Abstract
Choline is needed for the maintenance of the structural integrity and signaling functions of cell membranes, for neurotransmission, and for transport of lipids and as a source of methyl groups. Choline can be made de novo in the body, but some individuals must also obtain choline in the diet to prevent deficiency symptoms. A number of environmental and genetic factors influence dietary requirements for choline, and average intakes in the population vary widely. Therefore, certain individuals may be at greater risk of choline deficiency. Choline is critical during fetal development, particularly during the development of the brain, where it can influence neural tube closure and lifelong memory and learning functions.
Nutritional Importance of Choline
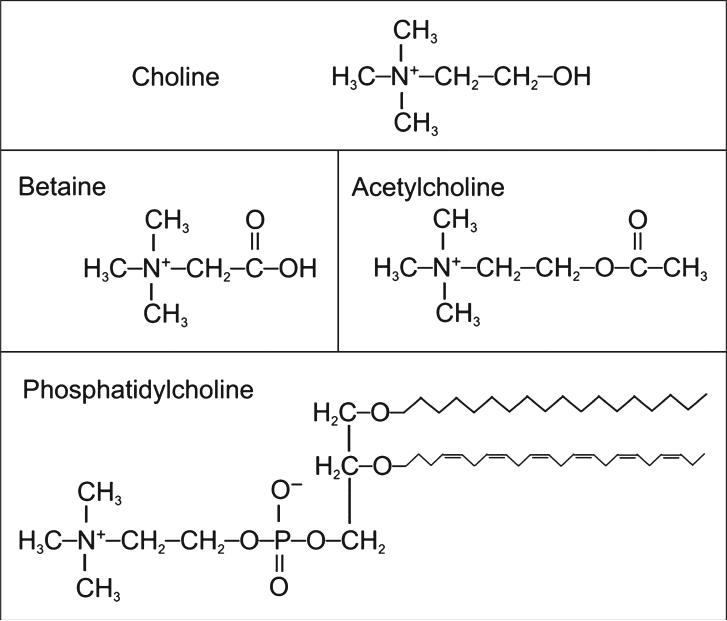
Choline is an essential nutrient for humans and is necessary for the normal function of all cells. As a critical component of the cell membrane, it ensures the structural integrity and signaling functions of the cell. Choline is also used for neurotransmission (as the metabolite, acetylcholine), is a major source of methyl donors, and is required for lipid transport from the liver.1 Considering these many diverse roles, choline deficiency can cause disorders in many bodily systems, including liver, muscle, and lymphocytes in humans and, additionally, the kidney, pancreas, and developing brain and nervous system in animals. A major role of choline is to serve as a precursor for the synthesis of membrane phospholipids, specifically phosphatidylcholine (shown in Figure 1). Phosphatidylcholine is the predominant phospholipid in mammalian membranes (>50%) and is critical for maintaining cell structure and facilitating cell signaling and transport across the membrane.2 Choline can also be found in sphingomyelin, another membrane phospholipid formed from phosphatidylcholine. Most of the phosphatidylcholine is synthesized from free choline using the cytidine diphospho-choline pathway.1 However, about 30% of phosphatidylcholine can be made by the sequential methylation of phosphatidylethanolamine (another membrane phospholipid) by the enzyme phosphatidylethanolamine methyltransferase (PEMT). By this pathway, the body can make choline de novo. However, this pathway is insufficient to supply all of the body's need for choline; thus, choline remains an essential nutrient.
Figure 1.
Structures of important choline-containing molecules.
Choline deficiency causes clinical illness in humans.
Only a small amount of dietary choline is metabolized to form acetylcholine, a neurotransmitter frequently used by the nerves controlling breathing, heart rate, and skeletal muscles. Acetylcholine is also important in the portions of the brain responsible for memory and mood.2 Because the rate of acetylcholine synthesis is most likely determined by choline availability (or, possibly, acetyl-CoA), dietary choline intake may directly affect cholinergic stimulation.
