Abstract
OBJECTIVE—Albuminuria can be caused by endothelial dysfunction as a result of ischemic nephropathy rather than classic diabetic nephropathy. We studied whether renal vascular resistance (resistive index [RI]) of the main renal arteries could be associated with albuminuria and further assessed the relationship between RI and aorta stiffness measured by brachial-ankle pulse-wave velocity (baPWV).
RESEARCH DESIGN AND METHODS—We consecutively studied 150 patients with type 2 diabetes and the absence of clinically overt renal artery stenosis. Renal function expressed as the estimated glomerular filtration rate (eGFR) was calculated using the modified formula of modification of diet in renal disease (MDRD). The RI [(peak systolic velocity –end-diastolic velocity)/peak systolic velocity] was measured with duplex Doppler ultrasonography.
RESULTS—When the presence of albuminuria (uAlb) was defined as urinary albumin-to-creatinine ratio (μg/mg · creatinine) >30, mean RI [(left RI + right RI)/2] was significantly higher in uAlb, compared with that in patients without uAlb. RI had significant associations with age (r = 0.398, P < 0.0001), diastolic blood pressure (r = −0.398, P < 0.0001), eGFR (r = −0.373, P < 0.0001), and baPWV (r = 0.223, P < 0.05), respectively. Multivariate logistic regression analysis showed that increased RI when defined as RI >0.72 (median) was significantly associated with age (P < 0.01, 95%CI 1.02–1.19), diastolic blood pressure (P < 0.01, 0.86–0.97), and uAlb (P < 0.01, 1.53–15.46), respectively. Moreover, RI was an independent risk factor for uAlb after adjustment of both diastolic blood pressure and eGFR.
CONCLUSIONS—Renal vascular resistance was associated with albuminuria and aorta stiffness. Increased RI may imply the presence of any type of underlying renal damage, including ischemic nephropathy.
Duplex Doppler ultrasonography was used to assess intrarenal hemodynamics. The resistive index (RI) calculated from blood flow velocities in vessels reflects renovascular resistance and is known to increase in various disorders (1–5). Moreover, vasoactive agents, such as angiotensin II or ACE inhibitors (6), are known to affect RI. Regarding mechanisms by which RI of intrarenal arterioles increase, we previously reported that arterio-arteriolosclerosis rather than interstitial fibrosis could play an important role (7). In addition, we reported that there was a direct relationship between RI and arteriolosclerosis in damaged kidneys, and RI at renal biopsy may be useful as one of the prognostic markers for renal outcome (7).
According to the annual report of the Japan Dialysis Treatment Society in 2006, the most frequent cause of end-stage renal disease is diabetes (8). Although diabetic nephropathy has been considered to be a microvascular complication, histopathological examination of renal biopsies showed not only typical diffuse or nodular lesions, but also arteriosclerotic glomerulosclerosis (9). It has been reported that RI in patients with renal dysfunction (chronic renal failure) secondary to type 2 diabetes were significantly increased compared to the patients with nondiabetic chronic renal failure (10). Furthermore, regardless of the status of microalbuminuria, which has been considered to be a risk factor for diabetic nephropathy and progression of renal insufficiency, glomerular filtration rate (GFR) was also reported to be correlated with RI (11). Indeed, there are several reports showing a correlation between RI and renal function (7,11,12). It is therefore conceivable that nonalbuminuric renal insufficiency (13) could be related to other pathogenetic disorders, such as ischemic nephropathy, rather than classic diabetic nephropathy. In this regard, it should be borne in mind that macroangiopathy, not microangiopathy, is likely to affect GFR because systemic atherosclerotic vascular disease adversely affects renal blood perfusion, resulting in a decrease of GFR, even if clinically overt renal artery stenosis is not evident. Ishimura et al. (12) have already reported that RI values are significantly correlated with both femoral and carotid arterial intima-media thickness (IMT) in type 2 diabetic patients with nephropathy and that intrarenal hemodynamics are affected by decreased GFR, probably through advanced arteriosclerosis. Recently, Ohta et al. (14) reported that increased RI of the main renal arteries is significantly correlated with the severity of systemic atherosclerosis. Furthermore, the intrarenal vascular resistance differs depending on the underlying renal disease and appears to increase to a greater extent in diabetic nephropathy (14). However, the relationship between RI and albuminuria remains unknown, despite albuminuria being a strong predictor of cardiovascular events caused by endothelial dysfunction (15).
