Abstract
OBJECTIVE—Low-dose insulin infusion has been shown to exert a prompt and powerful anti-inflammatory effect. Toll-like receptors (TLRs) are major determinants of the inflammatory response to viral and bacterial pathogens. We have now hypothesized that low-dose insulin infusion in obese type 2 diabetic patients suppresses TLR expression.
RESEARCH DESIGN AND METHODS—Ten type 2 diabetic patients were infused with a low dose of insulin (2 units/h) and dextrose to maintain normoglycemia for 4 h, while another 14 type 2 diabetic patients were infused with either dextrose or saline for 4 h and served as control subjects. Blood samples were collected before and at 2, 4, and 6 h. TLR expression was determined in mononuclear cells (MNCs).
RESULTS—Insulin infusion significantly suppressed TLR1, -2, -4, -7, and -9 mRNA expression in MNCs within 2 h of the infusion, with a maximum fall at 4 h by 24 ± 9%, 21 ± 5%, 30 ± 8%, 28 ± 5%, and 27 ± 10% (P < 0.05, for all), respectively, below the baseline. TLR2 protein was suppressed by 19 ± 7% (P < 0.05) below the baseline at 4 h. The DNA binding of PU.1, a major transcription factor regulating many TLR genes, was concomitantly suppressed by 24 ± 10% (P < 0.05) by 4 h in MNCs. There was no change in TLR expression or DNA binding by PU.1 following dextrose or saline infusion in the control groups.
CONCLUSIONS—Insulin suppresses the expression of several TLRs at the transcriptional level, possibly through its suppressive effect on PU.1.
We have shown previously that insulin exerts a prompt and powerful anti-inflammatory effect, including the suppression of nuclear factor-κB (NFκB) binding, reactive oxygen species generation, and p47phox expression by mononuclear cells (MNCs) (1,2) in humans. Insulin has also been shown to reduce the plasma concentrations of many proinflammatory mediators including C-reactive protein (CRP) and serum amyloid A (SAA) within 24 h in patients with acute myocardial infarction and in patients undergoing coronary artery bypass surgery (3,4).
Toll-like receptors (TLRs) are a variety of pathogen pattern recognition receptors that recognize bacterial and viral products and other pathogens (5). TLR4 recognizes endotoxin or lipopolysaccharide (LPS) and may be a mediator of endotoxin shock (6). TLR4 has also been shown to play an important role in the pathogenesis of atherosclerosis (7,8), diet-induced obesity, and the related insulin resistance (9,10). TLR2 (in a heterodimeric association with TLR1 or TLR6) recognizes certain lipopeptides, peptidoglycans, and other lipid moieties derived from gram-positive bacteria (5). Recently, TLR2 has been shown to mediate and aggravate myocardial tissue injury in ischemia reperfusion–based experimental animal models and that a deletion of TLR2 is associated with a reduction in the size of the experimental myocardial infarct (11). TLR2 deletion also results in the preservation of postischemic coronary endothelial function and the prevention of abnormal ventricular remodeling (12,13).
TLR7 and TLR9 recognize single-stranded viral RNA and microbial DNA, respectively. TLR9 also modulates adaptive immune responses including autoimmunity against DNA and chromatin (e.g., systemic lupus erythematosus) (14). A role for TLR9 and TLR2 has also been shown in the pathogenesis of experimental type 1 diabetes, specifically related to the autoimmune inflammation in the pancreatic islet (15,16).
Transcription of many TLRs is dependent upon myeloid-specific transcription factors including PU.1. PU.1 is a member of the ets gene family and is a master switch in the regulation of an array of genes involved in myeloid cell activation and differentiation, including TLR2, -4, and -9 (17,18). PU.1 binds to purine-rich regions of the TLR gene promoter to activate their transcription.
Recent data in humans have demonstrated that TLR2 and TLR4 expression and plasma LPS concentration are increased in patients with type 2 diabetes and that LPS concentration is related to plasma insulin concentration and insulin resistance (19). However, the possibility that insulin or macronutrient intake may modulate TLR expression has not been investigated. In view of the recent data on TLR involvement in insulin resistance and atherosclerosis and our previous work on the anti-inflammatory effect of insulin, it is important that the potential effects of insulin on TLRs be investigated.
