Abstract
Background
The purpose of this study was to determine the prevalence of claustrophobia in patients undergoing magnetic resonance imaging (MRI) after coronary artery bypass graft (CABG) surgery.
Methods
After IRB approval, we conducted a substudy of a prospective randomized controlled clinical trial of 311 patients evaluating administration of tranexamic acid and early saphenous vein graft patency with MRI after conventional CABG surgery. Chest tube drainage was measured at 6, 12, and 24 hours after surgery. The rate of transfusion and the amount of red blood cells (RBC), fresh frozen plasma (FFP), and platelets transfused were recorded.
Results
A total of 237(76%) patients underwent MRI after surgery. 39 (14%, [95% CI, 10.2 to 18.0]) patients experienced severe anxiety caused by a fear of enclosed space in the MRI coil necessitating termination of the procedure. Patients with claustrophobia were on average 5 years younger. They were more likely to have diabetes mellitus and hypertension. Patients with claustrophobia had increased chest tube drainage during the postoperative period. The rate of blood product transfusion was similar between the two groups but patients with claustrophobia who were transfused received significantly more RBC and FFP than patients without claustrophobia.
Conclusions
Postoperative claustrophobia and anxiety, leading to inability to undergo MRI, may be more common than previously described.
Keywords: Claustrophobia, magnetic resonance imaging, cardiac surgery, bleeding
Introduction
Magnetic resonance imaging (MRI) is increasingly used as a tool for assessing both ischemic brain injury as well as coronary graft patency after coronary revascularization surgery. Claustrophobia and anxiety related symptoms are frequent causes of failure in completing postoperative MRI. The reported incidence of anxiety-related reactions during MRI ranges from 4% to 30% in the general population with 3%–5% of patients unable to complete MRI examination (Hricak and Amparo 1984; Quirk et al 1989; Melendez and McCrank 1993).
Difficulty in completing the MRI assessment may lead to underestimation of the true incidence of neurological injury and graft patency after coronary artery bypass graft (CABG) surgery.
The aim of this study was to determine the prevalence of new onset claustrophobia in patients undergoing MRI after CABG surgery and to evaluate any contributing factors.
Materials and methods
This is a substudy of a prospective randomized controlled clinical trial of 311 patients scheduled for elective CABG surgery with cardiopulmonary bypass (CPB) evaluating the effects of tranexamic acid (TA) on early saphenous vein graft patency after surgery (Karski et al 2005). During the original study, all patients were preoperatively screened for signs of claustrophobia. The detailed interview about a fear of enclosed places was conducted. All patients were shown photographs of the MRI coil, and were given meticulous description and explanation of the MRI suite and the procedure itself. Patients with preoperative history of claustrophobia were excluded.
Other exclusion criteria were bleeding disorders, preoperative anemia, symptomatic peripheral vascular disease, connective tissue disease, age over 80 years, impaired renal and liver functions, history of psychiatric disorders and congestive heart failure, and known contraindications to MRI. Postoperative MRI was used to assess saphenous vein graft patency in the early postoperative period when patients were awake, extubated and hemodynamically stable.
Based on presence or absence of new onset claustrophobia during the MRI procedure, patients were classed into two groups: claustrophobia group and controls.
All patients received premedication with lorazepam 2 mg 1–2 h prior to surgery. Anesthetic technique was standardized to include fentanyl 10–20 mcg/kg, midazolam 0.1 mg/kg, pancuronium 0.15–0.20 mg/kg, and isoflurane 0.5%–1.5%. After surgery, patients were transferred to intensive care unit (ICU) for postoperative ventilation. Sedation was achieved with propofol infusion 0.5–4 mg/kg/h and morphine boluses. Patients were extubated according to the following criteria: patient responsive and cooperative, SaO2≥ 94% with FiO2 ≤ 60%, complete reversal of neuromuscular blockade, PaCO2 35–55 mmHg, stable hemodynamics, absence of uncontrolled arrhythmia, and nasopharyngeal temperature >36 °C.
Anticoagulation was achieved with heparin to maintain an activated clotting time (ACT) above 480 seconds. The CPB circuit was primed with 1.8 L of Ringer’s lactate solution and 50 cc of 20% mannitol. Albumin (25%) and synthetic colloids (Pentaspan®; Bristol-Myers Squibb) were added to the circuit as needed. Management of CPB included systemic temperature drift to 34 °C, alpha-stat pH management, targeted mean perfusion pressure between 50–70 mmHg, and pump flow rates of 2.0–2.4 L/min/m2. Myocardial protection was achieved with intermittent antegrade, and occasionally retrograde blood cardioplegia. After separation from CPB, heparin was neutralized with protamine 1 mg per 100 U of heparin, to achieve an ACT within 10% of the baseline.
