Abstract
OBJECTIVE—The purpose of this study was to determine the prevalence of and risk factors for diabetic neuropathy in a Canadian First Nation population.
RESEARCH DESIGN AND METHODS—This was a community-based screening study of 483 adults. Measures included glucose, A1C, cholesterol, triglycerides, homocysteine, hypertension, waist circumference, height, weight, and foot examinations. Neuropathy was defined as loss of protective sensation determined through application of a 10-g monofilament.
RESULTS—Twenty-two percent of participants had a previous diagnosis of diabetes, and 14% had new diabetes or impaired fasting glucose (IFG). The prevalence of neuropathy increased by glucose level: 5% among those with normal glucose levels, 8% among those with new IFG and diabetes, and 15% among those with established diabetes (P < 0.01). Those with neuropathy were more likely to have foot deformities (P < 0.01) and callus (P < 0.001) than those without neuropathy. Among those with dysglycemia (≥6.1 mmol/l), the mean number of foot problems for those with insensate feet was 3 compared with 0.3 among those with sensation (P < 0.001). In multivariate logistic regression female sex, low education, A1C, smoking, and homocysteine were independently associated with neuropathy, after controls for age.
CONCLUSIONS—Neuropathy prevalence is high, given the young age of our participants (mean 40 years) and was present among those with undiagnosed diabetes. The high number and type of foot problems places this population at increased risk for ulceration; the low level of foot care in the community increases the risk. Homocysteine is a risk factor that may be related to lifestyle and requires further investigation.
Diabetes-related foot complications are an increasingly common yet understudied problem (1) and include conditions such as neuropathy, foot deformities, ulcers, and lower-extremity amputation. The impact of diabetes-related foot complications on quality of life and health care costs is significant, especially for ulcers and amputation (2–4). The lifetime risk for foot ulceration among those with diabetes is 15% (5,6), and foot ulceration precedes amputation in 80% of patients (5). The major risk factors for diabetic foot ulceration are peripheral neuropathy, peripheral vascular disease, and previous foot ulcer (6). Of these, the most important contributory factor for foot ulceration is peripheral neuropathy; when neuropathy is absent, ulceration is rare (5,7). It is estimated that 80% of foot ulcers may be prevented through early detection (5). Screening for neuropathy has therefore been identified as an effective strategy in the prevention process (8). The purpose of this research was to determine the prevalence and determinants of diabetic neuropathy in a Canadian First Nation community that has a significantly greater rate of diabetes-related amputation than that for the general population (9).
Limited information exists on diabetes foot complications in Canadian First Nation populations. Depending on case definition, estimates of neuropathy range from 12% among a pediatric population to 46% among adults (9–12). Hanley et al. (10) reported the prevalence of neuropathy to be 46% among adult members of the Sandy Lake First Nation (northern Ontario), with neuropathy being defined as a score of ≥2 on a modified Michigan Neuropathy Screening Instrument. Reid et al. (11) performed foot examinations, interviews, and chart reviews on a sample (139 of 322) of individuals with diabetes in one northern Manitoba First Nation community and found that 82% had some form of diabetes-related foot problem. The average number of foot problems per individual was three. Neuropathy was found among 24% of participants, past or present foot ulcer in 15%, and 3% had had an amputation. Chuback et al. (12) completed chart reviews, interviews, and examinations on 110 Aboriginal attendees at an urban pediatric diabetes clinic in Manitoba and found a high prevalence of foot abnormalities (age range of participants was 12–17 years). Neuropathic symptoms (numbness, aching, and tingling) were found in 12% of participants. These results are especially disturbing, considering the short duration of diabetes in this pediatric population.
The study community reported in this article belongs to a tribal council for which the rate of diabetes-related amputation (6.2 per 1,000) is twice as great as that for other First Nations people in the province (3.1 per 1,000) and 30 times greater than the rate for non–First Nations people in the province (0.19 per 1,000) (9). Thus, diabetes-related foot problems are emerging as a serious complication of diabetes among First Nations people of Canada. We undertook a screening study in a community with a high rate of amputation to determine the burden of neuropathy and to initiate secondary prevention strategies.
