Abstract
OBJECTIVE—The purpose of this study was to examine the association between parental history of type 2 diabetes and glycemic control among diabetic urban African Americans.
RESEARCH DESIGN AND METHODS—Study participants included 359 African Americans with type 2 diabetes from Baltimore, Maryland, enrolled in Project Sugar 2. Participants underwent an interview-administrated questionnaire that asked about family history, sociodemographics, clinical characteristics, and knowledge and perception of adequate glycemic control. Regression analysis was used to determine the association between parental history of diabetes and glycemic control, as measured by A1C.
RESULTS—In the comparisons between participants with and without a parental history of diabetes, those with a positive parental history tended to be younger, have higher glucose levels, and have higher blood glucose levels before calling a doctor (all P < 0.05). After adjustments for age, sex, and BMI, there was a significant association (P = 0.02) between A1C and parental history with the mean A1C difference between those with a positive and a negative parental history being 0.58%. However, after adjustment for duration of diabetes, the association was no longer significant (P = 0.11). However, there was a tendency for individuals with two diabetic parents to have higher A1C (P = 0.011).
CONCLUSIONS—From these results, we conclude that among the urban African American participants who were aware of their parental history of diabetes, a positive parental history was associated with worse glycemic control, partly due to longer duration of diabetes. Parental history did not appear to be associated with better knowledge or perception of adequate glycemic control.
Many studies have investigated risk factors for type 2 diabetes (1). For instance, it has been well established that family history of type 2 diabetes has been found to be associated with an increased risk of developing the disease (2,3). However, few studies have evaluated the effects of family history on poor glycemic control, which has been found to be associated with many serious complications among individuals living with type 2 diabetes (4,5). Consequently, family history continues to be underutilized in disease prevention (6,7) and not often considered after the initial disease diagnosis.
Family history could have effects on glycemic control via genetic or behavioral mechanisms. For example, individuals with diabetes who have a parental history of type 2 diabetes may have worse glycemic control, in part due to the genetic risk factors of the disease, which may then influence the severity or duration of the condition. Alternatively, they could have better glycemic control, in part due to improved knowledge of the disease or health behaviors as a result of having affected family members. We conducted a study to quantify these effects and determine their overall effect on glycemic control.
This study has important clinical practice implications. If glycemic control is associated with family history, it is possible that given a patient's family history, clinicians will have insight into not only their risk of developing type 2 diabetes but also its severity.
RESEARCH DESIGN AND METHODS
The participants were 542 African Americans from Baltimore, Maryland, who were enrolled in Project Sugar 2, a randomized controlled trial to study the effects of nurse case manager and community health worker team interventions in improving diabetes control. Potential participants were identified using a university-affiliated managed care organization database and met the following criteria: aged ≥25 years, presence of type 2 diabetes (ICD-9 = 250), and no evidence of significant comorbid conditions likely to lead to death within the next 3–5 years (e.g., cancer, AIDS, end-stage renal disease, active tuberculosis, Alzheimer's disease, and congestive heart failure). These potential participants were then screened by telephone to determine whether or not they met the study's eligibility criteria: African American by self-report, Baltimore city resident, age ≥25 years, receiving care at one of the six clinic sites, and no active participation in other disease management programs of the managed care organization. Other exclusion criteria was determined during the baseline screening visit, including inability or unwillingness to give informed consent, inability to complete the baseline assessment (interview, clinical measures, and venipuncture), or having a severe psychiatric health condition. Of the 2,450 individuals who were screened, 542 agreed to enroll in the trial. Additional details of the recruitment process and characteristics of participants and nonparticipants can be found elsewhere (8). The roughly 3:1 female:male ratio shown in our study was also noted in the nonparticipants, most likely reflecting that women with diabetes are more likely than men with diabetes to be enrolled in primary care.
For the present analysis, we excluded 183 individuals because they either were missing a measured A1C value (n = 14) or were unaware of their parental diabetes history (n = 169). This left us with 359 participants. When we compared the included and excluded individuals, those included tended to be younger (56.2 vs. 60.2 years, P < 0.001) and have less education (15.9% vs. 24.6% with more than a high school education, P = 0.005). Other selected characteristics such as sex, type 2 diabetes duration, BMI, glucose levels, A1C, and presence of hypertension (defined as having a systolic or diastolic blood pressure ≥130 or 80 mmHg, respectively) were not significantly different.
