Abstract
Bovine pericardium is an important biomaterial with current application in glutaraldehyde-fixed bioprosthetic heart valves and possible future application as an unfixed biological scaffold for tissue engineering. The importance of both humoral and cell-mediated rejection responses toward fixed and unfixed xenogeneic tissues has become increasingly apparent. However, the full scope and specific identities of bovine pericardium proteins that can elicit an immune response remain largely unknown. In this study, an immunoproteomic approach was used to survey bovine pericardium proteins for their ability to elicit a humoral immune response in rabbits. A two-stage protein extraction protocol was used to separate bovine pericardium proteins into water- and lipid-soluble fractions. Two-dimensional gel electrophoresis was performed to separate the proteins from each fraction. Western blots were generated from two-dimensional gels of both bovine pericardium protein fractions. These blots were probed with serum from rabbits immunized with bovine pericardium and a secondary antibody was used to assess for IgG positivity. Western blots were compared to duplicate two-dimensional gels and proteins in matched spots were identified by tandem mass spectrometry. Thirty-one putative protein antigens were identified, eight of which are known to be antigenic from previous studies. All of the putative antigens demonstrated progressive staining intensity with increasing days of post-exposure serum. Identified antigenic proteins represented a variety of functional and structural protein types, and included both cellular and matrix proteins. The results of this study have implications for the use of bovine pericardium as a biomaterial in bioprostheses and tissue engineering applications, as well as xenotransplantation in general.
Keywords: Immunoproteomics, Scaffold, Tissue Engineering, Xenoantigens, Heart Valve
1. Introduction
Bovine pericardium (BP) is an important natural biomaterial with current application in glutaraldehyde-fixed bioprosthetic heart valves and possible future application as an unfixed biological scaffold for tissue engineering. The importance of the immune response to xenogeneic biomaterials such as BP is becoming increasingly apparent in both applications.
Fixation with glutaraldehyde was once thought to largely mitigate the immune response to connective tissue xenografts by irreversibly cross-linking graft matrix proteins [1]. It is now clear that both humoral and cell-mediated immune responses to glutaraldehyde-fixed xenografts occur [1-7]. Mounting evidence implicates chronic antibody formation and immune rejection in bioprosthetic heart valve degeneration and calcification [2, 3, 6, 7]. This raises the possibility that treatments, such as antigen removal or antigen masking, might be devised to reduce antigen-driven activation of the immune system and thereby improve the durability of bioprosthetic heart valves. Before antigenic proteins can be targeted for removal or masking, their identity must be known. However, the proteins responsible for triggering immune rejection in bioprosthetic heart valves remain largely unknown.
Unfixed BP has received attention as a potential xenogeneic biological scaffold for tissue engineering, including tissue engineered heart valves [8-13]. Because tissues are unfixed in this application, it is necessary to remove graft antigenicity prior to implantation. Numerous physical and chemical treatments designed to decrease the immunogenicity of unfixed xenogeneic biomaterials have been investigated [9, 11, 12, 14-26]. These treatments have generally been characterized as tissue “decellularization”, based on the assumption that antigens mediating an immune response to the graft would likely be cell-associated. It is now clear that the apparent elimination of intact cells based on light microscopic examination does not assure adequate removal of xenoantigens, nor mitigation of immune rejection [8, 27-29]. As a result, emphasis has shifted from tissue “decellularization” to “antigen removal” [30, 31]. Here again, the full scope of xenogeneic tissue antigenicity remains largely undefined with regard to the number, origin, and identity of potential xenoantigens. In this study, we applied an immunoproteomic approach to survey BP proteins for their ability to generate a xenogeneic IgG humoral immune response.
2. Materials and Methods
2.1. Tissue Harvest
BP was harvested aseptically from adult cattle shortly after death and transported to the laboratory in pH 7.4 phosphate-buffered saline with 0.1% (w/v) ethylenediamine tetraacetic acid, 100 KIU/mL aprotinin, 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B. Pericardial fat and loose connective tissue were removed. BP was cut into 1-cm2 pieces and stored at -80 °C in 85% Dulbecco’s modified eagles medium and 15% (v/v) dimethyl sulphoxide.
2.2. Antiserum Production
Anti-BP serum was generated in New Zealand White rabbits. Rabbits were used in accordance with the guidelines established by Colorado State University IACUC and the Guide for the Care and Use of Laboratory Animals [32]. BP (1 g) was placed in 5 mL of 10 mM pH 8.0 tris-HCl, 100 KIU/mL aprotinin, 1 mM dithiothreitol (DTT), 2 mM MgCl2, 10 mM KCl, and 0.5 mM Pefabloc (Roche Applied Science), and mechanically homogenized on ice. One mL of the homogenized BP suspension was injected subcutaneously into rabbits (n = 4) at days 0 (diluted in a 1:1 ratio with complete Freud’s adjuvant) and 14 (diluted in a 1:1 ratio with incomplete Freud’s adjuvant). Serum was collected from the rabbits at 0, 7, 14, 21, 42, 56, and 72 days, and stored at -80 °C.
2.3. Protein Extraction
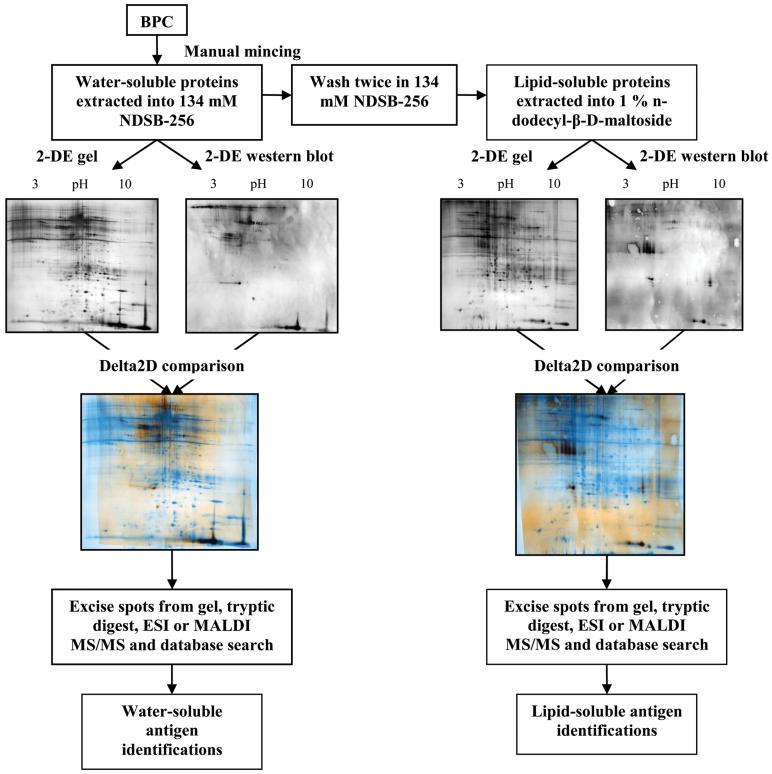
Protein extraction of BP was accomplished with a two-stage protocol to optimize collection of water- and lipid-soluble proteins (Fig. 1). Samples of BP were snap frozen in liquid nitrogen and minced into pieces approximately 0.5-1 mm on a side using sterile surgical instruments. Minced BP was placed in 1.5 mL cryogenic vials, containing 1 mL of 134 mM 3-(benzyldimethylammonio) propanesulphonate (NDSB-256) (Sigma-Aldrich), 10 mM pH 8.0 tris-HCl, 100 KIU/mL aprotinin, 1 mM DTT, 2 mM MgCl2, 10 mM KCl, and 0.5 mM Pefabloc. Vials were shaken on ice for 1 h and then centrifuged at 17,000×g, 4 °C for 25 min. The supernatant was collected and designated the water-soluble protein fraction. The pellet was washed twice in 0.5 mL of the same extraction solution by repeating the procedure described above. The supernatant from each wash was discarded. The pellet was resuspended in 0.5 mL of 1% n-dodecyl-β-D-maltoside (Sigma-Aldrich), 134 mM NDSB-256, 10 mM pH 8.0 tris-HCl, 100 KIU/mL aprotinin, 1 mM DTT, 2 mM MgCl2, 10 mM KCl, and 0.5 mM Pefabloc in nanopure water and shaken on ice for 1 h. Samples were centrifuged at 17,000×g, 4 °C for 25 min, the supernatant collected and designated the lipid-soluble protein fraction.
