Abstract
OBJECTIVE—We investigated how β-cell function and insulin sensitivity or resistance are affected by the type of blood sample collected or choice of insulin assay and homeostatis model assessment (HOMA) calculator (http://www.dtu.ox.ac.uk).
RESEARCH DESIGN AND METHODS—Insulin was measured using 11 different assays in serum and 1 assay in heparinized plasma. Fasting subjects with normoglycemia (n = 12), pre-diabetes, i.e., impaired fasting glucose or impaired glucose tolerance (n = 18), or type 2 diabetes (n = 67) were recruited. Patients treated with insulin or those who were insulin antibody–positive were excluded. HOMA estimates were calculated using specific insulin (SI) or radioimmunoassay (RIA) calculators (version 2.2).
RESULTS—All glucose values were within model (HOMA) limits but not all insulin results, as 4.3% were <20 pmol/l and 1% were >300 pmol/l. β-Cell function derived from different insulin assays ranged from 67 to 122% (median) for those with normoglycemia (P = 0.026), from 89 to 138% for those with pre-diabetes (P = 0.990), and from 50 to 81% for those with type 2 diabetes (P < 0.0001). Furthermore, insulin resistance ranged from 0.8 to 2.0 (P = 0.0007), from 1.9 to 3.2 (P = 0.842), and from 1.5 to 2.9 (P < 0.0001), respectively. This twofold variation in HOMA estimates from the various insulin assays studied in serum may be significant metabolically. Insulin was 15% lower in heparinized plasma (used in the original HOMA study) compared with serum, which is now more commonly used. β-Cell function differed by 11% and insulin resistance by 15% when estimates derived from specific insulin were calculated using the RIA rather than the SI calculator.
CONCLUSIONS—To enable comparison of HOMA estimates among individuals and different research studies, preanalytical factors and calculator selection should be standardized with insulin assays traceable to an insulin reference method procedure.
Homeostasis model assessment (HOMA) is widely used to calculate insulin resistance (HOMA-IR), and β-cell function (HOMA-β), from concomitant glucose and insulin (or C-peptide) in fasting subjects (1,2). There is growing recognition that interpretation of HOMA estimates (3) may not be appropriate in some circumstances because insufficient information is available on effects of preanalytical factors, insulin assays, or version of HOMA calculator selected. These parameters are defined within studies and are therefore stable but may differ between studies, making application of HOMA to individuals and comparison of research studies problematic.
The current HOMA specific insulin (SI) and radioimmunoassay (RIA) calculators (version 2.2) evolved from computer models that replaced equations derived from the original computer model published in 1985 (Table 1). The HOMA RIA calculator was originally developed for immunoassays not specific for insulin, whereas the HOMA SI calculator is recommended for use with assays specific for insulin, although no limits on any cross-reactivity with proinsulin(s) are specified.
Table 1.
Evolution of HOMA
| Year | Versions of HOMA | Information on insulin assay |
|---|---|---|
| 1985 | HOMA: initial publication comprising equations approximating to original computer model for estimation of HOMA-β and HOMA-S | For use in heparinized plasma with a competitive, insulin RIA not specific for insulin (mU/l) |
| 1998 | Update of HOMA: computer programs for HOMA-β and HOMA-S that account for variations in hepatic and peripheral glucose resistance, i.e., reduction in suppression of hepatic glucose output by hyperglycemia and also peripheral glucose-stimulated glucose uptake. Insulin secretion curve modified to allow for increases in response to plasma glucose >10 mmol/l. Model incorporates estimate of proinsulin secretion for use with RIA and SI assays and renal glucose losses for hyperglycemic subjects | For use with a competitive, immunoassay for “immunoreactive” insulin and specific insulin (SI) assay (mU/l) or C-peptide in heparinized plasma |
| 2004 | HOMA calculator version 2.1: released on 6 January 2004 | |
| 2004 | RIA and SI HOMA calculators version 2.2: released on 30 June 2004 for estimation of HOMA-IR in addition to HOMA-β and HOMA-S | RIA calculator for use in heparinized plasma with RIA/competitive immunoassays that cross-react with proinsulin(s) and SI calculator with SI assays (mU/l or pmol/l) |
| Future | HOMA-calculators: for estimation of all three HOMA variables | For use with human insulin assays (pmol/l) with defined specificity, traceable to an RMP and international standard, validated in serum and plasma |
A twofold difference in results from insulin assays was reported in 1996 (4,5) and confirmed recently in the U.K. and U.S. with newer insulin assays (6,7). The U.K. study found that insulin values from one specific insulin assay were 15% lower in heparinized plasma than in serum. Heparinized plasma was collected for the HOMA database (1) and is frequently used for research (3); however, serum is more commonly used in practice for insulin measurement. There is no advantage in the use of C-peptide rather than insulin measurements for HOMA, as results from different C-peptide assays vary and C-peptide is not stable when stored (8). In this study, HOMA estimates from 97 fasting subjects with normoglycemia, pre-diabetes, or type 2 diabetes were compared using 11 insulin assays to establish the extent to which the type of blood sample collected, insulin assay selected, or version of HOMA calculator used affect HOMA estimates.