RESEARCH DESIGN AND METHODS
Ten obese patients with type 2 diabetes, five female and five male subjects, were recruited for the insulin infusion study (age 47.9 ± 8.9 years, BMI 39.2 ± 6.5 kg/m2, and A1C 7.0 ± 0.8%). The subjects had well-controlled diabetes, with average A1C of 7 ± 0.81%. They were on stable oral antidiabetes medications. All patients were on metformin (1–2 g/day), and six patients were on sulfonylureas (5–10 mg/day glyburide or glipizide). None of the subjects was on insulin or thiazolidenedione therapy or taking any antioxidant or nonsteroidal anti-inflammatory drugs. After an overnight fast, subjects were infused with insulin (2 units/h), with 5% glucose and 20 mEq of potassium chloride for 4 h followed by 2 h of observation and wash-out. The glucose infusion rate was titrated to maintain blood glucose at a target level of 80–130 mg/dl. Blood glucose levels were measured every 15 min. None of the patients had any hypoglycemic symptoms. Another two groups of type 2 diabetic patients were infused with either 5% glucose (eight patients: four female and four male subjects, aged 45.8 ± 7.6 years, BMI 38.6 ± 7.2 kg/m2, and A1C 7.3 ± 0.9%) or normal saline (six patients: four female and two male subjects, aged 41.5 ± 8.2 years, BMI 36.9 ± 6.7 kg/m2, A1C 7.5 ± 1.1%) alone at a rate of 100 ml/h for 4 h and served as control subjects. Blood samples were collected at baseline and 2, 4, and 6 h. The protocol was approved by the human research committee of the State University of New York at Buffalo. An informed consent was signed by all subjects.
MNC isolation
Blood samples were collected in Na-EDTA and carefully layered on lympholyte medium (Cedarlane Laboratories, Hornby, ON). Samples were centrifuged and two bands separate out at the top of the red blood cells pellet. The MNC band was harvested and washed twice with Hank's balanced salt solution. This method provides yields >95% MNC preparation.
Quantification of TLR (-1, -2, -4, -7, and -9) mRNA in MNCs by RT-PCR
Total RNA was isolated using a commercially available RNAqueous 4PCR Kit (Ambion, Austin, TX). Real-time RT-PCR was performed using Cepheid Smart Cycler (Sunnyvale, CA), Sybergreen Master Mix (Qiagen, Valencia, CA), and gene-specific primers for TLRs (-1, -2, -4, -7, and -9) (Life Technologies, Rockville, MD). All values were normalized to the expression of a group of housekeeping genes, including actin, ubiquitin C, and cyclophilin A.
PU.1 DNA binding activity
Nuclear PU.1 DNA binding activity was measured by an electromobility shift assay. Nuclear extract was prepared from MNCs and by high salt extraction, as described previously (1). PU.1 assay was performed using specific binding site oligonucleotides corresponding to the PU.1 binding sites on TLR4 promoter (18): sense CGCTTTCACTTCCTCTCACCCTT and antisense AAGGGTGAGAGGAAGTGAAAGCG. The specificity of the band was confirmed by supershifting the band with specific antibody against PU.1 (Santa Cruz Biotechnology, Santa Cruz, CA) and by competition with cold oligonucleotides. PU.1 DNA binding was adjusted to Oct-1 DNA binding.
Western blotting
MNC total cell lysates were prepared as electrophoresis and immunoblotting was carried as described before (1). Monoclonal antibodies against TLR2, TLR4 (Abcam, Cambridge, MA), and actin (Santa Cruz Biotechnology) were used, and all values were corrected for loading to actin.
Plasma measurements
Glucose concentrations were measured in plasma by a YSI 2300 STAT Plus glucose analyzer (Yellow Springs, OH). Enzyme-linked immunosorbent assay was used to measure plasma concentrations of insulin (Diagnostic Systems Laboratories, Webster, TX), monocyte chemoattractant protein (MCP)-1, and soluble intercellular adhesion molecule (sICAM)-1 (R&D Systems, Minneapolis, MN).
