Abstract
OBJECTIVE—We investigated whether cardiovascular autonomic dysfunction was associated with glycemic control status over time in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS—From 1999 to 2000, cardiovascular autonomic nerve function testing (AFT) was performed on patients with type 2 diabetes (n = 1,021) and was followed-up in 2006 and February 2008. Tests for cardiovascular autonomic functions measured heart rate variability parameters (expiration-to-inspiration [E/I] ratio, responses to the Valsalva maneuver, and standing). AFT scores were determined from the results of the each test as follows: 0 for normal and 1 for abnormal. We began with those who had a score of 0 and assessed the changes in total score along with biannual A1C levels.
RESULTS—At follow-up, the development of cardiovascular autonomic dysfunction was 34.5% (E/I ratio 21.9%, Valsalva maneuver 77.8%, and posture 58.9%; n = 783). The development of cardiovascular autonomic dysfunction was higher in older patients (P < 0.001); in those with longer duration of diabetes (P < 0.001); of hypertension (P = 0.005), and of diabetic retinopathy (P < 0.001); and in those who had higher levels of microalbuminuria (P = 0.002). Logistic regression analysis revealed that the development of cardiovascular autonomic dysfunction was strongly associated with the mean A1C level during the follow-up period (mean A1C >9.0% vs. ≤7.0%, odds ratio 2.984, 95% CI 1.177–7.561; P = 0.021).
CONCLUSIONS—The development of cardiovascular autonomic dysfunction was independently associated with microvascular complications and glycemic control status during this 7.5-year follow-up in patients with type 2 diabetes.
Cardiovascular events are the main cause of death among patients with type 2 diabetes. Compared with their counterparts without diabetes, the relative risk of cardiovascular disease is about three times greater in such patients (1) and is associated with chronic diabetes complications. Thus, diabetic autonomic neuropathy (DAN) is closely related to death caused by cardiovascular disease and to all other causes of mortality in these patients (2,3). A recent meta-analysis also demonstrated that cardiovascular autonomic dysfunction, as measured by heart rate variability, correlated strongly with an increased risk of silent myocardial ischemia and mortality (4).
In contrast to other types of DAN, cardiovascular autonomic neuropathy (CAN) is a well-studied form of DAN (2,5) because it has a well-established diagnostic test (6). Cardiovascular autonomic nerve function testing (AFT) using heart rate variability is sensitive, noninvasive, and reproducible; therefore, it is easily applicable for screening a large number of diabetic patients even as outpatients (2,6). The reported prevalence of CAN varies dramatically from a low of 7.7% for patients with newly diagnosed type 1 diabetes to a high of 90% among potential recipients of a pancreas transplant (2,7,8).
Most diabetes complications can be prevented only if the glycemic status of patients with diabetes can be maintained within a nearly normal range (9). However, diabetes complications can develop despite intensive glycemic control (10,11). Poor glycemic control also plays a central role in the development and progression of autonomic dysfunction (12,13). However, much remains to be investigated about the natural course of CAN and related risk factors for its progression in patients with type 2 diabetes. If we could identify the contributing factors, early detection of CAN and prompt intervention would be clinically meaningful for the prevention of adverse cardiovascular outcomes in patients with type 2 diabetes.
In this longitudinal cohort study, we evaluated whether CAN would develop according to glycemic control status among patients with type 2 diabetes who initially showed normal cardiovascular autonomic nervous function. We also studied the factors related to the development of CAN.
RESEARCH DESIGN AND METHODS
From January 1999 to December 2000, AFT was performed on patients aged 25–75 years who had type 2 diabetes at the university-affiliated diabetes center of St. Vincent's Hospital in South Korea. Among these, patients with normal AFT results were recruited (n = 1,021) and received follow-up AFT from January 2006 to February 2008. Patients were excluded if they were >75 years old, if they were mentally ill or unable to undertake the test, if they had arrhythmia including atrial fibrillation, or if they had any severe illness, such as malignancy, severe infection, severe hypoglycemia, liver cirrhosis, heart failure, or alcoholism. Our institutional ethics committee approved the study.
