Abstract
Matriptase, a type 2 transmembrane serine protease, is predominately expressed by epithelial and carcinoma cells in which hepatocyte growth factor activator inhibitor 1 (HAI-1), a membrane-bound, Kunitz-type serine protease inhibitor, is also expressed. HAI-1 plays dual roles in the regulation of matriptase, as a conventional protease inhibitor and as a factor required for zymogen activation of matriptase. As a consequence, activation of matriptase is immediately followed by HAI-1-mediated inhibition, with the activated matriptase being sequestered into HAI-1 complexes. Matriptase is also expressed by peripheral blood leukocytes, such as monocytes and macrophages; however, in contrast to epithelial cells, monocytes and macrophages were reported not to express HAI-1, suggesting that these leukocytes possess alternate, HAI-1-independent mechanisms regulating the zymogen activation and protease inhibition of matriptase. In the present study, we characterized matriptase complexes of 110 kDa in human milk, which contained no HAI-1 and resisted dissociation in boiling SDS in the absence of reducing agents. These complexes were further purified and dissociated into 80-kDa and 45-kDa fragments by treatment with reducing agents. Proteomic and immunological methods identified the 45-kDa fragment as the noncatalytic domains of matriptase and the 80-kDa fragment as the matriptase serine protease domain covalently linked to one of three different secreted serpin inhibitors: antithrombin III, α1-antitrypsin, and α2-antiplasmin. Identification of matriptase-serpin inhibitor complexes provides evidence for the first time that the proteolytic activity of matriptase, from those cells that express no or low levels of HAI-1, may be controlled by secreted serpins.
Keywords: protease, type 2 transmembrane serine protease, protease inhibitor, ST-14, hepatocyte growth factor activator inhibitor 1
matriptase (also known as MT-SP1, TADG15, ST-14, and epithin) and hepatocyte growth factor activator inhibitor 1 (HAI-1) are a cognate pair of epithelium-derived, membrane-bound, serine protease, and Kunitz-type serine protease inhibitors (12, 17, 18, 30, 32). The functional relationship between matriptase and HAI-1 was initially recognized by the purification of matriptase-HAI-1 complexes from human milk (17). Matriptase-HAI-1 complexes were also detected in the conditioned media of cultured breast cancer cells and immortal mammary epithelial cells (3, 19). Subsequently, concordant expression of matriptase and HAI-1 was seen in human breast and ovarian cancer cell lines (23). Both proteins are broadly coexpressed by the epithelial component of nearly all epithelium-containing organ systems (10, 26). More recently, significant expression correlation between matriptase and HAI-1 at the RNA level was further emphasized by a transcriptional profiling study with the use of microarray data from 2,000 human normal and cancerous samples, including tumor samples from 18 different tissue types, 59 cancer-derived cell lines, and 86 types of nonpathogenic tissues (4).
In addition to this expression correlation and the formation of complexes in vivo and in vitro, an intimate functional linkage between matriptase and HAI-1 was clearly demonstrated during embryonic development and the tumorigenesis of epithelial cells (29). Matriptase and HAI-1 are coexpressed in chorionic trophoblasts during early development of the mouse placenta. Genetic ablation of HAI-1 causes placenta defects leading to embryonic death due to the loss of undifferentiated chorionic trophoblasts and impaired formation of the labyrinth layers (7, 29, 31). Genetic ablation of matriptase in HAI-1-deficient mouse embryos corrects the impaired processes of placentation caused by HAI-1 ablation (29). Therefore, coexpression of HAI-1 and matriptase is not required for placental development. However, HAI-1 must be expressed if matriptase is expressed, but placentation proceeds normally in embryos lacking both HAI-1 and matriptase. The close functional relationship between matriptase and HAI-1 is also observed in the skin of zebrafish (5). The loss of epithelial integrity in the HAI-1-deficient zebrafish epidermis can be rescued by simultaneous inactivation of matriptase. The functional linkage between matriptase and HAI-1 also has important implications for the development of cancer. Matriptase activity that is only partially opposed by endogenous HAI-1 causes increased carcinogen sensitivity and produces spontaneous tumorigenesis in the skin of keratin-5-matriptase transgenic mice (21). Matriptase-induced malignant transformation and its strong prooncogenic potential can, however, be counteracted by increasing epidermal HAI-1 expression (21). The close functional relationship of this cognate pair of protease and protease inhibitor has also been observed at the cellular and subcellular levels. Enforced expression of matriptase in breast cancer cell lines that do not naturally express the enzyme only results in the production of significant amounts of protein when HAI-1 is coexpressed (24, 27). In the absence of functional HAI-1, matriptase is only produced at very low levels and fails to traffic out of the endoplasmic reticulum/Golgi apparatus (24). In breast cancer cells that express both proteins, reducing expression of HAI-1 by small interfering RNA (siRNA)-mediated HAI-1 knockdown resulted in spontaneous zymogen activation of matriptase and significantly enhanced the zymogen activation of matriptase induced by sphingosine 1-phosphate (24). Unexpectedly, HAI-1 is required for matriptase activation (27), and, as a consequence, zymogen activation of matriptase is immediately followed by HAI-1-mediated inhibition of the active matriptase. Consistent with the dual roles of HAI-1 as a conventional protease inhibitor and as a cofactor for matriptase zymogen activation, activated matriptase has been consistently detected in HAI-1 complexes (3, 14).
