetes-induced increase of lipid peroxidation in sciatic nerves
Previous studies have established that nerve disorders in STZ-diabetic rats can be prevented and reversed by treatment with select neurotrophic factors. In order to determine whether this neuroprotection includes amelioration of oxidative stress, we evaluated the MDA+HAE levels in sciatic nerves from STZ-diabetic rats in which nerve function was restored by treatment with either prosaptide TX14(A) or NT-3 [9, 12]. MDA+HAE levels in sciatic nerves from untreated diabetic rats were significantly higher when compared to those obtained from control rats, but treatment with prosaptide TX14(A) or NT-3 neither prevented (Figure 5A) nor reversed (Figure 5B) the diabetes-induced increase of nerve lipid peroxidation.
Figure 5. Treatment with neurotrophic factors did not alter the diabetes-induced increase in nerve lipid peroxidation.
(A) Levels of MDA+HAE were measured in sciatic nerves from age-matched control rats (C); control rats treated with TX14(A) (C+Tx, 1 mg/kg, thrice weekly), STZ-diabetic rats (D); or STZ-diabetic rats treated with TX-14 (D+Tx, 1 mg/kg, thrice weekly). The duration of diabetes and treatment was 16 weeks. (B) Levels of MDA+HAE in sciatic nerves from age-matched control rats (C); STZ-diabetic rats (D); and STZ-diabetic rats treated with NT-3 (D+NT3; 1 mg/kg, thrice weekly). Treatment with NT-3 was initiated after 2 months of untreated diabetes and was continued for one additional month. Data are presented as mean ± SEM (N = 5–9 per group) and were analyzed by one-way ANOVA, after which multiple comparisons were made with the Bonferroni test. ** = p<0.01 and *** = p<0.001 versus control.
4. Discussion
In diabetes mellitus, oxidative stress is correlated with the development of complications in both type 1 and type 2 diabetic patients ([5] and references therein), with a decrease in anti-oxidant potential as well as increased lipid peroxidation and DNA oxidation accompanying disease progression. Numerous experimental studies have documented hyperglycemia-induced oxidative stress in peripheral nerve, dorsal root and sympathetic ganglia, and endothelial cells of rodent models of diabetes (comprehensively reviewed in [8]). A role for oxidative stress in the pathogenesis of diabetic neuropathy is further supported by experimental and clinical studies where various anti-oxidants, including glutathione and a precursor for glutathione biosynthesis [15, 16], lipid soluble anti-oxidants [17, 18], metal chelators [16, 19, 20], α-lipoic acid [21–24], and acetyl-L-carnitine [25–28], have been shown to ameliorate biochemical and functional nerve disorders. Despite widespread acceptance of oxidative stress having a role in the pathogenesis of diabetic neuropathy, the precise mechanisms involved remain unresolved.
Oxidative stress in diabetic nerve has generally been assessed by measuring just the accumulation of MDA and HAE lipid adducts, with lipid peroxidation reported as early as 3 weeks of STZ-induced diabetes [29]. In the present study, we confirmed the increased lipid peroxidation in nerve from diabetic rats from week 1 of hyperglycemia onwards and extended this finding to include a parallel increase in DNA oxidation. The lipid content of nerve is likely dominated by myelin lipids and oxidative damage to myelin may plausibly contribute to nerve disorders in diabetic polyneuropathy. The DNA within a nerve trunk derives from Schwann cells, endothelial cells, perineurial cells and assorted stromal cells, as well as from mitochondria within all cell types. The extent to which oxidative damage to DNA can alter protein expression has not yet been widely explored, but all of these cell types show some dysfunction during diabetes. Damage to mitochondrial DNA also has the potential to impact many aspects of nerve metabolism and function [30], and there is emerging evidence that mitochondrial dysfunction is an early pathogenic event in diabetic neuropathy [31–33]. Interestingly, increases in DNP-derivatized proteins, a marker for protein oxidation, were not evident at this time or even after 1 year of diabetes (Cunha, unpublished observations). Although this might reflect a faster turnover of damaged cellular proteins relative to lipids or DNA, certain nerve proteins such as collagen are long-lived [34] and presumably subject to oxidative modification. Given similar results after both 3 months and 1 year, it is not likely that the absence of increased DNP-derivatized proteins in nerves from STZ-diabetic rats is dependent on the duration of diabetes. The lack of increased protein oxidation might be marker-dependent, and it remains to be seen whether other markers of oxidative or nitrosative stress yield similar results.
