Abstract
OBJECTIVE—Our objective was to perform a quantitative review of prospective studies examining the association between the metabolic syndrome and incident diabetes.
RESEARCH DESIGN AND METHODS—Using the title terms “diabetes” and “metabolic syndrome” in PubMed, we searched for articles published since 1998.
RESULTS—Based on the results from 16 cohorts, we performed a meta-analysis of estimates of relative risk (RR) and incident diabetes. The random-effects summary RRs were 5.17 (95% CI 3.99–6.69) for the 1999 World Health Organization definition (ten cohorts); 4.45 (2.41–8.22) for the 1999 European Group for the Study of Insulin Resistance definition (four cohorts); 3.53 (2.84–4.39) for the 2001 National Cholesterol Education Program definition (thirteen cohorts); 5.12 (3.26–8.05) for the 2005 American Heart Association/National Heart, Lung, and Blood Institute definition (five cohorts); and 4.42 (3.30–5.92) for the 2005 International Diabetes Federation definition (nine cohorts). The fixed-effects summary RR for the 2004 National Heart, Lung, and Blood Institute/American Heart Association definition was 5.16 (4.43–6.00) (six cohorts). Higher number of abnormal components was strongly related to incident diabetes. Compared with participants without an abnormality, estimates of RR for those with four or more abnormal components ranged from 10.88 to 24.4. Limited evidence suggests fasting glucose alone may be as good as metabolic syndrome for diabetes prediction.
CONCLUSIONS—The metabolic syndrome, however defined, has a stronger association with incident diabetes than that previously demonstrated for coronary heart disease. Its clinical value for diabetes prediction remains uncertain.
Since major organizations such as the World Health Organization (WHO), the European Group for the Study of Insulin Resistance (EGIR), and the National Cholesterol Education Program (NCEP) released definitions of the metabolic syndrome, it has received a great deal of attention in the scientific literature. Much has been learned about the many facets of the syndrome, including its prevalence, incidence, and risks of leading to the development other conditions such as cardiovascular disease and diabetes.
Because of the controversy that has enveloped the concept of the metabolic syndrome, a thorough understanding of the association between the syndrome and diabetes—one of the main risks for people with the metabolic syndrome—is critical to furthering the debate about the syndrome's scientific relevance. At the time of a previous quantitative review, only a limited number of prospective studies of the metabolic syndrome and incident diabetes were available (1). Since that review, additional definitions of the syndrome have joined the previous ones, and the results of more prospective studies have been published. Therefore, the main objective of this study was to provide an updated quantitative review of the estimates of relative risk (RR) from prospective studies of the association between the metabolic syndrome and incident diabetes. In addition, we summarize other pertinent findings of these prospective studies. We also try to place the results into clinical context by comparing metabolic syndrome assessment with other, potentially simpler methods of assessing risk of incident diabetes.
RESEARCH DESIGN AND METHODS
A literature search using the title terms “metabolic syndrome” and “diabetes” was conducted in PubMed. We limited the search to articles that were written in English. Because the WHO was the first to release its definition, in 1998, we started our search for articles published that year and continued through April 2008. We reviewed titles and abstracts of the search results and retrieved promising articles. In addition, we augmented our search with reviews of the reference lists of retrieved articles.