RESEARCH DESIGN AND METHODS
Ethics approval was obtained from the South East Wales Local Research Ethics Committee, and the study complied with the current revision of the Declaration of Helsinki. All patients gave written informed consent. A comparison of measurements from different insulin assays in 150 samples from fasting (n = 99) and nonfasting subjects to reflect the range for insulin in patients with diabetes has been published previously (6).
Fasting subjects (n = 99), not stratified by age or sex, were recruited over 12-weeks with two excluded as their glucose results at the time of the study did not match designated glycemic status. Participants with type 2 diabetes were either being seen for a routine clinic appointment or diagnostic oral glucose tolerance test. Those with pre-diabetes or normoglycemia were identified from patient records or with the oral glucose tolerance test.
Blood samples were collected from fasting subjects, aged 18–75 years, either nondiabetic (n = 12; 12%), with pre-diabetes, i.e., impaired fasting glucose or impaired glucose tolerance (n = 18; 19%), or with type 2 diabetes (n = 67; 69%) defined by the 1999 World Health Organization criteria (9). Patients requiring insulin treatment or known to be insulin antibody–positive were excluded. Anonymized demographic data were collected.
Collection of blood and laboratory measurements
Blood samples were collected at the Diabetes Research Unit, Cardiff University, Cardiff, U.K. (6). Plasma glucose was measured in fluoride oxalate samples within 10 min of sampling using a Yellow Springs analyzer (YSI2300; Yellow Springs Instruments, Aldershot, U.K.). Serum or heparinized plasma samples were initially stored at −20°C for 1–2 days to ensure rapid freezing and then transferred to −70°C, procedures routine for this laboratory and known not to affect insulin results. Samples were dispatched on dry ice to laboratories in the U.K. and U.S. No hemolyzed or lipemic samples were collected. Samples were stored on receipt at −20°C (or −70°C if available).
Insulin was measured in serum by 11 different insulin assays (6), shown in Table 2, over 5 days or runs and in heparinized plasma by one assay only. The insulin assays are itemized in the order followed in the tables and figure with mean coefficients of variation (trilevel quality controls analyzed on five occasions) (6) as follows: for serum, 1) Abbott (AxSYM Insulin), 3.8%; 2) Bayer (ADVIA Centaur Insulin), 5.6%; 3) Biosource-Invitrogen (Insulin EASIA), 5.6%; 4) DakoCytomation (Insulin assay), 6.7%; 5) DPC (Immulite 1000), 4.7%; 6) Linco (U.S.) (ELISA), 6.4%; 7) Linco (U.S.) (RIA), 4.5%; 8) Mercodia (Iso-Insulin assay [not specific for insulin]), 3.9%; 9) MLT immunochemiluminometric assay), 5.1%; 10) Roche (Insulin E170), 1.4%; 11) Tosoh (Tosoh ST AIA-Pack IRI), 5.5%; and 12) Tosoh (U.S.) (Tosoh ST AIA-Pack IRI), 2.5%; and for plasma, 13) Biosource-Invitrogen (Insulin EASIA). Only the Mercodia Iso-Insulin 10-1128-01 assay was quoted by the manufacturer to have significant cross-reactivity with intact proinsulin (54%).