Statistical analysis
Statistical analysis was conducted using SigmaStat software (SPSS, Chicago, IL). All data are represented as means ± SE. Statistical analysis from baselines was carried out using Holm-Sidak one-way repeated-measures ANOVA. Dunnett's two-factor repeated-measures ANOVA method was used for multiple comparisons between different groups.
RESULTS
Insulin and glucose concentrations during infusions
Plasma insulin concentration increased by 166% over baseline (from 20.9 ± 10.9 μU/ml to 50.5 ± 22.4 μU/ml; P < 0.001) during the insulin infusion, while it fell slightly in the dextrose groups from 27.6 ± 5.6 μU/ml to 22.9 ± 6.5 μU/ml at 4 h (P = NS) and in the normal saline group from 20.6 ± 5.5 μU/ml to 17.9 ± 4.7 μU/ml at 4 h (P = NS). The mean blood glucose concentrations changed from 122 ± 15 mg/dl at baseline to 111 ± 10 mg/dl at 4 h following insulin infusion, which was not significantly different from that observed in the control groups (133 ± 40 mg/dl at baseline to 125 ± 29 mg/dl at 4 h in the dextrose group and from 135 ± 13 mg/dl at baseline to 109 ± 13 mg/dl at 4 h in the saline group).
Suppressive effect of insulin on TLR mRNA and protein expression in diabetic patients
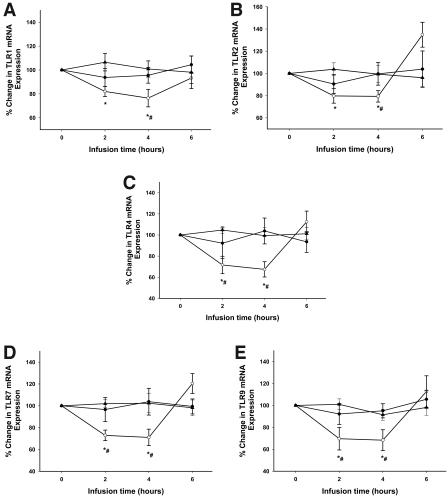
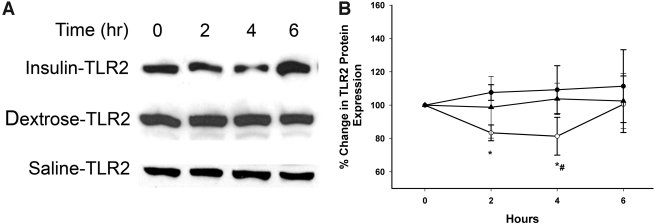
Insulin infusion suppressed the mRNA expression of TLR1, -2, -4, -7, and -9 in MNCs within 2 h, with a maximum fall at 4 h by 24 ± 9%, 21 ± 5%, 30 ± 8%, 28 ± 5%, and 27 ± 10%, respectively, below baseline (P < 0.05 for all) (Fig. 1). The expression of mRNA for all five TLRs reverted to baseline 2 h after the end of the infusion. There was no significant change in mRNA expression of TLR6 and TLR8 (data not shown). TLR2 protein was suppressed by 19 ± 7% below baseline at 4 h following insulin infusion (P < 0.05) when compared with baseline and to control subjects (Fig. 2), while TLR4 protein levels were reduced by only 8 ± 5% (P = NS). There was no significant change in TLR expression in the control group. We were unable to detect the other TLR proteins possibly due to the lower expression levels of these genes in the MNCs and the relatively low sensitivity of the Western blotting technique.
Figure 1.
TLR mRNA expression by RT-PCR following insulin or dextrose infusion in type 2 diabetic subjects. TLR1 (A), TLR2 (B), TLR4 (C), TLR7 (D), and TLR9 (E) mRNA expression in MNCs of type 2 diabetic subjects following 2 units/h insulin infusion compared with dextrose and saline infusion controls. *P < 0.05, repeated-measures ANOVA compared with baseline; #P < 0.05, two-way repeated-measures ANOVA compared with control groups. ○, insulin; •, dextrose; ▴, saline.
Figure 2.