Red blood cell concentrates were transfused to maintain hematocrit concentration at or above 20% during CPB, and at or above 24%–27% after CPB. Indication for platelet transfusion included a platelet count of <50 × 103/mm3, and a platelet count <80 × 103/mm3 in the presence of ongoing hemorrhage after complete reversal of heparin with protamine. Fresh frozen plasma was transfused to bleeding patients if the INR > 1.5 after complete neutralization of heparin with protamine. After surgery, chest tube drainage was collected in a sterile cardiotomy reservoir and autotransfused if drainage exceeded 150 ml for the first 6 hours after surgery. Total chest tube drainage was measured at 6, 12, and 24 hours after surgery. The rate of transfusion as well as the amount of red blood cells (RBC), fresh frozen plasma (FFP), and platelet transfusion were recorded for up to 24 hours after surgery.
Statistical analyses were performed using the SPSS system (SPSS Inc., Chicago, IL). Comparability of both groups was tested with chi-square statistics on qualitative variables and one-way analysis of variance on quantitative variables. P value of 0.05 was considered statistically significant.
Results
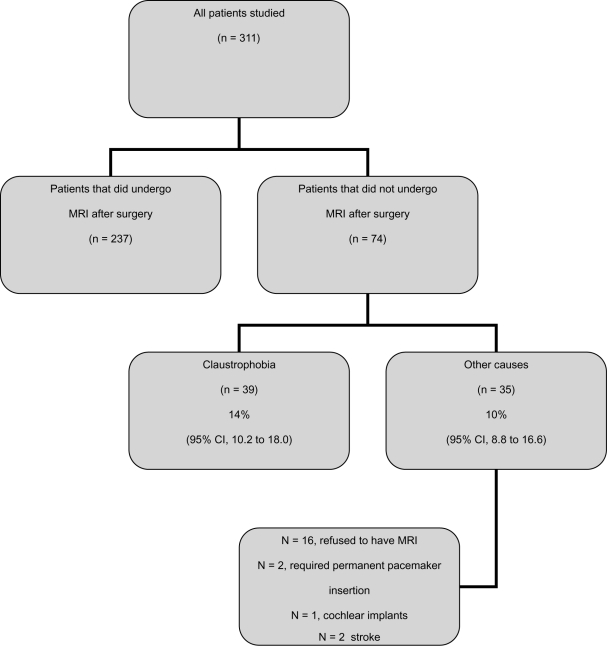
A total of 237 (76%) patients underwent coronary MRI procedure 5–30 days after surgery. There was no difference between the claustrophobia and controls groups with respect to the timing of MRI procedure. Median time for claustrophobia group was 13 days (range, 5–30 days), and 10 days for controls (range, 5–30 days), p = 0.84. None of these patients received any sedation before, during or after MRI assessment. 74 (24%) patients did not undergo MRI procedure; 39 (14%, [95% CI, 10.2 to 18.0]) patients started MRI assessment but were unable to finish it due to severe anxiety caused by a fear of enclosed space in the MRI coil. However, as soon as these patients were removed from the MRI coil, the anxiety subsided without requirements for any medication. 35 (10%; [95% CI, 8.8 to 16.6]) patients did not have MRI for a variety of reasons (16 patients refused to have MRI; 2 patients required permanent pacemaker insertion; 1 patient had cochlear implants; 2 patients had stroke; 4 patients died; and 10 patients had no MRI slot available) (Figure 1).
Figure 1.
Schematic diagram.
