Abstract
OBJECTIVE—To quantify the magnitude and pattern of cognitive difficulties in pediatric type 1 diabetes as well as the effects associated with earlier disease onset and severe hypoglycemia.
RESEARCH DESIGN AND METHODS—Pediatric studies of cognitive function since 1985 were identified for study inclusion using MEDLINE and PsycInfo. Effect size (ES, Cohen's d) between the diabetic and control groups, expressed in SD units, were calculated within cognitive domains to standardize meta-analysis test performance.
RESULTS—The meta-analysis sample of 2,144 children consisted of 1,393 study subjects with type 1 diabetes and 751 control subjects from 19 studies. Overall, type 1 diabetes was associated with slightly lower overall cognition (ES −0.13), with small differences compared with control subjects across a broad range of domains, excluding learning and memory, which were similar for both groups. Learning and memory skills, both verbal and visual (−0.28 and −0.25), were more affected for children with early-onset diabetes (EOD) than late-onset diabetes (LOD), along with attention/executive function skills (−0.27). Compared with nondiabetic control subjects, EOD effects were larger, up to one-half SD lower, particularly for learning and memory (−0.49). Generally, seizures were associated with a negligible overall cognition ES of −0.06, with slight and inconsistent cognitive effects found on some measures, possibly reflecting the opposing effects of poorer versus better metabolic control.
CONCLUSIONS—Pediatric diabetes generally relates to mildly lower cognitive scores across most cognitive domains. Cognitive effects are most pronounced and pervasive for EOD, with moderately lower performance compared with control subjects. Seizures are generally related to nominal, inconsistent performance differences.
Type 1 diabetes affects ∼1 in 500 children. Growing consensus indicates that children with type 1 diabetes, compared with control subjects, are at risk of developing cognitive difficulties (1). However, research results are inconsistent regarding the magnitude and pattern of cognitive difficulties due to heterogeneous samples, sampling procedures, cognitive abilities assessed, and study designs (1,2). Debate remains over the extent and type of pediatric diabetes cognitive difficulties—general cognitive or specific neuropsychological—and their associated risk factors.
Using meta-analysis to synthesize data across studies, this article aims to determine whether there is evidence of cognitive dysfunction in children with type 1 diabetes compared with demographically similar children without diabetes. Examination of effect size (ES, Cohen's d) highlights differences across various cognitive domains and the magnitude of those differences. A second aim of the current study is to determine whether some children with type 1 diabetes have an elevated risk of cognitive dysfunction. Earlier age of diabetes onset is identified in the literature as one of the strongest risk factors associated with disrupted cognitive functioning (3,4,5,6). To examine the impact of early-onset diabetes (EOD) on cognitive abilities, a second meta-analysis is conducted to compare children classified by authors as having earlier age at onset, which may range anywhere from 4 to 7 years depending on the author, to later age at onset (late-onset diabetes [LOD]). Examination of ES determines the scope and magnitude of differences, if any, across cognitive domains. After the relative comparison of EOD and LOD, each group is compared separately with children without diabetes to better assess the true magnitude of cognitive effects for each group compared with children without a chronic disease.
A clear picture of EOD effects is obscured because EOD may be a surrogate for recurrent severe hypoglycemia; young children with diabetes have a greater risk of severe hypoglycemia (7). Like EOD, severe hypoglycemia is also associated with poorer performance on measures of cognitive function, particularly memory (8), although these findings have not been consistent across studies (9,10). Therefore, ancillary analyses will be conducted to further explore the possible effects of severe hypoglycemia on cognitive function. Finally, EOD may be a surrogate for longer disease duration, since children with earlier onset correspondingly have longer disease duration than age-matched children diagnosed later. However, there are a limited number of studies available that specifically examine the impact of disease duration on cognitive function, such that these effects are not examined in the present study.
