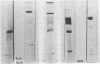
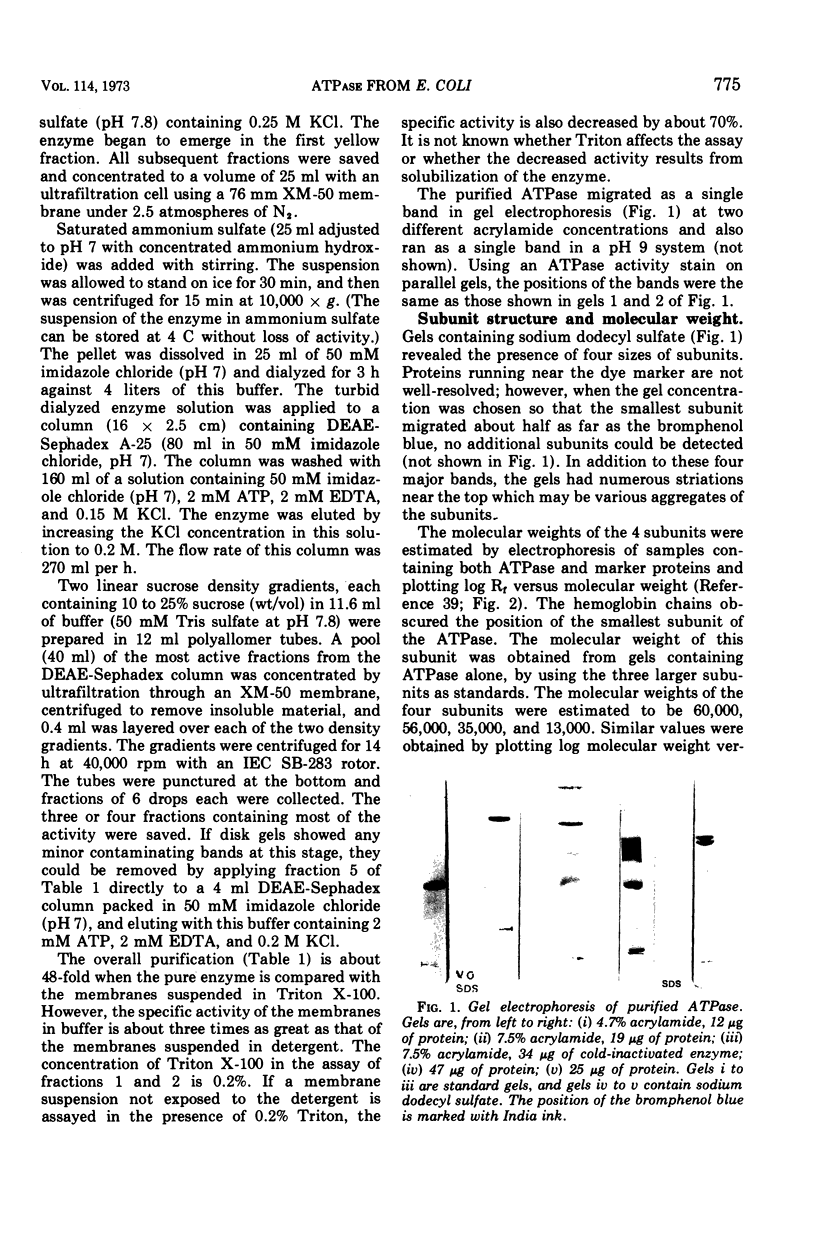
Abstract
The membrane adenosine triphosphatase (E.C. 3.6.1.3) from Escherichia coli has been solubilized with Triton X-100 and purified to near homogeneity. The purified enzyme has a sedimentation coefficient of 12.9S in a sucrose gradient, corresponding to a molecular weight of about 360,000. On electrophoresis in gels containing sodium dodecyl sulfate, it dissociates into subunits with apparent molecular weights of 60,000, 56,000, 35,000, and 13,000. The purified enzyme loses activity and breaks down into subunits when stored in the cold. Guanosine 5′-triphosphate and inosine 5′-triphosphate are alternative substrates. Ca2+ and, to a small extent, Co2+ or Ni2+ will substitute for Mg2+ in the reaction. The Km for Mg-adenosine triphosphate of the membrane-bound enzyme is 0.23 mM, and for the pure enzyme it is 0.29 mM. Azide is a noncompetitive inhibitor of both the membrane-bound enzyme and the pure enzyme. Pi is a noncompetitive inhibitor of the solubilized enzyme. An antibody to the purified enzyme was obtained from rabbits. The antibody inhibits the solubilized enzyme and virtually all of the adenosine triphosphate hydrolysis by membranes from cells grown aerobically or anaerobically. The antibody also inhibits the adenosine triphosphate-stimulated pyridine nucleotide transhydrogenase (E.C. 1.6.1.1) of the E. coli membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams A., Baron C. The isolation and subunit structure of streptococcal membrane adenosine triphosphatase. Biochemistry. 1967 Jan;6(1):225–229. doi: 10.1021/bi00853a035. [DOI] [PubMed] [Google Scholar]
- Abrams A., Smith J. B., Baron C. Carbodiimide-resistant membrane adenosine triphosphatase in mutants of Streptococcus faecalis. I. Studies of the mechanism of resistance. J Biol Chem. 1972 Mar 10;247(5):1484–1488. [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Davies P. L., Hou C. Function of energy-dependent transhydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1248–1255. doi: 10.1016/0006-291x(72)90969-2. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide-adenine dinucleotide oxidation in Escherichia coli particles. 3. Cellular location of menadione reductase and ATPase activities. Can J Biochem. 1967 Jul;45(7):1107–1124. doi: 10.1139/o67-128. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. I. Purification, homogeneity, and physical properties. J Biol Chem. 1971 Aug 25;246(16):4987–4994. [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. L., Bragg P. D. Properties of a soluble Ca 2+ - and Mg 2+ -activated ATPase released from Escherichia coli membranes. Biochim Biophys Acta. 1972 Apr 14;266(1):273–284. doi: 10.1016/0005-2736(72)90142-3. [DOI] [PubMed] [Google Scholar]