In light of the pivotal role of HAI-1 in the expression, zymogen activation, and inhibition of matriptase and in light of the data from the animal models, it is perhaps not surprising that a growing body of evidence shows that the tight expression correlation and close functional linkage of matriptase and HAI-1 may be altered or lost during the progression of human ovarian cancer. Both matriptase and HAI-1 are expressed by ovarian surface epithelial cells (25). However, expression of matriptase in the absence of HAI-1 or with very low levels of HAI-1 was observed in primary human ovarian carcinomas, particularly in advanced disease (25). Similar imbalances between matriptase and HAI-1 expression have been noted in other cancers of epithelial origin, particularly in the context of more advanced or aggressive disease, suggesting that this imbalance may play a significant role in carcinogenesis and tumor progression (28).
It is, therefore, surprising that there are some normal cell types that have been reported to express matriptase in the absence of HAI-1. Matriptase messenger RNA (mRNA) has been detected in peripheral blood leukocytes (30). The THP-1 human monocytic cell line has been reported to express matriptase but no HAI-1 (11). Matriptase mRNA is present in mouse peritoneal macrophages but not in bone marrow macrophages; however, HAI-1 is not detectable in macrophages of either type (4). These data and the finding that some tumors of epithelial origin apparently express matriptase in the absence of HAI-1 suggest that there must be a HAI-1-independent mechanism at work that can facilitate the expression, trafficking, activation, and inhibition of matriptase in these cells. HAI-1-independent matriptase expression by cells may lead to the release of active, non-HAI-1-complexed matriptase.
In the present study, we identified novel matriptase-containing complexes in human milk that contain no HAI-1. Further purification of these complexes followed by analysis with mass spectrometry-based protein identification and confirmation by immunoblotting studies demonstrated that they contain secreted serpin-type serine protease inhibitors, including antithrombin III (ATIII), α1-antitrypsin (α1AT), and α2-antiplasmin (α2AP). The existence of these matriptase-serpin complexes in human milk suggests that serpins may play an important role in regulating the activity of matriptase, particularly in the context of cell types that express matriptase in the absence of measurable HAI-1.
MATERIALS AND METHODS
Chemicals and reagents.
Human plasma ATIII was purchased from Innovative Research (Southfield, MI). Human plasma α1AT was purchased from Sigma (St. Louis, MO). Human plasma α2AP was purchased from Haematologic Technologies (Essex Junction, VT). CM-Sepharose and activated Sepharose beads were obtained from GE Healthcare (Piscataway, NJ). All other chemical reagents were obtained from Sigma unless otherwise specified.
Monoclonal antibodies.
Human matriptase protein was detected with either the M32 monoclonal antibody (mAb), which recognizes the third LDL receptor class A domain of matriptase in both the latent (one chain) and activated (two-chain) forms of the protease, or with the M69 mAb, which recognizes an epitope present only in the activated (two-chain) form of the enzyme (1, 2). Human HAI-1 was detected with the use of the HAI-1-specific mAb M19 (17). ATIII and α2AP polyclone antibodies were purchased from R&D Systems (Minneapolis, MN). α1AT polyclone antibody was purchased from Bethyl Laboratories (Montgomery, TX).