Family history of diabetes
Family history was characterized through an interview-administrated questionnaire. Participants were asked about the medical histories of their biological mother and father, including details concerning whether they were alive or deceased and their diabetes status. We then grouped the individuals who knew their family history, depending on whether they had zero, one, or two parents affected with diabetes. In addition, we classified the individuals as having negative parental history if both parents had never had diabetes or positive parental history if at least one parent had diabetes.
Glycemic control
A1C is a general measure of diabetes control and an indicator of an individual's blood glucose control over the past 2–3 months (9). A1C of <7% is a recommended aim for individuals with diabetes (9), with higher A1C corresponding to a higher mean plasma glucose level, indicating worse glycemic control (10). In this study, A1C was measured using venipuncture-drawn blood samples and high-pressure liquid chromatography.
Confounders/mediators
Potential confounders or mediators of the association between family history of diabetes and glycemic control, age, sex, duration of diabetes, and knowledge and perception of adequate glycemic control were determined by questionnaire. BMI was calculated using measured height and weight.
Data analysis
Student's t tests and χ2 tests were used to evaluate the differences in characteristics between the participants either included or excluded from this study. These tests were also used to compare selected characteristics and perceptions of the included participants having a positive parental diabetes history with those included and having a negative history. Multiple linear regression models were used to determine the crude association between A1C and type 2 diabetes parental history with the β-coefficients representing the difference in mean A1C between having a negative and positive diabetes parental history. These models were then adjusted to account for potential confounders/mediators in stages: stage 1, age and sex; stage 2, plus BMI; and stage 3, plus diabetes duration. All data analysis was completed using Stata statistical software (StataCorp, College Station, TX), and significance was determined by an α level of 0.05.
RESULTS
Selected characteristics of the study participants
Selected sociodemographic, behavioral, and clinical characteristics of the 359 included study participants are shown in Table 1. The 150 participants with a negative parental history had a mean age of 59.2 years, which was significantly older than the age of the 209 participants with a positive parental history (mean age 53.9 years). Random (nonfasting) glucose levels were significantly higher in the participants with a positive parental history (P = 0.004). Type 2 diabetes duration was slightly higher in the participants with a positive parental history, but that finding was not significantly significant. Participants with a negative family history and those with a positive parental history were predominantly women (24.7% of men had a negative parental history and 27.3% of men had a positive parental history). The majority of both groups had an education level between 8 and 12 years (no significant difference). These characteristics and others such as BMI, waist-to-hip ratio, cholesterol and HDL cholesterol concentrations, hypertension, diastolic blood pressure, and systolic blood pressure were not significantly different by parental history of diabetes.
Table 1.
Selected characteristics of diabetes of 359 Project Sugar 2 participants by parental history of diabetes
| Negative parental history | Positive parental history | P value | |
|---|---|---|---|
| n | 150 | 209 | |
| Age (years) | 59.2 ± 11.1 | 53.9 ± 10.6 | <0.001 |
| Sex (% males) | 24.7 | 27.3 | 0.58 |
| Education | |||
| >8 years | 4.7 | 4.3 | |
| 8–12 years | 74.7 | 67.9 | 0.31 |
| >12 years | 20.7 | 27.8 | |
| Diabetes duration (year) | 7.2 ± 7.5 | 8.7 ± 8.6 | 0.10 |
| Blood glucose (mg/dl) | 142.0 ± 69.4 | 166.3 ± 85.1 | 0.004 |
| BMI (kg/m2) | 34.0 ± 8.0 | 34.8 ± 9.0 | 0.35 |
| Waist-to-hip ratio | 0.90 (0.06) | 0.90 ± 0.07 | 0.65 |
| Cholesterol (mg/dl) | 188.7 ± 38.2 | 191.7 ± 52.6 | 0.56 |
| HDL cholesterol (mg/dl) | 52.9 ± 15.4 | 50.5 ± 14.7 | 0.14 |
| Hypertension (% yes) | 74.0 | 70.3 | 0.45 |
| Diastolic blood pressure (mmHg) | 78 ± 10.4 | 80 ± 11.9 | 0.14 |
| Systolic blood pressure (mmHg) | 137 ± 20.6 | 136 ± 20.8 | 0.53 |
| “What is your level of perfect blood sugar (mg/dl)?”* | 122.9 ± 33.8 | 125.0 ± 35.7 | 0.60 |
| “How high would your blood sugar have to be before you called your doctor (mg/dl)?”* | 259.6 ± 115.8 | 287.0 ± 108.2 | 0.04 |
Data are means ± SD.
