Abstract
The synthesis of a 24-membered macrocyclic hexaoxazole via ring-closing metathesis is described. The target compound selectively stabilizes G-quadruplex DNA with no detectable stabilization of duplex DNA. An MTT cytotoxicity assay indicated that this unsaturated macrocyclic hexaoxazole exhibits significant cytotoxicity towards P388, RPMI 8402, and KB3-1 cell lines with IC50 values of 45, 25, and 38 nM respectively.
The isolation and structure elucidation of telomestatin has sparked interest in the identification of compounds that can selectively stabilize G-quadruplex DNA.1 These compounds have potential as a novel class of anticancer agents.2 Recently the synthesis of the macrocyclic hexaoxazole HXDV was disclosed.3 HXDV is a 24-membered macrocycle that stabilizes G-quadruplexes presumably by π-stacking and hydrogen bonding interactions. HXDV is more readily synthesized than telomestatin. Despite the simpler structure, HXDV is extraordinarily selective at stabilizing G-quadruplex vs. duplex DNA, and is at least as potent as telomestatin as a cytotoxic agent.4 These results prompted us to investigate whether modifications could be made to the macrocyclic framework while retaining selectivity for G-quadruplex stabilization and antitumor activity. In this account a method is presented for the synthesis of macrocyclic hexaoxazoles via a ring closing metathesis reaction.
Previous studies in our laboratory confirmed that 24-membered macrocycles comprised of two teroxazole moieties joined by at least one valine residue displayed extraordinary levels of G-quadruplex stabilization with no detectable levels of duplex DNA stabilization.5 It was reasoned that two teroxazole units joined at their 2”- and 4-positions by a valine residue might be capable of undergoing a ring-closing reaction if the remaining 2”- and 4-positions were functionalized as allyl and vinyl respectively. Ring-closing metathesis has become a reliable method for the formation of a wide variety of cyclic compounds from five-membered rings to extremely large rings of 36–72 atoms.6 Certainly 24-membered rings have been synthesized previously by this method, although there is usually considerably more conformational flexibility in the acyclic precursors than in the present example.7 Recently, the application of the cross metathesis reaction to isolated vinyl oxazoles was reported.8 We report herein the first example of a ring-closing metathesis being applied to the synthesis of a macrocyclic hexaoxazole.
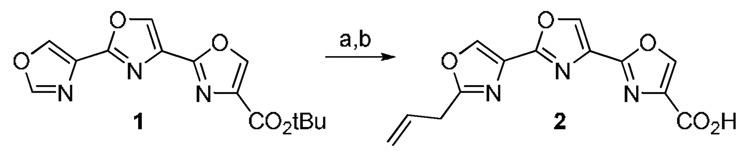
The synthesis of 2”-allylteroxazole-4-carboxylic acid is shown in Scheme 1. Teroxazole 1 is prepared in a similar manner as previously reported for 3, but starting with oxazole-4-carboxylic acid.3,9 Deprotonation at the 2”-position was effected with LiHMDS although the resulting lithio derivative could not be directly alkylated with allyl bromide. Fortunately, the cuprate derived from 2”-lithio 1 did react with allyl bromide to give the 2”-allyl derivative in 37% yield. The tert-butyl group was removed to give 2 in 89% yield upon treatment with TFA at 0 °C.10
Scheme 1.
(a) LiHMDS, THF, −42 °C; CuI, allyl bromide, −42 °C to rt, 37%; (b) TFA, anisole/CH2Cl2 (1:5), 0 °C, 89%.
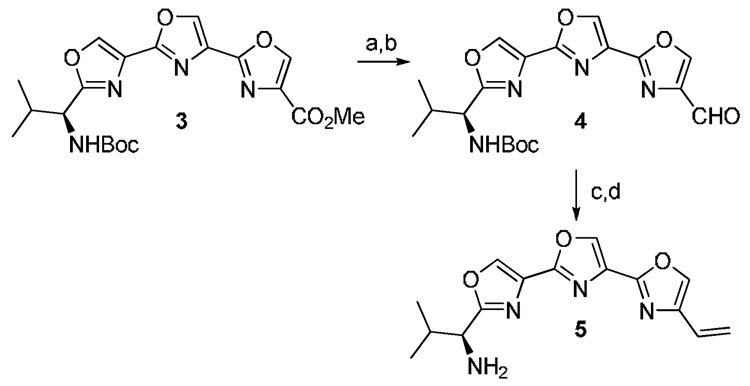
The preparation of 4-vinylteroxazole 5 (Scheme 2) was performed by routine transformation of the known 3. Reduction of the ester with NaBH4/LiCl followed by Swern oxidation afforded aldehyde 4. A Wittig reaction of 4 with methylenetriphenylphosphorane followed by TFA catalyzed removal of the Boc group gave intermediate 5 in 54% yield for the two steps.10
Scheme 2.