Table 2.
HOMA estimates by insulin assay and glycemic status
| Insulin assay | Normoglycemia
|
Pre-diabetes
|
Type 2 diabetes
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| HOMA-β (%)* | HOMA-S(%)† | HOMA-IR‡ | HOMA-β(%)* | HOMA-S(%)† | HOMA-IR‡ | HOMA-β(%)* | HOMA-S(%)† | HOMA-IR‡ | |
| n | 12 | 12 | 12 | 18 | 18 | 18 | 67 | 67 | 67 |
| 1 | 104.1 (67.3–156.8) | 62.8 (46.3–112.9) | 1.59 (0.89–2.16) | 119.7 (77.0–164.1) | 37.9 (25.7–69.9) | 2.68 (1.43–3.89) | 70.3 (40.9–94.2) | 41.4 (25.7–75.7) | 2.42 (1.32–3.89) |
| 2 | 86.7 (60.4–136.5) | 78.2 (58.0–131.9) | 1.28 (0.76–1.83) | 131.1 (72.1–157.3) | 31.3 (27.3–84.5) | 3.20 (1.18–3.66) | 66.7 (36.5–100.3) | 41.5 (25.4–90.9) | 2.41 (1.10–3.94) |
| 3 | 122.4 (104.0–177.9) | 49.8 (41.7–62.6) | 2.01 (1.61–2.40) | 131.7 (97.7–158.7) | 33.5 (26.2–52.0) | 3.00 (1.92–3.82) | 80.5 (53.4–111.4) | 34.4 (23.8–50.4) | 2.91 (1.98–4.20) |
| 4 | 79.6 (56.3–118.0) | 92.1 (65.2–155.2) | 1.09 (0.65–1.54) | 99.0 (63.0–127.1) | 48.2 (36.4–94.2) | 2.11 (1.06–2.75) | 52.5 (35.3–76.7) | 57.3 (35.2–93.0) | 1.75 (1.08–2.84) |
| 5 | 80.7 (60.5–139.0) | 84.5 (58.9–134.4) | 1.19 (0.75–1.70) | 110.9 (65.6–140.0) | 39.4 (30.7–98.5) | 2.56 (1.02–3.26) | 60.9 (36.3–88.7) | 49.0 (31.9–78.4) | 2.04 (1.28–3.13) |
| 6 | 67.4 (54.2–96.2) | 121.9 (93.3–164.8) | 0.82 (0.61–1.08) | 89.4 (58.5–116.3) | 52.0 (42.5–103.2) | 1.93 (0.97–2.35) | 49.5 (30.4–69.1) | 64.1 (42.4–114.7) | 1.56 (0.87–2.36) |
| 7 | 121.9 (96.7–169.9) | 54.9 (42.9–65.1) | 1.83 (1.54–2.38) | 137.6 (98.4–178.5) | 32.5 (23.0–59.1) | 3.08 (1.69–4.35) | 80.2 (55.5–107.8) | 34.5 (23.0–53.0) | 2.90 (1.89–4.35) |
| 8‡ | 72.4 (51.1–105.9) | 105.3 (80.3–183.5) | 0.95 (0.55–1.27) | 98.9 (62.1–131.8) | 49.1 (37.8–110.6) | 2.04 (0.90–2.65) | 57.1 (31.8–86.9) | 53.0 (29.3–109.6) | 1.89 (0.91–3.41) |
| 9 | 81.1 (55.2–124.7) | 96.2 (68.9–162.5) | 1.04 (0.62–1.48) | 100.8 (66.5–123.8) | 53.0 (37.7–91.2) | 1.89 (1.10–2.65) | 56.8 (33.1–79.2) | 61.0 (32.9–103.9) | 1.64 (0.96–3.04) |
| 10 | 81.9 (62.0–127.1) | 82.7 (64.3–126.9) | 1.21 (0.79–1.61) | 116.2 (68.8–157.9) | 36.1 (28.1–82.0) | 2.77 (1.22–3.56) | 63.9 (36.9–97.0) | 46.0 (26.6–77.8) | 2.17 (1.29–3.76) |
| 11 | 75.7 (59.8–114.6) | 96.2 (75.6–133.7) | 1.04 (0.75–1.36) | 101.8 (61.1–131.6) | 44.2 (34.7–104.2) | 2.27 (0.96–2.88) | 55.8 (31.0–78.1) | 55.9 (32.4–111.4) | 1.79 (0.90–3.09) |
| 12 | 73.1 (50.2–104.9) | 100.0 (83.2–197.9) | 1.00 (0.51–1.22) | 93.5 (52.1–138.3) | 46.4 (37.5–122.0) | 2.16 (0.82–2.67) | 51.0 (30.2–79.6) | 64.6 (34.9–132.9) | 1.55 (0.75–2.87) |