Fig. 1.
Workflow scheme for immunoproteomic identification of bovine pericardium xenoantigens.
The water-soluble fraction was concentrated using Centricon Ultracel YM-3 (cut-off 3000 Da) centrifugal filters (Millipore) at 6,500×g, 4 °C for 3 h and stored at -80 °C until required. The lipid-soluble fraction was concentrated with the same filters at 6,500×g, 4 °C for 90 min. Delipidation was achieved by ethanol precipitation of the concentrated lipid-soluble fraction. Lipid-soluble extracts were precipitated with nine volumes of ice-cold 100% ethanol. Samples were incubated at -4 °C for 60 min, and then centrifuged at 17,000×g for 25 min at 4 °C. The supernatant was discarded, the pellet air dried and resuspended in 9 M Urea, 3% CHAPS, 1% DTT, 1% triton X-100, 1% NDSB-256 in nanopure water and stored at -80 °C until required [33]. Total protein concentrations were determined using a DC protein assay kit (Bio-Rad) with bovine serum albumin (Sigma-Aldrich) as the standard.
2.4. One-Dimensional SDS-PAGE and Western Blot
SDS-PAGE was performed using an Xcell II, Surelock Mini-Cell electrophoresis system with 4-12% tris-glycine polyacrylamide gels (Invitrogen) [34]. In all cases, 37 μg of water-soluble protein and 15 μg of lipid-soluble protein and 15 μg of lipid-soluble protein were loaded in separate lanes on the gel. One-dimensional (1-D) western blotting onto a 0.2-μm pore size, nitrocellulose membrane was performed using an Xcell II blot module in accordance with the manufacturer’s recommendations (Invitrogen). Blots were blocked for 30 min with 5% non-fat dry milk in 10 mM pH 8.8 tris-buffered saline plus 0.5% Tween 20 (TBST). Blots (n = 16) were stained overnight at 4 °C, with a 1:10 dilution of rabbit serum either pre- (day 0) or post- (day 14, 21, 28, 42, 56 or 72) exposure to BP, in 5% milk in 10 mM TBST. Blots were washed 3 times for 10 min in 5% milk in TBST. The secondary antibody was a 1:5000 dilution of horseradish peroxidase-conjugated mouse monoclonal anti-rabbit IgG light chain specific antibody (Jackson ImmunoResearch Laboratories Inc), in 10 mM pH 8.8 tris-buffered saline. Blots were washed five times for 10 min in TBST. Digital images of blots were created using Supersignal Femto chemiluminescent reagent (Invitrogen) and recorded with a charge-coupled camera (CCD) bioimaging system (UVP Inc).
2.5. Two-Dimensional Gel Electrophoresis and Western Blot
Two-dimensional gel electrophoresis (2-DE) was performed in the first dimension using pH 3-10 non-linear immobilized pH gradient (IPG) ReadyStrips™ (Bio-Rad). IPG rehydration of water-soluble extracts was performed overnight, with 500 μg of protein diluted in an aqueous solution of 8 M urea, 2% CHAPS, 0.3% DTT, and 2% Ampholyte 3-10NL. For overnight IPG rehydration of lipid-soluble extracts, 200 μg of protein was diluted in a solution of 8 M urea, 1% CHAPS, 0.2% DTT, 1% triton X-100, 3% NDSB-256, 1.5% ASB 14, and 2% Ampholyte 3-10NL. Protein isoelectric focusing (IEF) was performed using a Multiphor II electrophoresis system (GE Healthcare) at 20 °C, with an initial 1 min linear increase in voltage to 500 V, followed by a linear increase in voltage to 3500 V over 5 h and then a constant voltage of 3500 V for 17.5 h.
Following IEF, IPG strips were reduced by submersion in a solution of 2% DTT w/v, 6 M urea, 30% glycerol v/v, and 0.1% SDS w/v for 15 min and then alkylated by submersion in a solution of 2.5% iodoacetamide (IAA), 6 M urea, 30% glycerol v/v, 0.1% SDS w/v, and a trace of bromophenol blue for 5 min. Strips were immediately loaded onto cast 12% polyacrylamide gels (20 cm × 20.5 cm × 1 mm) and electrophoresis performed at 200 V for 6 h in a Dodeca cell (Bio-Rad) with 0.1% SDS in 25 mM pH 8.3 tris-HCl, 192 mM glycine running buffer [35]. Gels were stained with a modified acidic silver staining protocol (Silver Stain PlusOne, AmershamPharmacia) [36, 37]. Digital gel images were recorded using a UVP CCD bioimaging system.
Western blotting of 2-DE gels was performed using a wet blot electrophoresis transfer system (Trans-Blot, Bio-Rad). Western blotting was performed onto 0.2-μm nitrocellulose membranes with a running buffer of 25 mM tris-HCl, 192 mM glycine at 100 V and 1 A for 1 h. Blots were stained and imaged as for 1-D western blots.
2.6. Image Analysis and Protein Identification
Resolved spots on two-dimensional (2-D) western blots were matched to the corresponding 2-DE gels using Delta 2D digital image analysis software (Decodon GmbH) (Fig. 1). Matched spots were excised from the gel, subjected to tryptic digestion and desalting. Tryptic digestion was accomplished by incubation of the gel spots with 0.04 μg sequencing grade modified trypsin (Promega) in 40 mM ABC overnight at 37 °C. Peptides were extracted from the gel spots by sequential incubation with 40 μL of 40 mM ammonium bicarbonate (ABC) for 15 min at room temperature, 5% formic acid for 15 min at 37 °C and 100% acetonitrile (ACN) for 15 min at 37 °C. The supernatant from the overnight tryptic digestion and those from all subsequent peptide extraction steps were collected and pooled. The pooled supernatants were dried using an SPD SpeedVac® (Thermo Electron Corporation) to a final volume of 5-10 μL. Resultant peptides were analyzed either by electrospray ionization-quadrupole/ion trap (ESI-Q/Trap) or matrix-assisted laser desorption/ionization-tandem time-of-flight (MALDI-TOF/TOF) mass spectrometry.
