Abstract
OBJECTIVE—To determine the contribution of maternal glucose and lipids to intrauterine metabolic environment and fetal growth in pregnancies with gestational diabetes mellitus (GDM).
RESEARCH DESIGN AND METHODS—In 150 pregnancies, serum triglycerides (TGs), cholesterol, free fatty acids (FFAs), glycerol, insulin, and glucose were determined in maternal serum and cord blood during the 3rd trimester. Maternal glucose values came from oral glucose tolerance testing and glucose profiles. Measurements of fetal abdominal circumference (AC) were performed simultaneously with maternal blood sampling and birth weight, and BMI and neonatal fat mass were obtained following delivery.
RESULTS—Maternal TGs and FFAs correlated with fetal AC size (at 28 weeks: triglycerides, P = 0.001; FFAs, P = 0.02), and at delivery they correlated with all neonatal anthropometric measures (FFA: birth weight, P = 0.002; BMI, P = 0.001; fat mass, P = 0.01). After adjustment for confounding variables, maternal FFAs and TGs at delivery remained the only parameters independently related to newborns large for gestational age (LGA) (P = 0.008 and P = 0.04, respectively). Maternal FFA levels were higher in mothers with LGA newborns than in those with appropriate for gestational age (AGA) newborns (362.8 ± 101.7 vs. 252.4 ± 10.1, P = 0.002). Maternal levels of TGs, FFAs, and glycerol at delivery correlated with those in cord blood (P = 0.003, P = 0.004, and P = 0.005, respectively). Fetal triglyceride and cholesterol levels were negatively correlated with newborn birth weight (P = 0.001), BMI (P = 0.004), and fat mass (P = 0.001). TGs were significantly higher in small for gestational age (SGA) newborns compared with AGA or LGA newborns, while insulin-to-glucose ratio and FFAs were the highest in LGA newborns.
CONCLUSIONS—In well-controlled GDM pregnancies, maternal lipids are strong predictors for fetal lipids and fetal growth. Infants with abnormal growth seem to be exposed to a distinct intrauterine environment compared with those with appropriate growth.
There is strong evidential support for the “fetal origins” hypothesis, which connects adulthood hypertension, insulin resistance, and dyslipidemia to adverse intrauterine conditions during gestation that might be associated with disproportionate fetal growth. An increased risk for adult metabolic disorders is well documented for subjects born growth retarded (1). In addition, there are substantial data indicating that accelerated fetal growth predisposes to later obesity (2), especially in diabetic pregnancies (3). Therefore, normalization of fetal growth is a principle in the management of pregnancies with diabetes. Therapeutic strategies that focus on tight glucose control have often limited success in avoiding accelerated growth and may even result in growth restriction. In overweight pregnant women, fetal growth seems to be determined only to a small extent by maternal glucose values (4), and normalization of fetal growth may only be achieved by the addition of insulin therapy despite apparently good glucose control with diet (5).
In view of increasing evidence that obesity, diabetes and cardiovascular diseases in later life may have prenatal antecedents, investigation of the determinants of intrauterine environment and fetal growth have become an important area of research. Variation of birth weight is strongly determined by neonatal fat mass, and it is likely that fetal growth disorders might also result from variations in maternal and fetal lipid metabolism. In nondiabetic pregnancies, maternal triglycerides (TGs) have been shown to be correlated with birth weight (6–9).
Our study aimed to determine the potential relationship of maternal serum glucose and lipids to intrauterine metabolic environment and fetal growth during late pregnancy in well-controlled gestational diabetic women. Therefore, we investigated the correlation of maternal serum lipid and glucose parameters, measured at different time points in the 3rd trimester of pregnancy, with fetal and neonatal anthropometric parameters and with the correspondent parameters in cord blood representing the current intrauterine metabolic environment of newborns that are small (SGA), appropriate (AGA), and large for gestational age (LGA) from pregnancies complicated by gestational diabetes mellitus (GDM).