At the beginning of the study, patient height, body weight, and systolic and diastolic blood pressure were measured. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or as any use of antihypertensive medications. Fasting and postprandial plasma glucose levels were measured using an automated enzymatic method and A1C levels were determined by high-performance liquid chromatography with a reference range of 4.4–6.4%. A1C levels were measured every 6 months to evaluate the status of glycemic control during the follow-up period. Blood lipid concentrations for total cholesterol, triglycerides, and HDL cholesterol were measured enzymatically using an automatic analyzer (model 736-40; Hitachi, Tokyo, Japan). Biochemical laboratory tests were performed at baseline and at the follow-up time points.
Cardiovascular AFT was performed by one examiner using the Ewing method, which includes tests for heart rate variability, such as the expiration-to-inspiration (E/I) ratio and responses to the Valsalva maneuver and to a postural change from lying to standing (2,6,14). Patients were asked to fast for 12 h before AFT and to avoid taking antidepressants, neuroleptics, caffeine, nicotine, or antihistamines. The E/I ratio was calculated as the mean of the longest R-R interval during expiration divided by the mean of the shortest R-R interval during inspiration. The ratio of postural change was the ratio of the longest R-R interval during beats 20–40 after standing to the shortest R-R interval during beats 5–25 after standing. For the heart rate response to the Valsalva maneuver, the ratio of the longest R-R interval to the shortest R-R interval was checked during forced exhalation into the mouthpiece of a manometer to 40 mmHg for 15 s (2,14).
An E/I ratio below the age-related reference value, a Valsalva ratio <1.2, and a posture ratio <1.03 were considered abnormal (14). Each of the three items was scored as 0 for normal or 1 for abnormal, to a maximum score of 3. Normal AFT was defined as a score of 0, and CAN was defined on the basis of at least one abnormal standard test. Progression of CAN was defined as an increase in a follow-up AFT score from the baseline value (a score of 0) to a score of 1–3; such patients were designated the “progressor” group. “Nonprogressors” were defined as type 2 diabetic patients whose CAN scores did not show any increase at the follow-up time point.
The urinary albumin excretion (UAE) rate was measured by enzyme immunoassay from a single 24-h urine collection (15) using immunoturbidimetry (Eiken, Tokyo, Japan). Patients were classified into three groups: those with no diabetic nephropathy (UAE <20 μg/min), those with microalbuminuria (MAU) defined as a UAE of 20–200 μg/min, and those with overt proteinuria with UAE >200 μg/min. Diabetic retinopathy was assessed from retinal photographs at baseline, and the findings were reviewed by one ophthalmologist.
Statistical analyses
All of the results are expressed as means ± SD or median (range). Statistical analyses were performed using SPSS statistical software (SPSS, Chicago, IL). P < 0.05 was considered significant. Because of their skewed distribution, triglyceride and HDL cholesterol concentrations and microalbuminuria levels were compared after logarithmic transformation. χ2 tests were used to test the univariate relationships of categorical variables, and independent Student's t tests were used for continuous variables. We used multiple logistic regression analysis to test associations between the outcome (progression of AFT at follow-up) and potential explanatory variables. The relationships were analyzed after adjustment for the following risk factors: age, duration of diabetes, presence of hypertension, smoking, diabetic foot ulceration, and diabetic retinopathy and nephropathy. The results are given as odds ratios with 95% CIs.
RESULTS
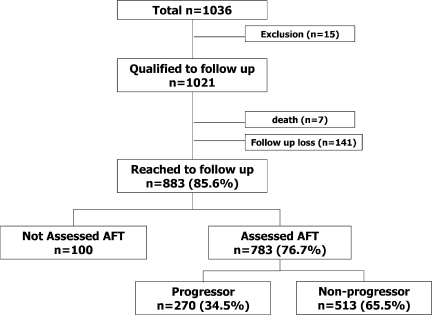
Of the 1,021 patients who had normal cardiovascular AFT results, 783 (76.7%) completed a follow-up AFT evaluation (Fig. 1). At baseline, the total study population consisted of 392 men and 629 women with mean ± SD age of 50.2 ± 10.7 years. Duration of diabetes was 7.1 ± 6.5 years, and A1C level was 8.6 ± 2.2%. The 783 participants who were evaluated did not differ from the 248 patients who were not assessed, with respect to age (50.7 ± 10.4 vs. 51.7 ± 11.6 years, P = 0.190), sex ratio (P = 0.649), presence of hypertension (P = 0.279), duration of diabetes (7.7 ± 6.7 vs. 7.4 ± 7.7 years, P = 0.390), or A1C level (8.7 ± 2.0% vs. 8.5 ± 2.4%, P = 0.915). During follow-up, seven patients died: four women and three men. The causes of death were sepsis in two patients, myocardial infarction in two patients, cerebral hemorrhage in one patient, and malignancy in two patients. The median follow-up period was 7.5 years.