| 13‖ | 116.4 (97.2–168.2) | 52.7 (40.8–70.1) | 1.90 (1.43–2.45) | 124.7 (102.1–150.4) | 33.7 (29.8–54.6) | 2.97 (1.83–3.36) | 70.2 (51.2–95.4) | 39.8 (28.5–55.2) | 2.51 (1.81–3.51) |
Data are medians(IQR). P values for serum only. Normoglycemia: *P = 0.0262, †P = 0.0004, ‡P = 0.0007; pre-diabetes: *P = 0.0990, †P = 0.7525, ‡P = 0.8421; type 2 diabetes: *P < 0.0001, †P = < 0.00001, ‡P < 0.0001. ‡RIA calculator used; otherwise SI calculator. ‖Plasma; otherwise serum.
Statistical analysis
Data were analyzed using SAS (10) with SI and RIA calculators (version 2.2, 30 June 2004; http://www.dtu.ox.ac.uk) for HOMA estimates. HOMA version 2 was recalibrated to give HOMA-β and insulin sensitivity (HOMA-S) of 100% in normal young adults when assays available then were used for insulin, specific insulin, or C-peptide. In this study, the SI calculator was used for 10 assays quoted as specific for insulin and the RIA calculator was used for one assay stated to cross-react with proinsulin. As HOMA is based on steady-state physiology, the model includes limits for glucose. Values outside these limits, 3–25 mmol/l for both calculators (30 June 2004; http://www.dtu.ox.ac.uk), are considered non–steady state and HOMA is considered to be inappropriate. Input limits for insulin for the SI calculator are 20–300 pmol/l and for RIA calculator are 20–400 pmol/l. For Table 2, insulin values outside these ranges were set to the values of the appropriate limit. Corresponding results for HOMA estimates when these results are excluded are found in supplemental Table 1 (available in an online appendix at http://dx.doi.org/10.2337/dc08-0097). Median score tests, i.e., median one-way nonparametric ANOVA, were used in Table 2 to test the significance of differences in a HOMA estimate derived from different insulin assays in serum for a particular glycemic state.
RESULTS
Normoglycemic participants (n = 12) were aged 45.8 ± 14.6 (mean ± SD) years but were older if they were pre-diabetic (n = 18; 61.8 ± 10.5 years) or had type 2 diabetes (n = 67; 59.8 ± 9.5 years; P < 0.0001). Most participants were white Caucasian with one of different ethnic origin in the pre-diabetic group and two in the group with type 2 diabetes. Half of the normoglycemic subjects, one-third of those with pre-diabetes, and 51 of 67 with type 2 diabetes were male. BMI was 27.9 ± 5.2 kg/m2 in the normoglycemic group, was 31.4 ± 4.9 kg/m2 in those with pre-diabetes, and was similar in those with diabetes at 31.8 ± 5.8 kg/m2. The median duration of diabetes was 4.5 (interquartile range [IQR] 0.5–7.0) years, with 25 patients being treated with diet alone and 42 taking oral antihyperglycemic agents.