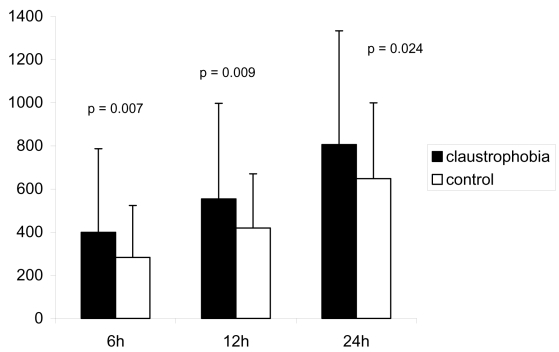
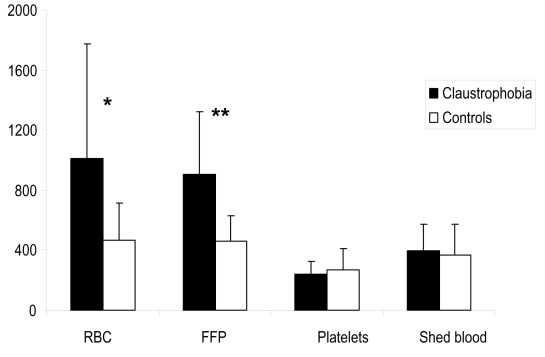
Patients with claustrophobia were on average 5 years younger. They were more likely to have diabetes mellitus and hypertension. There was no difference in gender, body surface area, left ventricle function, preoperative creatinine, and hematological parameters between the two groups. History of active smoking, self-reported alcohol consumption, and benzodiazepine treatment were similar between the two groups (Table 1). Patients with claustrophobia had increased chest tube drainage during the 6, 12, and 24 hours postoperatively (Figure 2). The rate of blood product transfusion was similar between the two groups (Table 2). However, patients with claustrophobia who were transfused received significantly more volume of RBC and FFP than patients without claustrophobia (Figure 3). There was no difference in the discharge plasma hemoglobin concentration between the claustrophobia and control groups, respectively (93.5 ± 17.5 vs. 95.5 ± 13.8; p = 0.4). The rates of postoperative myocardial infarction, atrial fibrillation, stroke, and duration of hospital length of stay were similar between the two groups.
Table 1.
Demographic variables and surgical characteristics. Data is expressed as mean ± standard deviation or number of patients (%)
| Claustrophobia group (n = 39) | Controls (n = 237) | P value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, yr | 55.8 ± 9.8 | 60.2 ± 8.2 | 0.005 |
| Male, n (%) | 33 (85) | 213 (90) | NS |
| Body surface area, m2 | 1.9 ± 0.18 | 1.9 ± 0.18 | NS |
| Left ventricular grade (1–4) | 1.9 ± 0.18 | 1.9 ± 0.17 | NS |
| Active smoking, n (%) | 3 (7.6) | 42 (17.5) | NS |
| Alcohol consumption (>2 drink/day), n (%) | 5 (12.5) | 52 (21) | NS |
| Coexisting illness, n (%) | |||
| Diabetes | 17 (43) | 64 (27) | 0.05 |
| Hypertension | 27 (69) | 123 (52) | 0.045 |
| Peripheral vascular disease | 11 (28) | 76 (32) | NS |
| Myocardial infarction | 18 (46) | 114 (48) | NS |
| Stroke | 1 (2.5) | 5 (2.1) | NS |
| Preoperative medication, n (%) | |||
| Beta blockers | 24 (61) | 140 (59) | NS |
| Calcium channel blockers | 17 (44) | 96 (40) | NS |
| Aspirin | 31 (79) | 194 (82) | NS |
| NSAIDs | 1 (2.5) | 4 (1.7) | NS |
| Benzodiazepines | 2 (5) | 22 (9.2) | NS |
| Preoperative laboratory variables | |||
| Hemoglobin, g/dL | 140.7 ± 14.7 | 142.9 ± 11.4 | NS |
| Platelet count, 103/mm3 | 229 ± 66.7 | 226 ± 55.0 | NS |
| Prothrombin time, sec | 11.0 ± 1.3 | 10.9 ± 1.2 | NS |
| Partial thromboplastin time, sec | 32.9 ± 21.5 | 31.4 ± 20.4 | NS |
| Creatinine, mg/dL | 88.7 ± 16.7 | 93.2 ± 18.4 | NS |
| Intraoperative variables | |||
| CPB time (min) | 83.5 ± 19.8 | 80.1 ± 20.4 | NS |
| Cross clamp time (min) | 65.2 ± 16.1 | 63.8 ± 17.7 | NS |
| Minimum temperature during cardiopulmonary bypass, °C | 33.8 ± 0.8 | 33.5 ± 1.2 | NS |
| Saphenous vein grafts, n | 2.8 ± 0.72 | 2.7 ± 0.72 | NS |
Abbreviations: NS, not significant; NSAIDs, nonsteroidal anti-inflammatory drugs.
Figure 2.
Comparison of chest tube drainage at 6, 12, and 24 hours after surgery.
Table 2.