n = 284; 116 with a negative parental history and 168 with a positive parental history.
Association between A1C and parental history
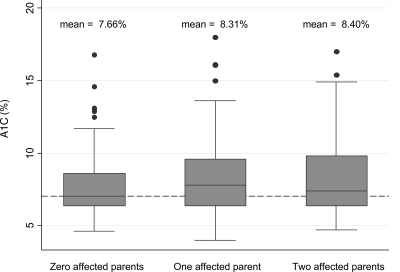
In the box plot graph shown in Fig. 1, individuals with affected parents tended to have significantly higher A1C (unadjusted P = 0.011). In addition, with an increasing number of affected parents, the mean A1C increased. After adjustment for age and sex, the relationship persisted. However, after further adjustment for duration of diabetes, the P value became nonsignificant (P = 0.09).
Figure 1.
A1C by parental history of diabetes in 359 participants in Project Sugar 2. P = 0.011.
Linear regression models of the association between A1C and parental history are shown in Table 2. There was a strong significant association between A1C and parental history (β = 0.67 and P = 0.006). Even with adjustments for age, sex, and BMI, the association was still significant (β = 0.58 and P = 0.02). However, after consideration of duration of diabetes as a factor, this association was no longer significant (β = 0.40 and P = 0.11).
Table 2.
Linear regression models of the crude and adjusted association between A1C and parental history of diabetes in 359 Project Sugar 2 participants
| β-coefficient (SEM) | P value | |
|---|---|---|
| Unadjusted model | 0.67 (0.24) | 0.006 |
| Adjusted for age and sex | 0.56 (0.25) | 0.02 |
| + BMI | 0.58 (0.25) | 0.02 |
| + Type 2 diabetes duration | 0.40 (0.25) | 0.11 |
Knowledge and perception of adequate glycemic control
Of the 359 participants, 284 answered questions based on their knowledge and perception of glycemic control (Table 1). Individuals with a positive parental diabetes history tended to report slightly higher blood glucose levels before calling a doctor (287.02 vs. 259.59 mg/dl, P = 0.04). Perception of perfect blood glucose levels did not differ significantly between those with negative or positive diabetes parental history.
CONCLUSIONS
Our findings point to several conclusions about this population of urban African Americans with type 2 diabetes. It is evident that a high proportion of urban African Americans are not completely aware of the diabetes statuses of their parents. However, among those who knew their parental diabetes history, a positive parental history of type 2 diabetes was associated with worse glycemic control, as indicated by longer duration of diabetes. This positive parental history of diabetes was not associated with better knowledge or perception of adequate glycemic control.
A strong association between A1C and parental history was shown, even with adjustments for age, sex, and BMI, indicating that these were not factors that significantly influenced the relationship. However, duration of diabetes caused the association to become no longer significant.
It is possible that the association between a positive parental history of diabetes and worse glycemic control is largely accounted for by the association of parental history with longer diabetes duration. Therefore, the fact that urban African Americans with type 2 diabetes and a positive parental history of diabetes tended to have worse glycemic control may be related to development of diabetes at an earlier age, causing a longer duration of the condition. The age of an individual when the disease is diagnosed is an important factor in determining further family history risk (7).
Those with a positive parental history tended to report higher blood glucose levels before calling a doctor, which may indicate that they underestimate the seriousness of their diabetes (11). For instance, these individuals may be accustomed to seeing family members having higher blood glucose levels and may be less concerned about the severity of the disease than those who do not have as much family experience with diabetes. Also, we found that individuals with a positive parental history have higher blood glucose levels, indicating that there may be differences in care and self-management.