The data for this article are from a larger screening study for diabetes and diabetes complications conducted in 2003 among adult members of the Sandy Bay First Nation. Sandy Bay First Nation is located in Manitoba, Canada. The community is located about 200 km from Winnipeg, the nearest major urban center, and is accessible by road year-round. The on-reserve population as of December 2001 was 2,968, of which 35% are aged <18.
RESEARCH DESIGN AND METHODS
As part of a larger screening study for diabetes and diabetes complications, all nonpregnant community members aged ≥18 years were invited to participate. A total of 483 community members participated, 36% of all eligible adults (n = 1,356). Full foot examinations were completed for 467 participants.
Assessment of neuropathy
Neuropathy was defined as the loss of protective sensation determined through application of the 10-g Semmes-Weinstein monofilament wire system. Using a standardized questionnaire, a registered nurse ascertained experience of foot pain, numbness, and tingling and then completed a foot examination for deformities, calluses, preulcers, ulcers, toenail integrity, and amputation. After this examination, the nurse applied the monofilament to 10 sites on the foot. Participants who were unable to sense the monofilament on one or more sites were defined as insensate (13). Individuals who had a previous foot ulcer (n = 1) or toe amputation (n = 3) were placed in the neuropathy category.
Risk factors for neuropathy
Glucose.
Fasting samples were obtained after an overnight 12-h fast. Glucose levels were determined using the hexokinase/glucose-6-phosphate dehydro- genase assay. A1C determination was based on the turbidimetric inhibition immunoassay for hemolyzed whole blood. Participants with fasting glucose values ≥5.8 mmol/l were requested to return for a second sample, with the average of the two samples being used in analysis. Diabetes was defined as a fasting blood glucose (FBG) ≥7.0 mmol/l. Impaired fasting glucose (IFG) was defined as FBG of 6.1–6.9 mmol/l.
Lipids and amino acids.
Total cholesterol and triglycerides were determined via enzymatic colorimetric methods (CHOD-PAP and GPO-PAP; Boehringer Mannheim, Mannheim, Germany). HDL cholesterol was measured by a direct enzymatic method (Boehringer Mannheim). LDL was calculated via the following formula but not on samples that had visible chylomicrons or triglycerides >4.52 mmol/l:
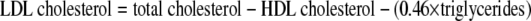 |
Apolipoproteins A and B were measured by the Beckman APA and APB tests, respectively (Beckman Array Systems). Quantitative determination of APA and APB was completed by rate nephelometry. Total fasting homocysteine was determined by high-performance liquid chromatography with fluorometric detection.
Hypertension.
Hypertension was defined systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg or a previous diagnosis with medications.
Anthropometric measures.
Height, weight, and waist and hip circumferences were measured using standard techniques (14).
Smoking.
Current and past smoking status and number of cigarettes smoked per day were determined using a standardized questionnaire. Pack-years was calculated as number of packs per day (one pack = 20 cigarettes) multiplied by number of years smoked.
Demographic information.
Standard demographic information and diabetes history were derived via questionnaire.
Analysis
Demographic, anthropomorphic, and health risk characteristics were compared by sex using χ2 tests for categorical variables and Kruskal-Wallis nonparametric tests for continuous variables. Means ± SD are reported. χ2 tests for linear association were used to assess differences in foot problems by glycemic status. χ2 tests were used to assess differences in foot problems by protective sensation. Tests were two-tailed with P < 0.05 taken as significant. Multivariate logistic regression was used to estimate odds ratios for the presence of neuropathy with 95% CIs. P values were two-tailed. Statistical analyses were performed using SPSS (version 15; SPSS, Chicago, IL) and SAS (version 8; SAS Institute, Cary, NC).