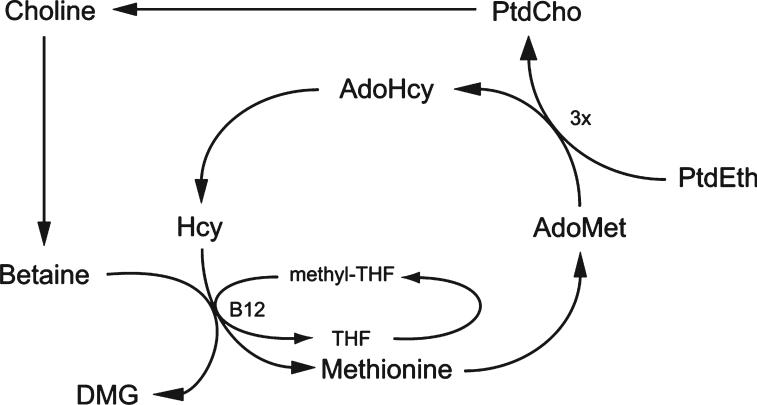
Choline can be metabolized to betaine, which is used as an osmolyte in the kidney and also as a source of methyl groups. There are at least 50 known methylation reactions identified in mammals, with the largest consumers of methyl groups being biosynthetic reactions such as biosynthesis of creatine and phosphatidylcholine.3 However, DNA is also methylated, and this modification can influence gene expression. Alterations in DNA methylation have been linked to diseases such as cancer and syndromes involving chromosomal instabilities.4 DNA methylation is also responsible for imprinting during the prenatal period,5 which may influence disease susceptibility later in life. As a methyl donor, betaine metabolism is intimately connected to the metabolism of folate, vitamin B12, and methionine, as shown in Figure 2. The role of each of these nutrients in 1 carbon metabolism and their dependence on each other reinforce the physiological importance of methyl group availability.
Figure 2.
Interrelationship of choline, homocysteine, and folate metabolism. PtdCho indicates phosphatidylcholine; PtdEth, phosphatidylethanolamine; Hcy, homocysteine; AdoMet, s-adenosylmethionine; AdoHcy, s-adenosylhomocysteine; THF, tetrahydrofolate; DMG, dimethylglycine.
Phosphatidylcholine is a critical component of very-low-density lipoproteins, which are responsible for transporting triglycerides out of the liver. One of the first clinical signs of dietary choline deficiency is the development of fatty liver (hepatosteatosis) resulting from the lack of phosphatidylcholine to package and export very-low-density lipoproteins.6 Prolonged deficiency of dietary choline in rodents can lead to the spontaneous development of hepatocarcinoma.7 Choline deficiency is the only nutrient deficiency shown to induce the development of spontaneous carcinoma. Liver damage in humans resulting from inadequate dietary choline can also be detected by elevations in serum aminotransferases.8 Similarly, elevations in muscle enzymes (eg, serum creatine phosphokinase) can occur in humans during choline deficiency.9 Muscle membrane integrity seems to be compromised, which may contribute to cell death and leakage of enzymes into the blood.10
Choline and betaine are methyl donors involved in 1 carbon metabolism with vitamin B12, folate, and methionine.
Dietary Requirements and Intake
The human requirement for dietary choline was officially recognized in 1998 with the establishment of adequate intake recommendations by the Food and Nutrition Board of the US Institute of Medicine.11 Before this time, choline was not considered essential because it could be synthesized endogenously via the PEMT pathway. However, studies in patients receiving low-choline solutions intravenously determined that endogenous synthesis was insufficient to prevent liver and muscle dysfunction characteristic of choline deficiency.8,12 The adequate intake and upper limit (UL) values for dietary choline are listed in Table 1. The UL was determined as the lowest level for observed adverse effects (hypotension).
Table 1.