The data also show that participants with a negative family history tended to be older than those with a positive parental history. This may be because older individuals are more likely to forget their parental history, and those who were not aware of their parental history were classified as having a negative history. Another possibility is that participants with a negative parental history tend to live longer. This is especially probable given the possibility that those with negative histories may have less severe diabetes because they tend to have a slightly shorter duration of the disease and lower fasting glucose levels. Genetic risk factors may have contributed to the significantly higher glucose levels present in those with positive histories, giving these individuals more severe cases of diabetes.
Our study has several strengths. It is one of the first studies to investigate the association between parental history of type 2 diabetes and glycemic control among African Americans. Although the association between parental history and the risk of diabetes has been well established (12), this study is unique as it relates parental history to glycemic control. This association adds further evidence of genetics contributing not only to diabetes incidence (13) but also to hyperglycemia (14) and increased severity of disease. In addition, this study includes a large sample of urban African Americans and is clinic based, indicating that the conclusions drawn here may be applicable to this population as a whole.
The study also has a few limitations. A large proportion of the participants were unable to provide parental history information. These individuals were then dropped from the study, decreasing the sample size. Even with the included participants, there is the possibility of family history misclassification. Finally, there are limited data on participants’ health behaviors. From this study, we are unable to tell whether other health-related actions influenced the association between parental history and glycemic control.
In this study, there is evidence of an association between parental history of type 2 diabetes and glycemic control in urban African Americans with diabetes. This finding can have important clinical implications in how clinicians use parental history in treating individuals with diabetes. Because the sample size of this study was small, larger datasets are needed to confirm this relationship.
Acknowledgments
This project was funded by grants from the National Institutes of Health (R01-DK48117 and R00052). T.L.G. was funded by a grant from the National Heart, Lung, and Blood Institute (K01-HL084700), and F.L.B. was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K24-DK6222).
Parts of this study were presented in abstract form at the 63rd annual meeting of the American Diabetes Association, New Orleans, Louisiana, 13–17 June 2003.
We acknowledge the efforts of the Project Sugar 2 research staff and The Johns Hopkins General Clinical Research Center. We also acknowledge the Project Sugar 2 participants whose cooperation made this research possible.
Published ahead of print at http://care.diabetesjournals.org on 5 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 26(Suppl. 1):S5–S20, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, Edwards KL: Family history of diabetes as a potential public health tool. Am J Prev Med 24:152–159, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Meigs JB, Cupples LA, Wilson PW: Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes 49:2201–2207, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Gaster B, Hirsch IB: The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med 158:134–140, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE: Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 124:90–96, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Yoon PW, Scheuner MT, Peterson-Oehlke KL, Gwinn M, Faucett A, Khoury MJ: Can family history be used as a tool for public health and preventive medicine? Genet Med 4:304–310, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Yoon PW, Scheuner MT, Khoury MJ: Research priorities for evaluating family history in the prevention of common chronic diseases. Am J Prev Med 24:128–135, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Gary TL, Batts-Turner M, Bone LR, Yeh HC, Wang NY, Hill-Briggs F, Levine DM, Powe NR, Hill MN, Saudek C, McGuire M, Brancati FL: A randomized controlled trial of the effects of nurse case manager and community health worker team interventions in urban African-Americans with type 2 diabetes. Control Clin Trials 25:53–66, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Standards of medical care in diabetes. Diabetes Care 27(Suppl. 1):S15–S35, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB: Tests of glycemia in diabetes. Diabetes Care 27:1761–1773, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Pierce M, Harding D, Ridout D, Keen H, Bradley C: Risk and prevention of type II diabetes: offspring's views. Br J Gen Pract 51:194–199, 2001 [PMC free article] [PubMed] [Google Scholar]
- 12.Klein BE, Klein R, Moss SE, Cruickshanks KJ: Parental history of diabetes in a population-based study. Diabetes Care 19:827–830, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Busch CP, Hegele RA: Genetic determinants of type 2 diabetes mellitus. Clin Genet 60:243–254, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT: Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 362:1275–1281, 2003 [DOI] [PubMed] [Google Scholar]