(a) NaBH4, LiCl, THF/MeOH (1:1) 73 %; (b) oxalyl chloride, DMSO, CH2Cl2, Et3N, −78 °C, 88%; (c) Ph3PCH3Br, n-BuLi, THF, 0 °C to rt, 74%; (d) TFA, CH2Cl2, 0 °C, 73%.
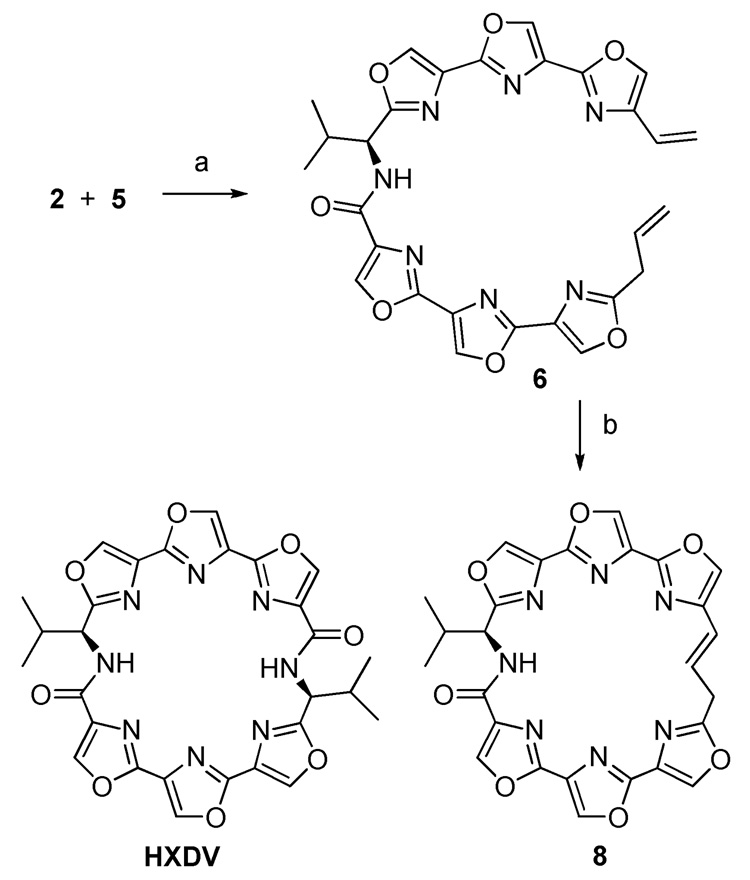
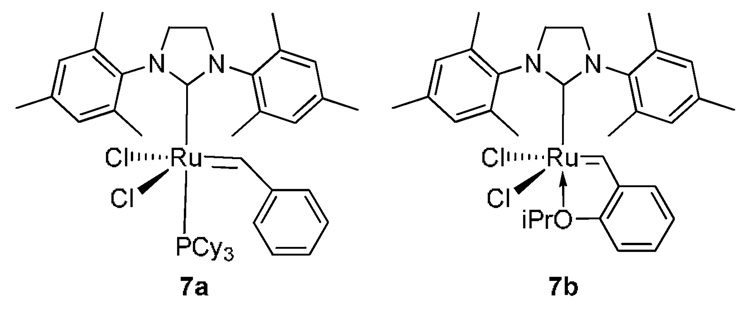
2”-Allylteroxazole 2 and 4-vinylteroxazole 5 were coupled in 69% yield using EDC and HOBt in the absence of added base (Scheme 3). The allyl group was found to be extremely sensitive to isomerization to a 1-propenyl derivative in the presence of triethylamine or Hunig’s base. Amide 6 was now set up to test the viability of the ring-closing metathesis reaction. The second generation Grubb’s catalysts 7a and the Hoveyda catalyst 7b were evaluated for their ability to promote the macrocyclization (Figure 1).11,12 Both catalysts gave the desired macrocycle 8 although 7b gave slightly higher yields (32% vs. 24% using 7a). The reactions were remarkably clean with no evidence of any cross-metathesis by-products. The geometry of the newly-formed olefinic linkage is E- as determined from the vicinal coupling constant J = 15.6 Hz.