Figure 1.
Study enrollment and follow-up.
At follow-up, 307 (39.2%) patients had hypertension and all of them were taking antihypertensive medication. Thirty-two (10.4%) patients were treated for hypertension with calcium channel blockers, 6 (2.0%) with β-blockers, 140 (45.6%) with ACE inhibitors or angiotensin receptor blockers, 4 (1.3%) with thiazides, and 125 (40.7%) with any combination of antihypertensive agents. Baseline sex ratios and BMIs were similar, and there was no difference in the treatment of diabetes (P = 0.773) or hypertension (P = 0.326) in the progressor and nonprogressor groups. However, the patients with progression were older (P < 0.001) with a longer duration of diabetes (P < 0.001), more were smokers (P = 0.045), and more had hypertension (P < 0.001). Moreover, the progressor group had higher levels of fasting glucose (P = 0.004), postprandial glucose (P = 0.010), and microalbuminuria (P = 0.002) than patients without progression (Table 1). However, there were no differences in total cholesterol, triglyceride, or HDL cholesterol levels between the two groups. The patients with progression also showed higher frequencies of diabetic nephropathy (P = 0.004) and diabetic retinopathy (P < 0.001) during the observation period than the patients in the nonprogressor group (Table 1).
Table 1.
Clinical characteristics according to progression of CAN
| Progressor | Nonprogressor | P value | |
|---|---|---|---|
| n | 270 | 513 | |
| Age (years) | 53.2 ± 9.7 | 48.8 ± 9.8 | <0.001 |
| Sex (male/female) | 105/165 | 215/298 | 0.170 |
| Duration (years) | 8.30 ± 6.9 | 6.27 ± 5.7 | <0.001 |
| BMI (kg/m2) | 25.1 ± 3.6 | 24.7 ± 3.2 | 0.195 |
| Hypertension | 124 (45.9) | 183 (35.7) | 0.005 |
| Smoking | 55 (20.3) | 74 (14.4) | 0.045 |
| Retinopathy at baseline | 97 (35.9) | 101 (19.7) | <0.001 |
| Nephropathy at baseline | 0.004 | ||
| Normal | 199 (73.7) | 426 (83.0) | |
| Microalbuminuria | 55 (20.4) | 74 (14.4) | |
| Overt proteinuria | 16 (5.9) | 13 (2.6) | |
| Diabetes treatment | 0.773 | ||
| Diet and exercise | 13 (4.8) | 22 (4.3) | |
| Oral agents | 150 (55.6) | 289 (56.3) | |
| Insulin | 43 (15.9) | 86 (16.8) | |
| Insulin + oral agents | 64 (23.7) | 116 (22.6) | |
| Baseline | |||
| FPG (mmol/l) | 9.52 ± 4.2 | 8.76 ± 3.0 | 0.004 |
| Creatinine (μmol/l) | 72 ± 22 | 72 ± 18 | 0.972 |
| Total cholesterol (mmol/l) | 4.73 ± 0.9 | 4.74 ± 1.2 | 0.827 |
| Triglyceride (mmol/l) | 1.54 (0.54–8.24) | 1.50 (0.41–8.64) | 0.995 |
| HDL cholesterol (mmol/l) | 10.6 (0.44–1.78) | 1.09 (0.47–2.12) | 0.110 |
| A1C (%) | 8.88 ± 2.0 | 8.39 ± 1.9 | 0.001 |
| Postprandial glucose (mmol/l) | 16.2 ± 5.6 | 15.1 ± 5.3 | 0.010 |
| Microalbuminuria (μg/min) | 12.4 (0.5–2634) | 10.0 (0.5–2398) | 0.002 |
| Follow-up | |||
| FPG (mmol/l) | 8.55 ± 3.1 | 8.34 ± 2.8 | 0.331 |
| Creatinine (μmol/l) | 90 ± 65 | 81 ± 21 | 0.006 |
| Total cholesterol (mmol/l) | 4.71 ± 1.0 | 4.62 ± 0.8 | 0.200 |
| Triglyceride (mmol/l) | 1.42 (0.34–9.15) | 1.40 (0.32–8.03) | 0.911 |
| HDL cholesterol (mmol/l) | 1.14 (0.34–2.79) | 1.14 (0.59–2.43) | 0.624 |
| A1C (%) | 8.33 ± 1.7 | 8.03 ± 1.6 | 0.017 |
| Postprandial glucose (mmol/l) | 14.4 ± 4.8 | 13.6 ± 4.9 | 0.024 |
| Microalbuminuria (μg/min) | 17.3 (0.5–3620) | 13.9 (5301.8) | 0.004 |
| Mean A1C (%) | 8.4 ± 1.4 | 7.9 ± 1.3 | <0.001 |
Data are means ± SD, n (%), or median (range). P < 0.05 was considered significant. FPG, fasting plasma glucose.