The focus of this article is the prospective association between the metabolic syndrome and incident diabetes in studies that are more or less population based. Therefore, we did not include studies that recruited participants with specific conditions such as cardiovascular disease. To be included in the analyses, articles had to include estimates of RR and CIs based on one of the major definitions of the metabolic syndrome (2–7). Articles that presented estimates of RR only in graphical form were not included in the quantitative analyses because of the difficulty in accurately deriving the estimate of RR and its CI. When multiple estimates were available for a particular cohort, only the estimate that most thoroughly adjusted for covariates or that presented estimates for men and women combined was selected. Some studies did not have all criteria required to define the metabolic syndrome according to established criteria, and, consequently, researchers modified definitions of the metabolic syndrome. For example, several studies used BMI instead of waist circumference (8–10). Two reviews relating metabolic syndrome to risk for vascular events noted similar RR estimates for studies based on use of BMI, waist circumference, or waist-to-hip ratio (1,11). Furthermore, one cohort did not have concentrations of HDL cholesterol (12,13). We included these studies in our summary. In addition, the WHO definition technically requires that participants undergo an oral glucose tolerance test (OGTT), have insulin resistance based on a gold-standard technique (specifically, the hyperinsulinemic-euglycemic clamp), and undergo a measurement of microalbuminuria. Most studies were unable to satisfy these criteria. Therefore, we included studies that did not conduct an OGTT, used a surrogate measure of insulin resistance (such as fasting concentration of insulin or homeostasis model assessment of insulin resistance [HOMA-IR]), and did not measure microalbuminuria. We abstracted the following data elements: lead author's name, year of publication, study name and location, sample size, follow-up time, sex composition and age of subjects (mean, median, or range), exclusion criteria, metabolic syndrome definition (including any changes), definition of diabetes, number of incident events, estimate of RR (odds ratio [OR], RR, or hazard ratio), 95% CI, and variables used to adjust estimates of RR.
SEs for the estimates of RR were estimated from the CIs. For each study, a weight was calculated as the inverse of the variance (1/SE2). Heterogeneity among studies was assessed using the Q statistic (14). If no heterogeneity was present (P of Q statistic ≥0.10), fixed-effects estimates of RR were calculated according to the inverse variance method (15). If heterogeneity was present (P of Q statistic <0.10), random-effects estimates of RR were calculated using the approach of Der Simonian and Laird (14). The influence of single studies on the summary estimates was examined graphically by checking how the elimination of each study affected the resulting summary estimate of RR (16). Evidence for publication bias was assessed by examining funnel plots and assessing funnel plot asymmetry (17). Analyses were conducted using Stata 9.2.
RESULTS
For our quantitative analyses, we included articles that are based on 16 cohorts (supplemental table available in an online appendix at http://dx.doi.org/10.2337/dc08-0423) (8–10,12,13,18–32). These 16 cohorts included over 2,604 incident events among 42,419 participants, depending on which of the multiple publications of the same cohort were included in the counts. Three cohorts included only men, whereas the other 13 included both men and women. Follow-up times ranged from 2.3 to 20 years. Two studies were not included because results were presented only in graphical form (33) or confidence limits were not provided (34).
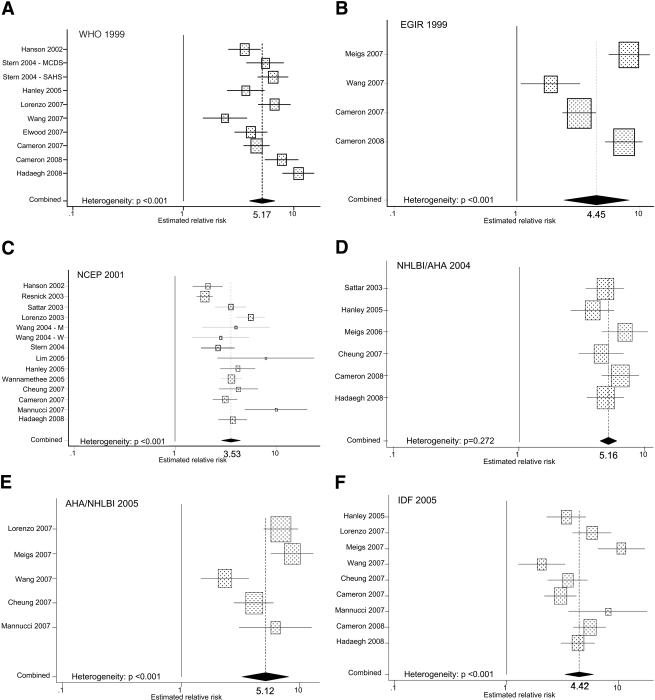
Ten cohorts yielded estimates of RR for the association of the metabolic syndrome and incident diabetes defined using the WHO 1999 definition, and the random-effects summary RR was 5.17 (95% CI 3.99–6.69) (Fig. 1). Four cohorts yielded estimates of RR for the EGIR 1999 definition, and the random-effects summary RR was 4.45 (2.41–8.22). Thirteen cohorts provided an estimate of RR for the NCEP 2001 definition, and the random-effects summary RR was 3.53 (2.84–4.39). Six cohorts reported estimates of RR for the National Heart, Lung, and Blood Institute/American Heart Association (NHLBI/AHA) 2004 definition, and the fixed-effects summary RR was 5.16 (4.43–6.00). Five cohorts reported estimated RRs for the AHA/NHLBI 2005 definition, and the random-effects summary RR was 5.12 (3.26–8.05). Nine cohorts produced estimates of RRs for the International Diabetes Federation (IDF) 2005 definition, and the random-effects summary RR was 4.42 (3.30–5.92). Funnel plot asymmetry was detected only for the analysis involving the NCEP 2001 definition (Egger's test: P = 0.028).