- Evans D. J. Membrane Mg-(Ca)-Activated Adenosine Triphosphatase of Escherichia coli: Characterization in the Membrane-Bound and Solubilized States. J Bacteriol. 1970 Dec;104(3):1203–1212. doi: 10.1128/jb.104.3.1203-1212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessenden-Raden J. M. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XX. Characterization of ASU-particles. J Biol Chem. 1969 Dec 25;244(24):6662–6667. [PubMed] [Google Scholar]
- Fessenden J. M., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XI. Stimulation of oxidative phosphorylation by coupling factors and oligomycin; inhibition by an antibody against coupling factor 1. J Biol Chem. 1966 May 25;241(10):2483–2489. [PubMed] [Google Scholar]
- Fisher R. J., Lam K. W., Sanadi D. R. Escherichia coli nicotinamide adenine nucleotide transhydrogenase driven by aerobic oxidation and its enhancement by an energy transfer factor from rat-liver mitochondria. Biochem Biophys Res Commun. 1970;39(6):1021–1025. doi: 10.1016/0006-291x(70)90660-1. [DOI] [PubMed] [Google Scholar]
- Forrest G., Edelstein S. J. On the subunit structure of the cold labile adenosine triphosphatase of mitochondria. J Biol Chem. 1970 Dec 10;245(23):6468–6470. [PubMed] [Google Scholar]
- Guarraia L. J., Peck H. D., Jr Dinitrophenol-stimulated adenosine triphosphatase activity in extracts of Desulfovibrio gigas. J Bacteriol. 1971 Jun;106(3):890–895. doi: 10.1128/jb.106.3.890-895.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Dio 9 and chlorhexidine: inhibitors of membrane-bound ATPase and of cation transport in Streptococcus faecalis. Biochim Biophys Acta. 1969 Jun 3;183(1):129–136. doi: 10.1016/0005-2736(69)90136-9. [DOI] [PubMed] [Google Scholar]
- Ishida M., Mizushima S. The membrane ATPase of Bacillus megaterium. II. Purification of membrane ATPases and their recombination with membrane. J Biochem. 1969 Aug;66(2):133–138. doi: 10.1093/oxfordjournals.jbchem.a129128. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. X. Correlation of morphology and function in submitochondrial particles. J Biol Chem. 1966 May 25;241(10):2475–2482. [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Energy linked nicotinamide adenine dinucleotide transhydrogenase in a mutant of Escherichia coli K12 lacking membrane Mg(2+)&z.sbnd;Ca(2+)-activated adenosine triphosphatase. FEBS Lett. 1972 May 1;22(2):197–199. doi: 10.1016/0014-5793(72)80043-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemme B., Klemme J. H., San Pietro A. PPase, ATPase, and photophosphorylation in chromatophores of Rhodospirillum rubrum: inactivation by phospholipase A; reconstitution by phospholipids. Arch Biochem Biophys. 1971 May;144(1):339–342. doi: 10.1016/0003-9861(71)90486-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Anraku Y. Membrane-bound adenosine triphosphatase of Escherichia coli. I. Partial purification and properties. J Biochem. 