Fasting plasma glucose was 5.2 ± 0.5 mmol/l, and median serum insulin (measured by 11 insulin assays) was 59 (IQR 38–78) pmol/l in normoglycemic subjects, 6.2 ± 0.4 mmol/l and 117 (54–142) pmol/l for subjects with pre-diabetes, and 8.4 ± 2.2 mmol/l and 86 (46–142) pmol/l for type 2 diabetic subjects. A P value of 0.07 was obtained from a Kruskal-Wallis test for insulin across the groups. HOMA-β was highest in those with pre-diabetes at 109% (71–149), lower in those with normoglycemia at 85% (64–128), and lowest in those with type 2 diabetes at 63% (38–88) (P = 0.0002). HOMA-IR was 1.30 (0.84–1.65) in normoglycemic subjects, highest in subjects with pre-diabetes at 2.58 (1.24–3.12), and slightly lower in diabetic subjects at 2.13 (1.19–3.54) (P = 0.022). Values for HOMA-S were 82% (67–137) for normoglycemic subjects, 40% (33–84) in those with pre-diabetes, and 50% (29–93) in those with diabetes (P = 0.025).
Percentage of patients with data outside input limits for HOMA calculators
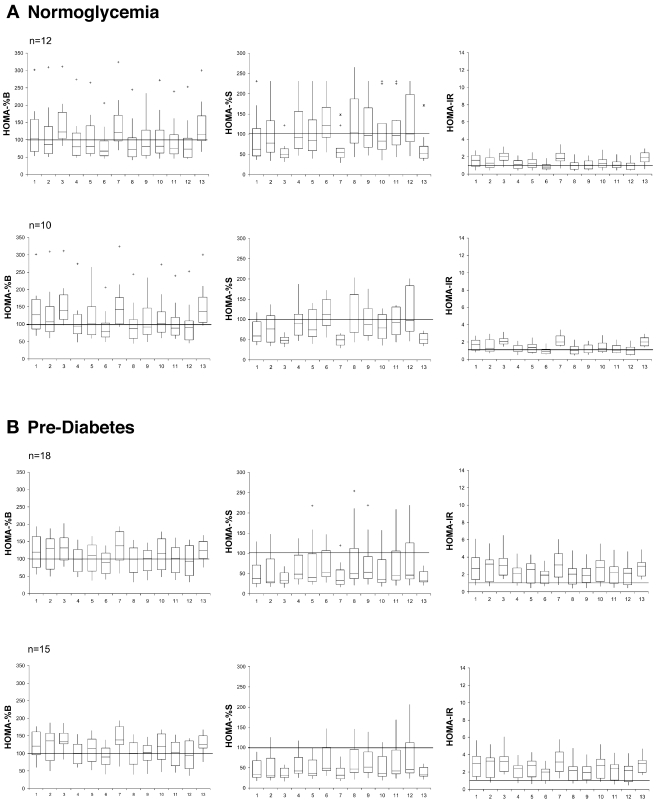
All glucose values obtained were appropriate for the HOMA calculator version 2.2. However, 4.3% of insulin results (55 in total) were <20 pmol/l, and 1% (12 in total) were >300 pmol/l and, therefore, were not accepted. The actual numbers of insulin values outside limits for each assay can be seen in supplemental Table 2 (available in the online appendix). HOMA estimates are presented in Fig. 1; both inclusion of participants with insulin values set to HOMA limits if outside these limits and exclusion if outside limits are shown.
Figure 1.
HOMA estimates by assay (order as in Table 2) in participants with normoglycemia, pre-diabetes, or type 2 diabetes. Plots on the top include all participants and plots directly below include only those with data within HOMA limits from all assays. Box and whisker plots are shown with bottom and top edges of the box located at sample 25th and 75th percentiles, the center horizontal line drawn at the 50th percentile (median), and vertical lines or whiskers drawn from the box to the most extreme point within 1.5 IQRs. More extreme values are marked with + (≥1.5 and <3 IQR) or * (≥3 IQR). The SI calculator was used for all assays except Mercodia.
Choice of HOMA calculator
For illustrative purposes only, the RIA calculator rather than SI calculator was used with assays specific for insulin; estimates of HOMA-β differed by 11% (P < 0.0001) and of HOMA-IR by 15% (P < 0.0001).
HOMA estimates from serum and plasma
The corresponding values for estimates in heparinized plasma versus serum can be seen in Table 2. It is important to note that the insulin assay used in plasma had a high bias compared with the other assays (6), which was reflected in the estimates.
HOMA estimates vary depending on insulin assay
The distribution of HOMA estimates for each insulin assay is presented by glycemic status in Fig. 1 and Table 2. HOMA estimates varied by up to twofold, depending on which insulin assay was used.