Figure 1.
Estimates of RR from prospective studies examining the associations between the metabolic syndrome and incident diabetes.
Comparing the summary RRs for the different definitions shown above is to some degree problematic because of differences in study populations and analytic approaches. Thus, a fairer comparison is between results from sets of studies that examined the same definitions and calculated estimated RRs in the same manner. Five studies compared the WHO 1999 and NCEP 2001 definitions (12,18,23,29,32). The random-effects summary RR was 4.40 (95% CI 2.87–6.75) for WHO 1999 and the fixed-effects summary RR was 3.12 (2.68–3.64) for NCEP 2001. Three studies compared the WHO 1999, NCEP 2001, and IDF 2005 definitions (23,29,32). The random-effects summary RR was 5.73 (3.04–10.79) for the WHO 1999 definition, and the fixed-effects summary RRs were 3.50 (2.91–4.22) for the NCEP 2001 definition and 3.51 (2.83–4.36) for the IDF 2005 definition. Five studies compared the NCEP 2001 and IDF 2005 definitions. The random-effects and fixed-effects summary RRs were 4.10 (3.13–5.39) and 3.72 (2.97–4.67), respectively (23,27,29,30,32). Five studies compared the AHA/NHLBI 2005 and IDF 2005 definitions, yielding random-effects summary RRs of 5.12 (3.26–8.05) and 5.00 (2.84–8.78), respectively (13,25–27,30). Four studies of the EGIR 1999 and IDF 2005 definitions produced random-effects summary RRs of 4.45 (2.41–8.22) and 4.33 (2.34–8.01), respectively (13,26,29,31). Two studies included the NCEP 2001, AHA/NHLBI 2005, and IDF 2005 definitions (27,29). Summary RRs were 6.16 (2.67–14.20) (random-effects), 4.51 (3.22–6.30) (fixed-effects), and 4.15 (2.88–5.96) (fixed-effects), respectively. Two studies included the WHO 1999, EGIR 1999, and NCEP 2001 definitions (12,29). The fixed-effects summary RRs were 4.09 (3.25–5.14), 2.78 (2.15–3.58), and 3.13 (2.46–3.99), respectively. Three studies compared the NCEP 2001, NHLBI/AHA 2004, and IDF 2005 definitions (23,27,32). The fixed-effects summary RRs were 3.95 (3.18–4.90), 4.44 (3.57–5.52), and 3.77 (3.03–4.70), respectively. Four studies provided results for the WHO 1999 and IDF 2005 definitions (23,29,31,32). The random-effects summary RRs were 6.19 (3.85–9.95) and 3.95 (3.01–5.19), respectively. Finally, three studies produced information about the WHO 1999 and EGIR 1999 definitions (12,29,31). The random-effects summary RRs were 4.47 (2.79–7.18) and 3.47 (1.81–6.63), respectively.
Sensitivity, specificity, positive predictive value, negative predictive value, and receiver operating characteristic curve
Estimates of sensitivity and specificity were available for ten cohorts (10,20,21,25,27,29,31–33,35), and estimates of positive and negative predictive values were available for six cohorts (25,27,29,31,32,35). Sensitivity ranged from 0.224 to 0.722, and specificity ranged from 0.613 to 0.939 (Table 1). Positive predictive values ranged from 0.078 to 0.36 and negative predictive values range from 0.90 to 0.983. Receiver operating characteristic curves were generated for six cohorts with the area under the receiver operating characteristic curve (aROC), ranging from 0.68 to 0.85 (10,20,23,26,32,35). For studies comparing existing definitions, differences among the aROCs were small and generally nonsignificant (20,23,26,35).