RESEARCH DESIGN AND METHODS
The study population was derived from a prior intervention study that compared different management strategies in GDM (10). All women had GDM diagnosed based on a 75-g oral glucose tolerance test (OGTT) with determination of three glucose values in capillary blood using the hexokinase method; at least two values had to exceed the Carpenter & Coustan criteria, which are endorsed by the American Diabetes Association (95/180/155 mg/dl) for measurements in venous plasma. With respect to lower glucose concentrations in capillary compared with venous blood, the threshold for fasting glucose was modified into 90 mg/dl, while postchallenge capillary glucose levels correspond with those in venous blood (11). The women were given dietary instruction and performed self-monitoring of blood glucose. Additional insulin therapy was given either based principally on maternal glucose levels or on fetal growth, as described previously (10). Data regarding maternal and neonatal characteristics, maternal glucose values from the diagnostic OGTT, and mean fasting and postprandial values from glucose profiles measured twice weekly were extracted from the database of the primary study.
Maternal blood samples were scheduled to be taken at entry to the study (∼28 weeks of gestation) and at 32, 36, and 39 weeks. Serum samples were frozen and stored at −80°C until analysis. Cord blood samples were taken immediately following delivery, and serum was stored at −80°C. Insulin was determined by ELISA (Pharmacia, Uppsala, Sweden); TGs, free fatty acids (FFAs), and cholesterol were measured by commercial kits (Menarini Diagnostic, Florence, Italy, for TGs and cholesterol; and Wako Chemical, Neuss, Germany, for FFAs). Glycerol was determined using a fluorometric method.
Fetal growth was measured by ultrasound performed at the same time as the maternal blood sampling. For analysis, we focused on abdominal circumference (AC), since the growth differences of the AC are mainly determined by the thickness of insulin-sensitive subcutaneous fat. Birth weight and length were obtained shortly after delivery, and neonatal skinfold thickness at the flank was measured within 48 h. Neonatal fat mass was calculated by a formula derived from Catalano et al. (12) that includes birth weight and length and flank skinfolds measurement: 0.30055 (birth weight) + 0.0453 (flank skinfold) –0.03237 (length) + 0.54657. Correlation with fat mass values obtained by total body electric conductivity, which is considered the gold standard, has been reported to be very high (R2 = 0.78, P = 0.001) (12). Infants with birth weight <10th percentile were classified as SGA, and those with birth weight >90th percentile as LGA based on gestational age and sex-adjusted birth weight percentiles derived from a German national database (13).
Data management and analysis was performed using SPSS 12.0 (Chicago, IL). Results are expressed as means ± SD. Relationships between variables were analyzed by bivariate correlation applying Spearman's correlation test. Differences between groups were analyzed using ANOVA with Bonferroni adjustment. Multiple logistic regression analysis was performed to determine maternal parameters independently associated with birth weight. All statistical tests were two-tailed, and a P value <0.05 was considered significant.
RESULTS
From the original study population (10), 150 mother-newborn pairs were selected based on availability of complete maternal blood and cord blood samples. Maternal and neonatal characteristics are shown in Table 1, and metabolic parameters measured in maternal blood taken close to delivery (between 36 and 39 weeks of gestation, in most cases 1 week to delivery) and in cord blood are shown in Table 2. The remaining women from the primary study who could not be included in the presented study due to missing cord blood samples were not significantly different from the women included regarding maternal or newborn characteristics.
Table 1.
Maternal and neonatal characteristics of pregnancies with GDM
| Maternal characteristics | |
| Age (years) | 31.2 ± 4.9 |
| Parity | 2.05 ± 1.2 |
| Prepregnancy BMI (kg/m2) | 27.8 ± 6.2 |
| Gestational age at diagnosis (weeks) | 25.9 ± 4.4 |
| Gestational age at entry (weeks) | 28.3 ± 2.4 |
| OGTT fasting (mg/dl) | 94.3 ± 13.9 |
| 1 h | 202.3 ± 26.9 |
| 2 h | 158.3 ± 31.8 |
| Glucose profiles at entry (mg/dl) | |
| Mean fasting glucose | 87.9 ± 10.9 |
| Mean postprandial glucose | 113.5 ± 15.3 |
| Glucose profiles close to delivery (mg/dl) | |
| Mean fasting glucose | 83.5 ± 10.7 |
| Mean postprandial glucose | 109.2 ± 13.7 |
| Neonatal characteristics | |
| Gestational age at delivery (weeks) | 39.2 ± 1.4 |
| Birth weight (g) | 3,389.81 ± 503 |
| BMI (kg/m2) | 13.0 ± 1.3 |
| Fat mass (g) | 432.1 ± 162.6 |
| LGA (%) | 13.1 |
| SGA (%) | 12.9 |
| C-section rate (%) | 11.7 |
Data are means ± SD.