At follow-up, 270 patients (34.5%) showed abnormal cardiovascular AFT scores (total score ≥1, the progressor group). The results of the deep breathing, Valsalva maneuver, and lying-to-standing tests were abnormal in 59 (21.9%), 210 (77.8%), and 159 (58.9%) patients, respectively. In terms of total score, 138 (51.1%) patients had a score of 1, 106 (39.3%) had a score of 2, and 26 (9.6%) had a score of 3. The distribution of abnormal E/I ratio, Valsalva maneuver, and posture ratios in patients with a total score of 1 (n = 138) at follow-up was 12 (8.7%), 83 (60.1%), and 43 (31.2%) patients, respectively.
Even among the 133 patients with newly diagnosed diabetes at baseline, 43 (32.3%) showed abnormal AFT results at follow-up, with total scores of ≥1. These included an abnormal E/I score in 4 (9%), an abnormal Valsalva ratio score in 34 (79%), and an abnormal posture score in 21 (49%) (Table 2).
Table 2.
Changes in cardiovascular AFT results in the progressor group
| Progressor group
|
||
|---|---|---|
| Total | Newly diagnosed diabetes | |
| n | 270 | 43 |
| Abnormal E/I ratio | 59 (21.9) | 4 (9.3) |
| Abnormal Valsalva maneuver | 210 (77.8) | 34 (79.1) |
| Abnormal posture | 159 (58.9) | 21 (48.8) |
| Total score at follow-up | ||
| 1 | 138 (51.1) | 28 (65.1) |
| 2 | 106 (39.3) | 14 (32.6) |
| 3 | 26 (9.6) | 1 (2.3) |
Data are n (%).
Logistic regression analysis revealed that the mean value of biannually measured A1C was correlated significantly with the future development of CAN. Compared with the patients whose mean A1C value was ≤7.0%, having a level of >11.0% was significantly associated with the progression of CAN (Table 3). The conventional risk factors, such as age (P < 0.001), diabetic retinopathy (P = 0.036), and diabetic nephropathy (P = 0.035) at baseline predicted the progression of CAN after adjustments were made for baseline age, hypertension, smoking habit, and the duration of diabetes. Duration of diabetes or having a smoking habit was not associated with the progression of AFT. In this study, uncontrolled glycemic status was an independent risk factor for the development of CAN.
Table 3.
Multiple logistic regression analysis
| Odds ratio (95% CI) | P value | |
|---|---|---|
| Age (years) | <0.001 | |
| ≤40 | 1.000 | |
| 41–50 | 1.787 (1.047–3.049) | 0.033 |
| 51–60 | 2.679 (1.550–4.630) | <0.001 |
| >60 | 3.867 (2.133–7.011) | <0.001 |
| Diabetes duration (years) | ||
| ≤5 | 1.000 | 0.744 |
| 6–10 | 0.855 (0.585–1.249) | 0.417 |
| 11–15 | 0.966 (0.607–1.538) | 0.966 |
| >15 | 1.147 (0.620–2.122) | 0.662 |
| Hypertension (yes vs. no) | 1.196 (0.857–1.667) | 0.292 |
| Smoking (yes vs. no) | 1.173 (0.763–1.804) | 0.466 |
| Retinopathy (yes vs. no) | 1.513 (1.028–2.226) | 0.036 |
| Diabetic nephropathy (yes vs. no) | 1.515 (1.031–2.228) | 0.035 |
| Mean A1C (%) | 0.001 | |
| ≤7.0 | 1.000 | |
| 7.01–9.0 | 1.138 (0.898–2.134) | 0.141 |
| 9.01–11.0 | 2.565 (1.526–4.312) | <0.001 |
| >11.0 | 2.752 (1.075–7.048) | 0.035 |
CONCLUSIONS
This was a longitudinal observational study designed to investigate factors that might influence the progression of CAN in patients with type 2 diabetes. We demonstrate that CAN progressed according to glycemic control status and that it was associated with preexisting chronic diabetes complications.