RESULTS
Table 1 contains demographic and health risk information. Men and women were equally represented. The sample was representative of the eligible adult community population for age and sex (Table 2). The level of employment is low for both sexes. Women were significantly more likely than men to report having completed at least grade 9. The majority of participants were current smokers. Mean pack-years smoked was about 8 for both sexes. Approximately 40% of men and women have hypertension. Although obesity is a major problem in both men and women, the problem is significantly greater among women (15). Of the participants, 29% had diabetes; 7% (35 of 483) were new diagnoses, and 22% (105 of 483) had a previous diagnosis of diabetes upon entry to the study. A further 7% were found to have IFG. No significant differences in glycemic status were found between men and women. The mean age of those with dysglycemia (44.51 ± 11.97 years) was significantly greater than for those with normal glucose values (34.01 ± 10.77 years; P < 0.001), with dysglycemia being defined as FBG ≥6.1 mmol/l or previous diagnosis of diabetes.
Table 1.
Characteristics and health risk profile of the study population by sex
| Variable | Male | Female | P value |
|---|---|---|---|
| n | 230 (48) | 253 (52) | |
| Age (years) | 37.3 ± 12.5 | 38.2 ± 12.1 | 0.373 |
| Age-group (years) | |||
| 18–29 | 73 (32) | 70 (28) | 0.728 |
| 30–39 | 65 (28) | 79 (31) | |
| 40–49 | 49 (21) | 59 (23) | |
| 50+ | 43 (19) | 45 (18) | |
| Education | |||
| Grade 9 or higher | 89 (39) | 131 (54) | <0.01 |
| <Grade 9 | 138 (61) | 111 (46) | |
| Employment status | |||
| Employed | 60 (26) | 77 (31) | 0.265 |
| Not employed | 169 (74) | 170 (69) | |
| Speak aboriginal language* | |||
| Yes | 204 (89.5) | 204 (83) | 0.061 |
| No | 24 (10.5) | 41 (17) | |
| Current smoker | |||
| Yes | 165 (73) | 184 (75) | 0.529 |
| No | 62 (27) | 60 (25) | |
| Pack-years smoked† | 8.02 ± 12.46 | 7.48 ± 10.68 | 0.921 |
| Hypertension‡ | |||
| Yes | 93 (41) | 108 (44) | 0.446 |
| No | 135 (59) | 136 (56) | |
| BMI (kg/m2) | 29.65 ± 5.92 | 33.32 ± 7.49 | <0.001 |
| Large waist§ | |||
| Yes | 120 (53) | 193 (81) | <0.001 |
| No | 106 (47) | 45 (19) | |
| Glycemic status | |||
| Normoglycemic | 150 (65) | 161 (64) | 0.444 |
| Impaired fasting glucose‖ | 18 (8) | 14 (5) | |
| Diabetes‖ | 62 (27) | 78 (31) |
Data are n (%) or means ± SD.
Local language is Saulteaux.
Number of packs per day (1 pack = 20 cigarettes) × number of years smoked.
Systolic >140 mmHg or diastolic >90 mmHg.
Men ≥102 cm; women ≥88 cm.
Fasting glucose 6.1–6.9 mmol/l. Fasting glucose ≥7.0 mmol/l or previous diagnosis.
Table 2.
Eligible and study populations by sex and age-group
| Eligible population*
|
Study participants
|
|||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Age-group (years) | ||||
| 18–29 | 258 (35) | 191 (31) | 73 (32) | 70 (28) |
| 30–39 | 195 (27) | 187 (30) | 65 (28) | 79 (31) |
| 40–49 | 148 (20) | 131 (21) | 49 (21) | 59 (23) |
| 50–59 | 84 (11) | 67 (11) | 29 (13) | 32 (13) |
| 60+ | 49 (7) | 46 (7) | 14 (6) | 13 (5) |
| Total | 734 | 622 | 230 | 253 |
Data are n (%).