1972 Mar;71(3):387–399. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambeth D. O., Lardy H. A. Purification and properties of rat-liver-mitochondrial adenosine triphosphatase. Eur J Biochem. 1971 Oct 14;22(3):355–363. doi: 10.1111/j.1432-1033.1971.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Lambeth D. O., Lardy H. A., Senior A. E., Brooks J. C. Reassessment of the molecular weight of mitochondrial ATPase from beef heart. FEBS Lett. 1971 Oct 1;17(2):330–332. doi: 10.1016/0014-5793(71)80179-5. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mirsky R., Barlow V. Purification and properties of ATPase from the cytoplasmic membrane of Bacillus megaterium KM. Biochim Biophys Acta. 1971 Sep 14;241(3):835–845. doi: 10.1016/0005-2736(71)90011-3. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation and properties of outer and cytoplasmic membranes in Escherichia coli. Biochim Biophys Acta. 1969;193(2):268–276. doi: 10.1016/0005-2736(69)90188-6. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Paetkau V., Lardy H. A. Phosphofructokinase. Correlation of physical and enzymatic properties. J Biol Chem. 1967 May 10;242(9):2035–2042. [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S., Warner R. C. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J Biol Chem. 1965 Dec;240(12):4694–4702. [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Schatz G., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XIV. J Biol Chem. 1967 May 25;242(10):2552–2560. [PubMed] [Google Scholar]
- Schnebli H. P., Vatter A. E., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Molecular weight, subunit structure, and amino acid composition. J Biol Chem. 1970 Mar 10;245(5):1122–1127. [PubMed] [Google Scholar]
- Senior A. E., Brooks J. C. Studies on the mitochondrial oligomycin-insensitivt ATPase. I. An improved method of purification and the behavior of the enzyme in solutions of various depolymerizing agents. Arch Biochem Biophys. 1970 Sep;140(1):257–266. doi: 10.1016/0003-9861(70)90030-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Hybridization of membranes by sonic irradiation. Biochemistry. 1971 Aug 17;10(17):3309–3313. doi: 10.1021/bi00793a023. [DOI] [PubMed] [Google Scholar]
- Vigers G. A., Ziegler F. D. Azide inhibition of mitochondrial ATPase. Biochem Biophys Res Commun. 1968 Jan 11;30(1):83–88. doi: 10.1016/0006-291x(68)90716-x. [DOI] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Necklen D. K. The redox environment and microbial physiology. I. The transition from anaerobiosis to aerobiosis in continuous cultures of facultative anaerobes. Biochim Biophys Acta. 1971 Dec 7;253(2):352–359. doi: 10.1016/0005-2728(71)90039-9. [DOI] [PubMed] [Google Scholar]