Table 1.
Sensitivity, specificity, positive predictive value, negative predictive value, and aROC reported from prospective studies reporting on the association between the metabolic syndrome and incident diabetes
| Reference number | Year | Metabolic syndrome definition | Sensitivity | Specificity | PPV | NPV | aROC |
|---|---|---|---|---|---|---|---|
| 33 | 2002 | Modified WHO 1998, WHR ≥0.91 | 0.67 | 0.80 | |||
| Modified WHO 1998, WC ≥94 cm | 0.57 | 0.83 | |||||
| NCEP 2001, WC >102 cm | 0.41 | 0.90 | |||||
| NCEP 2001, WC >94 cm | 0.49 | 0.84 | |||||
| 20 | 2003 | NCEP 2001 | 0.528 | 0.849 | 0.308 | 0.934 | 0.776 |
| Modified WHO 1999 | 0.428 | 0.872 | 0.304 | 0.921 | 0.762 | ||
| 21 | 2004 | NCEP 2001 (SAHS) | 0.662 | 0.722 | |||
| NCEP 2001 (MCDS) | 0.624 | 0.613 | |||||
| 35 | 2005 | NCEP 2001 | 0.50 | 0.82 | 0.36 | 0.90 | 0.75 |
| NCEP 2001, FPG 100–125 mg/dl | 0.64 | 0.74 | 0.32 | 0.91 | 0.75 | ||
| NCEP 2001, augmented | 0.81 | 0.61 | 0.29 | 0.94 | 0.78 | ||
| 23 | 2005 | NCEP 2001 | 0.69 | ||||
| WHO 1999 | 0.68 | ||||||
| IDF 2005 | 0.68 | ||||||
| 10 | 2005 | Number of abnormalities over 10 years | 0.626 | 0.747 | 0.72 | ||
| Number of abnormalities over 20 years | 0.539 | 0.757 | 0.70 | ||||
| 25 | 2007 | IDF 2005 | 0.703 | 0.746 | |||
| AHA/NHLBI 2005 | 0.611 | 0.831 | |||||
| Modified WHO 1999 | 0.549 | 0.866 | |||||
| 26 | 2007 | AHA/NHLBI 2005 | 0.82–0.85 | ||||
| IDF 2005 | 0.82–0.85 | ||||||
| EGIR 1999 | 0.78–0.84 | ||||||
| 27 | 2007 | NCEP 2001 | 0.258 | 0.939 | 0.246 | 0.943 | |
| NHLBI/AHA 2004 | 0.342 | 0.919 | 0.244 | 0.948 | |||
| AHA/NHLBI 2005 | 0.419 | 0.875 | 0.205 | 0.951 | |||
| IDF 2005 | 0.317 | 0.902 | 0.199 | 0.945 | |||
| 29 | 2007 | Modified WHO 1999 | 0.421 | 0.882 | 0.268 | 0.937 | |
| NCEP 2001 | 0.389 | 0.850 | 0.208 | 0.932 | |||
| EGIR 1999 | 0.224 | 0.921 | 0.224 | 0.921 | |||
| IDF 2005 | 0.240 | 0.907 | 0.209 | 0.921 | |||
| IDF 2005 with WHO obesity | 0.464 | 0.838 | 0.226 | 0.939 | |||
| NCEP 2001 with WHO obesity | 0.553 | 0.793 | 0.214 | 0.946 | |||
| IDF 2005 with reduced WC | 0.498 | 0.792 | 0.196 | 0.939 | |||
| 31 | 2008 | WHO 1999 | 0.564 | 0.829 | 0.115 | 0.980 | |
| NCEP 2004 | 0.622 | 0.764 | 0.099 | 0.981 | |||
| EGIR 1999 | 0.464 | 0.859 | 0.116 | 0.976 | |||
| IDF 2005 | 0.649 | 0.713 | 0.082 | 0.981 | |||
| 32 | 2008 | Modified WHO 1999 | 0.5479 | 0.9081 | 0.1973 | 0.9799 | |
| NCEP 2001 | 0.6075 | 0.7327 | 0.0863 | 0.9782 | 0.72 | ||
| IDF 2005 | 0.7219 | 0.6468 | 0.0779 | 0.9824 | |||
| NHLBI/AHA 2004 | 0.7043 | 0.6999 | 0.0889 | 0.9827 | 0.74 |
ADA, American Diabetes Association; FPG, fasting plasma glucose; MCDS, Mexico City Diabetes Study; NPV, negative predictive value; PPV, positive predictive value; SAHS, San Antonio Heart Study; WC, waist circumference; WHR, waist-to-hip ratio.