Dietary Reference Intakes for Choline
| Population and Age | Adequate Intake | Tolerable Upper Limit (UL) |
|---|---|---|
| Infants, mo | ||
| 0−6 | 125 mg/d, 18 mg/kg | Not possible to establish |
| 6−12 | 150 mg/d | Not possible to establish |
| Children, y | ||
| 1−3 | 200 mg/d | 1,000 mg/d |
| 4−8 | 250 mg/d | 1,000 mg/d |
| 9−13 | 375 mg/d | 2,000 mg/d |
| Men, y | ||
| 14−18 | 550 mg/d | 3,000 mg/d |
| ≥19 | 550 mg/d | 3,500 mg/d |
| Women, y | ||
| 14−18 | 400 mg/d | 3,000 mg/d |
| ≥19 | 425 mg/d | 3,500 mg/d |
| Pregnant | 450 mg/d | Age-appropriate UL |
| Lactating | 550 mg/d | Age-appropriate UL |
Data from the Institute of Medicine, National Academy of Sciences.11
The average choline intake for humans on an ad libitum diet is about 600 mg/d for men and 450 mg/d for women. However, food frequency questionnaires usually result in estimates of choline intake that are lower (250−500 mg/d). Choline is found in a wide variety of foods (http://www.nal.usda.gov/fnic/foodcomp/data/Choline/Choline.html).13 Because phosphatidylcholine is found abundantly in mammalian cell membranes, some of the best sources of choline include eggs and organ meats. Legumes and wheat germ are also good sources. Human milk is a rich source of choline, but soy- and casein-derived infant formulas sometimes contain less choline than human milk does unless choline is added to the formula during processing.14 Lecithin, which is another name for phosphatidylcholine, is often used as an emulsifier in commercial food processing and can increase the choline content of the food product.
Factors Influencing the Choline Requirement
Sex
Choline requirements seem to differ by sex and stage of life.1 Men and postmenopausal women are more likely than premenopausal women to develop signs of organ dysfunction on a choline-deficient diet. The reason for this disparity is currently under investigation, but it is most likely caused by estrogen's ability to enhance de novo synthesis of choline. Female rats make more phosphatidylcholine via the PEMT pathway than do male rats,15 and this increase in PEMT activity is linked to estrogen status.16 Furthermore, a study in castrated rats observed an increase in hepatic PEMT activity after treatment with estradiol.17
Pregnancy and Lactation
Choline is important for the brain development of the fetus and the maintenance of homocysteine concentrations during pregnancy.1 Maternal plasma choline can become depleted because of increased transport from mother to fetus. The placenta and amniotic fluid store large amounts of choline, presumably to ensure that adequate choline is delivered to the fetus, and plasma choline concentrations are up to 7-fold higher in fetuses and neonates than in the mother.1 Breast milk also has high concentrations of choline.14 Although estrogen levels rise in pregnancy, which may allow for increased de novo synthesis of choline, dietary intake of choline is still critical.18 Shaw and colleagues19 found an increased incidence of neural tube defects in women consuming less than 300 mg choline per day during pregnancy compared with women consuming more than 500 mg/d.
The demand for choline is increased in pregnant and lactating women, as well as postmenopausal women and men.