CONCLUSIONS
HOMA is widely used as a research tool in epidemiological studies in which invasive techniques for modeling such as intravenous glucose tolerance testing are not practical (3). HOMA is not applicable for individual patient care as insulin results from different laboratories may vary (4). We have compiled a checklist of procedures to follow when HOMA is used (Table 3). In this study, the first five were fulfilled but only one blood sample was collected. For the original model, three blood samples were collected 5 min apart to account for insulin pulsatility, although HOMA estimates have been published subsequently from one sample in large research or epidemiological studies.
Table 3.
Questions to ask before using HOMA version 2.2
| Questions | Answers |
|---|---|
| Do subjects need to be fasting? | Yes. |
| Can patients treated with insulin be included? | No. |
| Should patients take oral hypoglycemic agents on day of HOMA? | Yes, but after blood collection for HOMA. |
| Can insulin antibody–positive patients be included? | No, as insulin antibodies affect insulin measurements. |
| Can hemolyzed samples be used? | No, as insulin is degraded by enzymes released by red blood cells. |
| How should blood be collected? | Three heparinized blood samples 5 min apart for HOMA database, but single samples are now taken in some circumstances. HOMA estimates from serum and heparinized plasma differ and cannot be compared directly. |
| Can any insulin immunoassay be used? | HOMA estimates from different insulin assays cannot be compared directly as they can differ by up to 100%. Particular assays were not identified for the different versions of HOMA. Assay performance, both internal and external, should be evaluated because limitations documented (7). |
| Should assay be specific for insulin? | Most assays are specific for insulin, a requirement for traceability. The RIA calculator is available for nonspecific assays, but use of these assays is limited by lack of data on cross-reactivity with split proinsulin(s) and extent. |
| How should insulin or fasting plasma glucose (FPG) values be entered if outside limits for HOMA calculators? | Insulin values should be set to lower or upper limits for HOMA calculators as estimates of central tendency and dispersion will be affected if subjects are excluded if insulin values outside these limits. With current insulin assays, 4% of insulin values were below the HOMA lower limit and 1% were above the upper limit. FPG below the lower limit should not be used as hypoglycemia is a non–steady state not suitable for this steady-state model, or there has been a problem with the sample or its measurement. |
| Is there a reference material and method for human insulin commutable to current assays? | Currently all assays are standardised with the same reference preparation (IRP 66/304); however, results still vary by up to a factor of 2. Candidate RMPs have been published. |
| Are there reference ranges for HOMA estimates for different insulin assays? | No, HOMA estimates differ, depending on the insulin assay used. HOMA estimates are reported as a percentage of “normal;” therefore, normality should be defined for a population. |
When HOMA estimates from this study are compared with those reported previously, it is important to note that they reflect the population studied, i.e., a small sample size with participants being mostly white Caucasian, being older than those recruited for HOMA (version 2), and having higher BMI. From an analytical point of view, differences in conversion factors quoted for an insulin assay, ranging from 6.0 to 7.46 (6), will also contribute to variation. The HOMA calculator version 2.2 accepts international or molar units for insulin and uses a conversion factor of 6.9 (microunits per milliliter to picomoles per liter). The American Diabetes Association Working Group recommends a factor of 6.0 (7). The study does not address the question of long-term stability of assays as insulin was measured over a very short period using a particular batch of reagents and calibrator.
All glucose results were within limits for HOMA version 2.2, but 5% of insulin values fell outside with 4% below the lower limit (supplemental Table 1). It is interesting to note that there is no common policy among HOMA users on how to handle insulin results outside limits. Values can be excluded from the statistical analysis or set to limits; this may have affected results of published studies and have clinical implications. Until a new version of HOMA is developed that accepts the majority of insulin values generated by current assays, the quandary will continue. When reporting HOMA estimates, authors should state how these results are treated in the statistical analysis section of their article.
HOMA estimates from serum and heparinized plasma differ, reflecting the lower insulin values found in plasma (6). Although HOMA calculators are based on heparinized plasma collected under research conditions, serum is more widely used. Other sample types, e.g., EDTA plasma, may offer some benefits in terms of sample stability (11,12), but more information is required on the effects of anticoagulants and clot accelerators on insulin immunoassays .