Community members who met eligibility criteria (n = 1,356).
Neuropathy
Neuropathic foot problems by glycemic status are listed in Table 3. Overall, 34 participants (7%) had neuropathy. A significant progression in symptom occurrence is noted for all categories of neuropathic problems by glycemic status. Neuropathy was present in those with normal glucose levels (5%), was increased in those with newly discovered IFG or diabetes (8%), and was highest in those with established diabetes (15%). Of the participants, 34 (7%) reported numbness and tingling and 38 (8%) reported foot pain; 6 participants reported both. Women were more likely to report numbness and/or tingling (P < 0.05). Of the women, 9% (21 of 240) had insensate feet compared with 6% (13 of 227) of men. Significant differences in age were found between those with and without neuropathy. The mean age of those with neuropathy was 42.5 ± 12.5 years, whereas the mean age of those without neuropathy was 36.7 ± 11.9 years (P < 0.01). Among the participants who reported a previous diagnosis of diabetes, no significant differences in time since diagnosis were found between those with and without neuropathy and only 22% had previously had their feet examined by a health care provider before the screening study.
Table 3.
Neuropathic foot problems by glycemic status
| Normoglycemia | New IFG or diabetes | Previous diabetes | P value* | |
|---|---|---|---|---|
| Neuropathy† | ||||
| Present | 14 (5) | 5 (8) | 15 (15) | <0.01 |
| Absent | 286 (95) | 61 (92) | 86 (85) | |
| Pain | ||||
| Present | 18 (6) | 6 (9) | 14 (14) | <0.05 |
| Absent | 282 (94) | 60 (91) | 87 (86) | |
| Numbness, tingling | ||||
| Present | 14 (5) | 8 (12) | 12 (12) | <0.01 |
| Absent | 286 (95) | 58 (88) | 88 (88) | |
| Previous foot examination | ||||
| Yes | NA | NA | 22 (22) | NA |
| No | 78 (78) |
Data are n (%).
χ2 test for linear association.
Inability to sense 10-g monofilament on ≥1 site/10. NA, not applicable.
Participants with insensate feet were significantly more likely to have foot deformities (hallux valgus, hammer toes, claw toes, and bony protuberances) (P < 0.01) and calluses (P < 0.001) than were those with sensation. Of those with insensate feet, 18% (6 of 34) had an abnormal foot shape versus 5% (20 of 427) of those with sensation. Of those with insensate feet, 41% (14 of 34) had plantar calluses versus 12% (52 of 433) of those with sensation. In addition, the mean number of foot problems among dysglycemic participants with insensate feet (3.15) was significantly greater than that among those with sensation (0.27), with foot problems defined as pain, numbness, tingling, callus, nail pathological changes, foot deformity, preulcer, current or past ulcer, and amputation (P < 0.001).
Using multivariate logistic regression, we sought to determine factors associated with neuropathy (Table 4). Variables included in the modeling process were those that were significantly associated with neuropathy in bivariate χ2 testing. These variables were age (P < 0.01), education (P < 0.01), employment status (P < 0.01), hypertension (P < 0.05), A1C (P < 0.01), apolipoprotein A (P < 0.05), homocysteine (P < 0.05), and pack-years smoked (P < 0.01). Backwards stepwise logistic regression was performed using the preceding variables and sex. The final logistic regression model for presence of neuropathy included sex (female sex), education (completion of less than grade 9), A1C, pack-years smoked, and homocysteine.
Table 4.