Clinical Status
Choline requirements are also important to consider in clinical practice. Up to 84% of patients on total parenteral nutrition experience low plasma choline concentrations as well as fatty liver and liver damage.12 In many patients, fatty liver was resolved with choline supplementation and returned when standard total parenteral nutrition was reinstituted.12
Folate, Vitamin B12, and Methionine Nutriture
As mentioned previously, choline, folate, vitamin B12, and methionine metabolisms are interrelated (Figure 2). Thus, a disturbance in the availability or metabolism of 1 nutrient results in compensatory changes in the others. For example, animals and humans who are choline deficient have a higher demand for dietary folate because a greater supply of methyl-tetrahydrofolate is required to regenerate methionine from homocysteine.20 Likewise, a folate-deficient diet increases the demand for choline,21 and if dietary intake is inadequate, choline will become depleted. A deficiency in vitamin B12, required for the recycling of folate, would likely increase the demand for choline as well. Elevated plasma homocysteine levels are frequently seen when any of these deficiencies occurs.1
Gene Polymorphisms
When placed on a low-choline diet, only 68% of individuals developed signs of organ dysfunction characteristic of choline deficiency.9 This suggests that genetic variability among individuals may influence susceptibility to choline deficiency. Several of the enzymes responsible for methyl group metabolism are encoded for by genes that have single nucleotide polymorphisms (SNPs) that may alter gene expression or enzyme function. A very common genetic variation in folate metabolism that has been linked to risk of neural tube defects is the 5,10-methylenetetrahydrofolate dehydrogenase 1958A (MTHFD1) allele (rs2236225). Premenopausal women carrying this polymorphism were found to be 15 times more likely than noncarriers to develop signs of choline deficiency on a low-choline diet.22 It is likely that the variant MTHFD1 gene decreases the availability of methyl-tetrahydrofolate, thereby increasing the demand for choline as a methyl donor.
Common variations in genes involved in choline metabolism have also been found to influence susceptibility to choline deficiency. Of particular interest is an SNP located in the gene encoding for PEMT, (rs 12325817) the enzyme responsible for de novo choline synthesis. More than half of the population has at least 1 allele with this polymorphism, and women with this SNP were 7 times more likely to develop signs of choline deficiency when dietary intake of choline was insufficient.9 It is likely that this SNP results in a partial loss of PEMT enzyme function and a decrease in de novo choline synthesis. Interestingly, this SNP was not associated with increased risk of choline deficiency in men, suggesting that this SNP may be involved in estrogen's ability to induce PEMT. Another polymorphism found in choline dehydrogenase (CHDH), the enzyme responsible for the conversion of choline to betaine, conferred protection from choline deficiency when dietary intake is inadequate.9 This protection may result from the conservation of choline for membrane synthesis as its conversion to betaine is reduced.
Gene polymorphisms also increase risk of choline deficiency.
Importance of Choline During Brain Development
Folate is important for proper closure of the neural tube in the early stages of brain development. In fact, 50% or more of neural tube defects can be prevented by adequate dietary folate intake in the mother. Because folate metabolism and choline metabolism are closely related, a recent retrospective case-control study examined the relationship of dietary choline intake and incidence of neural tube defects.19 Women in the lowest quartile of choline intake (<300 mg/d) had 4 times the risk of having a baby with a neural tube defect than did women in the highest quartile of intake (>500 mg/d). Similar effects of choline intake and risk of orofacial clefts were observed.23 Thus, low choline intake may increase the risk of neural tube defects. Folate and choline both play an important role in methyl group availability; therefore, methylation reactions may be the mechanism by which these 2 nutrients affect neural tube closure.
Recently, a thorough review of the literature by McCann et al24 highlighted 34 studies in rodents that examined the relationship of choline during development and cognitive outcomes. The evidence strongly suggests that choline supplementation during pregnancy contributes to changes in neurological function in the fetus and an improvement in postnatal cognitive and behavioral tests. There is also evidence that choline deficiency leads to decrements in some measures of learning and memory.25 There are no current studies of choline supplementation and effects on brain development in humans during the perinatal period; thus, the relevance of rodent studies to the human condition remains to be determined.
The area of the brain that seems to be the most sensitive to choline availability for appropriate development is the hippocampus.2 This region of the brain plays an important role in memory and learning and is one of the few areas of the brain where nerve cells continue to multiply slowly throughout adulthood. In rodents, the time period where changes in choline availability most significantly alter the development of the hippocampus is days 11 to 18 of gestation and postnatal days 16 to 30.25 These periods of development coincide with periods of neurogenesis and synaptogenesis. During neurogenesis, neuronal precursor cells proliferate, migrate, and differentiate to neurons. Supplemental choline during this critical period enhances proliferation and differentiation, whereas choline deficiency decreases proliferation and differentiation. Choline deficiency also increases the rate of neuronal cell death.1 In humans, hippocampal development begins around the 56th day of pregnancy and continues until 4 years after birth. Although the effects of choline on fetal hippocampal development have been examined exclusively in rodent models, the development of the brain is similar in rodents and humans.