TLR2 protein expression by Western blotting following insulin, dextrose, or saline infusion in type 2 diabetes. A: Representative TLR2 immunoblot in total cell lysate from MNCs of diabetic subjects following 2 units/h insulin infusion compared with dextrose and saline infusion controls. B: TLR2 protein densitometry. ○, insulin; •, dextrose; ▴, saline. *P < 0.05, repeated-measures ANOVA compared with baseline; #P < 0.05, two-way repeated-measures ANOVA compared with control groups.
MyD88 and CD14 are essential proteins involved in TLR signal transduction and LPS binding; therefore, their expression following insulin infusion was also investigated. MyD88 and CD14 expression was not significantly altered following insulin infusion (data not shown).
Effect of insulin infusion on PU.1
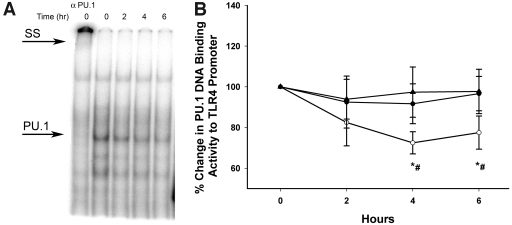
The binding of PU.1 to a specific and necessary motif (PU-12) on the TLR4 promoter in MNCs was suppressed by 24 ± 10% at 4 h following insulin infusion (P < 0.05) compared with baseline and control subjects (Fig. 3). This suppression did not revert to baseline at 6 h.
Figure 3.
PU.1 DNA binding activity to specific oligonucleotide on the TLR4 promoter following insulin dextrose and saline infusions in type 2 diabetic subjects by electromobility shift assay. A: Representative electromobility shift assay gel for PU.1 binding activity to PU-12 motif in TLR4 promoter in nuclear extracts from MNCs of type 2 diabetic subjects following 2 units/h insulin infusion. SS, supershift of PU.1–PU-12 complex from 0 h sample by a specific PU.1 antibody. B: Densitometry of PU.1 binding activity on TLR4 promoter. ○, insulin; •, dextrose; ▴, saline. *P < 0.05, repeated-measures ANOVA compared with baseline; #P < 0.05, two-way repeated-measures ANOVA compared with control groups.
Effect of insulin infusion on proinflammatory mediators
Following insulin infusion, plasma concentrations of MCP-1 fell significantly at 2 h and continued to fall at 4 h by 15 ± 4% below baseline (from 270 ± 43 ng/ml to 228 ± 32 ng/ml; P = 0.026; repeated-measures ANOVA). Plasma concentrations of sICAM-1 were also suppressed significantly by 4 h following insulin infusion by 10 ± 3% below baseline (from 301 ± 41 ng/ml to 263 ± 24 ng/ml; P = 0.017; repeated-measures ANOVA). The fall in MCP-1 and sICAM-1 concentrations was also significant (P < 0.05) when compared with the control group by two-way repeated-measures ANOVA.
CONCLUSIONS
Our data clearly show for the first time a potent and rapid suppressive effect of a low dose of insulin infusion on the expression of TLR1, -2, -4, -7, and -9 by 20–30%, evident at 2 h, continuing until 4 h, and reverting back to baseline 2 h after the cessation of infusion. This low-dose infusion of insulin has previously been shown to exert a rapid and profound anti-inflammatory effect within 2 h, as reflected in the suppression of NFκB binding and an increase in inhibitor of κBα expression. The fall in TLR2 mRNA was also associated with a reduction in the expression of TLR2 protein. The absence of a clear suppression of TLR4 protein level may be due to the relatively short period of infusion. This issue should be addressed in the future by longer periods of infusion. Our data also demonstrate clearly for the first time that insulin rapidly suppresses the DNA binding of PU.1 to a specific sequence of TLR4 gene promoter. PU.1 is a key transcription factor in the regulation of TLR transcription, and, thus, its suppression is reflected in the suppression of many of TLRs to a similar extent. The pattern of fall in TLR mRNA expression was similar to that observed with other proinflammatory mediators like MCP-1 and sICAM-1, which fell at 2 h, remained low for the duration of the infusion, and returned to baseline at 6 h, 2 h after the cessation of the insulin infusion. This is also consistent with our previous data on the suppressive effect of insulin on other indexes of inflammation. Thus, there is a remarkable consistency in the pharmacodynamics of the various aspects of the anti-inflammatory effects of insulin.