Blood product transfusion rates in patients with and without claustrophobia. Data is expressed as number of patients (%)
| Claustrophobia group (n = 39) | Controls (n = 237) | P value | |
|---|---|---|---|
| Red blood cells | 7 (18) | 45 (19) | NS |
| Platelets | 2 (5) | 4 (1.7) | NS |
| Fresh frozen plasma | 3 (7) | 10 (4) | NS |
| Shed blood | 8 (20) | 28 (12) | NS |
Abbreviation: NS, not significant.
Figure 3.
Comparison of the amount (mL) of blood product transfusion.
Notes: *p = 0.001; **p = 0.008.
Abbreviations: RBC, red blood cells; FFP, fresh frozen plasma.
Discussion
The main finding of this study was that the overall incidence of claustrophobia leading to cancellation of postoperative MRI was 14% (39/276). The rate of claustrophobia in our study considerably exceeded the previously reported rates of anxiety reactions in adult patients referred for MRI procedures either for clinical or research purposes varying from 1.2% to 5%. (Hricak and Amparo 1984; Brennan et al 1988; Quirk et al 1989; Kilborn and Labbe 1990; Melendez and McCrank 1993).
The clinical range of neurological abnormalities after cardiac surgery varies from mild behavioral changes to clinically apparent stroke. Claustrophobia is classed as an anxiety related disorder. Anxiety is often a consequence of cardiac surgery with the reported incidence in the first week after CABG surgery of 37% (Andrew et al 2000). The pathophysiology of new onset anxiety after cardiac surgery is uncertain. However, one can hypothesize that postoperative anxiety disorders including claustrophobia may be a manifestation of mild neurological impairment secondary to embolism, brain hypoperfusion, cerebral edema, and inflammatory response associated with CPB. Yin and colleagues (2007) found that patients undergoing CABG surgery with CPB suffered from more anxiety than patients without CPB.
Most of the previous studies on MRI-associated anxiety and claustrophobia have been concentrating on psychological aspects of these conditions (Quirk et al 1989; Kilborn and Labbe 1990; Melendez and McCrank 1993).
However, new onset claustrophobia after cardiac surgery with CPB could be potentially triggered by other mechanisms like neurological injury or inflammatory response. Another mechanism of postoperative anxiety might be linked to perioperative use of different sedative medications (eg, benzodiazepines, narcotic analgesics). However, postoperative sedation and pain control was standardized according to the current institutional practice. Furthermore, there was no difference with respect to preoperative use of benzodiazepines, alcohol consumption and smoking between the two groups. It has been previously reported that administration of benzodiazepine sedation prior to MRI procedure considerably decreased the incidence of claustrophobia (Francis and Pennell 2000). However, none of the patients in our study received any sedation prior to MRI. The anxiety subsided without any medication as soon as the patients were removed from the MRI coil.
Interestingly, patients who developed claustrophobia were transfused significantly greater volume of RBC and FFP than the controls. Furthermore, there was a trend towards increased rate of shed blood transfusion in patients with claustrophobia. Unprocessed shed blood contains high levels of cellular debris and lipid microparticulates (Booke et al 1997), which have been shown to cause microembolization to the brain blood vessels (Brooker et al 1998). Recently, we showed that processing of shed blood with a continuous flow cell-saver resulted in better preservation of cognitive function in patients undergoing CABG surgery (Djaiani et al 2007). It is recognized that allogenic blood transfusion and reinfusion of shed blood exacerbate inflammatory response mediated by cardiac surgery and CPB. Fransen and colleagues (1999) reported that packed red cell transfusion during cardiac surgery exacerbated the perioperative release of inflammatory mediators. Westerberg and colleagues (2004) have demonstrated that plasma concentrations of tumor necrosis factor-α, interleukin-6, and C3a were elevated in all patients after CABG surgery with CPB, but significantly more in patients receiving autotransfusion postoperatively. There is growing body of evidence indicating that activation of inflammatory response mediators is involved in the pathophysiology of anxiety-related reactions (Maes et al 1998, 1999; Bluthe 2000a). Studies in animals and humans clearly have demonstrated that activation of proinflammatory cytokines, may induce anhedonia, depressive symptoms, and anxiety (Maes et al 1998, 1999; Bluthe et al 2000a, 2000b). Furthermore, Maes and colleagues (2000) has demonstrated that women with postpartum anxiety had significantly higher serum interleukin-6 and interleukin-1 receptor antagonist levels than parturients without anxiety. It is possible that one of the mechanisms of postoperative anxiety and claustrophobia may be associated with activation of inflammatory system triggered by transfusion of blood products and shed blood.