RESEARCH DESIGN AND METHODS
Study selection
MEDLINE and PsycInfo databases (from 1985 through 2008) were used to identify cognitive performance studies in 1) children with type 1 diabetes versus control children without diabetes and 2) in children with type 1 diabetes divided into disease risk groups of a) EOD versus LOD and b) seizures versus without seizures. Key words used to search the literature were: cognition, attention, memory, learning, executive function, neurocognitive, neuropsychological, and intelligence. These descriptors were combined with the terms diabetes, type 1 diabetes, insulin-dependent diabetes mellitus, children, youth, and adolescents for the literature search.
Inclusion criteria
Studies in the overall meta-analytic review fulfilled the following criteria: 1) results published or available in English between 1985 and 2008, 2) children under 18 years of age when diagnosed with type 1 diabetes, 3) at least one measure of cognitive performance, 4) original data reported with sufficient information to allow calculation of ES (i.e., group means, SDs, etc.), and 5) a defined control group matched for at least age. A total of 1,029 children with type 1 diabetes and 751 nondiabetic control subjects from 15 studies were evaluated for the overall diabetes versus control comparisons (3,5,6,8,11–21) (online appendix Table 1, available at http://dx.doi.org/10.2337/dc07-2132).
Additional studies that met the above criteria and evaluated disease subgroups for age of diabetes onset or seizures were part of subgroup analyses (3–6,8–10,12,18–22). In the onset analysis, 768 children with diabetes from seven studies were divided into an EOD group (n = 232) or an LOD group (n = 536), based on each authors’ criteria. Seizure subgroups were comprised of 900 children with diabetes from nine studies with 310 in a seizure group and 590 in a no seizure group.
Before calculation of ES, results from each domain in each study were standardized. Means and pooled SDs were used to calculate Cohen's d as the ES for the diabetes and control groups. One study presented data as least-squared adjusted means (6), and raw data were obtained and recalculated to fit the meta-analysis format. Three other published studies utilized test scores that could not be standardized and were therefore excluded (J. Hagan, personal communication). Several authors (including Hershey, Holmes, Northam, Rovet, and Ryan) reported similar data from the same subjects in multiple publications; in each instance, data from the latest longitudinal report or the largest dataset were included.
Cognitive domains
Tasks from individual studies were assigned to one of six broad cognitive domains based on classification from current neuropsychological texts (23), as follows: intelligence, learning and memory, psychomotor activity and speed of information processing, attention/executive function, academic achievement, and visual motor integration. For more specific analyses, these domains are further divided into subcategories: Intelligence is subdivided into crystallized intelligence measuring acquired knowledge and fluid intelligence, which assesses a person's ability to use strategies to apply unfamiliar information to new situations. The learning and memory domain is divided into verbal and visual modalities, i.e., verbal or visual learning and memory. Because of the focus on these skills in the pediatric literature and in the classroom (12), the latter is further subdivided into components of verbal or visual learning (acquisition and storage of new modality-specific information with repeated exposure) and verbal or visual memory (immediate memory for new modality-specific information). Psychomotor activity and speed of information processing contains the following categories: psychomotor efficiency and motor speed. Psychomotor efficiency includes cognitively demanding information processing tasks and processing speed (i.e., Symbol Search), and motor speed includes measures of fine motor speed (i.e., Grooved Pegboard). All tasks in these two subcategories are timed, and performance is measured based on how many items are completed correctly. The attention/executive function domain consists of simple and complex attentional tasks, abstract problem solving, and decision making. Academic achievement encompasses academic skill development across broad areas of classroom performance including math and reading. Visual motor integration measures ability to coordinate visual perceptual input with fine motor output where performance is based on the quality of the written output and is not timed (e.g., Beery-Buktenica's Developmental Test of Visual-Motor Integration). Tasks that could not be classified were not included.