Maternal supplementation or deficiency in choline in laboratory animals can cause permanent alterations in the developing brain that will last for the lifetime of the offspring. Several studies have examined offspring of choline-supplemented dams up to 2 years of age.24 Even animals exposed to supplemental choline for only 6 days during gestation showed enhanced performance in spatial and memory tasks at 2 years of age compared with nonsupplemented animals.25 Neuronal structure and biochemical markers are also changed for the life of the animal when exposed to supplemental choline in utero. Neurons of the hippocampus have a larger cell body and a greater number of dendritic branches, and long-term potentiation, a marker of learning and memory, is enhanced.2 Alternatively, animals deprived of choline during days 11 to 18 of gestation had diminished long-term potentiation. Choline supplementation during the perinatal period also improved cognitive deficits resulting from prenatal exposure to alcohol, suggesting that choline may prevent some of the structural or functional changes associated with alcohol exposure.26
Summary
Choline is now recognized as an essential nutrient for humans. However, continuing research is needed to determine how sex, genetic variation, life stage, and other environmental factors may influence an individual's requirement for this nutrient. Choline may be especially important during pregnancy when it modulates proliferation of stem cells needed to form a normal fetus. Furthermore, it may influence brain development throughout gestation, and these influences may continue throughout the life span. Choline deficiency has been associated with liver and muscle damage and increases in homocysteine (a risk factor for heart disease) after a methionine load.27 Recent reports suggest that choline metabolism may also play a role in diabetes, cancer, and cystic fibrosis.28–30
The Food and Drug Administration Launches New Web Page to Enhance Online Consumer Health Information.
The Food and Drug And Administration recently announced 2 new initiatives to enhance online communications. A Web page, “Consumer Health Information for You and Your Family” (http://www.fda.gov/consumer), provides comprehensive and timely consumer information. A free monthly e-newsletter, “FDA Consumer Health Information” (http://www.fda.gov/consumer/consumerenews.html), will alert consumers to content contained on the page.
Acknowledgments
This work was funded by grants from the National Institutes of Health (NIH; DK55865, AG09525, and ES012997) and the US Department of Agriculture (2004−01,833). Support for this work was also provided by grants from the NIH to the University of North Carolina (UNC) Clinical Nutrition Research Unit (DK56350), the UNC General Clinical Research Center (RR00046), and the Center for Environmental Health and Susceptibility (ES10126).
Biography
Lisa M. Sanders, PhD, RD, is a postdoctoral fellow of the Department of Nutrition at the University of North Carolina at Chapel Hill.
Steven H. Zeisel, MD, PhD, is a Kenan Distinguished University Professor of Nutrition and Pediatrics in the School of Public Health and School of Medicine, Nutrition Research Institute, at the University of North Carolina at Chapel Hill. Dr Steven Zeisel holds many positions within the Schools of Public Health and Medicine at the University of North Carolina at Chapel Hill. He currently serves as Kenan Distinguished University Professor in Nutrition, Associate Dean for Research, and is the director of 1 of 8 national centers of excellence in human clinical nutrition research funded by the National Institutes of Health. Dr Zeisel was also recently named director of the UNC School of Public Health's Nutrition Research Institute at the newly formed North Carolina Research Campus in Kannapolis, NC. Dr Zeisel conducts an active research program that includes clinical research studies on nutrient requirements, nutrigenomics, molecular nutrition, brain development, and human requirements for the nutrient choline. Dr Zeisel is the author of more that 220 publications and reviews. Dr Zeisel received his MD from Harvard Medical School, his PhD in nutrition from MIT, and his residency training in pediatrics from Yale. Dr Zeisel is a member of several professional societies and serves on many boards. These include American Society for Nutritional Sciences, American College of Nutrition, Society for Pediatric Research, and the American Council on Science and Health, just to name a few.