The decision on which HOMA calculator to use may not be self-evident. The RIA model was developed with an RIA that demonstrated 100% cross-reactivity with proinsulin, but the RIA in this study is reported to be specific for insulin. Likewise, the SI calculator is recommended for assays that do not cross-react with proinsulin(s), yet recently an American Diabetes Association Task Force demonstrated that some immunometric assays described as “specific” show significant cross-reactivity with proinsulins (7). Because of the lack of availability of different proinsulin species, published data on cross-reactivity are sparse. Later versions of HOMA incorporate an estimate of proinsulin secretion for specific insulin assays, although this will not account for variation in the extent and nature of the cross-reactivity. Depending on glycemic status, proinsulin will account for 10 to 20% of “immunoreactive insulin” (13). When mean values from specific insulin assays were entered into the RIA calculator rather than the SI calculator, estimates were different from those obtained from the SI calculator, but the differences were rectified when mean specific insulin plus total proinsulin were entered into the RIA calculator (14).
Differences in HOMA estimates due to sample type and calculator version are small compared with the twofold difference in extremes of values reported by insulin assays. Difference plots for an insulin assay (6) versus the mean of assays (as no reference measurement procedure [RMP] was available) showed offsets or positive or negative concentration-dependent differences. This has been confirmed by a recent study (16) comparing immunoassays to isotope dilution, liquid chromatography/tandem mass spectrometry. A corresponding pattern of differences in HOMA estimates was found in this study with the direction depending on the nature of the transform required for the particular estimate (15) (supplemental material available in the online appendix). Ranking of insulin results was maintained in the different insulin assays (r2 0.983–0.997) (6) with this level of ranking broadly maintained for HOMA estimates in this study.
All insulin assays were quoted as being calibrated using International Reference Preparation (IRP) 66/304, but differences in calibration procedures and IRP commutability, assay format, specificity, and conversion factors may have contributed to assay bias (6). In the absence of an RMP, various tactics have been introduced to reduce variation, e.g., using a central laboratory with the same methodology throughout (17,18), sample exchanges (19), and ranking HOMA estimates from different assays (20), although these have limited applicability. To achieve standardization for insulin, an RMP and commutable reference material or certified serum calibrator producing traceable results to a defined measurand expressed in SI units are required. Progress has been made with publication of an isotope dilution, liquid chromatography/tandem mass spectrometry method (21,16) for serum insulin. However, establishment of traceability for HOMA has yet to be attained.
In common with other means of assessing insulin resistance or β-cell function, comparison among studies is problematic. Estimates cannot be compared if different insulin assays are involved nor can cut points be established across populations. In addition, they are affected by sample type and calculator version. The SI calculator recommended for assays that do not cross-react with proinsulin includes a factor to account for physiological actions of proinsulin. Not all immunometric assays demonstrate this degree of specificity (7). The RIA calculator should be used for assays that cross-react 100% with proinsulin whatever the assay architecture. Although direct comparison of numerical results of HOMA estimates may not be valid, it is valid to look at relative changes or to compare their distribution across populations.
In summary, until a new HOMA version based on an insulin assay traceable to an RMP with appropriate standards for serum and plasma is developed, this study provides the most reliable information on estimates from different sources. Selection of the appropriate HOMA calculator for the insulin assay is essential.
Supplementary Material
Acknowledgments
We thank Diabetes UK, the diagnostic companies named below, and University Hospital Birmingham Charitable Funds for funding. Participating Laboratories U.K.: Birmingham Children's Hospital; Clinical Biochemistry, University Hospital Birmingham; Derriford Hospital, Plymouth; Diabetes Research Unit, Cardiff University; Royal Surrey County Hospital, Guildford; St George's Hospital, Tooting; Walsall Manor Hospital; Laboratories U.S.: Diabetes Diagnostic Laboratory, Columbia, University of Missouri Health Care; and Linco Research, St. Charles, MO; Diagnostic companies: Abbott; Bayer (now Siemens Medical Solutions Diagnostics); DakoCytomation; DPC (now Siemens Medical Solutions Diagnostics); Linco (now Millipore); Biosource-Invitrogen (previously Medgenix); Mercodia; Molecular Light Technology MLT (now Invitron); Roche; and Tosoh.