Table 2.
Metabolic parameters in maternal serum close to delivery and in cord blood of all the offspring and according to categories of SGA, AGA, and LGA newborns from mothers with GDM
| Mother | Offspring
|
||||
|---|---|---|---|---|---|
| Total | SGA | AGA | LGA | ||
| n | 150 | 20 | 111 | 19 | |
| Glucose (mg/dl) | 84.2 ± 18.3 | 85.0 ± 21.4 | 82.6 ± 28.6a | 86.0 ± 21.0a | 80.3 ± 13.7a |
| Insulin (μU/ml) | 25.2 ± 21.7* | 10.2 ± 6.25 | 8.1 ± 10.0a | 8.6 ± 5.3a | 20.8 ± 6.9b |
| Insulin-to-glucose ratio | 0.29 ± 0.21† | 0.07 ± 0.002 | 0.06 ± 0.01a | 0.03 ± 0.005a | 0.26 ± 0.08b |
| TGs (mg/dl) | 265.9 ± 87.6† | 41.6 ± 21.8 | 62.1 ± 27.5a | 40.3 ± 22.6b | 32.0 ± 17.5b |
| Cholesterol (mg/dl) | 253.7 ± 55.6† | 63.5 ± 17.7 | 64.5 ± 14.4a | 63.3 ± 18.7a | 63.5 ± 12.4a |
| Glycerol (μmol/l) | 202.4 ± 100.3† | 76.1 ± 64.2 | 107.4 ± 123a | 70.5 ± 54.0a | 101.5 ± 83.2a |
| FFAs (μmol/l) | 262.5 ± 112.4† | 146.3 ± 88.2 | 189.0 ± 78.0a,b | 135.2 ± 75.8a | 213.0 ± 149.8b |
Data are means ± SE. For the difference between maternal serum and cord blood (total offspring):
P < 0.05 and
P < 0.001. For the difference between SGA, AGA, and LGA newborns: different superscript letters indicate P < 0.05.
Except for glucose, the concentration of all parameters determined in the mothers’ serum was significantly higher than in the correspondent cord blood serum. When all the individual paired samples were compared, only maternal levels of TGs, FFAs (Fig. 1), and glycerol taken close to delivery correlated significantly with those in cord blood (r = 0.19, P = 0.003; r = 0.28, P = 0.004; r = 0.26, P = 0.005, respectively). The relationship between the different maternal metabolic parameters during the 3rd trimester and fetal growth was evaluated by their correlation with the fetal AC measured at entry to the study (28.3 ± 2.4 weeks of gestation) and at 32 and 36 weeks. Both maternal TGs and FFAs correlated positively and significantly with fetal AC size at each of the studied time points (entry: TGs, r = 0.26, P = 0.001; FFAs, see Fig. 1). However, there were no significant correlations between fetal growth and any maternal glucose values either from the OGTT or from the self-monitoring glucose profiles performed at entry and at 32 and 36 weeks (data not shown). Maternal parameters from blood samples taken close to delivery were compared with neonatal anthropometric measures. Maternal plasma FFA and glycerol levels were significantly related to all anthropometric measures of newborns (glycerol vs. birth weight: r = 0.23, P = 0.3, vs. BMI: r = 0.24, P = 0.006, and vs. fat mass: r = 0.23, P = 0.01; for FFAs vs. birth weight: r = 0.27, P = 0.002, vs. BMI: r = 0.3, P = 0.001, and vs. fat mass: see Fig. 1). Maternal TGs had a positive correlation with newborn fat mass (r = 0.17, P = 0.03) but not with birth weight or neonatal BMI. After adjustment for maternal prepregnancy BMI, weight gain, age, parity, fasting, and postprandial glucose from the profiles at 36 weeks and close to delivery, only maternal FFAs and TGs remained independently related to LGA (adjusted P = 0.008 and P = 0.04, respectively). Maternal FFA levels were significantly higher in mothers with LGA infants than in mothers with AGA infants (362.8 ± 101.7 vs. 252.4 ± 10.1 μmol/l, P = 0.002).
Figure 1.