For ESI-Q/Trap analysis, extracted peptide samples were reconstituted in 10 μL of 0.5% formic acid with 2% acetonitrile. Nanoflow liquid chromatography was carried out by an LC Packings UltiMate integrated capillary high performance liquid chromatography system equipped with a Switchos valve switching unit (Dionex). For each sample, 6.4 μL were injected using a Famos auto sampler onto a PepMap C18 trap column (5 μm, 300 μm × 5 mm, Dionex) for on-line desalting and then separated on a PepMap C18 reverse phase nano column. Peptides eluted in a 15 min gradient of 5% to 40% acetonitrile in 0.1% formic acid at 250 nL/min into a 4000 Q Trap (ABI/MDS Sciex), a hybrid triple quadrupole linear ion trap mass spectrometer, that was equipped with a Micro Ion Spray Head II ion source. MS data acquisition was performed using Analyst 1.4.1 software (Applied Biosystems) in positive ion mode for information dependant acquisition (IDA) analysis. The nanospray voltage was set to 2.0 kV for all experiments. Nitrogen was used as the curtain gas, set to 10, and as the collision gas, set to high, with a heated interface at 175 °C. The declustering potential was set to 50 eV and Gas1 was set at 15 psi. After each survey scan between 400 m/z to 1600 m/z and an enhanced resolution scan, the three highest intensity ions with multiple charge states were selected for tandem MS (MS/MS) with rolling collision energy applied.
MS/MS spectra generated from ESI-Q/Trap analysis were interrogated using Mascot 2.2 (Matrix Science) and searched against the mammal taxonomy of the NCBI database (downloaded July 2007). The search parameters were set to allow for one missed cleavage, two variable modifications (methionine oxidation and cysteine carboxyamidomethylation), a peptide tolerance of 1.2 Da, and a MS/MS tolerance of 0.6 Da. Only peptides defined by a Mascot probability analysis (www.matrixscience.com/help/scoring_help.html#PBM) to be better than “identity” were considered and used for protein identifications. For protein identifications based on a single peptide match, the identity score criterion was made more stringent by also requiring a confidence interval of ≥ 95%.
For MALDI-TOF/TOF analysis, peptides were first desalted using ZipTips® C18 (Millipore) and then run as reported previously on a 4700 Proteomics Analyzer (Applied Biosystems).[38] Briefly, desalted digests were spotted onto target plates with 5 mg/mL alpha-cyano-4-hydroxycinnamic acid and 1 mg/mL ammonium phosphate in 50% ACN/0.1% TFA. MS was performed in positive ion reflector mode over a mass range of 700-4500 m/z, with 1200 laser shots per spot and internal calibration. Up to eight of the most intense peaks, excluding trypsin autolysis peaks, were selected from each MS spectrum for MS/MS. Tandem MS was performed in positive ion mode with 2700 laser shots, 1 kV collision energy and default calibration. GPS Explorer (v2.0 Applied Biosystems) was used as an interface between the raw data from the mass spectrometer and a local copy of Mascot search engine (v1.9 Matrix Science). A combined MS and MS/MS search was performed against a local copy of NCBInr (downloaded May 2007). Mascot searches were restricted to mammal taxonomy with 75 ppm MS mass tolerance, 0.3 Da MS/MS mass tolerance, trypsin specificity, two maximum missed cleavages, and the following three variable modifications: methionine oxidation and cysteine modifications by iodoacetamide and acrylamide. The criterion used to determine protein identification was a GPS Explorer confidence interval greater than 95%.
Localization of identified proteins was determined by a combination of peer-reviewed literature search, NCBI protein database search [39], PSORT subcellular localization prediction [40] and prediction of trans-membrane protein segments using HMMTOP [39-41] (Tables 1 and 2).
Table 1.
Bovine pericardium (BP) water-soluble fraction proteins identified by two-dimensional western blotting with rabbit serum collected before or after exposure to BP
| Spot Number | Protein Name | Accession No | Exposure Time Antibody First Detected | Protein Localization | MW | pI | Peptides Matched | Literature Evidence of Antigenicity |
|---|---|---|---|---|---|---|---|---|
| 1 | beta hemoglobin | gi|27819608 | 0 d | Cytoplasm | 15944.3 | 7.01 | 45 | [51] |
| 2 | UBFD1 protein | gi|151554567 | 0 d | Unknown | 33143.6 | 5.55 | 1 | None |
| 3 | albumin | gi|162648 | 0 d | Cytoplasm | 69278.5 | 5.82 | 19 | [52] |
| or 3 | hemoglobin beta-A chain | gi|122539 | 0 d | Cytoplasm | 15964.3 | 6.36 | 3 | None |
| 4 | albumin | gi|162648 | 0 d | Cytoplasm | 69278.5 | 5.82 | 19 | [52] |
| or 4 | hemoglobin beta-A chain | gi|122539 | 0 d | Cytoplasm | 15964.3 | 6.36 | 3 | None |
| 5 | albumin | gi|162648 | 0 d | Cytoplasm | 69278.5 | 5.82 | 19 | [52] |
| or 5 | hemoglobin beta-A chain | gi|122539 | 0 d | Cytoplasm | 15964.3 | 6.36 | 3 | None |
| 6 | hypothetical protein LOC540335 | gi|134085635 | 21 d | Unknown | 20765.6 | 5.09 | 3 | None |
| 7 | apolipoprotein A-1 | gi|245563 | 21 d | Cytoplasm or Membrane | 28414.8 | 5.57 | 16 | None |
| 8 | apolipoprotein A-1 | gi|245563 | 21 d | Cytoplasm or Membrane | 28414.8 | 5.57 | 9 | None |
| 9 | glutathione S-transferase M1 | gi|28461273 | 21 d | Nuclear or Cytoplasm | 25634.8 | 6.91 | 10 | None |
| 10 | peroxiredoxin 2 | gi|27807469 | 56 d | Cytoplasm | 21946.0 | 5.37 | 2 | None |
| 11 | alpha-1-acid glycoprotein | gi|94966811 | 56 d | Secreted | 23182.4 | 5.62 | 1 | None |