Immobilization of mAbs.
mAbs M19, M69, and 21-9 were covalently coupled to Sepharose 4B at 5 mg/ml gel following the manufacturer's instructions (GE Healthcare). Briefly, the mAbs were purified and dialyzed against the coupling buffer (0.1 M sodium bicarbonate containing 0.5 M sodium chloride) and incubated overnight at 4°C with cyanogen bromide-activated Sepharose 4B. The uncoupled mAbs were removed by washing the beads with the coupling buffer, and the residual coupling sites on beads were blocked by 1 M Tris buffer. The mAb-Sepharose beads were stored in PBS.
Purification of the 110-kDa matriptase complexes from human milk.
Frozen human milk from Georgetown University Medical Center Milk Bank was thawed and centrifuged to remove the milk fat and insoluble debris. The defatted milk was dialyzed against 10 mM phosphate buffer, pH 6.0, and loaded onto a CM-Sepharose FF column (2.5 × 20 cm; GE Health Science) equilibrated with 10 mM phosphate buffer, pH 6.0. The column was washed with 10 column volumes of equilibrium buffer. Proteins were eluted with a linear gradient of 0–0.5 M NaCl in 10 mM phosphate buffer, pH, 6.0, with a total volume of 500 ml. The column fractions containing 110-kDa complexes were collected and pooled. To remove the 95-kDa matriptase-HAI-1 complexes from the 110-kDa matriptase complex-enriched fractions, the samples were loaded onto a HAI-1 immunoaffinity column containing 1 ml mAb M19-Sepharose at a flow rate of 7 ml/h. The flow through, which contained the 110-kDa complexes, was then loaded onto a matriptase mAb 21-9 immunoaffinity column and the column washed with 1% Triton X-100 in PBS. Bound proteins then were eluted with 0.1 M glycine HCl, pH 2.4. Fractions were immediately neutralized by addition of 2 M Trizma base.
Western blotting.
Protein samples for Western blotting were diluted in 5× sample buffer. The sample buffer did not contain a reducing agent, and samples were not boiled before SDS-PAGE unless otherwise specified since reducing agents destroy the epitopes recognized by the mAbs and boiling disrupts matriptase-HAI-1 complexes. Proteins were resolved by 7.5% SDS-PAGE, transferred to Protran nitrocellulose membranes (Schleicher & Schuell, Keene, NH), and probed with the mAbs M32, M69, and M19 or the polyclonal antibodies directed against serpins. The binding of the primary antibody was detected with the use of horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and visualized using the Western Lightening Chemiluminescence Reagent Plus (Perkin-Elmer, Boston, MA).
Diagonal SDS-PAGE.
The 110-kDa complex was first boiled with SDS sample buffer in the absence of reducing agents and then resolved by electrophoresis. A gel strip was then sliced out from the gel used in the previous step and boiled with SDS sample buffer containing reducing agent. The reducing agent-treated strip was placed on the top of a new gel and subjected to electrophoresis in the second dimension, at 90 degrees to that in the first gel. Protein and protein fragments were assayed by colloidal Coomassie blue staining of the second gel.
Liquid chromatography/mass spectrometry analysis and identification of proteins.
The selected bands from the gels were initially excised, washed, destained, and trypsinized overnight at 37°C using standard protocol after DTT reduction and iodoacetamide alkylation. Liquid chromatography/mass spectrometry (LC/MS) analysis of tryptic peptides derived from protein samples were performed on Thermo Finnigan LCQ DECA XP mass spectrometer (ThermoFinnigan, San Jose, CA), which was connected to a nanoelectrospray ionizer. The Surveyor LC system with auto sampler (ThermoFinnigan) was used for peptide separation. The LC system was connected to a 10.5-cm fused silica reverse-phase C18 column (PicoFrit column; New Objective, Woburn, MA). The peptides were separated during 90 min of linear gradient of 5–90% acetonitrile/water mixture, containing 0.1% formic acid at a flow rate of 300 nl/min. MS scan events and HPLC solvent gradients were controlled by the Xcalibur software (ThermoFinnigan). The spectrums were accumulated in data-dependant acquisition mode, and the acquired MS/MS scans were searched against the human database (IPI) using SORCERER/SEQUEST search algorithm. Database search parameters were set to allow carboxyamidomethylation (+57.021464 Da) of cysteine residues and oxidation (15.99492 Da) of methionine as fixed modification. The best hit was selected on the basis of probability/percent of coverage and number of peptides or on the basis of Xcore.
RESULTS
Identification of matriptase complexes, which contain no HAI-1.