Increased lipid peroxidation and DNA oxidation in peripheral nerve was established after as little as 1 or 2 weeks of diabetes, respectively, and remained relatively constant for many months. The efficacy of insulin treatment indicates that the both lipid peroxidation and DNA oxidation are not a direct consequence of any oxidative damage directly associated with STZ, and that lipid peroxidation is reversible. The rapid onset of lipid peroxidation and DNA oxidation makes them a candidate for exerting an early role in the pathogenic cascade leading to nerve dysfunction, as it precedes functional and structural disorders reported in the nerve of diabetic rats, such as conduction slowing and reduced axonal caliber [35, 36]. Other early changes seen in the nerve of diabetic rats after onset of hyperglycemia include accumulation of polyol pathway products and reduced blood flow [37, 38], and there is considerable debate regarding putative associations between these disorders (see [8] and references therein).
ARI treatment prevents reduced nerve blood flow in diabetic rats, so that nerve ischemia appears secondary to flux through the polyol pathway [10, 39]. Because nerve lipid peroxidation is completely prevented and reversed by aldose reductase inhibition, the consumption of glucose by the polyol pathway likely precipitates hyperglycemic-induced oxidative stress and suggests that oxidative stress derived from other polyol-pathway-independent sources contributes negligibly to the measured increase in lipid peroxidation seen in the nerve of diabetic rats. One possible explanation for the beneficial effect of ARIs in this study could be due to the fact that inhibition of aldose reductase prevents activation of protein kinase C (PKC), one of the proteins associated with the diabetes-induced increase in oxidative stress (reviewed by Srivastava et al., 2005). Similar efficacy has been reported with structurally dissimilar ARIs [7, 25], indicating that the effect is unlikely to be a drug property independent of inhibition of aldose reductase. Thus, exaggerated flux through the polyol pathway is an early initiating event that promotes many subsequent pathogenic mechanisms in peripheral nerve.
The sensitivity of hyperglycemia-induced lipid peroxidation to ARIs points to processes associated with flux through aldose reductase as the source of this oxidative stress, including GSH depletion promoted by NADPH deficiency, polyol-induced osmotic stress or downstream consequences of flux through aldose reductase, such as non-enzymatic glycation and glycoxidation resulting from fructose production. While the exact mechanism behind the sensitivity of lipid peroxidation to aldose reductase inhibition is not yet clear [8], our measurements of lipid peroxidation in rats fed diets containing 40% D-galactose provide insight as to which part of the polyol pathway is involved. Because dulcitol, the product of galactose metabolism by aldose reductase, is not subsequently metabolized by sorbitol dehydrogenase, the absence of increased levels of lipid adducts in nerve from galactose-fed rats suggests that hyperglycemia-induced increased lipid peroxidation results from downstream consequences of flux through aldose reductase and not an imbalance in the NADPH:NADP+ ratio or polyol accumulation per se. Galactose-fed rats develop nerve disorders similar to those seen in STZ-diabetic rats and patients with diabetic neuropathy, including nerve conduction slowing, hyperalgesia, and Schwann cell damage, which are all prevented by ARI treatment [40–42]. Our present data suggests that lipid peroxidation does not contribute to these disorders in galactose-fed rats, and that increased flux through aldose reductase can generate nerve damage independent of oxidative stress mechanisms.
Expression of neurotrophic factors and their receptors is altered in the dorsal root ganglia, nerve, muscle and other tissues of animals with experimental diabetes [43–45], suggesting a role for impaired neurotrophic support in the pathogenesis of diabetic neuropathy. While treatment with a variety of neurotrophic factors has been shown to ameliorate diabetes-induced functional and structural nerve defects [12, 46, 47], whether ameliorating oxidative stress is part of the protective mechanism has not been directly addressed. This possibility is suggested by reports that growth factor treatment protects mitochondrial function and may prevent excess free radical production by mitochondria [31, 32, 48, 49]. However, treatment with either the prosaposin-derived neurotrophic peptide, TX14(A), or the neurotrophin, NT-3, both of which prevented and reversed nerve conduction deficits in STZ-induced diabetes [9, 12], did not have an impact on nerve lipid peroxidation levels. Our findings suggest that the efficacy of TX14(A) and NT-3 against disorders of nerve function and structure in diabetic rats is not dependent on reducing nerve lipid peroxidation, and that nerve conduction can be restored to normal even in the face of increased lipid peroxidation. Whether neurotrophic factors intercede in pathogenic cascades downstream of lipid peroxidation or act entirely independently of oxidative stress pathways remains to be determined.