Number of metabolic syndrome components
Several studies examined the risk associated with increasing number of components of the metabolic syndrome (Table 2). In these studies, subjects with zero abnormalities formed the reference group. Three studies based their criteria on those of the NCEP 2001 definition. The estimated RRs for participants in the West of Scotland Coronary Prevention Study (8) were 7.26 (95% CI: 2.25–23.40) for those with three abnormalities and 24.4 (7.53–79.60) for those with four or more abnormalities. Estimated RRs for participants with three abnormalities and four or more abnormalities were 4.56 (2.48–8.78) and 10.88 (5.77–20.50), respectively, in the British Regional Heart Study (10). In the Framingham Offspring Study, the estimated RR for participants with three or more abnormalities was 23.83 5.80–98.01) among men and 29.69 (9.10–96.85) among women (22). Furthermore, at least two other studies using criteria not based on one of the major definitions also showed substantially elevated estimated RRs associated with three and four or more of abnormalities (28,36).
Table 2.
Estimates of OR, RR, or hazard ratio (HR) using zero abnormalities as the reference category from prospective studies reporting on the association between the metabolic syndrome and incident diabetes
| Reference number | Year | Number of criteria | OR/RR/HR | 95% Lower limit | 95% Upper limit |
|---|---|---|---|---|---|
| 36 | 2002 | 3 | 9.37 | 2.22 | 39.59 |
| 4+ | 33.67 | 7.93 | 142.96 | ||
| 8 | 2003 | 3 | 7.26 | 2.25 | 23.4 |
| 4+ | 24.4 | 7.53 | 79.6 | ||
| 22 | 2005 (Men) | 3+ | 23.83 | 5.80 | 98.01 |
| 22 | 2005 (Women) | 3+ | 29.69 | 9.10 | 96.85 |
| 10 | 2005 | 3 | 4.56 | 2.48 | 8.78 |
| 4+ | 10.88 | 5.77 | 20.50 | ||
| 28 | 2007 | 3 | 7.05 | ||
| 4 | 13.39 | 4.26 | 42.08 |
CONCLUSIONS
The prospective studies show that the metabolic syndrome, regardless of how it is defined, is a significant predictor of incident diabetes in many different populations, including Native Americans, U.S. Hispanics, Mexicans, Turks, Iranians, Mauritians, Chinese, Europeans, and those of European descent. Although considerable heterogeneity existed among these studies, the average estimated summary RR of 3.5–5.2 for incident diabetes with any metabolic syndrome criteria is greater than the association of metabolic syndrome with cardiovascular events (∼1.5 to 2.0) (1,11). These data confirm that the metabolic syndrome is significantly more strongly associated with risk for incident diabetes, likely because some of its components (in particular fasting glucose and waist circumference) are more strongly associated with diabetes risk.
The heterogeneity among studies emanates from at least two sources: the origins of the study population and the approach in analyzing the study data. For example, with the NCEP 2001 definition, Native Americans cohorts produced a fixed-effects summary RR of 1.98 (95% CI 1.69–2.32) (18,19), Asian and Mauritius cohorts produced a fixed-effects summary RR of 3.53 (2.96–4.20) (9,12,27,29,32), and North American and European studies produced a random-effects summary RR of 4.13 (3.16–5.40) (8,10,20,21,23,30). The variables used to adjust estimated RRs varied considerably among studies, with some adjusting for nothing, others for multiple factors, and others potentially overadjusting with use of, for example, OGTTs.