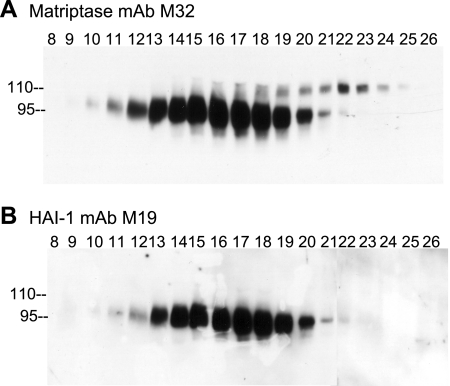
During lactation, mammary epithelial cells robustly activate matriptase immediately followed by HAI-1-mediated inhibition of active matriptase and the shedding into the milk of matriptase-HAI-1 complexes with a size of 95 kDa (17). Although the physiological role of matriptase zymogen activation during lactation remains to be determined, human milk is apparently one of the best systems in which to characterize the diverse roles of this type 2 transmembrane serine protease. When human milk was fractionated by CM-Sepharose chromatography, minor matriptase complexes with an apparent mass of 110 kDa were eluted and partially separated in different column fractions (Fig. 1A, fractions 18 through 24) from the majority of the matriptase detected in 95-kDa complexes (Fig. 1A, fractions 10 through 21). The 95-kDa matriptase protein bands were further confirmed to be HAI-1 complexes by blotting with the HAI-1 mAb M19 (Fig. 1B), consistent with our previous study (17). In contrast, the minor 110-kDa matriptase-containing complexes apparently do not contain HAI-1 since they were not detected by the HAI-1 mAb M19 (Fig. 1B).
Fig. 1.
Matriptase complexes in human milk. Human milk was fractionated by CM ionic exchange chromatography. The column fractions (8-26) were subjected to immunoblot analysis using matriptase monoclonal antibody (mAb) M32 (A) and hepatocyte growth factor activator inhibitor-1 (HAI-1) mAb M19 (B). Two distinct matriptase-containing complexes were detected by matriptase mAb M32, as indicated. The majority of matriptase was detected at 95 kDa in the column fractions 10-21. A small portion of matriptase was detected at 110 kDa in column fractions 18-24. HAI-1 mAb M19 detected only the 95-kDa but not the 110-kDa form.
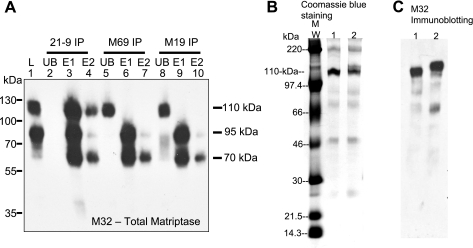
To characterize and compare the novel 110-kDa matriptase-containing complexes with the matriptase-HAI-1 complexes, fractions containing roughly equal amounts of both 95-kDa HAI-1 complexes and 110-kDa complexes (such as fraction 21 in Fig. 1 and Fig. 2A, lane 1) were subjected to immunoprecipitation experiments with the use of immobilized matriptase and HAI-1 mAbs (Fig. 2A). When both the 95-kDa and 110-kDa complexes were incubated with immobilized matriptase mAb 21-9 on Sepharose beads (18), both complexes bound efficiently to the beads and were immunodepleted from the solution (Fig. 2A, lane 2), confirming that both complexes contain matriptase. In contrast, immobilized HAI-1 mAb M19 only resulted in immunodepletion of the 95-kDa complexes and not the 110-kDa complexes [Fig. 2A, M19 immunoprecipitation (IP), unbound (UB), lane 8], confirming the results from immunoblot blot analyses in Fig. 1 that the 110-kDa complexes contained no HAI-1. In addition to the matriptase mAbs M32 and 21-9 and the HAI-1 mAb M19, we have developed unique mAbs that can distinguish the latent from the activated form of matriptase (1). These activated matriptase-specific mAbs have been used in our previous studies for the characterization of matriptase activation in vivo and in vitro (1, 2, 8, 13, 14, 16). When both the 110-kDa and 95-kDa matriptase complexes were incubated with Sepharose beads linked to the activated matriptase-specific mAb M69, only 95-kDa matriptase-HAI-1 complexes and not the 110-kDa complexes were immunodepleted from the solution (Fig. 2A, M69 IP, UB, lane 5), suggesting that the unique, activation-associated epitope recognized by mAb M69 is not present on the 110-kDa complexes. To confirm that the immunodepletion seen in these experiments was indeed due to the complexes binding to the antibody beads, the bound proteins were eluted and subjected to Western blot analysis. The beads were washed, and the bound proteins were eluted with 0.1 M glycine buffer, pH 2.4, and immediately neutralized by Trizma base. This elution procedure was repeated twice, yielding two samples for each set of beads (E1 and E2). Analysis of these eluates demonstrated that the 95-kDa matriptase-HAI-1 complexes were liberated from the beads as both the original 95-kDa complex and as a 70-kDa band size expected for uncomplexed matriptase (Fig. 2A, lanes 3, 4, 6, 7, 9, and 10), consistent with the features of a Kunitz inhibitor of HAI-1, which shows acid sensitivity and reversible binding and inhibition to matriptase (1). The 110-kDa complexes were also eluted from the matriptase mAb 21-9-Sepharose (Fig. 2A, lanes 3 and 4) but not from the mAb M19-Sepharose (Fig. 2A, lanes 9 and 10) or the mAb M69-Sepharose (Fig. 2A, lanes 6 and 7), consistent with the immunodepletion results. The differential binding of both matriptase-containing complexes to the HAI-1 mAb not only demonstrates the novelty of the 110-kDa matriptase-containing complexes but also provides the biochemical means for the separation and purification of the novel matriptase-containing complexes from the predominant HAI-1-containing complexes.