In summary, lipid and DNA, but not protein, adducts are increased in sciatic nerve of STZ-diabetic rats. Nerve lipid peroxidation and DNA oxidation are present after little as 1 and 2 weeks of diabetes, respectively, prior to the development of many of the functional and structural defects that characterize experimental diabetic neuropathy. Since both increased lipid peroxidation and DNA oxidation were prevented by insulin, oxidative stress in experimental diabetes is induced by hyperglycemia and is not a toxic effect of STZ. Because accumulation of lipid adducts is sensitive to aldose reductase inhibition in nerves from STZ-diabetic rats and is not present in nerves from galactose-fed rats, lipid peroxidation is likely a downstream consequence of flux through aldose reductase. Finally, the efficacy of TX14(A) and NT-3 at preventing or reversing STZ-induced nerve conduction deficits is not dependent on ameliorating increased lipid peroxidation.
Acknowledgements
We would like to thank Dr. Irina Obrosova and Carolina Aimore Bonin for technical advice. Supported by NIH awards HHSN267200612889C (NAC), DK057629 (NAC) and DK078374 (APM) and the Juvenile Diabetes Research Foundation (APM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw JE, Boulton AJ. The pathogenesis of diabetic foot problems: and overview. Diabetes. 1997;46:858–861. doi: 10.2337/diab.46.2.s58. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PK, Tomlinson DR. Diabetic and hypoglycemic neuropathy. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. 3rd ed. Philadelphia: WB Saunders; 1993. pp. 1219–1250. [Google Scholar]
- 3.Keen H. Insulin resistance and the prevention of diabetes mellitus. N Engl J Med. 1994;331:1226–1227. doi: 10.1056/NEJM199411033311812. [DOI] [PubMed] [Google Scholar]
- 4.Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6:475–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 5.Vincent AM, Russel JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 6.Cameron NE, Cotter MA. Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res Clin Pract. 1999;45:137–146. doi: 10.1016/s0168-8227(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 7.Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16:123–125. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 8.Obrosova IG. How does glucose generate oxidative stress in peripheral nerve? Int. Rev Neurobiol. 2002;50:3–35. doi: 10.1016/s0074-7742(02)50071-4. [DOI] [PubMed] [Google Scholar]
- 9.Mizisin AP, Steinhardt RC, O’Brien JS, Calcutt NA. TX14(A), a prosaposin-derived peptide, reverses established nerve disorders in streptozotocin-diabetic rats and prevents them in galactose-fed rats. J Neuropathol Exp Neurol. 2001;60:953–960. doi: 10.1093/jnen/60.10.953. [DOI] [PubMed] [Google Scholar]
- 10.Calcutt NA, Mizisin AP, Kalichman MW. Aldose reductase inhibition, Doppler flux and conduction in diabetic rat nerve. Eur J Pharmacol. 1994;251:27–33. doi: 10.1016/0014-2999(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 11.Sweeley CC, Bentley R, Makita M, Wells WW. Gas-liquid chromatography of trimethylsilyl derivates of sugars and related substances. J Am Chem Soc. 1963;85:2497–2507. [Google Scholar]
- 12.Mizisin AP, Calcutt NA, Tomlinson DR, Gallagher A, Fernyhough P. Neurotrophin-3 reverses nerve conduction velocity deficits in streptozotocin rats. J Peripher Nerv Syst. 1999;4:211–221. [PubMed] [Google Scholar]
- 13.Erdelmeier I, Gerard-Monnier D, Yadan JC, Chaudiere J. Reactions of N-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals: Mechanistic aspects of the colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1184–1194. doi: 10.1021/tx970180z. [DOI] [PubMed] [Google Scholar]
- 14.Ramos KM, Jiang Y, Svensson CI, Calcutt NA. Pathogenesis of spinally mediated hyperalgesia in diabetes. Diabetes. 2007;56:1569–1576. doi: 10.2337/db06-1269. [DOI] [PubMed] [Google Scholar]