Therefore, we assessed the relationship between RI of the main renal arteries and albuminuria. Moreover, we studied the severity of other macroangiopathy evidenced by an increase in aorta stiffness measured by brachial-ankle pulse-wave velocity (baPWV), carotid IMT, and ankle-brachial pressure index (ABI) in association with RI.
RESEARCH DESIGN AND METHODS
We consecutively studied 150 patients with type 2 diabetes attending the diabetes clinics in our hospital between March 2005 and June 2006. The diagnosis of diabetes was based on a previous history of diabetes or fulfillment of World Health Organization criteria (16). Patients with known renal arterial stenosis, or those with malignancy or systemic disorders, were excluded. The study protocol was approved by the Research and Ethics Committee of the Shonan Kamakura General Hospital, and informed consent was obtained.
Blood was drawn in the morning after an overnight fast of at least 12 h. Urinary albumin concentration was measured by enzyme-linked immunosorbent assay using fresh spot urine and was expressed as milligrams creatinine of urine. Normoalbuminuria was defined as urinary albumin-to-creatinine ratio <30 μg/mg in two or more urine samples and no more than one value ≥30 μg/mg. Microalbuminuria was defined as urinary albumin-to-creatinine ratio >30–299 μg/mg. Macroalbuminuria was defined by albumin-to-creatinine ratio >300 μg/mg. The presence of albuminuria (uAlb) was defined as urinary albumin-to-creatinine ratio (μg/mg · Cr) >30. Renal function expressed by the estimated glomerular filtration rate (eGFR) was calculated using the modified formula of modification of diet in renal disease (MDRD) (17):
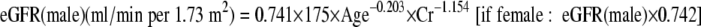 |
Comorbidity was recorded and ischemic heart disease was defined as the presence or any history of myocardial infarction, unstable angina, percutaneous coronary intervention, or coronary artery bypass graft surgery. Cerebrovascular disease was defined as the presence or any history of cerebral or cellebellar infarction or bleeding or subarachnoidal hemorrhage. Peripheral arterial disease was defined as the presence or any history of bypass surgery or percutaneous peripheral intervention or limb ischemia as evidenced by symptoms of intermittent claudication, resting pain, or gangrene.
Duplex Doppler ultrasonography
All measurements were made after an overnight fast in a supine position and during suspended respiration at the end of inspiration. Images were obtained with a duplex Doppler apparatus (Aloka SSD 2000; Aloka, Tokyo, Japan) with a 5-MHz convex array probe in both real-time/color-coded Doppler and pulse Doppler modes. The ultrasound probe was positioned gently on the flank in oblique projection, and the kidney was visualized as a longitudinal image. A Doppler beam was placed on the main tract of the renal arteries. RI was calculated by the built-in software as follows: RI = (peak systolic velocity − end-diastolic velocity)/peak systolic velocity.
The use of antihypertensive agents was not suspended before the resistive index measurement. Although the patients were not specifically screened for the presence of undiagnosed renal artery stenosis, we nevertheless measured the size of the nephrogram and blood flow at the hilum of each kidney. None of the patients who were included in the study had a hilar acceleration time >100 cm/s or had evidence of kidney atrophy <8 cm. The average of right RI and left RI with the difference between the two kidneys not <15% was used as a marker of renal artery resistance in each individual.
Measurements were performed by well-trained staff unaware of any information concerning the patients. Regarding reproducibility of measurements, interobserver and intraobserver variances were 4.0 and 5.1%, respectively (7).