Multivariate logistic regression analysis of factors associated with neuropathy
| β (SEM) | P value | Exp (β) (95% CI) | |
|---|---|---|---|
| Age | −0.016 (0.02) | 0.411 | 0.984 (0.946–1.023) |
| Sex | |||
| Male | 1.00 | ||
| Female | 0.989 (0.428) | <0.05 | 2.689 (1.163–6.220) |
| Education | |||
| <Grade 9 | 1.00 | ||
| ≥Grade 9 | −1.118 (0.466) | <0.05 | 0.327 (0.131–0.0814) |
| A1C | 0.267 (0.089) | <0.01 | 1.306 (1.097–1.556) |
| Homocysteine | 0.151 (0.045) | <0.01 | 1.163 (1.064–1.271) |
| Pack-years smoked | 0.048 (0.013) | <0.001 | 1.049 (1.023–1.077) |
The odds of having neuropathy were 2.7 times greater for women than for men and 3 times greater among those who completed less than grade 9 compared with those who completed grade 9 or higher. The odds of neuropathy increase for each percent increase in A1C; the risk for neuropathy is twice as great for someone with an A1C of 9 versus 6%. Neuropathy risk is three times greater for someone who smoked 30 pack-years than for someone who smoked 10 pack-years. Finally, the risk of neuropathy is twice as great for participants with a homocysteine level of 9 μmol/l versus those with a level of 13 μmol/l (hyperhomocysteinemia defined as >12 μmol/l) (16).
CONCLUSIONS
Of the participants with diabetes upon entry to the study, 15% had neuropathy, a number that is similar to recent estimates (17). However, the mean age of our participants (40.7 years) is younger than that of the comparison populations, and the mean time since diabetes diagnosis is comparatively short (8.75 years). The prevalence of neuropathy increased significantly by glucose level, a finding that is consistent with population-based studies (17) and current understanding on the role of glycemic control in the development and progression of neuropathy (7). Of the individuals with new IFG or diabetes diagnoses, 8% had evidence of neuropathy. Thus, complications were developing at the time of diagnosis. The short duration of disease before the onset of foot problems may indicate that diabetes complications occur more quickly among this population and/or that diabetes diagnoses are delayed. Participants with neuropathy were, on average, older than those without neuropathy. However, in multiple logistic modeling, glycemic control was a stronger predictor of neuropathy than age.
Individuals with neuropathy were more likely to have other foot problems compared with those without neuropathy. The presence of these foot problems increases the risk for foot ulceration among those with insensate feet, because of increased pressure loads and shearing forces (7,8). Therefore, foot examinations and treatment are a vital component of ulcer and amputation prevention (8).
In multivariate logistic regression, female sex, low education level, A1C, smoking, and homocysteine were independently associated with neuropathy, after controls for age. Completion of less than grade 9 may be a marker of lower socioeconomic status, which in turn may be associated with poorer access to health care services and self-care practices. Smoking has been found to be associated with neuropathy incidence among those with type 1 diabetes (18). Homocysteine, an amino acid that is produced from the metabolism of methionine (19), increased risk for neuropathy independent of age, sex, education level, A1C, and smoking. Homocysteine has been implicated in cardio- and cerebrovascular disease, primarily through impairment of endothelial functioning, leading to atherosclerosis and thrombosis (16,19–22). However, the etiological role of homocysteine in cardio- and cerebrovascular disease has not been established, so homocysteine may be a marker for other factors or may be related to other confounding factors (16). Ambrosch et al. (20) found a relationship between homocysteine and neuropathy among patients with type 2 diabetes. After addition of controls for creatinine, vitamins B12 and B6, and folate (all confounders for homocysteine), homocysteine was significantly associated with neuropathy.
The study is subject to limitations. First, the sample is relatively small. It is possible that study participants are systematically different from those who did not participate. However, the sample is representative of the eligible community population on demographic factors. Importantly, we did not only screen the “sickest” individuals in the community. When data were collected for this study, there were 275 community members with diabetes; 105 participated in the study. In addition, 10 individuals had amputations, and 15 individuals had end-stage renal disease. Three individuals with amputations participated in the study, whereas none of the individuals with end-stage renal disease participated. The prevalence and pattern of neuropathy found in our study are similar to those in other population-based studies. Thus, we have some confidence that our sample is representative of the eligible study population.