Statistical analyses
Cohen's d was calculated as a measure of ES (24). ES, which represents the standardized differences between diabetic and control groups expressed in SD units, was calculated for each cognitive domain in each study. The direction of an ES indicates better or poorer cognitive performance. In the meta-analysis, a combined d value is reported for each cognitive domain as an expression of the magnitude of associations across studies. The d values are weighted for sample size to adjust for possible bias due to unequal samples across studies. The number of participants that contributed data to each ES is noted in parentheses in Fig. 1A and B. In addition, the 95% CI, on the basis of the SE, provides an indication of the significance of the difference in performance between the diabetic and control groups.
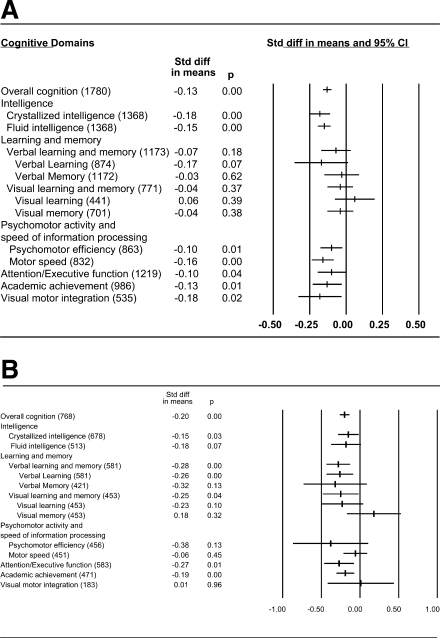
Figure 1.
Standardized ES —Panel A. Diabetes versus Control children meta-analytic standardized ES (Cohen's d) for the cognitive domains. Number of participants included in the calculation of each domain is listed in parentheses. Refs.: Overall Cognition = (3,5,6,8,11–21), Crystallized Intelligence = (3,5,6,11,12,14,16,17,19–21), Fluid Intelligence = (3,5,6,11,12,14,16,17,19–21), Verbal learning and memory = (3,6,8,12,15,19–21), Visual learning and memory = (3,6,8,12,19–21), Psychomotor Efficiency = (6,11,12,14,16,19,20), Motor Speed = (3,8,11–13,16,19,20), Attention/Executive Function = (3,6,8,11–14,16,17,19,20), Academic Achievement = (3,5,11,12,16,18,19), Visual Motor Integration = (5,11,12,19,20) —Panel B. Earlier versus Later Disease Onset group meta-analytic standardized ES (Cohen's d) for the cognitive domains. Number of participants included in the calculation of each domain is listed in parentheses. Refs.: Overall Cognition = (3–6,8–10,12,18–22), Crystallized Intelligence = (3–6,9,10,19–22), Fluid Intelligence = (3–6,9,10,19–22), Verbal learning and memory = (3,6,8–10,12,19–22), Visual learning and memory = (3,6,8–10,19–22), Psychomotor Efficiency = (6,10,19,20,22), Motor Speed = (3,8,19,20,22), Attention/Executive Function = (3,6,8–10,19,20,22), Academic Achievement = (3,5,18,19,22), Visual Motor Integration = (5,19,20).
An overall d value, in which all cognitive domains are pooled, was computed first as an index of general cognitive function (overall cognition). To address the possibility of publication bias, a fail-safe N also was calculated. This measure is used to estimate the number of unpublished nonsignificant cognitive domains necessary to falsify the significant overall cognition ES. In Fig. 1A, the fail-safe N is 187. For the overall cognition ES to be nonsignificant, an additional 187 nonsignificant cognitive domains would be necessary, suggesting that observed significant effects are not likely due to publication bias.
Statistical overview
Meta-analyses were performed separately for the difference between the diabetic and control groups and for the possible contribution of disease factors, such as age of diabetes onset to performance. The Comprehensive Meta-Analysis (CMA, version 2) statistical package (25) was used for all analyses and to create forest plots.
Subgroup meta-analyses
Subgroup meta-analyses examined the relative contribution of the following disease risk factors to performance in each of the cognitive domains reported in Fig. 1.