Aorta stiffness expressed as baPWV, ABI, and carotid IMT
Aortic stiffness can be assessed noninvasively by measurement of baPWV (18). Recently, a new and simple device to measure baPWV has been developed. The device measures baPWV using an oscillometric method (19). In this study, the baPWV was automatically calculated with the use of a Colin Waveform Analyzer (form PWV/ABI; Colin, Komaki, Japan). The instrument records baPWV, blood pressure, electrocardiogram, heart sounds, and ABI simultaneously. Validity, reproducibility, and clinical significance of noninvasive baPWV measurement have been reported elsewhere (19). ABI was estimated by ankle systolic blood pressure/brachial systolic blood pressure.
According to our previous report (20), both carotid arteries were examined using a 7.5-MHz linear array transducer with high-resolution B-mode ultrasonography (Aloka, Tokyo, Japan). The IMT was defined as the distance between the leading edge of the lumen-intima echo of the near wall and the leading edge of the media-adventitia echo. The maximum IMT was recorded in the right and left carotid arteries, respectively. In addition to RI, baPWV, IMT, and ABI were expressed as an average of right and left measurements, respectively.
Statistical analysis
All the data are expressed as means ± SD. Differences between the two groups were analyzed by an unpaired Student's t test. One-way ANOVA was used for multiple comparisons of more than three groups followed by Scheffe's test. Logistic multiple regression analysis was performed to determine more related variables for RI. Increased RI when defined as RI >0.72 (median) was used for logistic regression analysis. The F value for a candidate's entrance or removal from the discriminate function test was set at 4.0. The level of statistical significance was defined as P < 0.05. All the statistical analysis was performed with SAS/Windows (Statview version 5.0).
RESULTS
Basic characteristics of the 150 patients are shown in Table 1. Mean age ± SD was 66.1 ± 10.2 years, and A1C was 8.01 ± 1.81%. Serum creatinine and eGFR were 0.94 ± 0.43 mg/dl and 77.6 ± 22.0 ml/min per 1.73 m2, respectively. Systolic blood pressure (sBP) and diastolic blood pressure (dBP) were both relatively well controlled (sBP 139 ± 21 mmHg and dBP 79 ± 14 mmHg). Comorbidity and co-current use of drugs are also shown in Table 1. The median of urinary albumin excretion was 22.8 μg/mg · Cr. The RI of all patients was 0.720 ± 0.071. The baPWV, IMT, and ABI values were 1,894 ± 519 cm/s, 0.943 ± 0.123 mm, and 1.11 ± 0.12, respectively (Table 1).
Table 1.
Basic characteristics of the patients
| n | 150 |
| Sex (M/F) | 102/48 |
| Age (years) | 66.1 ± 10.2 |
| A1C (%) | 8.01 ± 1.81 |
| sBP (mmHg) | 139 ± 21 |
| dBP (mmHg) | 79 ± 14 |
| Total cholesterol (mg/dl) | 207 ± 40 |
| Triglycerids (mg/dl) | 170 ± 176 |
| HDL (mg/dl) | 54 ± 17 |
| Creatine (mg/dl) | 0.94 ± 0.43 |
| eGFR (ml/min per 1.73 m2) | 77.6 ± 22.0 |
| RI | 0.720 ± 0.006 |
| baPWV (cm/s) | 1,894 ± 510 |
| IMT (mm) | 0.94 ± 0.23 |
| Urinary albumin excretion (μg/mg · creatinine) | 215.1 ± 640 |
| Comorbidity | |
| Hypertension (%) | 72.0 |
| Hyperlipidemia (%) | 64.0 |
| Ischemic heart disease (%) | 35.3 |
| Cardiovascular disease (%) | 8.7 |
| Peripheral arterial disease (%) | 12.7 |
| Drugs | |
| Angiotensin receptor blockade (%) | 50.7 |
| ACE inhibitors (%) | 14.0 |
| Calcium-channel blockers (%) | 38.7 |
Data are means ± SD.