A second limitation is that we used fasting glucose to identify glucose intolerance, similar to other population-based epidemiologic studies. Had we completed 2-h glucose tolerance tests, we probably would have identified more glucose intolerance. It is possible that some individuals we classified as normoglycemic may have been dysglycemic. However, the relationship between glucose control and neuropathy was confirmed in the logistic regression analyses.
A third limitation is that our definition of neuropathy may have high sensitivity but lower specificity. Use of the 10-g monofilament is a validated procedure that has demonstrated ability to predict risk for ulceration and amputation (8,23–27). The protocol we followed has been shown to be associated with large-fiber neuropathy and increased risk for ulceration (27). Armstrong et al. (25) tested sensitivity and specificity of the 10-g monofilament procedure and found that specificity increased without a decrease in sensitivity up to four imperceptible sites. Our use of one or more insensate sites may have resulted in an overestimation of neuropathy prevalence. However, as our population is at high risk for ulceration and amputation, we chose a protocol with high sensitivity. That our findings are similar to those of recent population-based studies provides confidence in the protocol.
Finally, we did not control for other factors known to cause neuropathy (e.g., vitamin B12 and folate deficiencies, hypothyroidism, and alcohol intake) (28) or hyperhomocysteinemia (e.g., vitamin B6, chronic alcohol intake, smoking, caffeine, and certain medications) (16), so the relationship between homocysteine and neuropathy requires further investigation. However, although an influence of dietary and lifestyle factors may be possible in this population, given the low socioeconomic standing and self-reported food insecurity, it is unlikely that they completely explain the relationship between neuropathy and homocysteine. The prevalence of neuropathy was greatest among those with established diabetes, intermediate among those with newly discovered diabetes and IFG, and lowest among those with normal glucose status. If diet and lifestyle were the only factors involved in occurrence of neuropathy, a graded relationship with glycemic status would not necessarily be present. Nevertheless, lifestyle factors are implicated in hyperhomocysteinemia, so further investigation of this finding is warranted.
The burden of foot problems in Sandy Bay is high, and access to foot care and specialist services is limited. In a community with a high amputation rate, only 22% of those with diabetes had ever received a foot examination. We increased community capacity to identify those at greatest risk. We continue to work with the community on secondary prevention efforts.
Acknowledgments
This research was supported by the Canadian Institute for Health Research and the Manitoba Health Research Council.
We gratefully acknowledge the study participants, the Sandy Bay Diabetes Working Group, and the Sandy Bay First Nation leadership. We thank the anonymous reviewers for their insightful comments.
Published ahead of print at http://care.diabetesjournals.org on 28 May 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ulbrecht JS, Cavanagh PR, Caputo GM: Foot problems in diabetes: an overview. Clin Infect Dis 39 (Suppl 2):S73–S82, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh PR, Lipsky BA, Bradbury, Botek G: Treatment for diabetic foot ulcers. Lancet 366:1725–1735, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Meatherall BL, Garrett MR, Kaufert J, Martin BD, Fricke MW, Arneja AS, Duerksen F, Koulack J, Fong HM, Simonsen N, Nicolle LE, Trepman E, Embil JM: Disability and quality of life in Canadian Aboriginal and non-Aboriginal diabetic lower-extremity amputees. Arch Phys Med Rehabil 86:1594–1602, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs P, Blanchard JF, James RC, Depew N: Excess costs of diabetes in the Aboriginal population of Manitoba, Canada. Can J Public Health 91:298–301, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton AJM: The diabetic foot. In Textbook of Diabetic Neuropathy. Gries FA, Cameron NE, Low PA, Ziegler A, Eds. New York, Thieme, 2003, p. 295–305
