Abstract
OBJECTIVE—Neonatal diabetes is a heterogeneous group of disorders with diabetes manifestation in the first 6 months of life. The most common etiology in permanent neonatal diabetes is mutations of the ATP-sensitive K+ channel subunits; in transient neonatal diabetes, chromosome 6q24 abnormalities are the most common cause.
RESEARCH DESIGN AND METHODS—We report a sporadic case of diabetes without ketoacidosis diagnosed on the fourth day of life.
RESULTS—Analysis of the KCNJ11 gene found a novel R365H mutation in the proband and her unaffected father. The functional analysis did not support pathogenicity of this variant. When the patient's diabetes remitted in the seventh month of life, the 6q24 region was analyzed and a paternally inherited duplication was identified.
CONCLUSIONS—Our case reports a coincidental novel KCNJ11 variant in a patient with transient neonatal diabetes due to a 6q24 duplication, illustrating the difficulty in testing neonates before the clinical course of neonatal diabetes is known.
Neonatal diabetes is a heterogeneous group of disorders with diabetes manifestation before 6 months of life that most frequently has a monogenic etiology (1). In patients with permanent neonatal diabetes (i.e., without remission) (PND), mutations of the KATP channel subunits (encoded by the KCNJ11 and ABCC8 genes) and mutations of the insulin gene are the most common etiology (1). Transient neonatal diabetes (TND) usually resolves by 6 months of age, but more than 50% of TND patients relapse into diabetes during childhood or adulthood (2). Abnormalities of the imprinted region, 6q24, which encompasses the ZAC and HYMAI genes, cause ∼70% of TND cases (3). Mutations of KATP channel subunits are the second most common etiology (3).
RESEARCH DESIGN AND METHODS
We report on a patient who was born with a birth weight of 2,320 g at 38 weeks' postgestation (<3rd centile). Because of the low birth weight, she required hospitalization at the newborn care unit. She was diagnosed with diabetes without ketoacidosis on the fourth day of postnatal life (blood glucose 19.5 mmol/l). The initial treatment was intravenous insulin (0.04 units · kg−1 · h−1), followed by subcutaneous injections of NPH insulin (0.9 units · kg−1 · day−1).
RESULTS
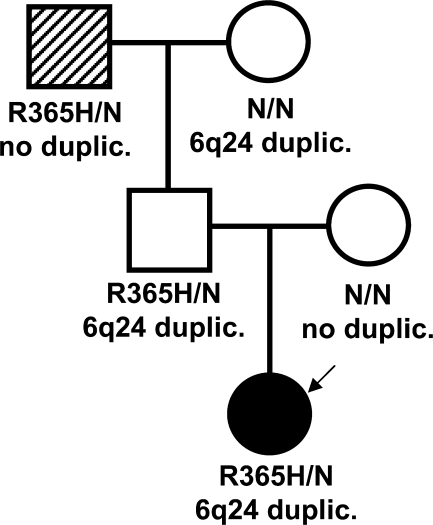
At the age of 2 weeks and following a clinical diagnosis of neonatal diabetes, analysis of the KCNJ11 gene was undertaken (3). We found a novel, heterozygous missense mutation, R365H (c.1094G>A; p.Arg365His), in the proband. The mutation was present in the unaffected father and paternal grandfather (Fig. 1). Standard oral glucose tolerance tests revealed a normal glucose tolerance with normal insulin and C-peptide values in the parents and paternal grandmother, but the paternal grandfather had impaired fasting glycemia combined with impaired glucose tolerance (0-h glycemia 5.8 mmol/l; 2-h glycemia 9.4 mmol/l).
Figure 1.
Pedigree of the family with coincidence of the novel R365H variant of the KCNJ11 gene and the 6q24 duplication. Nature of hyperglycemia: neonatal diabetes. The KCNJ11 genotype is shown under each symbol. The 6q24 status is shown below. An arrow points to the proband. The proband, the proband's father, and the proband's paternal grandfather are carriers of the R365H mutation. The proband also has a paternally inherited 6q24 duplication. The proband's father has a maternally derived 6q24 duplication. The paternal grandfather has both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), which seem to be associated with older age or type 2 diabetes diagnosis and not with the R365H mutation of the KCNJ11 gene. •, TND; ▒, IFG + IGT. NN, no mutation identified.
KATP channel mutations causing TND in the proband and adult-onset diabetes in a parent and/or grandparent have been previously reported (3). The R365H mutation identified in our family was thought likely to be pathogenic because the arginine residue at codon 365 is conserved in dog, rat, and mouse, was not present in 298 control chromosomes, and has not previously been reported in patients with hyperinsulinism. Therefore, in vitro functional studies of this mutation were undertaken. Channels containing the R365H mutation were expressed in Xenopus oocytes and their surface density, activation by the metabolic inhibitor azide, and blocking by the sulfonylurea tolbutamide were measured (4). Unexpectedly, there was no difference between the wild-type and mutant channels, which calls into question the pathogenicity of this mutation.