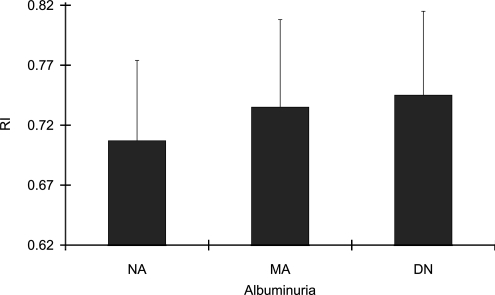
RI was significantly higher in patients with macroalbuminuria (0.745 ± 0.077), compared with that in patients with normoalbuminuria (0.707 ± 0.067) (P < 0.01), as shown in Fig. 1. Univariate regression analysis showed that RI had significant associations with age (r = 0.398, P < 0.0001), dBP (r = −0.398, P < 0.0001), eGFR (r = −0.373, P < 0.0001), and baPWV (r = 0.223, P < 0.05) (Table 2), respectively. Urinary albumin excretion, as a continuous variable, was not correlated with RI. Multivariate logistic regression analysis showed that independent of eGFR, RI was significantly associated with age (P < 0.01, 95% CI 1.02–1.18), dBP (P < 0.01, 0.86–0.97), and uAlb (when the presence of albuminuria was defined as urinary albumin-to-creatinine ratio >30 μg/mg:uAlb) (P < 0.01, 1.53–15.46) (Table 3), respectively. However, baPWV, IMT, and ABI as well as eGFR were not identified as independent risk factors for increased RI. Also, when uAlb was considered as a dependent variable, RI (P < 0.05) as well as dBP (P < 0.05) and eGFR (P < 0.01) remained significant as independent risk factors.
Figure 1.
RI was significantly higher in patients with macroalbuminuria (DN) (0.745 ± 0.077) compared with that in patients with normoalbuminuria (NA) (0.707 ± 0.067) (P < 0.01). MA, microalbuminuria.
Table 2.
Univariate regression analysis associated with RI
| R | P | |
|---|---|---|
| Age | 0.398 | <0.0001 |
| sBP | 0.136 | 0.126 |
| dBP | −0.398 | <0.0001 |
| A1C | 0.064 | 0.460 |
| Urinary albumin excretion | 0.0616 | 0.882 |
| eGFR | −0.373 | <000.1 |
| baPWV | 0.223 | 0.015 |
| IMT | 0.142 | 0.154 |
| ABI | 0.167 | 0.065 |
Table 3.
Multivariate logistic regression analysis associated with increased renal vascular resistance
| β | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|
| Age | 1.100 | 1.023 | 1.184 | 0.010 |
| sBP | 1.020 | 0.984 | 1.057 | 0.284 |
| dBP | 0.919 | 0.860 | 0.979 | 0.006 |
| A1C | 0.916 | 0.696 | 1.206 | 0.532 |
| uAlb | 4.862 | 1.529 | 15.45 | 0.007 |
| eGFR | 1.002 | 0.973 | 1.031 | 0.909 |
| baPWV | 0.999 | 0.989 | 1.000 | 0.169 |
| IMT | 2.179 | 0.229 | 20.72 | 0.497 |
| ABI | 20.77 | 0.242 | 1,783.1 | 0.182 |
R2 = 0.246.
CONCLUSIONS
In the present study, we showed that renal vascular resistance was higher in patients with macroalbuminuria. After an adjustment of eGFR, RI remained significant and was an independent risk factor for the presence of albuminuria. Therefore, RI is a useful marker for the presence of any type of nephropathy found in type 2 diabetes. When we consider the stages of diabetic nephropathy, it is plausible that there is a stage in which albuminuria and GFR do not always correspond to each other. Recent results regarding the pathophysiology of renal disease in type 2 diabetes have challenged the concept that a decline in renal function in patients with diabetes is always accompanied by increased albuminuria. MacIsaac et al. (11) have suggested that renal insufficiency, without albuminuria, is common in type 2 diabetes.