Correlation of maternal serum FFA with fetal growth and fetal FFA. A: Maternal FFA at study entry, ∼28 weeks of gestation, and fetal abdominal circumference at study entry. B: Maternal FFA close to delivery and neonatal fat mass. C: Maternal FFA close to delivery and fetal FFA measured in cord blood serum.
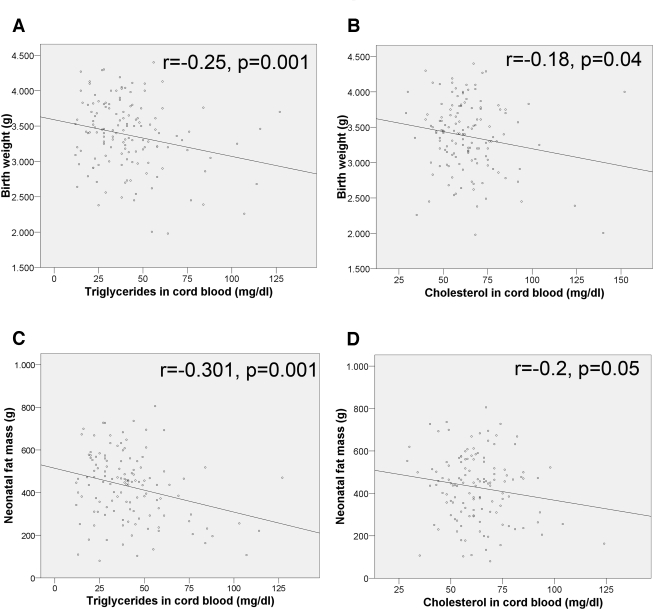
On the neonate side, cord TG and cholesterol levels correlated negatively and significantly with birth weight (Fig. 2), BMI (r = −0.24, P = 0.004), and fat mass (Fig. 2), whereas insulin and the insulin-to-glucose ratio correlated positively and significantly with birth weight (r = 0.23, P = 0.006, and r = 0.19, P = 0.03, respectively), BMI (r = 0.24, P = 0.005, and r = 0.21, P = 0.12), and fat mass (r = 0.21, P = 0.02; and r = 0.17, P = 0.48). Cord blood glucose, glycerol, and FFAs did not show any significant correlation with neonatal size. Cord blood TG levels of SGA newborns were significantly increased compared with those of AGA or LGA newborns (Table 2), whereas insulin, insulin-to-glucose ratio, and FFAs were the highest in LGA infants.
Figure 2.
Correlation of cord blood serum TGs and cholesterol with birth weight and neonatal fat mass.
CONCLUSIONS
The present findings demonstrate that circulating maternal lipids, but not glucose, correlate with fetal growth at different time points during the 3rd trimester in a population of well-controlled GDM pregnancies. Maternal FFAs and TG levels measured close to delivery predicted LGA birth weight independently of maternal BMI, and maternal FFA, TG, and glycerol concentrations correlated with those measured in cord blood serum. Cord blood of SGA newborns showed high TG levels, whereas LGA newborns showed high FFA concentrations, high insulin-to-glucose ratios, and low TG levels.
Both maternal TG and FFA levels correlated with fetal growth during pregnancy and with neonatal anthropometric measures, the best correlation found with neonatal fat mass. Maternal hypertriglyceridemia is a characteristic feature of pregnancy (14), and although maternal circulating TGs do not directly cross the placenta (15), the presence of lipoprotein receptors, fatty acid–binding proteins (16), and different lipase activities (17) in the placenta allows the efficient transfer of maternal fatty acids to the fetus. It had been previously shown that the concentration of TGs in the 3rd trimester is a stronger predictor of birth weight than glucose parameters (6–8). Published data have been limited to a one–time point measurement in conjunction with OGTT, whereas this study considers lipid and glucose data at different times during the 3rd trimester and close to delivery (∼1 week to delivery). Due to the strict primary protocol of the investigated study cohort, our study population presented a tight and stable glucose control, which may have attenuated the impact of variations in maternal glycemia on fetal growth. However, even at study entry, glucose values were not related to fetal growth, and from clinical experience we know that good glucose control does not necessarily prevent accelerated growth, especially in obese women. The success of additional insulin therapy to lower the macrosomia rate in obese women with normal glucose values, as demonstrated by Langer et al. (5), may be in part the result of the antilipolytic effect of insulin. This action would decrease maternal FFA and TG levels, reducing their potential effect on fetal fat mass. This may explain the normalization of macrosomia rates observed in studies that applied a fetal growth–based approach of individualized GDM therapy. Insulin therapy was given to women with accelerated fetal growth, as indicated by fetal AC >75th percentile despite maternal glucose being maintained within the target range (10,18). Our findings are in accordance with those of Kitijama et al. and Knopp et al. (6,7), who showed that high maternal TG levels predicted macrosomia independently of maternal BMI.