Scheme 3.
(a) EDC, HOBt, DMF, 0 °C to rt, 69%; (b) 7b, CH2Cl2, Δ, 96 h, 32%. Structure of HXDV shown for comparison.
Figure 1.
Metal carbene catalysts employed in this study.
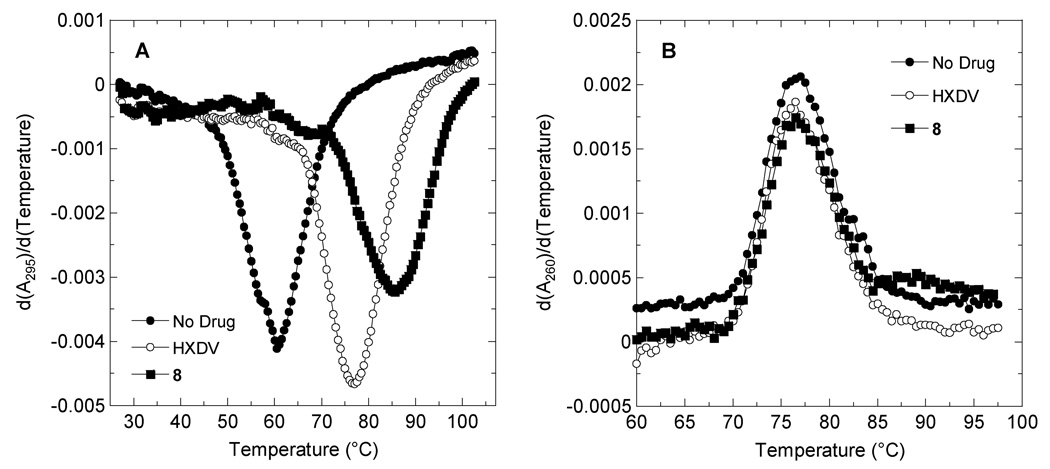
The unsaturated macrocycle 8 and HXDV were evaluated for their relative abilities to bind and thermally stabilize G-quadruplex and duplex DNA in the presence of K+ ions. We used salmon testes DNA (ST-DNA) as a representative model for duplex DNA. As a representative model for G-quadruplex DNA, we used the human telomeric DNA sequence d[T2G3(T2AG3)3A], which is known to form an intramolecular G-quadruplex in potassium solution.13 Figure 2 shows the UV melting profiles (depicted in their first-derivative forms) of d[T2G3(T2AG3)3A] and ST-DNA in the absence and presence of the two compounds. Note that the presence of neither compound alters the thermal stability of ST-DNA, with any differences between the transition temperatures (Ttran) corresponding to the maxima of the first-derivative melting profiles being within the experimental uncertainty. This observation is indicative of an absence of duplex DNA binding on the part of both compounds. In the case of HXDV, we used isothermal titration calorimetry to verify the lack of duplex DNA binding implied by the UV melting studies.14
Figure 2.
First derivatives of the UV melting profiles of d[T2G3(T2AG3)3A] (A) and ST-DNA (B) in the absence and presence of HXDV or compound 8. Profiles were acquired on an AVIV model 14NT-UV-VIS spectrophotometer using quartz cuvettes with a 1 cm pathlength. The temperature was raised in 0.5 °C increments and the samples were allowed to equilibrate for 1.5 minutes at each temperature setting, whereupon absorbances were recorded over a period of 5 seconds and averaged. When present, ST-DNA was used at a base pair concentration of 15 µM, while d[T2G3(T2AG3)3A] was used at a strand concentration of 4 µM. Macrocyclic ligands were used at a concentration of 15 µM in the ST-DNA experiments and 20 µM in the d[T2G3(T2AG3)3A] experiments. The solution conditions were 10 mM EPPS (pH 7.5) and sufficient KCl to bring the total K+ concentration to 50 mM. In the ST-DNA experiments, the acquisition wavelength was 260 nm, while being 295 nm in the d[T2G3(T2AG3)3A] experiments.