Increased RI is known to be correlated with renal dysfunction or urinary albumin excretion in hypertensive patients under treatment with ACE inhibitors (6). We showed that RI was correlated with macroalbuminuria. Recently, Nosadini et al. (21) showed that increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. The patients with RI >0.8 showed a significant deterioration in GFR. However, albumin excretion rate was similar in both the patients with RI above or below 0.8 at baseline, although overt proteinuria tended to develop more frequently in patients with RI >0.8. Therefore, the present study may indicate that RI was significantly correlated with albuminuria except in normoalbuminuric patients. Considering the finding that RI was not correlated with proteinuria in patients with idiopathic chronic glomerulonephritis (7), the results of the present study may also suggest the difference in the pathophysiological significance of albuminuria between both groups: those with chronic glomerulonephritis and those with diabetes. In diabetic patients, polyvascular disease based on systemic atherosclerotic disorders could affect renal injury through long-term intrarenal ischemia. Indeed, increased urinary albumin excretion can be a marker of coexisting coronary artery disease in patients with peripheral arterial disease (22). Atherosclerosis affects different vascular systems resulting in polyvascular disease. Recently, the REACH (Reduction of Atherothrombosis for Continued Health) study revealed that coronary, cerebral, and peripheral arterial diseases are superimposed on one another (23). In the patients with diabetes, endothelial cells of the renal or intrarenal artery, in addition to glomeruli, would be more affected by atherosclerosis than those in the patients with chronic glomerulonephritis. On the other hand, albuminuria in the patients with chronic glomerulonephritis may be more likely derived from glomerular capillary damage alone without (or with less) damage to the main renal artery. This may be the reason why RI was not correlated with proteinuria in the patients with chronic glomerulonephritis.
Renal injury in diabetic patients includes various pathophysiological disorders. In this regard, we need to be aware of ischemic nephropathy resulting from diminished renal blood perfusion, even if clinically overt renal artery stenosis is absent. Ischemic nephropathy originally refers to the reduction of GFR that is caused by hemodynamically significant renal artery obstruction (24). However, the major clinical questions confronting the nephrologist in considering the diagnosis of ischemic nephropathy include, “Which clinical or laboratory features are most useful in its detection?” The finding that increased RI of renal arteries is significantly correlated with albuminuria would provide a clue to study the clinical prognosis of less severe but long-term ischemic nephropathy. Endothelial cells in glomerular capillary could be easily injured by mechanical stress including ischemia. It has been recently reported that even in proteinuric subjects without diabetes, there is evidence of macrovascular endothelial dysfunction remote from the kidney and of low-grade inflammation that is associated with microvascular endothelial dysfunction (25).
In conclusion, increased RI may imply the presence of any type of underlying renal damage, including ischemic nephropathy resulting in endothelial dysfunction in type 2 diabetes.