Fig. 2.
Immunological characterization and purification of 110-kDa matriptase complexes. A: immunological comparison of the 2 milk-derived matriptase complexes. The pooled 110-kDa complex-enriched CM column fractions (19-25), which contained similar levels of both 110- and 95-kDa matriptase complexes (lane 1, L), were subjected to immunoprecipitation (IP) with immobilized matriptase mAb 21-9, activated matriptase mAb M69, and HAI-1 mAb M19, respectively, as indicated (21-9 IP, M69 IP, and M19 IP). The unbound (UB) fractions were collected (lanes 2, 5, and 8). After being washed, the proteins, captured by immobilized mAbs beads, were eluted by glycine buffer, pH 2.4, and immediately neutralized. Two sequential elutions were performed (lanes 3, 6, and 9 for elution 1, E1; lanes 4, 7, and 10 for elution 2, E2). All these samples were analyzed by immunoblot with matriptase mAb M32. The 95-kDa matriptase-HAI-1 complexes were immunodepleted by these 3 immobilized mAbs and disappeared from the unbound fractions (lanes 2, 5, and 8). The 95-kDa matriptase-HAI-1 complexes were eluted as 95-kDa complexes and dissociated 70-kDa form (lanes 3, 4, 6, 7, 9, and 10). The 110-kDa matriptase-containing complexes were immunodepleted only by mAb M32-Sepharose beads (lane 2) but not by mAb M69-Sepharose beads (lane 5) and M19-Sepharose beads (lane 8). A matriptase species with a size of 90 kDa was seen in the unbound fractions after removal of 95-kDa matriptase-HAI-1 complexes by anti-HAI-1 mAb M19 (lane 8). The 90-kDa matriptase complexes turned out also to be matriptase-HAI-1 complexes, which contained a degraded HAI-1 fragment that lost the epitope recognized by M19 mAb (data not shown). B and C: immunoaffinity purification of 110-kDa matriptase-containing complexes. The 110-kDa matriptase complex-enriched CM fractions (from Fig. 1) were passed through HAI-1 mAb M19-Sepharose column to remove the 95-kDa matriptase-HAI-1 complexes. The 110-kDa complexes were then purified by immunoaffinity chromatography with matriptase mAb 21-9. The eluted proteins were mixed with SDS sample buffer containing no reducing agents and incubated at room temperature (lanes 1) or 95°C (lanes 2) for 5 min before SDS-PAGE. The eluted proteins in SDS gel were either stained with Coomassie blue (B) or analyzed by immunoblot using matriptase mAb M32 (C). MW, molecular weight markers. The 110-kDa matriptase complexes were eluted as major proteins from immunoaffinity column and were stable in boiling SDS sample buffer (lanes 2). Several minor proteins were also seen in eluted fractions, including the dissociated, active matriptase species (70 kDa), 90-kDa matriptase-HAI-1 complexes (also seen in A, lane 8), and polymeric immunoglobulin receptor. The 90-kDa complexes contain matriptase and a shorter HAI-1 fragment in which the epitope recognized by M19 mAb was lost (data not shown). The 90-kDa matriptase-HAI-1 complexes were completely dissociated after boiling and detected as 70-kDa bands by matriptase mAb M32 mAb (C, lane 2).