Although a variety of diabetes risk prediction models exist, some not requiring any laboratory measures, three direct comparisons of the predictive ability of the metabolic syndrome and a single diabetes risk score, the Diabetes Risk Score, have been conducted (21,29,31). This prediction model includes age, sex, ethnicity, fasting glucose, systolic blood pressure, HDL cholesterol, BMI, and family history of diabetes (37). Thus, four components of the metabolic syndrome as per the NCEP 2001 definition and subsequent revisions (with BMI in lieu of waist circumference) are represented in continuous form in this model. Both in the San Antonio Heart Study and the Mexico City Diabetes Study, the sensitivity of the Diabetes Risk Score was significantly larger than that of metabolic syndrome diagnosis at a given specificity (21). In a study conducted in Mauritius, Cameron et al. concluded that the Diabetes Predicting Model provided somewhat better prediction than the metabolic syndrome (29). Using data of the AusDiab study, Cameron et al. reached a similar conclusion (31).
Furthermore, the concept of risk scores using routinely available or easily collectable data has emerged as an appealing tool for predicting both undiagnosed prevalent diabetes and the risk for future incident diabetes. The Finnish Diabetes Risk Score (FINDRISC)—using categorical variables for age, BMI, waist circumference, history of antihypertensive drugs or high blood glucose, physical activity, and daily consumption of fruit, berries, and vegetables—achieved an aROC between 0.85 and 0.87 for 10-year incident diabetes (38), exceeding the aROC reported in all the metabolic syndrome studies reporting these data (Table 2). However, results from the AusDiab study showed that a modified version of FINDRISC (score >10) had a worse positive predictive value and specificity at a comparable sensitivity than the metabolic syndrome using the NCEP 2001 and IDF 2005 definitions (31). Similarly, the recent German Diabetes Risk Score (comprising age, height, waist circumference, history of hypertension, physical activity, smoking, and dietary factors) has reported an aROC for incident diabetes of between 0.82 and 0.84 (39).
Of the components of the metabolic syndrome, impaired fasting glucose is generally thought to be the strongest predictor of diabetes (8,12,13,22,27,29,31,40). Like that of Cameron et al., most studies reported that concentrations of fasting glucose provided similar or, as in the AusDiab study, better prediction of diabetes than metabolic syndrome diagnosis (31). However, Lorenzo et al. (25) showed that the metabolic syndrome provided additional prediction beyond that provided by impaired fasting glucose alone in the San Antonio Heart Study.
Little research has been conducted examining the predictive ability of the various combinations of metabolic syndrome components in definitions such as the NCEP 2001, NHLBI/AHA 2004, and AHA/NHLBI 2005. One possible reason for this is that many studies have not had sufficient incident events of diabetes to evaluate the many combinations. For example, the NCEP 2001 definition and the two subsequent updates yield 16 combinations of three or more cardiometabolic abnormalities. Using data from the Framingham Offspring Study, Wilson et al. (22) did examine a number of combinations of the five NHLBI/AHA 2004 components and concluded that the RRs of combinations of three abnormalities were not larger than combinations of two factors or, indeed, elevated fasting glucose alone.
Another issue that has received little attention is whether the metabolic syndrome represents more than the sum of its parts. Cameron et al. (29) found that only the WHO 1999 definition—not the NCEP 2001, EGIR 1999, or IDF 2005 definitions—significantly added to the prediction of incident diabetes in models that included the individual components of the metabolic syndrome. Using data from the West of Scotland Coronary Prevention Study, Sattar et al. (8) concluded that the metabolic syndrome was not a significant predictor of incident diabetes once its components were accounted for.
A number of researchers have examined the effect of changing aspects of the definition of the metabolic syndrome on the predictive ability of the syndrome for diabetes. Lowering waist circumference or glucose thresholds generally increased the prevalence of the metabolic syndrome and the sensitivity but at the expense of specificity (8,32,33,35).