- 15.Bravenboer B, Kappelle AC, Hamers FP, van Buren T, Erkelens DW, Gipen WH. Potential use of glutathione for the prevention and treatment of diabetic neuropathy in the streptozotocin-induced diabetic rat. Diabetologia. 1992;35:813–817. doi: 10.1007/BF00399926. [DOI] [PubMed] [Google Scholar]
- 16.Love A, Cotter MA, Cameron NE. Nerve function and regeneration in diabetic and galactosemic rats: antioxidant and metal chelator effects. Eur J Pharmacol. 1996;31:433–439. doi: 10.1016/s0014-2999(96)00528-6. [DOI] [PubMed] [Google Scholar]
- 17.Cameron NE, Cotter MA, Maxfield EK. Anti-oxidant treatment prevents the development of peripheral nerve dysfunction in streptozotocin-diabetic rats. Diabetologia. 1993;36:299–304. doi: 10.1007/BF00400231. [DOI] [PubMed] [Google Scholar]
- 18.Cameron NE, Cotter MA, Dines KC, Maxfield EK, Carey F, Mirrlees DJ. Aldose reductase inhibition, nerve perfusion, oxygenation and function in STZ-diabetic rats: dose-response considerations and independence from a myo-inositol mechanism. Diabetologia. 1994;37:651–663. doi: 10.1007/BF00417688. [DOI] [PubMed] [Google Scholar]
- 19.Cameron NE, Cotter MA. Neurovascular dysfunction in diabetic rats: potential contribution of autooxidation and free radical examined using transition metal chelating agents. J Clin Invest. 1995;96:1159–1163. doi: 10.1172/JCI118104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron NE, Cotter MA. Effects of extracellular metal chelator on neurovascular function in diabetic rats. Diabetologia. 2001;44:621–628. doi: 10.1007/s001250051669. [DOI] [PubMed] [Google Scholar]
- 21.Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995;18:1160–1167. doi: 10.2337/diacare.18.8.1160. [DOI] [PubMed] [Google Scholar]
- 22.Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46:S38–S42. doi: 10.2337/diab.46.2.s38. [DOI] [PubMed] [Google Scholar]
- 23.Cameron NE, Cotter MA, Horrobin DH, Tritschler HJ. Effects of alpha-lipoic acid on neurovascular function in diabetic rats: interaction with essential fatty acids. Diabetologia. 1998;41:390–399. doi: 10.1007/s001250050921. [DOI] [PubMed] [Google Scholar]
- 24.Stevens MJ, Obrosova IG, Cao X, van Huysen C, Greene DA. Effect of DL-a-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1016. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 25.Lowitt S, Malone JI, Salem AF, Korthals J, Benford S. Acetyl-L-carnitine corrects the altered peripheral nerve function of experimental diabetes. Metabolism. 1995;44:677–680. doi: 10.1016/0026-0495(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 26.Cotter MA, Cameron NE, Keegan A, Dines KC. Effects of acetyl-L-carnitine on peripheral nerve function and vascular supply in experimental diabetes. Metabolism. 1995;44:1209–1214. doi: 10.1016/0026-0495(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 27.Sima AA, Ristic H, Merry A, Kamijo M, Lattimer SA, Stevens MJ, Greene DA. Primary preventive and secondary interventionary effects of acetyl-L-carnitine on diabetic neuropathy in the biobreeding Worcester rat. J Clin Invest. 1996;97:1900–1907. doi: 10.1172/JCI118621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Grandis D, Minardi C. Acetyl-L:-carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long-term, randomized, double-blind, placebo-controlled study. Drugs R D. 2002;3:223–231. doi: 10.2165/00126839-200203040-00001. [DOI] [PubMed] [Google Scholar]
- 29.Obrosova IG, Van Huysen C, Fathallah L, Cao Xc, Stevens MJ, Greene DA. Evaluation of alpha(1)-adrenoceptor antagonist on diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2000;14:1548–1558. doi: 10.1096/fj.14.11.1548. [DOI] [PubMed] [Google Scholar]
- 30.Manfredi G, Beal MF. The role of mitochondria in the pathogenesis of neurodegenerative diseases. Brain Pathol. 2000;10:462–472. doi: 10.1111/j.1750-3639.2000.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasan S, Stevens M, Wiley JW. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 32.Huang TJ, Price SA, Chilton L, Calcutt NA, Tomlinson DR, Verkhratsky A, Fernyhough P. Insulin prevents depolarization of the mitochondrial inner membrane in sensory neurons of Type I diabetic rats in the presence of sustained hyperglycemia. Diabetes. 2003;52:2129–2136. doi: 10.2337/diabetes.52.8.2129. [DOI] [PubMed] [Google Scholar]