In striking contrast to their negligible impacts on ST-DNA thermal stability, both compounds increase the thermal stability of d[T2G3(T2AG3)3A]. Thus, like HXDV, compound 8 binds to G-quadruplex DNA with a high degree of specificity. Significantly, the unsaturated macrocycle 8 thermally stabilizes d[T2G3(T2AG3)3A] to a greater extent than HXDV (ΔTtran = 24.5 °C and 16.0 °C for compound 8 and HXDV, respectively). The greater ΔTtran afforded by compound 8 relative to HXDV may reflect a correspondingly enhanced affinity for the host quadruplex, as the relative degree to which a ligand thermally stabilizes a nucleic acid target often correlates with its relative binding affinity.
Evaluation of the cytotoxic activity of unsaturated macrocycle 8 was performed using an MTT assay (Table 1). Among the cell lines employed for this assay was murine leukemia P388, a human lymphoblastoma RPMI 8402, human nasopharyngeal carcinoma KB3-1, and KB3-1 cell lines that over-express for the efflux transporters MDR1 (KBV-1), and BCRP (KBH5.0). The data indicate that compound 8 displays potent cytotoxic activity against P388, RPMI 8402, and KB3-1 with IC50 values of 45, 25, and 38 nM respectively. In each case the IC50 values determined for compound 8 represents an order of magnitude improvement over those observed for the macrocyclic hexaoxazole HXDV. The data also suggest that both 8 and HXDV are substrates for MDR1, but not BCRP.
Table 1.
Cytotoxic activity of unsaturated macrocycle 8.a
| Compound | P388 | RPMI 8402 | KB3-1 | KBV-1 | KBH5.0 |
|---|---|---|---|---|---|
| 8 | 0.045 | 0.025 | 0.038 | 3.3 | 0.043 |
| HXDV | 0.5 | 0.41 | 0.3 | 8.0 | 0.5 |
IC50 values are reported in □M concentrations and represent the average values from replicate assays. The exposure time of the cells to the test compounds was 4 days.
In summary, ring closing metathesis offers a rapid method for the synthesis of 24-membered macrocyclic hexaoxazoles from suitably substituted teroxazole precursors. The resulting unsaturated macrocycle 8 selectively stabilizes G-quadruplex DNA without any detectable stabilization of duplex DNA. The degree of G-quadruplex stabilization was greater than previously observed using HXDV. The replacement of one of the valine linkages of HXDV by a propenyl group led to an order of magnitude enhancement in cytotoxic potency when assayed against several tumor cell lines. These results when taken together suggest that the ring-closing metathesis approach has the potential for development of novel G-quadruplex stabilizers with enhanced anticancer activity.