EOD versus LOD.
EOD was defined by the authors of the original studies and included subjects diagnosed before 4 years of age (5,6), 5 years of age (3,18,19,22), and 7 years of age (4). For the purpose of this meta-analysis, performance of children with EOD was defined inclusively as younger than age 7 years (n = 232) and was compared with those with later onset, LOD (n = 536).
EOD and LOD versus control.
A subset of five onset studies with matched nondiabetic control subjects was evaluated in two supplemental meta-analyses to determine representative cognitive profiles and the relative magnitude of ES for children with EOD (n = 166) and LOD (n = 322) in comparison with healthy control children (n = 414) (3,5,6,18,19).
Severe hypoglycemia.
Severe hypoglycemia was defined by Epidemiology of Diabetes Interventions and Complications criterion (26) of at least one seizure (n = 310 vs. no seizures, n = 590). Two studies (9,21) categorized hypoglycemic episodes as severe if patients required glucagon or the assistance of others, consistent with the earlier Diabetes Control and Complications Trial definition (27).
RESULTS
Altogether 15 studies of pediatric diabetes and cognitive function were identified in the literature that met criteria for inclusion in the meta-analysis shown in Fig. 1A. The overall diabetes/control meta-analysis sample (N = 1,780) consisted of 1,029 children with diabetes (mean age 12.96 years) and 751 control subjects (mean age 12.55 years). Most studies matched subjects according to participant age. Seven studies matched participants according to parents’ education, occupation, or both, usually by use of sibling control subjects. The average age of diabetes onset was 6.6 years (range 4.13–11.8), and the average diabetes duration was 5.23 years (0.5–8.9).
Children with type 1 diabetes demonstrated slightly lower performance than control subjects (overall cognition −0.13) in all cognitive domains, except learning and memory. Lower scores were found in intelligence (crystallized and fluid), psychomotor activity and speed of information processing (psychomotor efficiency and motor speed), attention/executive function, visual motor integration, and academic achievement. Most ES's are small (d < −0.2) according to Cohen's criteria (24). The ES translates into slightly lower group test scores of 1–3 standard score points that are not likely to be clinically detectable (standard score mean ± SD: 100 ± 15). The cognitive performance of both groups is generally age appropriate, consistent with previous reports (3,11) (Fig. 1A).
Subgroup meta-analyses
All cognitive domains listed in Fig. 1 were evaluated for each of the subgroup comparisons (onset and seizure). Average age of diabetes onset for the subgroup analyses is 6.3 years (range 4.1–8.6) with a mean diabetes duration of 5.9 years (2.6–8.9). Children in the subgroup analyses were approximately the same age (mean 12.59 years) as those in the overall sample, had the same average age of diabetes onset (mean 6.3 years), and slightly longer diabetes duration (5.9 vs. 5.2 years, respectively).
Earlier onset of diabetes: EOD versus LOD.
Fig. 1, Panel B, shows that children with EOD performed more poorly than LOD children in Overall Cognition, ES of −0.20. In contrast to the overall diabetes effect of intact learning and memory in Panel A of Fig. 1, EOD is associated with strongest effects in lower Verbal (−0.28) and Visual (−0.25) Learning and Memory, Attention/executive function (−0.27), Academic achievement (−0.19) and lower Crystallized Intelligence (−0.15). A trend toward lower Fluid Intelligence (−0.18) also is found. ES's for early onset are larger than those for the overall diabetes versus control meta-analysis, but still within the small range, according to Cohen's criteria (24). (Panel B, Fig. 1)
Earlier onset of diabetes: EOD and LOD versus control.
To better determine the pattern and magnitude of cognitive profiles associated with EOD and LOD subgroups, a comparison between each onset group and nondiabetic controls was undertaken. Two additional MA's were conducted with a subset of five studies that contained onset groups and nondiabetic controls (3,5,6,18,19). Comparable LOD = Control performance could suggest that the overall diabetes ES's in Panel A of the figure are a function of lower EOD scores that skew the diabetes scores. Due to the tests available for these analyses, it was not possible to subdivide the Learning and Memory categories beyond modality effects.