This study has several limitations. First, we did not formally measure level of preoperative anxiety in our patients, the factor that can significantly influence postoperative anxiety related reactions. However, all patients were screened for signs of claustrophobia. The detailed interview about a fear of enclosed places was conducted. All patients were shown photographs of the MRI coil, and were given meticulous description and explanation of the MRI suite and the procedure itself. Furthermore, patients with preoperative history of claustrophobia were excluded. Second, we did not perform a formal neuropsychological examination of patients with claustrophobia. However, the present sample size of 39 patients with claustrophobia was too small to make a definitive statement regarding the neurocognitive function. Third, there were 16 patients who refused to have the MRI procedure after surgery. It is possible that these patients did not want to undergo MRI procedure due to elevated anxiety levels, and consequently the prevalence of claustrophobia could have even been higher than currently reported. Fourth, we did not measure plasma levels of inflammatory markers perioperatively. Consequently, the causality of an association between inflammatory response, organic brain injury and postoperative anxiety related reactions would require further testing in future studies.
In summary, the overall rate of new onset claustrophobia leading to cancellation of MRI after cardiac surgery was 14% (95% CI, 10.2 to 18.0). Based on our observations, postoperative claustrophobia and anxiety leading to inability to complete MRI procedure may be more common than previously described. Further investigations are required to identify contributing factors and potential treatment modalities in reducing the incidence of new onset claustrophobia.
References
- Andrew MJ, Baker RA, Kneebone AC, et al. Mood state as a predictor of neuropsychological deficits following cardiac surgery. J Psychosom Res. 2000;48:537–46. doi: 10.1016/s0022-3999(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Laye S, Michaud B, et al. Role of interleukin-1beta and tumour necrosis factor-alpha in lipopolysaccharide-induced sickness behaviour: a study with interleukin-1 type I receptor-deficient mice. Eur J Neurosci. 2000;12:4447–56. [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, et al. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol Behav. 2000;70:367–73. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Booke M, Fobker M, Fingerhut D, et al. Fat elimination during intraoperative autotransfusion: an in vitro investigation. Anesth Analg. 1997;85:959–62. doi: 10.1097/00000539-199711000-00002. [DOI] [PubMed] [Google Scholar]
- Brennan SC, Redd WH, Jacobsen PB, et al. Anxiety and panic during magnetic resonance scans. Lancet. 1988;2:512. doi: 10.1016/s0140-6736(88)90159-6. [DOI] [PubMed] [Google Scholar]
- Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–5. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- Djaiani G, Fedorko L, Borger MA, et al. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–95. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- Francis JM, Pennell DJ. Treatment of claustrophobia for cardiovascular magnetic resonance: use and effectiveness of mild sedation. J Cardiovasc Magn Reson. 2000;2:139–41. doi: 10.3109/10976640009148683. [DOI] [PubMed] [Google Scholar]
- Fransen E, Maessen J, Dentener M, et al. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–9. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- Hricak H, Amparo EG. Body MRI. Alleviation of claustrophobia by prone positioning. Radiology. 1984;152:819. doi: 10.1148/radiology.152.3.6463267. [DOI] [PubMed] [Google Scholar]
- Karski J, Djaiani G, Fedorko L, et al. Perioperative use of antifibrinolytics does not compromise early graft patency after coronary revascularization surgery. J Thorac Cardiovasc Surg. 2005;130:309–14. doi: 10.1016/j.jtcvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Kilborn LC, Labbe EE. Magnetic resonance imaging scanning procedures: development of phobic response during scan and at one-month follow-up. J Behav Med. 1990;13:391–401. doi: 10.1007/BF00844886. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–18. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Delmeire L, et al. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry. 1999;45:833–9. doi: 10.1016/s0006-3223(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25:121–37. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 1993;270:745–7. doi: 10.1001/jama.1993.03510060091039. [DOI] [PubMed] [Google Scholar]
- Quirk ME, Letendre AJ, Ciottone RA, et al. Anxiety in patients undergoing MR imaging. Radiology. 1989;170:463–6. doi: 10.1148/radiology.170.2.2911670. [DOI] [PubMed] [Google Scholar]
- Westerberg M, Bengtsson A, Jeppsson A. Coronary surgery without cardiotomy suction and autotransfusion reduces the postoperative systemic inflammatory response. Ann Thorac Surg. 2004;78:54–9. doi: 10.1016/j.athoracsur.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Yin YQ, Luo AL, Guo XY, et al. Postoperative neuropsychological change and its underlying mechanism in patients undergoing coronary artery bypass grafting. Chin Med J (Engl) 2007;120:1951–7. [PubMed] [Google Scholar]