Acknowledgements
This study was supported by generous funding from Celgene Corp. and NIH Grants CA098127 (EJL) and CA097123 (DSP). Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant P41RR0954.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Shin-ya K, Wierzba K, Matsuo K, Ohtani T, Yamada Y, Furihata K, Hayakawa Y, Seto H. J. Am. Chem. Soc. 2001;123:1262. doi: 10.1021/ja005780q. [DOI] [PubMed] [Google Scholar]
- 2.(a) Han H, Hurley LH. Trends Pharmacol. Sci. 2000;21:136. doi: 10.1016/s0165-6147(00)01457-7. [DOI] [PubMed] [Google Scholar]; (b) Hurley LH, Wheelhouse RT, Sun D, Kerwin SM, Salazar M, Federoff OY, Han FX, Han H, Izbicka E, Von Hoff DD. Pharmacol. Ther. 2000;85:141. doi: 10.1016/s0163-7258(99)00068-6. [DOI] [PubMed] [Google Scholar]; (c) Neidle S, Read MA. Biopolymers. 2001;56:195. doi: 10.1002/1097-0282(2000)56:3<195::AID-BIP10009>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (d) Hurley LH. Biochem. Soc. Trans. 2001;29:692. doi: 10.1042/0300-5127:0290692. [DOI] [PubMed] [Google Scholar]; (e) Perry PJ, Jenkins TS. Mini-Rev. Med. Chem. 2001;1:31. doi: 10.2174/1389557013407304. [DOI] [PubMed] [Google Scholar]
- 3.Minhas GS, Pilch DS, Kerrigan JE, LaVoie EJ, Rice JE. Bioorg. Med. Chem. Lett. 2006;16:3891. doi: 10.1016/j.bmcl.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri CM, Srinivasan AR, Rzuczek SG, Rice JE, LaVoie EJ, Pilch DS. Nucleic Acids Res. 2007;35:3272. doi: 10.1093/nar/gkm188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rzuczek SG, Pilch DS, LaVoie EJ, Rice JE. Bioorg. Med. Chem. Lett. 2008;18:913. doi: 10.1016/j.bmcl.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Grubbs RH. Tetrahedron. 2004;60:7117. [Google Scholar]; (b) Hoveyda AH, Zhugralin AR. Nature. 2007;450:243. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Bulger PG, Sarlah D. Ang. Chem. Int. Ed. 2005;44:4490. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]; (d) Arakawa K, Eguchi T, Kakinuma K. J. Org. Chem. 1998;63:4741. [Google Scholar]
- 7.(a) Malhas RN, Ibrahim YA. Synthesis. 2006;19:3261. [Google Scholar]; (b) Zhong S, Mondon M, Pilard S, Len C. Tetrahedron Lett. 2006;47:6221. [Google Scholar]; (c) Roethle PA, Chen IT, Trauner D. J. Am. Chem. Soc. 2007;129:8960. doi: 10.1021/ja0733033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Anzo T, Suzuki A, Sawamura K, Motozaki T, hatta M, Takao K-i, Tadano K-i. Tetrahedron Lett. 2007;48:8442. [Google Scholar]
- 8.Hoffman TJ, Rigby JH, Arseniyadis S, Cossy J. J. Org. Chem. 2008;73:2400. doi: 10.1021/jo702305g. [DOI] [PubMed] [Google Scholar]
- 9.Shafer CM, Molinski TF. Heterocycles. 2000;53:1167. [Google Scholar]