- 6.Reiber GE, McFarland LV: Epidemiology and health care costs for diabetic foot problems. In The Diabetic Foot. 2nd ed. Veves A, Giurini JM, LoFerfo FW, Eds. Totowa, NJ, Humana Press, 2006, p. 39–50
- 7.Tesfaye S: Diabetic neuropathy. In The Diabetic Foot. 2nd ed. Veves A, Giurini JM, LoFerfo FW, Eds. Totowa, NJ, Humana Press, 2006, p. 105–129
- 8.Wu S, Armstrong DG, Lavery LA, Harkless LB: Clinical examination of the diabetic foot and the identification of the at-risk patient. In The Diabetic Foot. 2nd ed. Veves A, Giurini JM, LoFerfo FW, Eds. Totowa, NJ, Humana Press, 2006, p. 201–226
- 9.Martens P, Ed. The Health and Health Care Use of Registered First Nations People Living in Manitoba. Winnipeg, Manitoba Centre for Health Policy, 2002
- 10.Hanley AJG, Harris SB, Mamakeesick M, Goodwin K, Fiddler E, Hegele RA, Spence JD, House AA, Brown E, Schoales B, McLaughlin JR, Klein R, Zinman B: Complications of type 2 diabetes among Aboriginal Canadians. Diabetes Care 28:2054–2057, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Reid KS, Martin BD, Duerksen F, Nicolle LE, Garrett M, Simonsen JN, Trepman E, Embil JM: Diabetic foot complications in a northern Canadian Aboriginal community. Foot Ankle 27:1065–1073, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chuback J, Embil JM, Sellers E, Trepman E, Cheang M, Dean H: Foot abnormalities in Canadian Aboriginal adolescents with type 2 diabetes. Diabet Med 24:747–752, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DG: The 10-g monofilament: the diagnostic divining rod for the diabetic foot (Editorial)? Diabetes Care 23:887, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Canadian Society for Exercise Physiology (CSEP): The Canadian Physical Activity, Fitness and Lifestyle Appraisal. Ottawa, Canadian Society for Exercise Physiology, 1996
- 15.World Health Organization: Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation on Obesity. Geneva, World Health Org., 2000 [PubMed]
- 16.Kaul S, Zadeh AA, Shah PK: Homocysteine hypothesis for atherothrombotic cardiovascular disease. J Am Coll Cardiol 48:914–923, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Gregg EW, Gu Q, Williams D, de Rekeneire N, Cheng YJ, Geiss L, Engelgau M: Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among US adults aged 40 or older. Diabetes Res Clin Pract 77:485–488, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Tesfaye S, Chaturvedi N, Eaton SEM, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH: Vascular risk factors and diabetic neuropathy. N Engl J Med 352:341–350, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Maron BA, Loscalzo J: Homocysteine. Clin Lab Med 26:591–609, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ambrosch A, Dierkes J, Lobmann R, Kühne W, König W, Luley C, Lehnert H: Relation between homocysteinaemia and diabetic neuropathy in patients with type 2 diabetes mellitus. Diabet Med 18:185–192, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Rasouli ML, Nasir K, Blumenthal RS, Park R, Aziz DC, Budoff MJ: Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis 181:159–165, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S: The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the multi-ethnic study of atherosclerosis (MESA). J Am Coll Cardiol 48:1190–1197 [DOI] [PubMed]
- 23.Boyko E, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG: A prospective study of risk factors for diabetic foot ulcer: the Seattle Diabetic Foot Study. Diabetes Care 22:1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini J, Veves A: Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 23:606–611, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Armstrong DG, Lavery LA, Vela SA, Quebedeaux TL, Fleischli JG: Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 158:289–292, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Rith-Najarian SJ, Stolusky T, Gohdes DM: Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. Diabetes Care 15:1386–1389, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Armstrong DG, Lavery LA: Clinical Care of the Diabetic Foot. Alexandria, VA, American Diabetes Association, 2005
- 28.Perkins BA: Demystifying diabetic neuropathy. Can Diabetes 20:3–7, 2007 [Google Scholar]