Purification and biochemical characterization of milk-derived 110-kDa matriptase complexes.
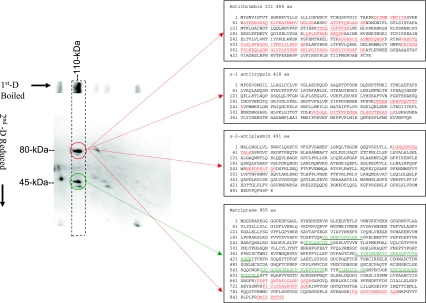
We further purified the novel matriptase-containing complexes from the 110-kDa complex-enriched CM-Sepharose column fractions by using a matriptase mAb 21-9-Sepharose immunoaffinity column after removal of the matriptase-HAI-1 complexes with an immobilized HAI-1 mAb M19 immunoaffinity column (Fig. 2B). The fractions eluted from the matriptase-immunoaffinity column were analyzed by a one-dimensional SDS PAGE under both nonboiled and boiled conditions (Fig. 2B) and then a two-dimensional, diagonal gel electrophoresis under reducing conditions (Fig. 3). The 110-kDa complexes represented the major protein band in the eluted fraction (Fig. 2B, lane 1). Boiling of the sample with SDS did not cause dissociation of the complexes but resulted in a slightly slower rate of migration on SDS-PAGE (Fig. 2B, lane 2). Western blot analysis confirmed that the 110-kDa protein bands were matriptase-containing complexes (Fig. 2C). These analyses suggest that there was a covalent linkage between the matriptase and its binding partner(s) in these complexes, distinct from the noncovalent interactions between matriptase and HAI-1, which can be dissociated by boiling (17) or by acidic pH (1). Interestingly, after boiling in the presence of SDS, we observed that the 110-kDa band apparently contained more than one species (Fig. 2B, lane 2). To analyze the constituents of the 110-kDa complexes, a strip of SDS polyacrylamide gel containing the 110-kDa species was sliced from the gel, chemically reduced using 50 mM DTT, and then subjected to electrophoresis in a second SDS polyacrylamide gel (diagonal gel electrophoresis). Diagonal gel electrophoresis is a useful tool to analyze protein complexes since those proteins whose rates of migration are the same or similar before and after exposure to reducing agents will appear on a diagonal line; however, protein complexes whose subunits dissociate will yield bands on the same path on the gel under the diagonal line. After this procedure, the 110-kDa complexes were dissociated into bands of 80 kDa and 45 kDa (Fig. 3). These data suggest that 110-kDa matriptase complexes contain two disulfide-linked fragments of 80 kDa and 45 kDa.
Fig. 3.
Analysis and identification of the milk-derived 110-kDa complexes. Left: analysis of 110-kDa matriptase complexes by diagonal gel electrophoresis. Immunoaffinity-purified 110-kDa matriptase complexes (see Fig. 2) were mixed with SDS sample buffer containing no reducing agents, incubated at 95°C for 5 min, and then resolved by SDS-PAGE (1st D Boiled). A gel strip was then sliced out, boiled in 1× SDS sample buffer in the presence of reducing agents, and electrophoresed on a second SDS gel (2nd D Reduced). The gels were stained with Coomassie blue. After this procedure, the 110-kDa matriptase complexes were converted to 80-kDa and 45-kDa protein spots, as indicated in the dashed rectangle. Right: proteomic protein identification of the subunits of the 110-kDa matriptase-containing complexes. Both 80-kDa and 45-kDa protein spots derived from 110-kDa matriptase complexes were excised, trypsinized, and then subjected to liquid chromatography-tandem mass spectrometry analysis (LC-MS/MS) for protein identification. Database search revealed that there were 11 tryptic fragments derived from the 45-kDa protein spots matched to 6 stretches of amino acid sequences at the noncatalytic domain of matriptase. These 6 stretches of amino acid sequences are double-underlined. The 80-kDa spots yielded many tryptic fragments, which matched to at least 4 different proteins. Among these tryptic fragments, 5 fragments matched to 4 stretches of amino acid sequences of the serine protease domain of matriptase; 30 fragments matched to 8 stretches of amino acid sequences of antithrombin III (ATIII); 3 fragments matched to 2 stretches of amino acid sequences of α1-antitrypsin (α1AT); 2 fragments matched to 2 stretches of amino acid sequences of α2 antiplasmin (α2AP). These stretches of amino acid sequences matched to the 80-kDa protein spots are underlined. The deduced amino acid sequences were obtained from GenBank, and the Accession Numbers are AAD42765 for matriptase, AAB40025 for ATIII, AAB59371 for α1AT, and CAA85350 for α2AP.