Recent work has shown that TLR4, and possibly TLR2, might mediate diet-induced obesity and insulin resistance and might, therefore, be involved in the pathogenesis of type 2 diabetes. It has been shown that TLR4−/− mice are protected from high-fat diet–induced insulin resistance (9,10). Also, RNA interference–mediated inhibition of TLR2 expression in muscle cells inhibited palmitate-induced insulin resistance (20). TLR2 and TLR4 expression is increased in adipose tissue of type 2 diabetic subjects (19) and in the MNCs of type 1 diabetic subjects (21). The expression of TLR4 in MNCs in type 1 diabetic subjects is related significantly to A1C, and the incubation of MNCs with glucose increases TLR4 expression (21). LPS concentration is also higher in type 2 diabetic subjects and related to plasma insulin concentration and insulin resistance (19). These facts support a role of the TLR pathway in the pathogenesis of type 1 and type 2 diabetes and the pathogenesis of diabetes complications. This is consistent with the observations that inflammatory mediators interfere with insulin signaling and might play a significant role in the development of insulin resistance. Thus, the suppression of TLR4 and TLR2 expression following a low-dose insulin infusion points toward a potential suppressive role for insulin on insulin resistance and atherosclerosis. Furthermore, since TLR9 is also involved in the pathogenesis of autoimmune mechanisms related to type 1 diabetes in experimental animals and TLR2 is involved in β-cell death and the suppression of TLR9, TLR2 may potentially be relevant to the prevention of type 1 diabetes (15,16).
PU.1 is the transcription factor that binds to a purine-rich region of the TLR gene promoters in order to activate the transcription of TLRs (17,18). Binding and activation would result in increased synthesis of TLRs at the transcriptional level. The fact that there is a suppression of PU.1 in parallel with the reduction in mRNA of TLR1, -2, -4, -7, and -9 within 2 h of starting insulin infusion suggests strongly that the suppression of transcription of TLRs by insulin is prompt and is probably mediated by the suppression of this key transcription factor. This observation expands our understanding of the anti-inflammatory effect of insulin.
Since TLR4 mediates the inflammatory response to endotoxin, it is possible that insulin may potentially reduce the inflammatory response to endotoxin by reducing the receptor population binding to endotoxin, provided we can demonstrate a reduction in TLR4 protein expression with longer infusions in the future. This effect would be in addition to the more direct anti-inflammatory effect of its own through the suppression of NFκB and early growth response factor-1, two major proinflammatory transcription factors. Such an additional effect may be of considerable importance since inflammation triggers an increase in TLR expression and thus provides a positive feedback for inflammation. This positive feedback, which may lead to a more protracted and intense inflammation, would potentially be prevented by insulin. A direct anti-inflammatory effect of insulin and its additional ability to suppress TLR expression provide it a profoundly potent combination in combating inflammatory processes. The suppression of TLR2 expression at mRNA and protein level by insulin is relevant to gram-positive bacterial infections. These actions of insulin may have contributed to the beneficial effects of insulin observed by Langouche et al. (22) in patients in critical care. It is also relevant that a similar low dose of insulin infusion causes a reduction of 40% in plasma CRP and SAA concentrations within 24 h in acutely ill patients with acute myocardial infarction (3) and in patients undergoing coronary artery bypass surgery (4). Whether a significant part of this suppression of CRP and SAA and the cardioprotection observed in acute myocardial infarction receives a contribution from a reduction in TLR2 expression needs to be carefully assessed in the future.
Recent work (23) has shown that in atherosclerosis, the expression of TLR1, -2, and -4 in the arterial intima is increased, especially in areas with inflammatory infiltration. The increase in TLR2 and TLR4 expression is associated with an increase in intranuclear NFκB. Several TLR4 ligands, such as oxidized LDL, human heat shock protein-60 and -70, and peptidoglycan are found in atherosclerotic plaques. They may activate NFκB and cause a release of cytokines and matrix metalloproteinases. This indicates that insulin action on TLR expression might play a role in atherosclerosis suppression and potential prevention of plaque rupture.