Diabetes is an important risk factor for development of cardiovascular and cerebrovascular diseases (16,17). DAN is a serious and common complication of diabetes associated with an increased risk of cardiovascular mortality. Many studies have shown consistently that there is an increased risk of cardiovascular mortality among patients with an abnormal CAN assessment, compared with those with normal assessment (18,19). Therefore, early detection of high-risk diabetic patients and aggressive intervention should be promising therapeutic strategies.
In 2005, a consensus statement from the American Diabetes Association (ADA) recommended the battery of tests for the assessment of CAN including heart rate variability (HRV), E/I ratio, response to the Valsalva maneuver, and standing up (2,14). HRV methods are age dependent but independent of the intrinsic heart rate (2,14,20). Until now, measuring HRV has been a standard screening method in the diagnosis of autonomic dysfunction. AFT is easy to perform and is a reproducible test using a simple device at the patient's bedside (21,22).
There have been limited observations with regard to the progression of CAN in patients with type 2 diabetes. Poor glycemic control plays a central role in its development and progression (12,13). Moreover, strict glycemic control can slow the progression and delay the appearance of abnormal AFTs (10,23). In the European Diabetes (EURODIAB) Prospective Complication Study, the incidence of neuropathy was 23.5%, and the change in A1C value during a follow-up period of 7 years was independently associated with the incidence of neuropathy (11). The Diabetes Control and Complications Trial (DCCT) Research Group also documented that intensive therapy can slow the progression and the development of an abnormal autonomic test. However, these well-performed prospective studies were only conducted on patients with type 1 diabetes.
Similar to the EURODIAB study, we found that ∼35% of our patients showed progression of CAN. Even in the patients with newly diagnosed type 2 diabetes, ∼30% showed abnormal AFT results after 7.5 years. Glycemic control was an important risk indicator in this progression. We used the mean A1C value, measured every 6 months for 7.5 years, as a representative index of glycemic control status. Blood glucose control, as determined by A1C levels, seemed to be improved more during follow-up in the progressor group than in the nonprogressor group. However, the mean A1C values during the observation period were significantly higher in the progressor group. We suggest that, as for type 1 diabetes, glycemic control could influence the development and progression of CAN in patients with type 2 diabetes.
In addition, previous reports about CAN were designed to test only the E/I ratio or orthostatic hypotension. However, in our results, each of the three AFT tests showed a different rate of progression; a partial measurement of AFT offers only limited information about CAN. With use of the ADA criteria for the diagnosis of CAN in this study, the frequency of abnormal AFT results were, in order, the Valsalva maneuver, posture, and the E/I ratio among patients whose initial AFT results were normal. We suggest that the Valsalva ratio is the most sensitive parameter of the three values.
Interestingly, the duration of diabetes was not significantly associated with the development of CAN. Although diabetes duration has been reported to play an important role in patients with type 1 diabetes (12), the influence of diabetes duration seems to play a less important role in type 2 diabetes. Consistent with previous studies, we found an association between CAN and microvascular complications (24,25). CAN progression is correlated with diabetic retinopathy, diabetic nephropathy, and an increased urinary microalbumin excretion rate. This finding suggests that clinicians should pay more attention to patients with diabetic retinopathy or nephropathy, over and above the strict glycemic control required for the prevention of CAN.