EOD versus control.
When the scores of EOD children are compared to nondiabetic controls instead of the LOD disease contrast group, not surprisingly, the magnitude of the ES's are larger (i.e., Overall Cognition = −29). Most striking are individual ES's for lower EOD Verbal (−0.49) and Visual (−0.44) Learning and Memory, poorer Attention/Executive Function (−0.39), and lower Intelligence as reflected by both Crystallized (−0.35) and Fluid (−0.28) abilities. These ES's are moderate in magnitude and range up to 6.5 to seven points lower on standardized tests, which may be clinically meaningful and detectable in a classroom setting. Only two domain ES's failed to reach significance: Psychomotor Efficiency (−0.37) and Visual Motor Integration (−0.16) although they trended lower as well. Test variability in the measures utilized appears to account for failure of the moderately-sized Psychomotor Efficiency effect to reach significance. In sum, while many cognitive effects are present, learning and memory abilities, both verbal and visual, are the most negatively impacted in this subgroup of children.
LOD versus control.
While the magnitude of the difference is not as large, LOD performance was also lower than that of nondiabetic controls in Overall Cognition (−0.13). In fact, the magnitude of the LOD difference is consistent with the Overall Cognition ES for the Diabetes group in Fig. 1, Panel A, suggesting that general diabetes results in Panel A are not skewed by including EOD effects. Small LOD effects were found in both lower Crystallized (−0.20) and Fluid (−0.14) Intelligence, Visual Learning and Memory (−0.17), Motor Speed (−0.17) and Visual Motor Integration (−0.17), but not Verbal Learning and Memory (−0.03), Psychomotor efficiency (−0.04) or Academic achievement (−0.01).
Hypoglycemic seizures (+SZ).
Seizures are associated with a nominal Overall Cognition ES of −0.06 and small to nominally lower scores in four cognitive domains of Crystallized (−0.19) and Fluid Intelligence (−0.21), Academic achievement (−0.10) and Visual motor integration (−0.06). In contrast, nominally better visual memory (0.13) and learning (0.12) is present. Generally, seizures are associated with nominal or small cognitive effects that are inconsistent and should have little clinical impact for children with type 1 diabetes as a whole, although some individual children may be more adversely affected.
CONCLUSIONS
Children with TID performed slightly less well than controls on global measures of intelligence and on a broad range of specific neuropsychological skills such as attention/executive function, providing support for a relation between type 1 diabetes and slightly lower cognitive function. Score differences are found in all broad cognitive domains except learning and memory. The ES of −0.13 for Overall Cognition, however, converts into nominal score differences that are one to two points lower on average for children with diabetes versus controls. See Fig. 1, Panel A. A small difference of this magnitude is not clinically significant and indicates that overall group cognitive performance is generally intact and age appropriate. However, despite generally intact group scores, there is likely to be some variability in cognitive abilities across individual children that could range from some subtly affected individuals with circumscribed effects to others who may have moderate or even severe dysfunction. Further assessment of various disease risk factors and their associated cognitive effects may lead to better identification of those children most at-risk for more severe disruption.
The secondary analyses in this study identifies subgroups of children most at-risk for cognitive disruption. Findings indicate that children with earlier diabetes onset (EOD), before the age of 7, show evidence of greatest cognitive disruption, with an Overall Cognition ES of-0.20 or 3-points lower performance across multiple domains, compared to their disease contrast group with later onset (LOD). See Fig. 1, Panel B. Contrary to the overall Diabetes meta-analysis with no learning and memory effects, the EOD children show their largest ES's in both lower verbal and visual learning and memory, congruent with literature findings reported for both seizures and EOD (1,2,17). Other EOD effects include small differences in attention/executive function and lower crystallized intelligence (i.e., acquired information), but not fluid intelligence (visual/spatial) scores. Academic achievement also is slightly lower for EOD compared to LOD children. Generally, all of these effects are small, in the range of a quarter of a standard deviation.