| 12 | triosephosphate isomerase | gi|61888856 | 56 d | Cytoplasm | 26672.8 | 6.45 | 2 | [47] |
| 13 | hemoglobin chain A | gi|116812902 | 56 d | Cytoplasm | 15174.9 | 8.07 | 35 | [51] |
| 14 | proline synthase homolog | gi|77735663 | 56 d | Cytoplasm | 29943.3 | 6.72 | 8 | None |
| 15 | peroxiredoxin 3 | gi|75948233 | 56 d | Mitochondrial | 31256.2 | 7.10 | 11 | None |
| 16 | Ig gamma-2 chain C region (clone 32.2) | gi|89611 | 56 d | Membrane | 36019.8 | 8.04 | 11 | None |
| 17 | hemoglobin beta-A chain | gi|122539 | 56 d | Cytoplasm | 15964.3 | 6.36 | 5 | None |
| 18 | Enolase 1 | gi|87196501 | 56 d | Cytoplasm or Membrane | 47296.4 | 6.37 | 9 | [53, 54] |
| 19 | Enolase 1 | gi|87196501 | 56 d | Cytoplasm or Membrane | 47296.4 | 6.37 | 21 | [53, 54] |
| 20 | Enolase 1 | gi|87196501 | 56 d | Cytoplasm or Membrane | 47296.4 | 6.37 | 37 | [53, 54] |
| 21 | allergenbos d 6 | gi|1351907 | 56 d | Secreted | 69248.4 | 5.82 | 43 | [52] |
| or 21 | hypothetical LOC534509 | gi|77736171 | 56 d | Unknown | 52209.2 | 7.9 | 6 | None |
| 22 | alpha-1-acid glycoprotein | gi|94966811 | 56 d | Secreted | 23182.4 | 5.62 | 1 | None |
| 23 | haptoglobin | gi|83638561 | 56 d | Cytoplasm | 44830.6 | 7.83 | 7 | None |
| 24 | parkinson disease 7 | gi|62751849 | 56 d | Unknown | 20035.3 | 6.84 | 5 | None |
| 25 | allergen bos d 6 | gi|1351907 | 56 d | Secreted | 69248.4 | 5.82 | 18 | [52] |
| 26 | allergen bos d 6 | gi|1351907 | 56 d | Secreted | 69248.4 | 5.82 | 18 | [52] |
| 27 | hypothetical LOC532481 | gi|129277510 | 56 d | Unknown | 26176.5 | 6.59 | 10 | None |
| 28 | hypothetical LOC532481 | gi|129277510 | 56 d | Unknown | 26176.5 | 6.59 | 5 | None |
| 29 | hypothetical LOC532481 | gi|129277510 | 56 d | Unknown | 26176.5 | 6.59 | 17 | None |
| 30 | hypothetical LOC532481 | gi|129277510 | 56 d | Unknown | 26176.5 | 6.59 | 15 | None |
| 31 | hypothetical LOC532481 | gi|129277510 | 56 d | Unknown | 26176.5 | 6.59 | 3 | None |
| 32 | beta hemoglobin | gi|27819608 | 56 d | Cytoplasm | 15944.3 | 7.01 | 7 | [51] |
| 33 | haptoglobin | gi|83638561 | 56 d | Cytoplasm | 44830.6 | 7.83 | 7 | None |
Table 2.
Bovine pericardium (BP) lipid-soluble fraction proteins identified by two-dimensional western blotting with rabbit serum collected after exposure to BP
| Spot Number | Protein Name | Accession No | Exposure Time Antibody First Detected | Protein Localization | MW | pI | Peptides Matched | Literature Evidence of Antigenicity |
|---|---|---|---|---|---|---|---|---|
| 1 | beta hemoglobin | gi|27819608 | 21 d | Cytoplasm | 15944.3 | 7.01 | 22 | [51] |
| 2 | beta hemoglobin | gi|27819608 | 21 d | Cytoplasm | 15944.3 | 7.01 | 11 | [51] |
| 3 | apolipoprotein A-I | gi|245563 | 21 d | Cytoplasm or Membrane | 28414.8 | 5.57 | 21 | None |
| 4 | alpha-1-B glycoprotein | gi|114053019 | 21 d | Secreted | 53553.5 | 5.3 | 5 | None |
| 5 | alpha-1-B glycoprotein | gi|114053019 | 21 d | Secreted | 53553.5 | 5.3 | 5 | None |
| 6 | hemoglobin chain A | gi|116812902 | 72 d | Cytoplasm | 15174.9 | 8.07 | 16 | [51] |
| 7 | hemoglobin chain A | gi|116812902 | 72 d | Cytoplasm | 15174.9 | 8.07 | 8 | [51] |
| 8 | glutathione S-transferase pi | gi|29135329 | 72 d | Nuclear or Cytoplasm | 23613.1 | 6.89 | 2 | None |
| 9 | hypothetical LOC614712 | gi|77736475 | 72 d | Unknown | 18011.9 | 9.3 | 1 | None |
| 10 | hypothetical LOC614712 | gi|77736475 | 72 d | Unknown | 18011.9 | 9.3 | 1 | None |
| 11 | hypothetical LOC614712 | gi|77736475 | 72 d | Unknown | 18011.9 | 9.3 | 3 | None |
| 12 | hypothetical LOC514335 | gi|77735877 | 72 d | Unknown | 28699.1 | 8.76 | 4 | None |
| 13 | hypothetical LOC532481 | gi|129277510 | 72 d | Unknown | 26176.5 | 6.59 | 3 | None |
| 14 | apolipoprotein A-I precursor | gi|162678 | 72 d | Cytoplasm or Membrane | 30271.3 | 5.8 | 3 | None |
| 15 | apolipoprotein E | gi|312893 | 72 d | Secreted | 35869.7 | 5.44 | 3 | None |
| 16 | annexin V | gi|260137 | 72 d | Nucleus or Membrane | 35942.7 | 4.94 | 6 | [55, 56] |
| 17 | osteoglycin protein | gi|74354996 | 72 d | Matrix | 34197.4 | 5.43 | 5 | None |
| 18 | osteoglycin precursor | gi|129077 | 72 d | Matrix | 34209.4 | 5.43 | 7 | None |
| 19 | osteoglycin precursor | gi|129077 | 72 d | Matrix | 34209.4 | 5.43 | 8 | None |
| 20 | osteoglycin precursor | gi|129077 | 72 d | Matrix | 34209.4 | 5.43 | 9 | None |
| 21 | chain A dimethylarginine dimethylamino-hydrolase I | gi|109157267 | 72 d | Cytoplasm | 31157.6 | 5.67 | 5 | [57] |
| 22 | chain D bovine mitochondrial F-1 ATPase | gi|1827812 | 72 d | Membrane | 51705.1 | 5.00 | 12 | None |
| 23 | chain D bovine mitochondrial F-1 ATPase | gi|1827812 | 72 d | Membrane | 51705.1 | 5.00 | 20 | None |
| 24 | hypothetical LOC535804 | gi|115497132 | 72 d | Unknown | 56453.1 | 8.57 | 19 | None |
| 25 | hypothetical LOC535804 | gi|115497132 | 72 d | Unknown | 56453.1 | 8.57 | 19 | None |
| 26 | allergen bos d 6 | gi|1351907 | 72 d | Secreted | 69248.4 | 5.82 | 8 | [52] |
3. Results
3.1. One-Dimensional Western Blot
Bands were present on blots of water- and lipid-soluble BP proteins when incubated with serum from BP-injected rabbits (Fig. 2). The number and intensity of bands from both fractions increased with time post-exposure to BP (i.e. 21, 42, and 72 day post-exposure serum) suggesting a mounting IgG antibody response to BP proteins. A few bands were present on water-soluble BP protein blots when exposed to pre-immune serum, suggesting the possibility of natural rabbit antibodies against some BP proteins. All of these bands intensified on incubation with post-immune serum.
Fig. 2.