- 33.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194:279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Romen W, Lange HW, Hempel K, Keck T. Studies on collagen metabolism in rats. II. Turnover and amino acid composition of the collagen of glomerular basement menbrane in diabetes mellitus. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;36:313–320. [PubMed] [Google Scholar]
- 35.Greene DA, De Jesus PV, Winegrad AI. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975;55:1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakobsen J. Axonal dwindling in early experimental diabetes. II. A study of isolated nerves. Diabetologia. 1976;12:547–553. doi: 10.1007/BF01220630. [DOI] [PubMed] [Google Scholar]
- 37.Gabbay KH, Merola LO, Field RA. Sorbitol pathway: presence in nerve and cord with substrate accumulation in diabetes. Science. 1966;151:209–210. doi: 10.1126/science.151.3707.209. [DOI] [PubMed] [Google Scholar]
- 38.Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107:935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- 39.Cameron NE, Cotter MA, Dines KC, Maxfield EK, Carey F, Mirrlees DJ. Aldose reductase inhibition, nerve perfusion, oxygenation and function in streptozotocin-diabetic rats: dose response considerations and independence from myo-inositol mechanism. Diabetologia. 1994;37:651–663. doi: 10.1007/BF00417688. [DOI] [PubMed] [Google Scholar]
- 40.Willars GB, Calcutt NA, Tomlinson DR. Nerve conduction velocity and axonal transport of 6-phosphofructokinase activity in galactose-fed rats. J Neurol Sci. 1991;104:46–51. doi: 10.1016/0022-510x(91)90214-r. [DOI] [PubMed] [Google Scholar]
- 41.Calcutt NA, Malmberg AB, Yamamoto T, Yaksh TL. Tolrestat treatment prevents modification of the formalin test model of prolonged pain in hyperglycemic rats. Pain. 1994;58:413–420. doi: 10.1016/0304-3959(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 42.Mizisin AP, Powell HC. Schwann cell changes induced as early as one week after galactose intoxication. Acta Neuropathol (Berl) 1997;93:611–618. doi: 10.1007/s004010050659. [DOI] [PubMed] [Google Scholar]
- 43.Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res. 1990;26:258–267. doi: 10.1002/jnr.490260217. [DOI] [PubMed] [Google Scholar]
- 44.Fernyhough P, Diemel LT, Brewster WJ, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscles of experimentally diabetes. J Neurochem. 1995;65:1231–1237. doi: 10.1046/j.1471-4159.1995.64031231.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt RE, Dorsey DA, Roth KA, Parvin CA, Hounsom L, Tomlinson DR. Effect of streptozotocin-induced diabetes on NGF, P75(NTR) and TrkA content of prevertebral and parasympathetic ganglia. Brain Res. 2000;867:149–156. doi: 10.1016/s0006-8993(00)02281-2. [DOI] [PubMed] [Google Scholar]
- 46.Diemel LT, Brewster WJ, Fernyhough P, Tomlinson DR. Expression of neuropeptides in experimental diabetes; effects of treatment with nerve growth factor brain-derived neurotrophic factor. Brain Res Mol. 1994;21:171–175. doi: 10.1016/0169-328x(94)90391-3. [DOI] [PubMed] [Google Scholar]
- 47.Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997;46:S43–S49. doi: 10.2337/diab.46.2.s43. [DOI] [PubMed] [Google Scholar]
- 48.Fernyhough P, Huang TJ, Verkhratsky A. Mechanism of mitochondrial dysfunction in diabetic sensory neuropathy. J Peripher Nerve Syst. 2003;8:227–235. doi: 10.1111/j.1085-9489.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 49.Gustafsson H, Söderdahl T, Jönsson G, Bratteng Jo, Forsby A. Insulin-like growth factor type 1 prevents hyperglycemia-induced uncoupling protein 3 down regulation and oxidative stress. J Neurosci Res. 2004;77:285–291. doi: 10.1002/jnr.20142. [DOI] [PubMed] [Google Scholar]