Some of the most extensive efforts at improving the predictive ability of the metabolic syndrome, starting with the NCEP 2001 definition, were conducted by Hanley et al. (23) using data from the Insulin Resistance Atherosclerosis Study. To some degree, the numerous changes that the authors tested affected the prevalence of the metabolic syndrome, the ORs for incident diabetes, and the population-attributable risk. The lowest aROCs of 0.66 were observed for definitions that required fasting plasma glucose or elevated waist circumference as mandatory components. The highest aROC of 0.72 was observed for a definition that included the insulin sensitivity index as a sixth component.
A couple of investigations also compared the predictive ability of the metabolic syndrome with that of individual components of the metabolic syndrome or ones closely related to it. In the San Antonio Heart Study, Lorenzo et al. (20) reported that the aROC for NCEP 2001 was similar to that for the 2-h glucose value. Hanley et al. (23) examined the predictive ability of impaired glucose tolerance, quantitative insulin sensitivity check index, insulin sensitivity, the ratio of concentrations of triglycerides to HDL cholesterol, hypertriglyceridemic waist, and concentrations of C-reactive protein compared with NCEP 2001, WHO 1999, and IDF 2005 definitions of metabolic syndrome. The prevalence of these conditions ranged from a low of 18.4% for the hypertriglyceridemic waist to a high of 69.6% for a concentration of C-reactive protein ≥1 mg/l. A concentration of C-reactive protein ≥3 mg/l yielded the lowest OR for incident diabetes (1.83 [95% CI: 1.23–2.74]), aROC (0.60), and population-attributable risk (19.4%), whereas impaired glucose tolerance yielded the highest OR (5.42 [3.60–8.17]), aROC (0.72), and population-attributable risk (58.47%).
Our study illustrates that the metabolic syndrome is a fairly strong predictor of incident diabetes in many populations and that it predicts diabetes more strongly than it predicts coronary heart disease events. However, whether the metabolic syndrome was ever intended to be more strongly predictive of diabetes than cardiovascular disease is not entirely clear. Additional comparisons of the metabolic syndrome to simple, readily available non–laboratory-based questionnaires are needed because questionnaires appear better placed as initial screening tools to identify high-risk individuals, whose subsequent evaluation would incorporate the results from blood tests. Moreover, on the balance of current but limited evidence, it appears that the metabolic syndrome may not add to the prediction of incident diabetes beyond its components, in particular impaired fasting glucose, although more data are needed to confirm this. The most successful attempts to improve the predictive ability of the metabolic syndrome appear to have revolved around creating more of a continuous scale from its components, emphasizing loss of power from a dichotomous use of data. Current evidence casts substantial doubt on the clinical value of diagnosing the metabolic syndrome to identify those at elevated risk for diabetes.
Supplementary Material
Published ahead of print at http://care.diabetesjournals.org on 30 June 2008.