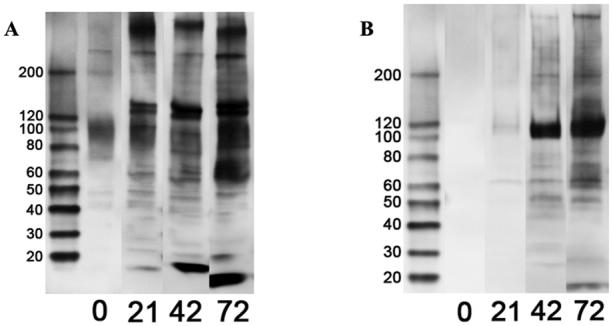
One-dimensional western blots of water-soluble (A) and lipid-soluble (B) bovine pericardium protein fractions treated with pre-immune (0-d) and 21-, 42-, and 72-d post-exposure anti-bovine pericardium rabbit serum.
3.2. Two-Dimensional Gels, Western Blots and Antigen Identifications
Two-dimensional gels of water- and lipid-soluble fractions (n = 8 for each fraction) of BP proteins were well resolved with minimal smearing in either direction (Figs. 3 and 4). Sequential treatment of corresponding 2-D western blots (n = 8 for each fraction) with pre-immune and 21-, 56-, and 72-day post-BP injection rabbit serum, demonstrated a few pre-immune and several new post-exposure spots. Similar to the results obtained on 1-D blots, spot number and intensity increased with increasing days of post-exposure serum on blots of both BP protein fractions (Fig. 5). Resolved spots on 2-D western blots matched well with corresponding silver-stained gels (Fig. 1).
Fig. 3.
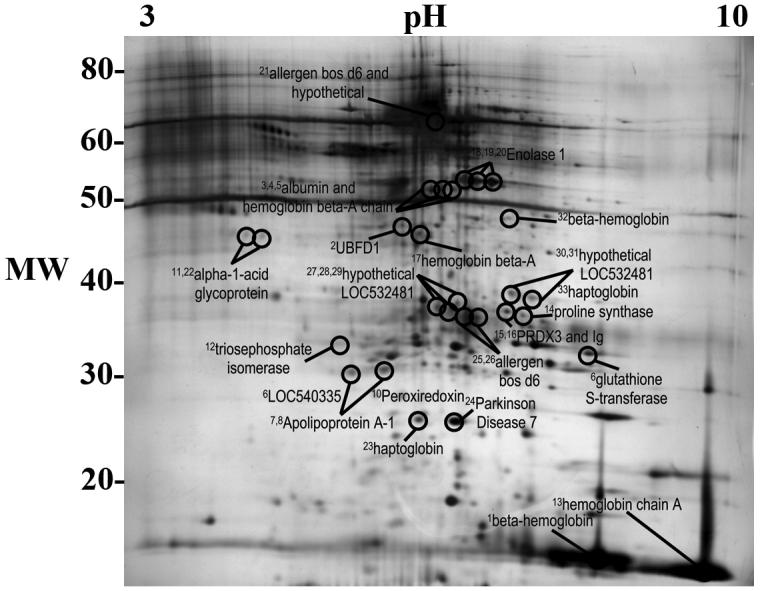
Two-dimensional gel of bovine pericardium water-soluble fraction proteins identified as antigens. Superscript numbers correspond to spot numbers in Table 1.
Fig. 4.
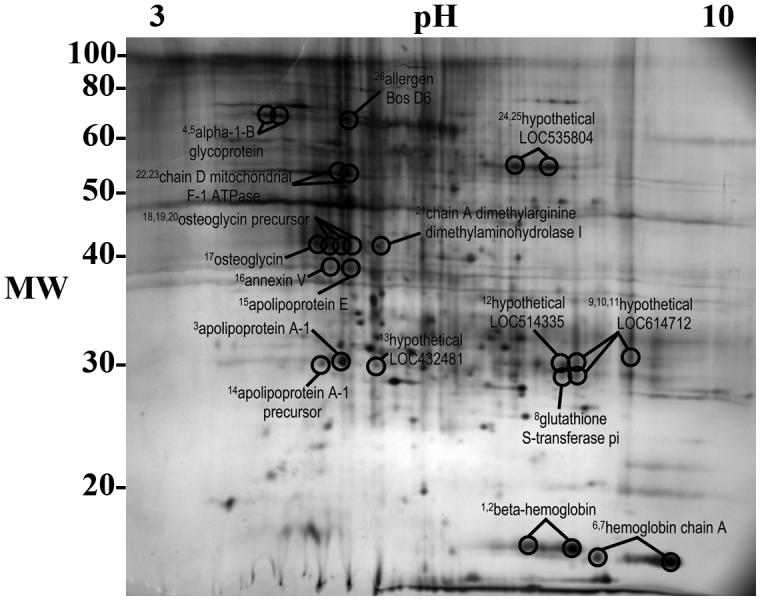
Two-dimensional gel of bovine pericardium lipid-soluble fraction proteins identified as antigens. Superscript numbers correspond to spot numbers in Table 2.
Fig. 5.
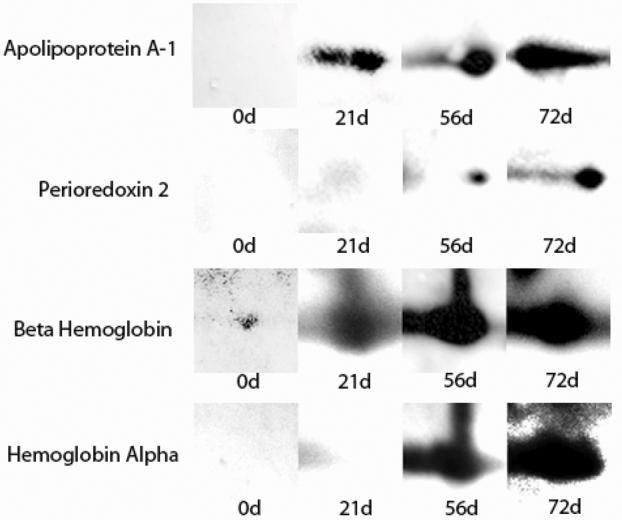
Sequential 2-D western blot spots of 4 bovine pericardium (BP) putative protein antigens resolved with pre-immune (0-d) and 21-, 56-, and 72-d post-exposure anti-BP rabbit serum.
3.3. Protein Identification
Proteins were identified from gel spots corresponding to resolved spots on western blots of both water- and lipid-soluble fractions of BP. Identified proteins are reported in Tables 1 and 2, and the location of gel spots from which proteins were identified are shown in Figs. 3 and 4. A total of 63 proteins were identified (37 from water-soluble and 26 from lipid-soluble gels), representing 31 unique antigens (20 from water-soluble and 11 from lipid-soluble gels) (Tables 1 and 2).
4. Discussion
Abundant evidence demonstrates that both glutaraldehyde-fixed and unfixed BP xenografts elicit an immune response [1-8, 27-31]. However, little is known about the nature and identity of BP proteins eliciting the immune response. In this study, we utilized an immunoproteomic approach to screen BP proteins for their ability to generate an IgG humoral immune response. Two-dimensional western blots of water- and lipid-soluble BP protein fractions were probed with pre- and post-exposure anti-BP rabbit serum. Antibody detection was limited to antibodies of the IgG subtype. This approach led to the identification of 31 putative antigenic proteins capable of eliciting a T cell-dependent antibody response, as revealed by IgG production [42, 43]. Similar approaches have been used recently to identify relevant antigenic proteins in oncologic, autoimmune, and infectious diseases [44-50]. To our knowledge, this study is the first to apply an immunoproteomic approach to identify potential antigens in xenotransplanted tissues.