Milk-derived 110-kDa matriptase complexes contain the secreted serpin inhibitors ATIII, α1AT, and α2AP.
Both the 80-kDa and 45-kDa protein bands were subjected to MS-based protein identification studies as described in the materials and methods (Fig. 3). The 80-kDa protein bands contained multiple proteins. Tryptic fragments derived from this protein band contain five peptides matched to matriptase, 30 peptides matched to ATIII, three peptides matched to α1AT, and two matched to α2AP. Eleven tryptic peptides derived from the 45-kDa protein bands were matched to matriptase (Fig. 3). Interestingly, the five tryptic peptides derived from the 80-kDa bands, which matched to matriptase, were all located within the serine protease domain of matriptase, whereas, the matriptase-matching tryptic peptides from the 45-kDa bands were all localized in the noncatalytic domains of matriptase. These data suggest that the 110-kDa complexes contain disulfide-linked, two-chain, activated matriptase. Considering the molecular mass of the three serpins identified (∼55 kDa for ATIII, ∼60 kDa for α1AT, and ∼70 kDa for α2AP) and of the serine protease domain of matriptase (25 kDa), it seems very likely that the 80-kDa bands are composed of three different complexes of the matriptase serine protease domain with one or other of these three serpins. Therefore, the 110-kDa complexes are likely to be a mixture of matriptase-ATIII, matriptase-α1AT, and matriptase-α2AP complexes.
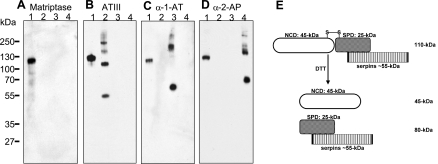
We further confirmed that the 110-kDa matriptase complexes are a mixture of matriptase associated with each of the three secreted serpins by immunoblot with ATIII, α1AT, and α2AP polyclonal antibodies and the matriptase mAb M32 (Fig. 4). The 110-kDa complexes and the various serpins were subjected to SDS-PAGE and then blotted with each of the four antibodies, respectively. The 110-kDa complexes were recognized by the matriptase mAb M32 and all three serpin polyclonal antibodies (Fig. 4, A–D, lane 1). The matriptase mAb M32 did not interact with these three serpin proteins (Fig. 4A, lanes 2, 3, and 4); the serpin polyclonal antibodies only interacted with their corresponding proteins and not the other serpins (Fig. 4, B–D, lanes 2, 3, and 4).
Fig. 4.
The milk-derived 110-kDa species are matriptase-serpin complexes (A–D). Immunological confirmation of 110-kDa matriptase complexes containing the secreted serpins, ATIII, α1AT, and α2AP. To confirm that the 110-kDa matriptase complexes contain secreted serpins, purified 110-kDa matriptase complexes (lanes 1), blood-derived ATIII preparation (lanes 2), α1AT preparation (lanes 3), and α2AP preparation (lanes 4) were examined by immunoblot analyses with matriptase mAb M32 (A, matriptase), antithrombin antibody (B, ATIII), α1AT antibody (C), and α2AP antibody (D). Roughly, 2 ng of 110-kDa complexes were used for A, B, and C, 100 ng for D. E: schematic representation of 110-kDa matriptase-serpin complexes and their dissociation by reducing agents. Milk-derived 110-kDa matriptase complexes contain secreted serpins covalently linked with activated matriptase in which the noncatalytic domain (NCD: 45 kDa) is linked with serine protease domain (SPD: 25 kDa) by a disulfide bond (S-S). Reducing agents, such as DTT, break the disulfide linkage but not the covalent bond linking the matriptase serine protease domain with the serpin inhibitors and convert the 110-kDa complexes into the NCD of matriptase with a size of 45-kDa and 80-kDa protein fragments, which contain the SPD of matriptase covalently linked to the secreted serpins.