Published ahead of print at http://care.diabetesjournals.org on 19 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Rifkin MD, Needleman L, Pasto ME: Evaluation of renal transplant rejection by duplex Doppler examination. Am J Roengenol 148:759–762, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Platt JF, Rubin JM, Ellis JH, DiPietro MA: Duplex Doppler US of the kidney: differentiation of obstructive from nonobstructive dilatation. Radiology 171:515–517, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Platt JF, Ellis JH, Rubin JM, DiPietro MA: Intrarenal arterial Doppler sonography in the detection of renal vein thrombosis of the native kidney. Am J Roengenol 162:1367–1370, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Platt JF, Ellis JH, Rubin JM, DiPietro MA, Sedman AB: Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease. Am J Roengenol 154:1223–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Patriquin HB, O'Regan S, Robitaille P, Paltiel H: Hemolytic-uremic syndrome: intrarenal arterial Doppler patterns as a useful guide to therapy. Radiology 172:625–628, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Leoncini G, Martinoli C, Viazzi F: Changes in renal resistive index and urinary albumin excretion in hypertensive patients under long-term treatment with lisinopril or nifedipine GITS. Nephron 90:169–173, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H, Suzuki S, Miura S: Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46:603–609, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Japanese Society for Dialysis Therapy: Report of the annual statistical survey of the Japanese Society for Dialysis Therapy in 2006. Tokyo. JSDT 12:1–186, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Gambara V, Mecca G, Remuzzi G, Bertani T: Heterologous nature of renal lesions in type II diabetes. J Am Soc Nephrol 3:1458–1466, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto N, Ishimura E, Taniwaki H, Emoto M, Shoji T, Kawaguchi T, Inaba M, Nishizawa Y: Diabetes mellitus worsens intrarenal hemodynamic abnormalities in nondialyzed patients with chronic renal failure. Nephron 86:44–51, 2000 [DOI] [PubMed] [Google Scholar]
- 11.MacIsaac RJ, Panagiotopoulos S, McNeil KJ, Smith TJ, Tsalamandris C, Hao H, Matthews PG, Thomas MC, Power DA, Jerums G: Is nonalbuminuric renal insufficiency in type II diabetes related to an increase in intrarenal vascular disease? Diabetes Care 29:1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ishimura E, Nishizawa Y, Kawagishi T, Okuno Y, Kogawa K, Fukumoto S, Maekawa K, Hosoi M, Inaba H, Emoto M, Morii H: Intrarenal hemodynamic abnormalities in diabetic nephropathy measured by duplex Doppler sonography. Kidney Int 51:1920–1927, 1997 [DOI] [PubMed] [Google Scholar]
- 13.MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G: Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 27:195–200, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Ohta Y, Fujii K, Arima H, Matsumura K, Tsuchihashi T, Tokumoto M, Tsuruya K, Kanai H, Iwase M, Hirakata H, Iida M: Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens 23:1905–1911, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hillege HL, Janssen WMT, Bak AAA, Diercks GFH, Grobbee DE, Crijns HJG, Van Gilst WH, DeZeeuw D, Jong PE: Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249:519–526, 2001 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization: Diabetes Mellitus: Report of a WHO Study Group. Geneva, World Health Org., 1985. (Tech. Rep. Ser., no. 727) [PubMed]
- 17.Levy AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation: Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lehmann ED: Pulse wave velocity as a marker of vascular disease. Lancet 348:741–744, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Horii S, Yamamoto Y: Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 25:359–364, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi S, Moriya H, Aso Kuniko, Ohtake T: Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int 63:1881–1887, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Nosadini R, Velussi M, Brocco E, Abaterusso C, Carraro A, Piarulli F, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Tonolo G: Increased renal arterial resistance predicts the course of renal function in type 2 diabetes with microalbuminuria. Diabetes 55:234-238, 2006 [PubMed] [Google Scholar]
- 22.Sonmez K, Eskisar AO, Demir D, Yazicioglu MV, Mutlu B, Dogan Y, Izgi A, Mansuroglu D, Bakal RB, Elonu OH, Turan F: Increased urinary albumin excretion rates can be a marker of coexisting coronary artery disease in patients with peripheral arterial disease. Angiology 57:15–20, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ohman EM, Bhatt DL, Steg PG, Goto S, Hirsch AT, Lian CS, Mas JL, Richard AJ, Rother J, Wilson PW, REACH Registry Investigators: The Reduction of Atherothrombosis for Continued Health (REACH) registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J 151:786.e1–786.e10, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jacobson H: Ischemic renal disease. Kidney Int 34:729–743, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Paisley KE, Beaman M, Tooke JE, Mohamed-Ali V, Lowe GDO, Shore AC: Endothelial dysfunction and inflammation in asymptomatic proteinuria. Kidney Int 63:624–633, 2003 [DOI] [PubMed] [Google Scholar]