Our results are supported by the fact that four of the putative antigens in the study (albumin, hemoglobin chain A, beta hemoglobin, and allergen bos d 6) have been previously identified as xenoantigens by other methods [51, 52]. Four of the other putative antigens identified here (enolase 1, triosephosphate isomerase, annexin V, and chain A dimethylarginine dimethylamino-hydrolase I) have been identified previously as targets of autogenous immune responses in either neoplasia or autoimmune diseases [47, 53-57]. Two additional putative antigens (peroxiredoxins II and III) have closely related isotypes (peroxiredoxin I and IV) that have previously been identified as antigens [45, 46, 58, 59]. Notably, twenty-three putative BP antigens have not been previously identified as antigenic. Five of these were hypothetical proteins of unknown function, not previously described at the protein level. All BP proteins identified as antigens demonstrated progressive staining intensity with increasing days of post-exposure serum, lending further support to the conclusion that antigen identifications in this study were the result of an active humoral immune response. Four putative antigens were identified by pre-exposure serum suggesting the presence of natural rabbit antibodies toward these antigens. Staining intensity for these four antigens increased progressively with post-exposure serum.
Several of the putative antigens identified in this study exhibit shared structural or functional properties, which may provide insight into the determinants of antigenicity. A number of the putative antigens identified were isoforms (e.g., peroxiredoxins II and III, or glutathione S-transferases M1 and pi), suggesting the possibility of a conserved antigen-determining region within each family. In addition, several putative BP antigens are characterized as antioxidant proteins. Interestingly, a similarly disproportionate number of antioxidant proteins have been identified as antigens in autoimmune disease [58].
A diverse functional range of antigenic BP proteins were identified, with cytoplasmic, mitochondrial, nuclear, membrane, secreted and extracellular matrix proteins all represented. The identification of the extracellular matrix protein osteoglycin as a putative BP antigen has important implications for the use of BP as a biomaterial. Osteoglycin is a keratin sulphate proteoglycan, involved in collagen fibrillogenesis [60, 61]. It has become increasingly clear that the assumption that xenoantigens are likely to be cell-associated is not valid. Our findings further emphasize that treatment of unfixed xenogeneic tissues for tissue engineering applications must shift from a paradigm of “decellularization” to one of “antigen removal” [9-12, 14-26]. Current or future antigen removal or antigen masking treatments will need to account for both cellular and extracellular matrix antigens.
Post-translational modifications (PTM) including phosphorylation, acylation, glycosylation, and alkylation can potentially contribute to or alter protein antigenicity [62-65]. Interestingly, some of the proteins identified as antigens in this study have been previously identified as antigenic in other settings, and, in each case, their antigenic domains have been mapped to protein sites [47, 51-53]. Furthermore, 15 of the putative antigens were identified from multiple closely associated spots, suggesting that varying degrees of PTM were present, but that antigenicity was not dependent on PTM [44, 45, 47, 49]. Additional studies will be necessary to both confirm xenogeneic antigenicity and identify the specific antigenic domain of each identified putative xenoantigen. Other approaches could also be applied to evaluate the presence of non-protein antigens in BP.
Some known antigens (e.g., collagen, elastin) that are present in BP, were not identified in the presented data [66, 67]. This finding is not surprising, as no current proteomic methodology exists which is capable of resolving the entire proteome. Other proteomic methodologies (e.g., shotgun, benzyldimethyl-n-hexadecylammonium chloride SDS-PAGE, agarose gel IEF) may provide data complementary to those presented here. Such approaches may have particular merit in resolving additional membrane, soluble matrix and high molecular weight proteins [68-70].
5. Conclusion
The immunoproteomic approach employed in this study identified 31 bovine pericardium proteins as putative xenogeneic antigens. Identified antigenic proteins represented a variety of functional and structural protein types, and included both cellular and extracellular proteins. The results of this study have implications for bovine pericardium as a biomaterial in bioprostheses and tissue engineering applications, as well as xenotransplantation in general.
Acknowledgements
Research supported by grant number R21 HL081107 from the National Heart Lung and Blood Institute (NHLBI) at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Human P, Zilla P. Inflammatory and immune processes: the neglected villain of bioprosthetic degeneration? J Long Term Eff Med Implants. 2001;11(34):199–220. [PubMed] [Google Scholar]
- 2.Dahm M, Lyman WD, Schwell AB, Factor SM, Frater RW. Immunogenicity of glutaraldehyde-tanned bovine pericardium. J Thorac Cardiovasc Surg. 1990 Jun;99(6):1082–1090. [PubMed] [Google Scholar]
- 3.Dahm M, Husmann M, Eckhard M, Prufer D, Groh E, Oelert H. Relevance of immunologic reactions for tissue failure of bioprosthetic heart valves. Ann Thorac Surg. 1995 Aug;60(2 Suppl):S348–352. doi: 10.1016/0003-4975(95)00291-r. [DOI] [PubMed] [Google Scholar]
- 4.Human P, Zilla P. Characterization of the immune response to valve bioprostheses and its role in primary tissue failure. Ann Thorac Surg. 2001 May;71(5 Suppl):S385–388. doi: 10.1016/s0003-4975(01)02492-4. [DOI] [PubMed] [Google Scholar]
- 5.Mirzaie M, Meyer T, Saalmuller A, Dalichau H. Influence of glutaraldehyde fixation on the detection of SLA-I and II antigens and calcification tendency in porcine cardiac tissue. Scand Cardiovasc J. 2000 Dec;34(6):589–592. doi: 10.1080/140174300750064530. [DOI] [PubMed] [Google Scholar]
- 6.Salgaller ML, Bajpai PK. Immunogenicity of glutaraldehyde-treated bovine pericardial tissue xenografts in rabbits. J Biomed Mater Res. 1985 Jan;19(1):1–12. doi: 10.1002/jbm.820190103. [DOI] [PubMed] [Google Scholar]
- 7.Manji RA, Zhu LF, Nijjar NK, Rayner DC, Korbutt GS, Churchill TA, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006 Jul 25;114(4):318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 8.Goncalves AC, Griffiths LG, Anthony RV, Orton EC. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J Heart Valve Dis. 2005 Mar;14(2):212–217. [PubMed] [Google Scholar]