REFERENCES
- 1.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeisel SH. Choline and brain development. In: Bowman BR, Russell RM, editors. Present Knowledge in Nutrition. 9th ed. Vol. 1. ILSI Press; Washington, DC: 2006. pp. 352–360. [Google Scholar]
- 3.Stead LM, Brosnan JT, Brosnan ME, Vance DE, Jacobs RL. Is it time to reevaluate methyl balance in humans? Am J Clin Nutr. 2006;83:5–10. doi: 10.1093/ajcn/83.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 5.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 7.Zeisel SH, Albright CD, Shin O-K, Mar M-H, Salganik RI, da Costa K-A. Choline deficiency selects for resistance to p53-independent apoptosis and causes tumorigenic transformation of rat hepatocytes. Carcinogenesis. 1997;18:731–738. doi: 10.1093/carcin/18.4.731. [DOI] [PubMed] [Google Scholar]
- 8.Zeisel SH, da Costa K-A, Franklin PD. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 9.da Costa K, Kozyreva OG, Song J, Galanko JA, Fischer LM, Zeisel SH. Common genetic polymorphisms have major effects on the human requirement for the nutrient choline. FASEB J. 2006;20:1336–1344. doi: 10.1096/fj.06-5734com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine, National Academy of Sciences . Choline. Dietary Reference Intakes for Folate, Thiamin, Riboflavin, Niacin, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Vol. 1. National Academy Press; Washington, DC: 1998. pp. 390–422. [PubMed] [Google Scholar]
- 12.Buchman AL, Ament ME, Sohel M. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. J Parenter Enteral Nutr. 2001;25:260–268. doi: 10.1177/0148607101025005260. [DOI] [PubMed] [Google Scholar]
- 13.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 14.Holmes-McNary M, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am J Clin Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 15.Noga AA, Vance DE. A gender-specific role for phosphatidylethanolamine N-methyltransferase-derived phosphatidylcholine in the regulation of plasma high density and very low density lipoproteins in mice. J Biol Chem. 2003;278:21851–21859. doi: 10.1074/jbc.M301982200. [DOI] [PubMed] [Google Scholar]
- 16.Drouva SV, LaPlante E, Leblanc P, Bechet JJ, Clauser H, Kordon C. Estradiol activates methylating enzyme(s) involved in the conversion of phosphatidylethanolamine to phosphatidylcholine in rat pituitary membranes. Endocrinology. 1986;119:2611–2622. doi: 10.1210/endo-119-6-2611. [DOI] [PubMed] [Google Scholar]
- 17.Young DL. Estradiol- and testosterone-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res. 1971;12:590–595. [PubMed] [Google Scholar]
- 18.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 19.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 20.Varela-Moreiras G, Selhub J, da Costa K, Zeisel SH. Effect of chronic choline deficiency in rats on liver folate content and distribution. J Nutr Biochem. 1992;3:519–522. [Google Scholar]
- 21.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129:712–717. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- 22.Kohlmeier M, da Costa KA, Fischer LM, Zeisel SH. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005;102:16025–16030. doi: 10.1073/pnas.0504285102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- 24.McCann JC, Hudes M, Ames BN. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci Biobehav Rev. 2006;30:696–712. doi: 10.1016/j.neubiorev.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 26.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–2020. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- 29.Glunde K, Ackerstaff E, Mori N, Jacobs MA, Bhujwalla ZM. Choline phospholipid metabolism in cancer: consequences for molecular pharmaceutical interventions. Mol Pharmacol. 2006;3:496–506. doi: 10.1021/mp060067e. [DOI] [PubMed] [Google Scholar]
- 30.Innis SM, Hasman D. Evidence of choline depletion and reduced betaine and dimethylglycine with increased homocysteine in plasma of children with cystic fibrosis. J Nutr. 2006;136:2226–2231. doi: 10.1093/jn/136.8.2226. [DOI] [PubMed] [Google Scholar]