In the meantime, the patient developed hypoglycemia on insulin treatment, and at the age of 7 months, the insulin therapy was discontinued. The nine-value 24-h glycemic profile 7 months after stopping insulin administration was normal (4.7–4.1–5.5–7.8–7.6–7.1–3.9–5.3–5.2 mmol/l), and A1C was 4.3% (within the Diabetes Control and Complications Trial range), (high-performance liquid chromatography, Variant II; Biorad, Hercules, CA, U.S.). Currently, with the subject at the age of 2 years, A1C is 5.1%. No glycosuria has been noted on repeated testing.
Thus, we had to revise our initial diagnosis of PND due to a KCNJ11 mutation (3). Genetic analysis of the methylation status within the TND critical region on chromosome 6 was performed using the methylation-specific PCR method, as previously described (5). A 6q24 duplication was identified, and family member testing demonstrated that the duplication was also present in the unaffected father and paternal grandmother (Fig. 1).
CONCLUSIONS
We report a novel KCNJ11 variant (R365H) and a 6q24 duplication in a proband with TND and her unaffected father. The paternally inherited 6q24 duplication is likely to be the etiological mutation as a consequence of overexpression of paternal genes within the duplicated chromosome 6q24 region. Expression of genes in this region is regulated by imprinting. The maternal allele is methylated and therefore inactive: only genes on the paternally derived chromosome are transcribed. Normal development depends on normal doses of gene transcripts. The proband′s father is unaffected because his 6q24 duplication is maternally inherited and, therefore, inactive (2). The impaired fasting glycemia and impaired glucose tolerance in the paternal grandfather may be influenced by his age (53 years), weight (BMI 28.5 kg/m2), and maternal history of type 2 diabetes. We conclude that the R365H is likely to be a rare variant of no clinical significance.
In PND patients, screening of the KCNJ11 mutations is recommended, and if it is negative, mutations in other genes should be investigated (e.g., insulin gene, ABCC8, etc.) (1,6). In the case of TND, KATP channel genes should be tested if 6q24 analysis is negative. Our case report highlights the difficulty in testing neonates before the clinical course (transient versus permanent) is known and when no supportive signs (e.g., macroglossia and umbilical hernia in 6q24 abnormalities [2]) are present. Thus, in these patients, we recommend screening for KCNJ11 gene mutations and 6q24 abnormalities simultaneously, as making the genetic diagnosis earlier may influence treatment (patients with KATP channel mutations are often sensitive to sulfonylureas, while patients with 6q24 abnormalities are usually treated with insulin [2,3]). Finally, when a novel mutation in a known gene is found, functional studies and investigations of other neonatal diabetes genes can play an important role.
Acknowledgments
This work was supported in part by research grants MZ.2005/15-NEDU-01 and APVV-51-014205 Slovakia (to I.K.), CENDO (to I.K.), BITCET (to I.K.), and the Wellcome Trust and European Union (to A.T.H. and F.M.A.). A.T.H. is a Wellcome Trust Research Leave Fellow.
We thank Associate Professor D. Chovancova, MD, PhD; Associate Professor J. Strnova, MD, PhD; B. Ukropcova, MD; A. Penesova, MD, PhD; and Alica Mitkova.
Published ahead of print at http://care.diabetesjournals.org on 12 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Stoy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S: Insulin Mutation Screening in 1044 Patients with Diabetes: Mutations in the INS gene are a Common Cause of Neonatal Diabetes but a Rare Cause of Diabetes Diagnosed in Childhood or Adulthood. Diabetes 57:1034–1042, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Temple IK, Shield JP: Transient neonatal diabetes, a disorder of imprinting. J Med Genet 39:872–875, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT: Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56:1930–1937, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proks P, Shimomura K, Craig TJ, Girard CA, Ashcroft FM: Mechanism of action of a sulphonylurea receptor SUR1 mutation (F132L) that causes DEND syndrome. Hum Mol Genet 16:2011–2019, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Mackay DJ, Temple IK, Shield JP, Robinson DO: Bisulphite sequencing of the transient neonatal diabetes mellitus DMR facilitates a novel diagnostic test but reveals no methylation anomalies in patients of unknown aetiology. Hum Genet 116:255–261, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Colombo C, Porzio O, Liu M, Massa O, Vasta M, Salardi S, Beccaria L, Monciotti C, Toni S, Pedersen O, Hansen T, Federici L, Pesavento R, Cadario F, Federici G, Ghirri P, Arvan P, Iafusco D, Barbetti F: the Early Onset Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetes (SIEDP): Seven mutations in the human insulin gene linked to permanent neonatal/infancy-onset diabetes mellitus. J Clin Invest. In press. [DOI] [PMC free article] [PubMed]