EOD and LOD versus control comparisons
To determine cognitive profiles uniquely associated with each onset group and to test the possibility that EOD effects might underlie the general diabetes effects in the overall meta-analysis (Fig. 1, Panel A), a subset of EOD and LOD children with matched controls was evaluated with further meta-analyses (3,5,6,18,19). When compared to nondiabetic controls, the EOD group displays a larger difference in lower overall cognition (−0.29) then compared to the LOD disease contrast group (−0.20). Healthy children without a chronic disease are the typical comparison group in a classroom setting, and EOD children show moderate ES's, almost 1/2 standard deviation lower, in crucial verbal (−0.49) and visual (−0.44) learning and memory skills. This ES translates into 6.5 to 7-point lower scores and may be detectable in a learning environment. Moderately lower attention and executive function (−0.39), a diverse set of higher order cognitive processes, such as planning, inhibitory control, and sustained attention, is also found in the EOD/Control comparison. Poorer attention and executive function abilities have been identified in children with learning disabilities and may impact learning in the classroom. A 6-point difference in selective deployment of attention and decision-making, skills required to learn salient information in academic settings, also might be detectable in a learning environment. Lower academic achievement is found (ES = −0.28) along with lower crystallized and fluid intelligence, which in toto indicate widespread and generally moderate cognitive effects in association with EOD youth compared to controls, with particularly negative effects for memory and learning.
Results also show that the scores of LOD children are lower than nondiabetic controls and, in fact, the LOD overall cognition ES of −0.13 mirrors that of the general diabetes/control difference in Fig. 1, Panel A. Children with LOD exhibited lower crystallized and fluid intelligence, as well as intact verbal but lower visual learning and memory. Lower visual motor integration skills also are found along with reduced psychomotor speed. In sum, it appears that children with later disease onset experience slight but detectable performance differences when compared to controls. However, effects are small, in the order of one to three points on standardized tests, which suggest that as a group, these findings are not clinically meaningful. However, as with the diabetes/control comparisons, there is likely to be variability across children, with some demonstrating no impairment and some demonstrating moderate to severe difficulties.
Seizures (+SZ) were related to the smallest Overall Cognition ES of −0.06 of all groups evaluated; a negligible (@-1 point) difference on average. Nevertheless, larger effects, although still small, were found in Crystallized and Fluid Intelligence (@-3 points) as well as nominally lower scores in visual motor integration and academic achievement (@ −1 point). In contrast, better visual memory and learning (@ +1.3 points) was found. This discrepancy in performance may be due to the opposing effects of those in relatively poorer versus better metabolic control, effects that cannot be measured in these analyses. Negligible or generally absent cognitive difficulties is congruent with results from a number of cross sectional studies (9,10) and the longitudinal DCCT and EDIC studies (26,27) but varies with some individual reports (8,21). While results of this MA suggest that hypoglycemic seizures appear to be relatively innocuous in terms of overall pediatric cognitive effects, with an average diabetes duration of 5.23 years, effects may be greater for children in chronically poorer metabolic control who have been shown to have lower scores in individual studies (5,26,27). Further, the present meta-analysis findings cannot rule out a synergistic effect when seizures occur in children with earlier diabetes onset (28) who may be more adversely affected.
Frequently, EOD children have a significant number of seizures due to the behavioral and medical challenges of disease management in children younger than age seven (5). This confound between EOD and seizures is such that at one point in the pediatric literature, EOD was hypothesized to be a proxy or masking variable for the presumed underlying effects of seizures (7). The present MA results, based on a large number of over 2,000 pediatric patients from many different research groups, suggest that the effects of seizures may be more benign than originally hypothesized for most children, and equally importantly, seizures do not appear to be a proxy or surrogate variable for EOD. Interactive effects may ultimately prove more likely (6,21).