- 9.Mendelson K, Schoen FJ. Heart valve tissue engineering: Concepts, approaches, progress, and challenges. Annals Of Biomedical Engineering. 2006 Dec;34(12):1799–1819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuenschwander S, Hoerstrup SP. Heart valve tissue engineering. Transpl Immunol. 2004 Apr;12(34):359–365. doi: 10.1016/j.trim.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000 Nov;21(22):2215–2231. doi: 10.1016/s0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt D, Stock UA, Hoerstrup SP. Tissue engineering of heart valves using decellularized xenogeneic or polymeric starter matrices. Philos Trans R Soc Lond B Biol Sci. 2007;362:1505–1512. doi: 10.1098/rstb.2007.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Z, Zhang E, Li Y, Chen H, Lai Y. Immunogenicity of acellular bovine pericardium in vivo. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2005 Dec;22(6):1203–1205. [PubMed] [Google Scholar]
- 14.Steinhoff G, Stock U, Karim N, Mertsching H, Timke A, Meliss RR, et al. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation. 2000 Nov 7;102(19 Suppl 3):III50–55. doi: 10.1161/01.cir.102.suppl_3.iii-50. [DOI] [PubMed] [Google Scholar]
- 15.Cebotari S, Mertsching H, Kallenbach K, Kostin S, Repin O, Batrinac A, et al. Construction of autologous human heart valves based on an acellular allograft matrix. Circulation. 2002 Sep 24;106(12 Suppl 1):I63–I68. [PubMed] [Google Scholar]
- 16.Bertipaglia B, Ortolani F, Petrelli L, Gerosa G, Spina M, Pauletto P, et al. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: the VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur) Ann Thorac Surg. 2003 Apr;75(4):1274–1282. doi: 10.1016/s0003-4975(02)04706-9. [DOI] [PubMed] [Google Scholar]
- 17.Schenke-Layland K, Opitz F, Gross M, Doring C, Halbhuber KJ, Schirrmeister F, et al. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals-an in vitro study. Cardiovasc Res. 2003 Dec 1;60(3):497–509. doi: 10.1016/j.cardiores.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Elkins RC, Dawson PE, Goldstein S, Walsh SP, Black KS. Decellularized human valve allografts. Ann Thorac Surg. 2001 May;71(5 Suppl):S428–432. doi: 10.1016/s0003-4975(01)02503-6. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien MF, Goldstein S, Walsh S, Black KS, Elkins R, Clarke D. The SynerGraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg. 1999 Oct;11(4 Suppl 1):194–200. [PubMed] [Google Scholar]
- 20.Goldstein S, Clarke DR, Walsh SP, Black KS, O’Brien MF. Transpecies heart valve transplant: advanced studies of a bioengineered xeno-autograft. Ann Thorac Surg. 2000 Dec;70(6):1962–1969. doi: 10.1016/s0003-4975(00)01812-9. [DOI] [PubMed] [Google Scholar]
- 21.Booth C, Korossis SA, Wilcox HE, Watterson KG, Kearney JN, Fisher J, et al. Tissue engineering of cardiac valve prostheses I: development and histological characterization of an acellular porcine scaffold. J Heart Valve Dis. 2002 Jul;11(4):457–462. [PubMed] [Google Scholar]
- 22.Korossis SA, Booth C, Wilcox HE, Watterson KG, Kearney JN, Fisher J, et al. Tissue engineering of cardiac valve prostheses II: biomechanical characterization of decellularized porcine aortic heart valves. J Heart Valve Dis. 2002 Jul;11(4):463–471. [PubMed] [Google Scholar]
- 23.Bader A, Schilling T, Teebken OE, Brandes G, Herden T, Steinhoff G, et al. Tissue engineering of heart valves--human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998 Sep;14(3):279–284. doi: 10.1016/s1010-7940(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 24.Taylor PM. Biological matrices and bionanotechnology. Philos Trans R Soc Lond B Biol Sci. 2007 Aug 29;362(1484):1313–1320. doi: 10.1098/rstb.2007.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang NT, Xie SZ, Wang SM, Gao HY, Wu CG, Pan LF. Construction of tissue-engineered heart valves by using decellularized scaffolds and endothelial progenitor cells. Chin Med J (Engl) 2007 Apr 20;120(8):696–702. [PubMed] [Google Scholar]
- 26.Zhao DE, Li RB, Liu WY, Wang G, Yu SQ, Zhang CW, et al. Tissue-engineered heart valve on acellular aortic valve scaffold: in-vivo study. Asian Cardiovasc Thorac Ann. 2003 Jun;11(2):153–156. doi: 10.1177/021849230301100214. [DOI] [PubMed] [Google Scholar]
- 27.Kasimir MT, Rieder E, Seebacher G, Nigisch A, Dekan B, Wolner E, et al. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis. 2006 Mar;15(2):278–286. discussion 286. [PubMed] [Google Scholar]
- 28.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg. 2003 Jun;23(6):1002–1006. doi: 10.1016/s1010-7940(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 29.Vesely I, Noseworthy R, Pringle G. The hybrid xenograft/autograft bioprosthetic heart valve: in vivo evaluation of tissue extraction. Ann Thorac Surg. 1995 Aug;60(2 Suppl):S359–364. doi: 10.1016/0003-4975(95)00206-z. [DOI] [PubMed] [Google Scholar]
- 30.Ueda Y, Torrianni MW, Coppin CM, Iwai S, Sawa Y, Matsuda H. Antigen clearing from porcine heart valves with preservation of structural integrity. Int J Artif Organs. 2006 Aug;29(8):781–789. doi: 10.1177/039139880602900808. [DOI] [PubMed] [Google Scholar]
- 31.Kasimir MT, Rieder E, Seebacher G, Wolner E, Weigel G, Simon P. Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng. 2005 Jul-Aug;11(78):1274–1280. doi: 10.1089/ten.2005.11.1274. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council. Institute of Laboratory Animal Resources Commission on Life Sciences . Guide for the Care and Use of Laboratory Animals. National Academy Press; Wash DC: 1996. [Google Scholar]
- 33.Hansson SF, Puchades M, Blennow K, Sjogren M, Davidsson P. Validation of a prefractionation method followed by two-dimensional electrophoresis - Applied to cerebrospinal fluid proteins from frontotemporal dementia patients. Proteome Sci. 2004 Nov 18;2(1):7. doi: 10.1186/1477-5956-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopfer U, Hopfer H, Jablonski K, Stahl RA, Wolf G. The novel WD-repeat protein Morg1 acts as a molecular scaffold for hypoxia-inducible factor prolyl hydroxylase 3 (PHD3) J Biol Chem. 2006 Mar 31;281(13):8645–8655. doi: 10.1074/jbc.M513751200. [DOI] [PubMed] [Google Scholar]
- 35.Lacerda CM, Choe LH, Reardon KF. Metaproteomic analysis of a bacterial community response to cadmium exposure. J Proteome Res. 2007 Mar;6(3):1145–1152. doi: 10.1021/pr060477v. [DOI] [PubMed] [Google Scholar]
- 36.Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, et al. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis. 2000 Nov;21(17):3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Rabilloud T, Charmont S. In: Proteome research: two-dimensional gel electrophoresis and identification methods. Rabilloud T, editor. Springer; Berlin; New York: 2000. [Google Scholar]
- 38.Choe LH, Aggarwal K, Franck Z, Lee KH. A comparison of the consistency of proteome quantitation using two-dimensional electrophoresis and shotgun isobaric tagging in Escherichia coli cells. Electrophoresis. 2005 Jun;26(12):2437–2449. doi: 10.1002/elps.200410336. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen SK, Harry JL, Sebastian L, Baker J, Traini MD, McCarthy JT, et al. Unseen proteome: mining below the tip of the iceberg to find low abundance and membrane proteins. J Proteome Res. 2003 May-Jun;2(3):303–311. doi: 10.1021/pr025588i. [DOI] [PubMed] [Google Scholar]