The potential importance of maternal lipids for the development of the fetus is underlined by the fact that both cord blood FFA and TG levels seemed to be tightly associated with maternal lipids. There is a paucity of studies published thus far comparing maternal and fetal lipid parameters. In contrast to our data obtained in a well-controlled GDM population, Merzouk et al. (19) reported that maternal triglyceride levels in late gestation had been a significant predictor of elevated fetal lipids only in a group of poorly controlled diabetic mothers, not in well-controlled type 1 diabetic mothers. In another study, the same authors reported corresponding lipoprotein abnormalities (elevated TGs, cholesterol, and apolipoprotein B100) only in obese mothers and their macrosomic infants (20).
The most striking finding from this study was that fetal TGs were negatively correlated with birth weight, resulting in significantly higher TG levels in SGA newborns compared with AGA or LGA infants. There are previous studies demonstrating elevated TG levels in cord blood of growth retarded newborns (21,22). A theoretical explanation for the inverse correlation between fetal TGs and birth weight might be reduced lipoprotein lipase (LPL) activity. LPL present in the capillary endothelium of extrahepatic tissues catalyzes the hydrolysis of TG-rich lipoproteins (namely chylomicrons and VLDL) and their products, and FFAs and glycerol are taken up by the adjacent tissue. Because it is known that the expression of LPL is strongly associated with the development of adipose tissue, we hypothesize that SGA newborns might have a low LPL activity responsible for their augmented circulating TG levels. By contrast, low TG levels found in LGA newborns would be the result of enhanced LPL activity derived from their increased adipose mass. Interestingly, in the pediatric literature there are also reports of high TG levels in the serum of SGA newborns taken a few days after delivery (23,24), especially in infants with a Ponderal index <10th percentile (24). This fits with our observations and suggests that SGA infants appear to have impaired utilization of circulating TGs, as a consequence of lacking peripheral adipose.
In relation to the question of whether infants with abnormalities in growth are exposed to a different intrauterine environment than those with appropriate growth, our data demonstrated that both newborns with impaired or accelerated growth show metabolic abnormalities but of different character. SGA newborns had been exposed to high levels of TGs, whereas LGA infants already showed intrauterine signs of insulin resistance indicated by the high insulin-to-glucose ratio. This could be responsible for the elevated FFA levels found in the LGA infants, which might reflect a reduced effect of insulin inhibiting lipolysis or augmented lipolytic activity due to their increased adipose tissue mass. How these metabolic patterns contribute to the known disposition of SGA and LGA newborns for obesity, diabetes, and cardiovascular disorders in later life remains to be examined further. From animal models, we know that induced hyperinsulinism in the area of the hypothalamus region during the perinatal phase leads to later obesity. Little is known about the long-term effects of intrauterine hypertriglyceridemia. We do know that blood lipoprotein abnormalities in childhood are predictive for those in adulthood (25), and it is likely that this is also true extremely early in life, a very sensitive time. In summary, these findings show the effect of a disturbed maternal lipid metabolism on the fetal metabolic environmental and fetal growth that may have long-term consequences.