Could the pervasive cognitive effects of EOD in the present meta-analysis result from method insensitivity to detect EOD differential or circumscribed effects? Probably not, because the meta-analysis is able to detect very circumscribed and small, even negligible, effects associated with hypoglycemic seizures. An important consideration though is that the additional EOD results are based in part on some of the earliest studies in the literature (3,5) although more recent studies reveal consistent findings (19), including a large-scale post-DCCT longitudinal study (6). Now, the next questions may be what mechanisms place EOD children at increased risk and what prevention steps are possible? While EOD status itself cannot presently be altered, its potentially negative interactions with other disease risk variables such as hypoglycemic seizures and chronic hyperglycemia can be minimized. EOD may produce a background vulnerability (28) that might amplify the negative effects of these other disease risks. Undesirable glucose excursions may be minimized with continuous glucose monitoring systems that warn of glucose perturbations to help achieve near-normal metabolic control.
While the present authors were unable to assess the impact of diabetes duration on cognitive function due to a lack of relevant studies, it is noteworthy that small, but reliable cognitive differences are documented in this meta-analysis of children who have an average disease duration of just 5.23 years. Cognitive effects, although mild, appear relatively quickly following diagnosis. Consistent with this appraisal, Northam et al. 's longitudinal evaluation of 90 newly diagnosed children also revealed cognitive changes over just a 6-year period (6). After only two years children with diabetes, particularly those with EOD (<4years), exhibited less improvement on measures of nonverbal visuospatial skills than those with LOD or controls (6). Taken together, the impact of diabetes upon pediatric cognition appears to begin shortly after diagnosis. Future studies should examine the impact of disease duration on cognition and identify if possible CNS or other biological changes may occur over time.
Limitations
This meta-analytic documentation of a conclusive, but small, relation between TID and cognitive function in a large pediatric sample is significant given the “noise” and variability present in most clinical research studies. However, many of the factors that contribute to variability among studies should be considered in the interpretation of the present results. First and foremost, many of the studies included in this meta-analysis were cross-sectional in nature. Derivation of causal inferences is problematic. More rigorous, longitudinal studies with glucose meters or continuous glucose monitoring systems to track glucose excursions may better examine the locus and progression of cognitive difficulties over time, particularly when coupled with neuroimaging studies. Secondly, the pattern of subgroup results appears to clearly implicate early disease onset with the greatest magnitude and scope of cognitive difficulties compared to controls. However, disease subgroup differences should be interpreted with caution since they are not independent effects. Future studies may find that it is the interaction among different disease variables that most strongly impacts cognition in children.
Acknowledgments
This work is supported in part by NIH grants DK56975 and DK070917, awarded to Clarissa S. Holmes. Portions of this manuscript were presented in abstract form at the 66th and 67th Scientific Sessions of the American Diabetes Association. We would like to thank Drs. Hagen, Hershey, Northam, Rovet, Ryan, and Wysocki who answered queries about their datasets.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Holmes CS, Fox MA, Cant MC, Lampert NL, Greer T: Disease and demographic risk factors for disrupted cognitive functioning in children with insulin-dependent diabetes mellitus (IDDM). School Psych Review 28:215–227, 1999 [Google Scholar]
- 2.Desrocher M, Rovet J: Neurocognitive correlates of type 1 diabetes mellitus in childhood. Child Neuropsychol 10:36–52, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ryan CM, Vega A, Drash A: Cognitive deficits in adolescents who developed diabetes early in life. Pediatrics 75:921–927, 1985 [PubMed] [Google Scholar]
- 4.Holmes CS, Richman LC: Cognitive profiles of children with insulin-dependent diabetes. Dev Behav Pediatr 6:323–326, 1985 [PubMed] [Google Scholar]