- 40.Sprenger J, Fink JL, Teasdale RD. Evaluation and comparison of mammalian subcellular localization prediction methods. BMC Bioinformatics. 2006;7(Suppl 5):S3. doi: 10.1186/1471-2105-7-S5-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998 Oct 23;283(2):489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 42.Mitchison NA. T-cell-B-cell cooperation. Nat Rev Immunol. 2004 Apr;4(4):308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 43.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 44.Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9824–9829. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita Y, Nakanishi T, Hiramatsu M, Mabuchi H, Miyamoto Y, Miyamoto A, et al. Proteomics-based approach identifying autoantibody against peroxiredoxin VI as a novel serum marker in esophageal squamous cell carcinoma. Clin Cancer Res. 2006 Nov 1;12(21):6415–6420. doi: 10.1158/1078-0432.CCR-06-1315. [DOI] [PubMed] [Google Scholar]
- 46.Chang JW, Lee SH, Jeong JY, Chae HZ, Kim YC, Park ZY, et al. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005 May 23;579(13):2873–2877. doi: 10.1016/j.febslet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 47.Xiang Y, Sekine T, Nakamura H, Imajoh-Ohmi S, Fukuda H, Nishioka K, et al. Proteomic surveillance of autoimmunity in osteoarthritis: identification of triosephosphate isomerase as an autoantigen in patients with osteoarthritis. Arthritis Rheum. 2004 May;50(5):1511–1521. doi: 10.1002/art.20189. [DOI] [PubMed] [Google Scholar]
- 48.He P, Naka T, Serada S, Fujimoto M, Tanaka T, Hashimoto S, et al. Proteomics-based identification of alpha-enolase as a tumor antigen in non-small lung cancer. Cancer Sci. 2007 Aug;98(8):1234–1240. doi: 10.1111/j.1349-7006.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mini R, Bernardini G, Salzano AM, Renzone G, Scaloni A, Figura N, et al. Comparative proteomics and immunoproteomics of Helicobacter pylori related to different gastric pathologies. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Mar 20;833(1):63–79. doi: 10.1016/j.jchromb.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 50.Krah A, Jungblut PR. Immunoproteomics. Methods Mol Med. 2004;94:19–32. doi: 10.1385/1-59259-679-7:19. [DOI] [PubMed] [Google Scholar]
- 51.Oshima M. Species-specific antigenic reactive regions in alpha and beta chains of human hemoglobin. Igaku Kenkyu. 1982 Dec;52(6):313–327. [PubMed] [Google Scholar]
- 52.Beretta B, Conti A, Fiocchi A, Gaiaschi A, Galli CL, Giuffrida MG, et al. Antigenic determinants of bovine serum albumin. Int Arch Allergy Immunol. 2001 Nov;126(3):188–195. doi: 10.1159/000049513. [DOI] [PubMed] [Google Scholar]
- 53.Terrier B, Degand N, Guilpain P, Servettaz A, Guillevin L, Mouthon L. Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun Rev. 2007 Jan;6(3):176–182. doi: 10.1016/j.autrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Bogdanos DP, Gilbert D, Bianchi I, Leoni S, Mitry RR, Ma Y, et al. Antibodies to soluble liver antigen and alpha-enolase in patients with autoimmune hepatitis. J Autoimmune Dis. 2004 Nov 19;1(1):4. doi: 10.1186/1740-2557-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aslan H, Pay S, Gok F, Baykal Y, Yilmaz MI, Sengul A, et al. Antiannexin V autoantibody in thrombophilic Behcet’s disease. Rheumatol Int. 2004 Mar;24(2):77–79. doi: 10.1007/s00296-002-0274-z. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Garcia MI, Fernandez JA, Rodriguez A, Fernandez MP, Gutierrez C, Torre-Alonso JC. Annexin V autoantibodies in rheumatoid arthritis. Ann Rheum Dis. 1996 Dec;55(12):895–900. doi: 10.1136/ard.55.12.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng X, Liao AJ, Tang HL, Yi L, Xie N, Su Q. Screening Human Gastric Carcinoma-associated Antigens by Serologic Proteome Analysis. Ai Zheng. 2007 Oct;26(10):1080–1084. [PubMed] [Google Scholar]
- 58.Karasawa R, Ozaki S, Nishioka K, Kato T. Autoantibodies to peroxiredoxin I and IV in patients with systemic autoimmune diseases. Microbiol Immunol. 2005;49(1):57–65. doi: 10.1111/j.1348-0421.2005.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 59.Iwata Y, Ogawa F, Komura K, Muroi E, Hara T, Shimizu K, et al. Autoantibody against peroxiredoxin I, an antioxidant enzyme, in patients with systemic sclerosis: possible association with oxidative stress. Rheumatology (Oxford) 2007 May;46(5):790–795. doi: 10.1093/rheumatology/kem010. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez B, Kampmann A, Pipp F, Zimmermann R, Schaper W. Osteoglycin expression and localization in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem. 2003 Apr;246(12):3–11. [PubMed] [Google Scholar]
- 61.Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem. 2004 Oct 1;279(40):41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- 62.Gumperz JE. The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic. 2006 Jan;7(1):2–13. doi: 10.1111/j.1600-0854.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 63.Bricard G, Porcelli SA. Antigen presentation by CD1 molecules and the generation of lipid-specific T cell immunity. Cell Mol Life Sci. 2007 Jul;64(14):1824–1840. doi: 10.1007/s00018-007-7007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ezzelarab M, Ayares D, Cooper DK. Carbohydrates in xenotransplantation. Immunol Cell Biol. 2005 Aug;83(4):396–404. doi: 10.1111/j.1440-1711.2005.01344.x. [DOI] [PubMed] [Google Scholar]
- 65.Kearns-Jonker M, Barteneva N, Mencel R, Hussain N, Shulkin I, Xu A, et al. Use of molecular modeling and site-directed mutagenesis to define the structural basis for the immune response to carbohydrate xenoantigens. BMC Immunol. 2007;8:3. doi: 10.1186/1471-2172-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Duivenvoorde LM, Louis-Plence P, Apparailly F, van der Voort EI, Huizinga TW, Jorgensen C, et al. Antigen-specific immunomodulation of collagen-induced arthritis with tumor necrosis factor-stimulated dendritic cells. Arthritis Rheum. 2004 Oct;50(10):3354–3364. doi: 10.1002/art.20513. [DOI] [PubMed] [Google Scholar]
- 67.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007 May;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 68.Bierczynska-Krzysik A, Kang SU, Silberrring J, Lubec G. Mass spectrometrical identification of brain proteins including highly insoluble and transmembrane proteins. Neurochem Int. 2006 Aug;49(3):245–255. doi: 10.1016/j.neuint.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 69.Oh-Ishi M, Maeda T. Disease proteomics of high-molecular-mass proteins by two-dimensional gel electrophoresis with agarose gels in the first dimension (Agarose 2-DE) J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Apr 15;849(12):211–222. doi: 10.1016/j.jchromb.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 70.Hess JL, Blazer L, Romer T, Faber L, Buller RM, Boyle MD. Immunoproteomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Feb 5;815(12):65–75. doi: 10.1016/j.jchromb.2004.07.047. [DOI] [PubMed] [Google Scholar]