Active pharmaceutical research aiming to reduce TLR4 expression to prevent the proinflammatory action of LPS is currently being undertaken. This includes an attempt to generate antibodies against TLR4. The rapid suppression of TLR2 and TLR4 expression by insulin implies that insulin can be used clinically in endotoxemia and gram-negative (TLR4) and gram-positive (TLR2) infections to limit their inflammatory effects. Furthermore, the involvement of TLR2 in ischemia-reperfusion injury (11) and that of TLR4 in atherogenesis further justifies the use of insulin in acute and chronic atherosclerotic syndromes. In this context, the ability of insulin to suppress CRP is also relevant, since CRP mediates injury during ischemia-reperfusion of the heart and synthetic small molecules (phasphotidyl choline derivatives), which bind to and block CRP action and reduce the size of myocardial injury (24).
In conclusion, insulin suppresses TLR expression and the activity of the transcription factor PU.1. The suppressive effect of insulin on TLRs also has important potential implications in the treatment of inflammatory conditions including endotoxemia, other infections, and acute coronary syndromes in which TLR2-related mechanisms are involved. The suppressive effect of insulin on TLR4 is also important in understanding the relationship of inflammation to obesity and insulin resistance and atherosclerosis.
Published ahead of print at http://care.diabetesjournals.org on 12 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S: Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Dandona P, Aljada A, Mohanty P, Ghanim H, Bandyopadhyay A, Chaudhuri A: Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. Diabetes Care 26:3310–3314, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri A, Janicke D, Wilson MF, Tripathy D, Garg R, Bandyopadhyay A, Calieri J, Hoffmeyer D, Syed T, Ghanim H, Aljada A, Dandona P: Anti-inflammatory and profibrinolytic effect of insulin in acute ST-segment-elevation myocardial infarction. Circulation 109:849–854, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Visser L, Zuurbier CJ, Hoek FJ, Opmeer BC, de Jonge E, de Mol BA, van Wezel HB: Glucose, insulin and potassium applied as perioperative hyperinsulinaemic normoglycaemic clamp: effects on inflammatory response during coronary artery surgery. Br J Anaesth 95:448–457, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Janssens S, Beyaert R: Role of toll-like receptors in pathogen recognition. Clin Microbiol Rev 16:637–646, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F: Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Stoll LL, Denning GM, Weintraub NL: Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol 24:2227–2236, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Li H, Sun B: Toll-like receptor 4 in atherosclerosis. J Cell Mol Med 11:88–95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS: TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW: Toll like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100:1589–1596, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Shishido T, Nozaki N, Takahashi H, Arimoto T, Niizeki T, Koyama Y, Abe J, Takeishi Y, Kubota I: Central role of endogenous toll-like receptor-2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem Biophys Res Commun 345:1446–1453, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V: Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol 27:1064–1071, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I: Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108:2905–2910, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Means TK, Luster AD: Toll-like receptor activation in the pathogenesis of systemic lupus erythematosus. Ann N Y Acad Sci 1062:242–251, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol 178:693–701, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS: Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 27:321–333, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M: Transcriptional regulation of the human toll-like receptor 2 gene in monocytes and macrophages. J Immunol 168:5629–5637, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Rehli M, Poltorak A, Schwarzfischer L, Krause SW, Andreesen R, Beutler B: PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem 275:9773–9781, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S: Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292:E740–E747, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Senn JJ: Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem 281:26865–26875, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I: Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 93:578–583, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G: Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest 115:2277–2286, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi E, Orihara K, Hamasaki S, Ishida S, Kataoka T, Ogawa M, Saihara K, Okui H, Fukudome T, Shinsato T, Shirasawa T, Ichiki H, Kubozono T, Ninomiya Y, Otsuji Y, Tei C: Association between toll-like receptors and the extent and severity of coronary artery disease in patients with stable angina. Coron Artery Dis 18:31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Pepys MB, Hirschfield GM, Tennent GA, Gallimore JR, Kahan MC, Bellotti V, Hawkins PN, Myers RM, Smith MD, Polara A, Cobb AJ, Ley SV, Aquilina JA, Robinson CV, Sharif I, Gray GA, Sabin CA, Jenvey MC, Kolstoe SE, Thompson D, Wood SP: Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 440:1217–1221, 2006 [DOI] [PubMed] [Google Scholar]