The findings and conclusions in this article are those of the authors and do not represent the official position of the Centers for Disease Control and Prevention.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Ford ES: Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28:1769–1778, 2005 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization: Definition, diagnosis, and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part I: diagnosis and classification of diabetes mellitus. Geneva, World Health Org., 1999. (WHO/NCO/NCS/99.2)
- 3.Balkau B, Charles MA: Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 16:442–443, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C: Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109:433–438, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Zimmet P, Shaw J: The metabolic syndrome–a new worldwide definition. Lancet 366:1059–1062, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F: Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J: Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 108:414–419, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Lim HS, Lip GY, Beevers DG, Blann AD: Factors predicting the development of metabolic syndrome and type II diabetes against a background of hypertension. Eur J Clin Invest 35:324–329, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Shaper AG, Lennon L, Morris RW: Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 165:2644–2650, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM: Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 49:403–414, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Wang JJ, Hu G, Miettinen ME, Tuomilehto J: The metabolic syndrome and incident diabetes: assessment of four suggested definitions of the metabolic syndrome in a Chinese population with high post-prandial glucose. Horm Metab Res 36:708–715, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Wang JJ, Li HB, Kinnunen L, Hu G, Jarvinen TM, Miettinen ME, Yuan S, Tuomilehto J: How well does the metabolic syndrome defined by five definitions predict incident diabetes and incident coronary heart disease in a Chinese population? Atherosclerosis 192:161–168, 2007 [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7:177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Greenland S: Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30:1–30, 1987 [DOI] [PubMed]
- 16.Tobias A: Assessing the influence of a single study in meta-analysis. Stata Tech Bull 47:15–17, 1999 [Google Scholar]
- 17.Egger M, Davey SG, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson RL, Imperatore G, Bennett PH, Knowler WC: Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 51:3120–3127, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Resnick HE, Jones K, Ruotolo G, Jain AK, Henderson J, Lu W, Howard BV: Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care 26:861–867, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM: The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 26:3153–3159, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Stern MP, Williams K, Gonzalez-Villalpando C, Hunt KJ, Haffner SM: Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 27:2676–2681, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB: Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112:3066–3072, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hanley AJ, Karter AJ, Williams K, Festa A, D’Agostino RB, Jr, Wagenknecht LE, Haffner SM: Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation 112:3713–3721, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB: Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 91:2906–2912, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo C, Williams K, Hunt KJ, Haffner SM: The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 30:8–13, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Meigs JB, Rutter MK, Sullivan LM, Fox CS, D’Agostino RB, Sr, Wilson PW: Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care 30:1219–1225, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Cheung BM, Wat NM, Man YB, Tam S, Thomas GN, Leung GM, Cheng CH, Woo J, Janus ED, Lau CP, Lam TH, Lam KS: Development of diabetes in Chinese with the metabolic syndrome: a 6-year prospective study. Diabetes Care 30:1430-1436, 2007 [DOI] [PubMed]
- 28.Elwood PC, Pickering JE, Fehily AM: Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health 61:695–698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron AJ, Zimmet PZ, Soderberg S, Alberti KG, Sicree R, Tuomilehto J, Chitson P, Shaw JE: The metabolic syndrome as a predictor of incident diabetes mellitus in Mauritius. Diabet Med 24:1460–1469, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mannucci E, Monami M, Cresci B, Pala L, Bardini G, Petracca MG, Dicembrini I, Pasqua A, Buiatti E, Rotella CM: National Cholesterol Education Program and International Diabetes Federation definitions of metabolic syndrome in the prediction of diabetes. Results from the FIrenze-Bagno A Ripoli study. Diabetes Obes Metab 10:430–435, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Cameron AJ, Magliano DJ, Zimmet PZ, Welborn TA, Colagiuri S, Tonkin AM, Shaw JE: The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med Epub ahead of print, 2008 [DOI] [PubMed]
- 32.Hadaegh F, Ghasemi A, Padyab M, Tohidi M, Azizi F: The metabolic syndrome and incident diabetes: Assessment of alternative definitions of the metabolic syndrome in an Iranian urban population. Diabetes Res Clin Pract 80:328–334, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA: Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol 156:1070-1077, 2002 [DOI] [PubMed]
- 34.Onat A, Hergenc G, Keles I, Dogan Y, Turkmen S, Sansoy V: Sex difference in development of diabetes and cardiovascular disease on the way from obesity and metabolic syndrome. Metabolism 54:800–808, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt MI, Duncan BB, Bang H, Pankow JS, Ballantyne CM, Golden SH, Folsom AR, Chambless LE: Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 28:2013–2018, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Klein BE, Klein R, Lee KE: Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 25:1790–1794, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Stern MP, Williams K, Haffner SM: Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 136:575–581, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Lindstrom J, Tuomilehto J: The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26:725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, Pfeiffer AF, Spranger J, Thamer C, Haring HU, Fritsche A, Joost HG: An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 30:510–515, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Macchia A, Levantesi G, Borrelli G, Franzosi MG, Maggioni AP, Marfisi R, Scarano M, Tavazzi L, Tognoni G, Valagussa F, Marchioli R: A clinically practicable diagnostic score for metabolic syndrome improves its predictivity of diabetes mellitus: the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)-Prevenzione scoring. Am Heart J 151:754, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.