Published ahead of print at http://care.diabetesjournals.org on 7 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Eriksson J, Forsen T, Tuomilehto J, Jaddoe V, Osmond C, Barker D: Effect of size at birth and childhood growth on the insulin resistance syndrome in elderly individuals. Diabetologia 45:342–348, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Harder T, Rodekamp E, Schellong K, Dudenhausen J, Plagemann A: Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol 165:849–857, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Schaefer-Graf UM, Pawliczek J, Passow D, Hartmann R, Rossi R, Bührer C, Harder T, Plagemann A, Vetter K, Kordonouri O: Birth weight and parental BMI predict overweight in children from mothers with gestational diabetes. Diabetes Care 28:1745–1750, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Schaefer-Graf UM, Kjos S, Kilavuz Ö, Plagemann A, Brauer M, Dudenhausen J, Vetter K: Determinants of fetal growth at different periods of pregnancies complicated by gestational diabetes or impaired glucose tolerance. Diabetes Care 26:193–198, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Langer O, Yogev Y, Xenakis E, Brustman L: Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol 192:1768–1776, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kitajima M, Oka S, Yasuhi IFM, Rii Y, Ishimaru T: Maternal serum triglyceride at 24–32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol 97:776–780, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Knopp RH, Magee MS, Walden CE, Bonet B, Benedetti TJ: Prediction of infant birth weight by GDM screening: importance of triglyceride. Diabetes Care 15:1605–1613, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Nolan C, Riley S, Sheedy M, Walstab J, Beischer N: Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care 18:1550–1556, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Di Cianni G, Miccoli R, Volpe L, Lencioni C, Ghio A, Giovannitti M, Cuccuru I, Pellegrini G, Chatzianagnostou K, Boldrini A, Del Prato S: Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 22:21–25, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Schaefer-Graf U, Kjos S, Fauzan O, Bühling K, Siebert G, Bührer C, Ladendorf B, Dudenhausen J, Vetter K: A randomized trial evaluating a predominately fetal growth-based strategy to guide management of gestational diabetes in Caucasian women. Diabetes Care 27:297–302, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Roggenbruck L, Kleinwechter HJ, Demandt N, Dörner KM: Diagnostics of gestational diabetes: which cutoff-values are valid for capillary whole blood. Clin Lab 50:403–408, 2004 [PubMed] [Google Scholar]
- 12.Catalano P, Thomas A, Avallone D, Amini S: Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol 173:1176–1181, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Voigt M, Schneider K, Jährig K: [Analysis of all births of the year 1992 in the Federal Republic of Germany] Geburth u Frauenheilk 56:550–558, 1996. [article in German] [Google Scholar]
- 14.Montelongo A, Lasunción M, Pallardo L, Herrera E: Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 1651–1659, 1992 [DOI] [PubMed]
- 15.Herrera E, Lasunción M: Maternal lipid metabolism and placental lipid transfer. Horm Res 65:59–64, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Cummings S, Hatley W, Simpson E: The binding of high and low density lipoproteins to human placental membrane fractions. J Clin Endocrinol Metab 54:903–908, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Bonet B, Brunzell J, Gown A, Knopp R: Metabolism of very-low-density lipoprotein triglyceride by human placental: the role of lipoprotein lipase. Metabolism 41:596–603, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Kjos S, Schaefer-Graf UM: Modified therapy for gestational diabetes using high-risk and low-risk fetal abdominal circumference growth to select strict versus relaxed maternal glycemic target. Diabetes Care 30 (Suppl. 2): S200–S205, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Merzouk H, Madani S, Korso N, Bouchenak M, Prost J, Belleville J: Maternal and fetal serum lipids and lipoprotein concentrations and compositions in type 1 diabetic pregnancy: relationship with maternal glycemic control. J Lab Clin Med 136:441–448, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Merzouk H, Meghelli-Bouchenak M, Prost J, Belleville J: Impaired serum lipids and lipoproteins in fetal macrosomia related to maternal obesity. Biol Neonate 77:17–24, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Rodie V, Caslake M, Stewart F, Sattar N, Ramsay J, Greer I, Freeman D: Fetal cord plasma lipoprotein status in complicated human pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Atherosclerosis 176:181–187, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Merzouk H, Meghelli-Bouchenak M, El-Korso N, Belleville J, Prost J: Low birth weight at term impairs cord serum lipoprotein compositions and concentrations. Eur J Pediatr 157:321–326, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Cui Y, Tong X, Hongmao Y, Song L: Glucose and lipid metabolism in small-for-gestational age infants at 72 hours of age. J Clin Endocrinol Metab 92:681–684, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Jones J, Gercel-Taylor C, Taylor D: Altered cord serum lipid levels associated with small for gestational age infants. Obstet Gynecol 93:527–531, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Bao W, Srinivasan S, Wattigney W, Bao W, Berenson G: Usefulness of childhood low-density lipoprotein cholesterol level in predicting adult dyslipidemia and other cardiovascular risks: the Bogalusa Heart Study. Arch Intern Med 24:1315–1320, 1996 [PubMed] [Google Scholar]