We also thank Professor Stephen Gough, Rachel Round, and Janet Smith.
Published ahead of print at http://care.diabetesjournals.org on 5 June 2008.
S.E.M. and S.D.L. contributed equally to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Matthews DR. Hosker JP: Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Wallace TM, Matthews DR: The assessment of insulin resistance in man. Diabet Med 19:527–534, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Sapin R: Insulin immunoassays: fast approaching 50 years of existence and still calling for standardization. Clin Chem 53:810–812, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Robbins DC, Andersen L, Bowsher R, Chance R, Dinesen B, Frank B, Gingerich R, Goldstein D, Wiedmeyer H-M, Haffner S, Hales CN, Jarett L, Polonsky K, Porte D, Skyler J, Webb G, Gallagher K: Report of the American Diabetes Association's Task Force on standardization of the insulin assay. Diabetes 45:242–256, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Manley SE, Stratton IM, Clark PM, Luzio SD: Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem 53:922–932, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Marcovina S, Bowsher RR, Miller WG, Staten M, Myers G, Caudill SP, Campbell SE, Steffes MW: Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin Chem 53:711–716, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Wiedmeyer HM, Polonsky KS, Myers GL, Little RR, Greenbaum CJ, Goldstein DE, Palmer JP: International comparison of C-peptide measurements. Clin Chem 53:784–787, 2007 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus [article online], 1999. Geneva, World Health Org. Department of Noncommunicable Disease Surveillance, 1999. Available from http:/www.who.int. Accessed May 2008.
- 10.SAS Institute: Statistical Analysis System. 6th ed. Cary, NC, SAS Institute, 1990
- 11.Vogeser M, Parhofer KG: Limited preanalytical requirements for insulin measurement. Clin Biochem 38:572–575, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Pfützner A, Pfützner AH, Kann PH, Stute R, Löbig M, Yang JW, Mistry J, Forst T: Clinical and laboratory evaluation of a new specific ELISA for intact proinsulin. Clin Lab 51:243–249, 2005 [PubMed] [Google Scholar]
- 13.Nagi DK, Knowler WC, Mohamed-Ali V, Bennett PH, Yudkin JS: Intact proinsulin, des 31, 32 proinsulin, and specific insulin concentrations among nondiabetic and diabetic subjects in populations at varying risk of type 2 diabetes. Diabetes Care 21:127–133, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Manley SE, Luzio SD, Clark P, Stratton IM: Which version of HOMA (Homeostasis Model Assessment) should be used with current insulin assays? (Abstract). Diabetes 55(Suppl. 1):2284, 2006 [Google Scholar]
- 15.Luzio SD, Clark PM, Stratton IM, Manley SE Comparison of HOMA estimates of insulin sensitivity and β-cell function depends on the bias of insulin assays (Abstract). Diabetes 54(Suppl. 1):1033-PO, 2005
- 16.Rodríguez-Cabaleiro D, Van Uytfanghe K, Stove V, Fiers T, Thienpont LM: Pilot study for the standardization of insulin immunoassays with isotope dilution-liquid chromatography/tandem mass spectrometry. Clin Chem 53:1462–1469, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Adler AI, Neil HA, Manley SE, Holman RR, Turner RC: Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom prospective diabetes study (UKPDS 47). Am Heart J 138:S353–S359, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR: UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 44:156–163, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Meigs JB, Haffner SM, Nathan DM, D’Agostino RB, Wilson PW: Sample exchange to compare insulin measurements between the San Antonio Heart Study and the Framingham Offspring Study. J Clin Epidemiol 54:1031–1036, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Heine R, Wareham NJ: DECODE Study Group: Are insulin resistance, impaired fasting glucose and impaired glucose tolerance all equally strongly related to age? Diabet Med 22:1476–1481, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Van Uytfanghe K, Rodríguez-Cabaleiro D, Stöckl D, Thienpont LM: New liquid chromatography/electrospray ionisation tandem mass spectrometry measurement procedure for quantitative analysis of human insulin in serum. Rapid Commun Mass Spectrom 21:819–821, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.