The limitations of our study are as follows. First, there have been no normal values established for the E/I ratio, Valsalva maneuver, and posture tests specific to normal Korean subjects or to Korean patients with diabetes. It remains to be clarified whether the normal values for Western populations can be applied to Korean subjects; however, we performed AFT with the same method by one examiner throughout. Second, tests of blood pressure response to standing, blood pressure response to a sustained handgrip, and the presence of orthostatic hypotension were not checked because these tests were not available in our Diabetes Center from 1999 to 2000. However, each of the three tests of cardiovascular autonomic function used had a sensitivity and specificity of >90% (2). Moreover, the ADA suggests that the three tests used here adequately fulfill their requirements because of their reliability, reproducibility, general mutual correlation, and correlation with the tests of peripheral somatic nerve function and well-established normal values. Third, we could not evaluate the clinical importance of the progression of CAN. However, our ongoing follow-up studies should provide some insight into this issue.
In summary, prolonged exposure to uncontrolled hyperglycemic conditions could predict future CAN, independent of the duration of diabetes among patients at baseline. Cardiovascular AFT should be monitored to pay attention to major potential cardiovascular complications even in asymptomatic patients, but especially among those for whom glycemic control is not maintained adequately.
Acknowledgments
This work was supported by a grant from the Seoul R&BD Program.
Published ahead of print at http://care.diabetesjournals.org on 28 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Goff DC Jr, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, Buse JB, Genuth S, Probstfield JL, Simons-Morton DG, ACCORD Study Group: Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 18:4i–20i, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Vinik AI, Maser RE, Mitchell BD, Freeman R: Diabetic autonomic neuropathy. Diabetes Care 26:1553–1579, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Wheeler SG, Ahroni JH, Boyko EJ: Prospective study of autonomic neuropathy as a predictor of mortality in patients with diabetes. Diabetes Res Clin Pract 58:131–138, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Maser RE, Mitchell BD, Vinik AI, Freeman R: The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 26:1895–1901, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Ziegler D: Diabetic cardiovascular autonomic neuropathy. Circulation 115:387–397, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ewing DJ, Martyn CN, Young RJ, Clarke BF: The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8:491–498, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D, Gries FA, Spuler M, Lessmann F: The epidemiology of diabetic neuropathy: Diabetic Cardiovascular Autonomic Neuropathy Multicenter Study Group. J Diabetes Complications 6:49–57, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Kennedy WR, Navarro X, Sutherland DE: Neuropathy profile of diabetic patients in a pancreas transplantation program. Neurology 45:773–780, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 12:405–412, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complication Trial Research Group: The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med 122:561–568, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH: EURODIAB Prospective Complications Study Group: Vascular risk factors and diabetic neuropathy. N Engl J Med 352:341–350, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Ziegler D: Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev 10:339–383, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Young RJ, Ewing DJ, Clarke BF: Nerve function and metabolic control in teenage diabetics. Diabetes 32:142–147, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28:956–962, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Kearney EM, Mount JN, Watts GF, Slavin BM, Kind P: Simple immunoturbidimetric method for determining urinary albumin at low concentrations using Cobas-Bio centrifugal analyser. J Clin Pathol 40:465–468, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH: Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 141:421–431, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR: Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 27:201–207, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Jermendy G: Clinical consequences of cardiovascular autonomic neuropathy in diabetic patients. Acta Diabetol 40(Suppl. 2):S370–S374, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Maser RE, Lenhard MJ: Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab 90:5896–5903, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Ziegler D, Laux G, Dannehl K, Spuler M, Muhlen H, Mayer P, Gries FA: Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med 9:166–175, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Dyrberg T, Benn J, Christiansen JS, Hilsted J, Nerup J: Prevalence of diabetic autonomic neuropathy measured by simple bedside tests. Diabetologia 20:190–194, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Mackay JD, Page MM, Cambridge J, Watkins PJ: Diabetic autonomic neuropathy: the diagnostic value of heart rate monitoring. Diabetologia 18:471–478, 1980 [DOI] [PubMed] [Google Scholar]
- 23.The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 41:416–423, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valensi P, Paries J, Attali JR, French Group for Research and Study of Diabetic Neuropathy: Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications—the French multicenter study. Metabolism 52:815–820, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Anan F, Nakagawa M, Yufu K, Ooie T, Nawata T, Shigematsu S, Hara M, Saikawa T, Yoshimatsu H: Microalbuminuria, cardiovascular autonomic dysfunction, and insulin resistance in patients with type 2 diabetes mellitus. Metabolism 53:1359–1364, 2004 [DOI] [PubMed] [Google Scholar]