- 10.The structures of all compounds were determined using 1H (200 MHz) and 13C-NMR (50 MHz) in CDCl3 unless otherwise noted. Results are reported as ppm downfield from internal TMS: 1: white solid, mp 283 °C (dec.); 1H NMR (CDCl3) δ 8.44 (s, 2H), 8.23 (s, 1H), 8.07 (d, 1H, J=0.74), 1.61 (s, 9H); 13C NMR (CDCl3) δ 159.1, 155.0, 151.1, 142.3, 138.8, 138.5, 135.0, 130.2, 128.8, 81.6, 27.3; IR (thin film, NaCl) 1721 cm−1; HRMS (ESI) m/z calculated for C14H13N3O5Na (M+Na) 326.0753; found 326.0750. 2: white solid, mp 283–285 °C (dec); IR (CHCl3) 3135, 1686 cm−1; 1H NMR (CDCl3+CD3OD) δ 8.38 (s, 1H) 8.27 (s, 1H), 8.26 (s, 1H), 5.91 (m, 1H), 5.19 (m, 2H), 3.55 (dt, 2H, J = 1.4, 6.6 Hz); HRMS calcd for C13H9N3O5H (M+H): 288.0620; found: 288.0622. 4: white solid, mp 196–198 °C; IR (CHCl3) 3373, 1690 cm−1; 1H NMR (CDCl3) δ 10.0 (s, 1H), 8.38 (s, 1H) 8.33 (s, 1H), 8.32 (s, 1H), 5.29 (d, 1H, J = 9.2 Hz), 4.83 (dd, 1H, J = 6.2, 8.8 Hz), 2.23 (m, 1H), 1.42 (s, 9H), 0.94 (d, 3H, J = 4.0 Hz), 0.92 (d, 3H, J = 3.0 Hz); 13C NMR (CDCl3) δ 183.1, 164.8, 155.5, 155.0, 154.4, 142.7, 140.7, 138.5, 138.4, 129.8, 128.7, 79.2, 53.4, 32.0, 27.4, 17.8, 17.1; HRMS calcd for C19H22N4O6Na (M+Na): 425.1437; found: 425.1432. 5: white solid, mp 125–127 °C; IR (CHCl3) 3381 cm−1; 1H NMR (CDCl3) δ 8.34 (s, 1H) 8.30 (s, 1H), 7.63 (s, 1H), 6.59 (dd, 1H, J = 11, 17.4 Hz), 6.06 (d, 1H, J = 17.4 Hz), 5.37 (d, 1H, J = 11 Hz), 3.92 (d, 1H, J = 6.6 Hz), 2.17 (m, 1H), 1.00 (d, 3H, J = 5.6 Hz), 0.96 (d, 3H, J = 7.0 Hz); 13C NMR (CDCl3) δ 167.4, 155.2, 153.9, 139.6, 138.3, 137.4, 134.1, 130.7, 128.7, 123.8, 116.0, 55.0, 32.5, 18.1, 17.1; HRMS calcd for C15H16N4O3H (M+H): 301.1301; found: 301.1269. 6: white solid, mp 235–237 °C (dec); IR (CHCl3) 3363, 1653 cm−1; 1H NMR (CDCl3) δ 8.34 (s, 2H) 8.33 (s, 1H), 8.30 (s, 1H), 8.26 (s, 1H), 7.62 (s, 1H), 7.59 (d, 1H, J = 10.4 Hz), 6.57 (dd, 1H, J = 11, 17.4 Hz), 6.01 (m, 2H), 5.32 (m, 4H), 3.64 (d, 2H, J = 6.2 Hz), 2.41 (m, 1H), 1.07 (d, 3H, J = 7.0 Hz), 1.00 (d, 3H, J = 6.6 Hz); 13C NMR (CDCl3) δ 163.5, 163.3, 159.0, 155.6, 155.0, 153.9, 153.7, 140.7, 139.6, 138.6, 138.5, 138.2, 137.5, 135.9, 134.1, 130.7, 130.0, 129.5, 129.1, 128.8, 123.8, 118.3, 116.0, 51.6, 31.9, 31.6, 18.0, 17.5; HRMS calcd for C28H23N7O7H (M+H): 570.1737; found: 570.1732. 8: white solid, mp 195–197 °C (dec); [α]D = −53.8° (c 0.433, CHCl3); IR (CHCl3) 3149, 1669, 1658 cm−1; 1H NMR (CD2Cl2, 400 MHz) δ 8.29 (d, 1H, J = 8.4 Hz), 8.258 (s, 1H) 8.254 (s, 1H), 8.23 (s, 1H), 8.20 (s, 1H), 8.18 (s, 1H), 7.60 (m, 1H), 7.56 (s, 1H), 6.49 (d, 1H, J = 15.6 Hz), 5.32 (m, 1H), 3.84 (ddd, 1H, J = 2.4, 2.8, 19.4 Hz), 3.73 (ddd, 1H, J = 8.4, 8.8, 20.2 Hz), 2.39 (m, 1H), 1.02 (d, 3H, J = 6.8 Hz), 0.94 (d, 3H, J = 6.8 Hz); 13C NMR (CDCl3) δ 163.4, 163.1, 159.1, 155.7, 154.9, 153.9, 153.8, 140.1, 139.0, 137.6, 137.40, 137.38, 136.29, 136.27, 132.7, 130.8, 130.2, 129.0, 128.9, 127.7, 118.0, 52.2, 33.0, 28.8, 17.7, 17.1; HRMS calcd for C26H19N7O7H (M+H): 542.1424; found: 542.1429.
- 11.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 12.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168. [Google Scholar]
- 13.Luu KN, Phan AT, Kuryavyi V, Lacroix L, Patel DJ. J. Am. Chem. Soc. 2006;128:9963. doi: 10.1021/ja062791w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilch DS, Barbieri CM, Rzuczek SG, Lavoie EJ, Rice JE. Biochimie. 2008 doi: 10.1016/j.biochi.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]