- 5.Rovet J, Ehrlich R, Hoppe M: Specific intellectual deficits in children with early onset diabetes mellitus. Child Dev 59:226–234, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warner GL, Werther GA: Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 24:1541–1546, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ryan C, Becker D: Hypoglycmia in children with type 1 diabetes mellitus:risk factors, cognitive function, and management. Endocrinol Metab Clin North Am 28:883–900, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Hershey T, Lillie R, Sadler M, White NH: Severe hypoglycemia and long-term spatial memory in children with type 1 diabetes mellitus: a retrospective study. J Int Neuropsychol Soc 9:740–750, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Wysocki T, Harris MA, Mauras N, Fox L, Taylor A, Jackson SC, White NH: Absence of adverse effects of severe hypoglycemia on cognitive function in school-aged children with diabetes over 18 months. Diabetes Care 26:1100–1105, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Strudwick SK, Carne C, Gardiner J, Foster JK, Davis E, Jones, TW: Cognitive functioning in children with early onset type 1 diabetes and severe hypoglycemia. J Pediatr 147:680–685, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Rovet JF, Ehrlich RM, Czuchta D: Intellectual characteristics of diabetic children at diagnosis and one year later. J Pediatr Psychol 15:775–788, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Kaufman FR, Epport K, Engilman R, Halvorson M: Neurocognitive functioning in children diagnosed with diabetes before age 10 years. J Diabetes Complications 13:31–38, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Reich JN, Kaspar C, Puczynski MS, Puczynski S, Cleland J, Dellángela K, Emanuele MA: Effect of hypoglycemic episode on neuropsychological functioning in diabetic children. J Clin Exper Neuropsychol 12:613–626, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Holmes CS, Dunlap WS, Chen RS, Cornwell JM: Gender differences in the learning status of diabetic children. J Consult Clin Psychol 60:698–704, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Kovacs M, Ryan C, Obrosky DS: Verbal intellectual and visual memory performance of youths with childhood-onset insulin-dependent diabetes mellitus. J Pediatr Psychol 19:475–483, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Crawford SG, Kaplan BJ, Field LL: Absence of an association between insulin-dependent diabetes mellitus and developmental learning difficulties. Hereditas 122:73–78, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Hershey T, Bhargava N, Sadler M, White NH, Craft S: Conventional versus intensive diabetes therapy in children with type 1 diabetes. Diabetes Care 22:1318–1324, 1999 [DOI] [PubMed] [Google Scholar]
- 18.McCarthy AM, Lindgren S, Mengeling MA, Tsalikian E, Engvall JC: Effects of diabetes on learning in children. Pediatrics 109:1–10, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Fox MA, Chen RS, Holmes CS: Gender differences in memory and learning in children with insulin-dependent diabetes mellitus (IDDM) over a four-year follow-up interval. J PediatrPsychol 28:569–578, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hannonen R, Tupola S, Ahonen T, Riikonen R: Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycemia. Dev Med Child Neurology 45:262–268, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey, T: Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatric Diabetes 9:87–95, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Holmes CS, Chen RS, Streisand R, Marschall DE, Souter S, Swift EE, Peterson CC: Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. J Pediatr Psychol 31:770–784, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lezak MD: Neuropsychological assessment: 3rd Edition. New York: Oxford Press, 1995
- 24.Cohen J: Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum, 1988
- 25.BioStat: Comprehensive Meta-Analysis Software User Guide, Version 2. Englewood, NJ, 2006, (www.meta-analysis.com).
- 26.The Diabetes Complications and Clinical Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Long-term effects of diabetes and its treatment on cognitive function. N Engl J Med 356, 1842–52, 2007 [DOI] [PMC free article] [PubMed]
- 27.The DCCT Research Group: Effects of intensive diabetes treatment on the development and progression of long term complications in adolescents with insulin dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 125:177–188, 1994 [DOI] [PubMed] [Google Scholar]
- 28.Ryan, CM: Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatric Diabetes 7:289–297, 2006 [DOI] [PubMed] [Google Scholar]