In contrast to Kunitz-type serine protease inhibitors such as HAI-1, serpin-type serine protease inhibitors, including ATIII, α1AT, and α2AP, inhibit proteases by forming covalent-linked complexes in a suicide mode (9, 34). This unique feature of serpin-type inhibitors is consistent with the resistance to dissociation by boiling SDS in the absence of reducing agents exhibited by the 110-kDa matriptase-serpin complexes (Fig. 2). Interestingly, chemical reduction of the 110-kDa matriptase-serpin complexes cleaved the disulfide-linkage between the serine protease domain and noncatalytic domain of matriptase in the complexes and resulted in the release of the noncatalytic domains of matriptase, seen as the 45-kDa protein band in the diagonal gel electrophoresis study (Fig. 3). After releasing the 45-kDa matriptase noncatalytic domain, the 110-kDa complexes were converted into 80-kDa complexes consisting of the matriptase serine protease domain covalently linked with either ATIII, α1AT, or α2AP. In Fig. 4E, we summarize the structure of 110-kDa complexes and the conversion into their subunits by chemical reduction.
Matriptase and its inhibitors in human milk.
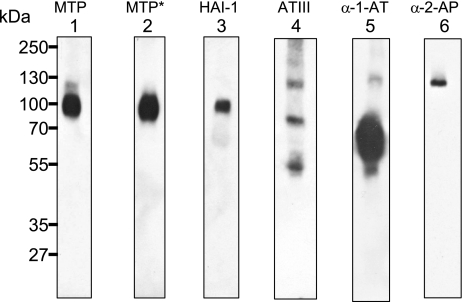
We further analyzed the expression status of matriptase and its endogenous inhibitors, including HAI-1, ATIII, α1AT, and α2AP in defatted human milk (Fig. 5). Matriptase was detected predominantly in its 95-kDa HAI-1 complexes and also as a minor species at 110-kDa, which may represent a mixture of matriptase complexes with the individual secreted serpins (Fig. 5, lane 1). There was no free (noncomplexed) matriptase, either in its latent or activated form, detected in human milk. The activated matriptase-specific mAb M69, only detected the 95-kDa matriptase-HAI-1 complexes (Fig. 5, lane 2), further confirmed that there was no free, active matriptase in human milk. HAI-1 was detected only in 95-kDa matriptase complex, demonstrating that there was also no free (noncomplexed) HAI-1 (Fig. 5, lane 3). In contrast, free (noncomplexed) ATIII and α1AT were detected in human milk in addition to the complexed forms (Fig. 5, lanes 4 and 5).
Fig. 5.
Expression analysis of matriptase and its endogenous inhibitors, HAI-1, ATIII, α1AT, and α2AP. Defatted human milk was subjected to Western blot analyses using matriptase mAb M32 (MTP), activated matriptase mAb M69 (MTP*), HAI-1 mAb M19 (HAI-1), ATIII antibody, α1AT antibody, and α2AP.
Hematopoietic cells express matriptase with no or very low levels of HAI-1.
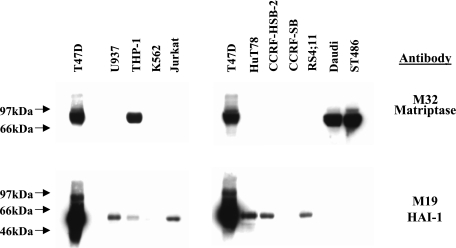
In search of cell types that utilize different mechanisms that do not involve HAI-1 for the regulation of matriptase, we turned our attention to hemopoietic cells, some of which previously were reported to express matriptase in the absence of HAI-1 (4, 11). Western blot analysis of human leukemia and lymphoma cell lines for the expression of matriptase and HAI-1 was performed (Fig. 6). T-47D human breast cancer cells, which express high levels of both matriptase and HAI-1, were used as a control. Western blotting with the matriptase mAb M32 revealed that THP-1, human monocytic cells, and two Burkitt's lymphoma cells, Daudi and ST486, were positive for the protease, whereas the seven others were negative (Fig. 6). Although low levels of HAI-1 were detected in some cells that express no matriptase, neither Daudi nor ST486 cell lines coexpressed HAI-1 with matriptase. THP-1 cells express HAI-1 at very low levels compared with the T-47D cells and even the other HAI-1-positive leukemia cell lines. These expression analyses suggest that some hematopoietic cells are likely using HAI-1 as an independent mechanism for the regulation of matriptase and that the secreted serpins identified in the present study may serve as endogenous inhibitors for matriptase derived from these hematopoietic cells.
Fig. 6.
Expression of matriptase and HAI-1 protein in human leukemia cell lines. Twenty-five micrograms of whole cell lysates from a variety of human cell lines, as indicated, were resolved by SDS-PAGE and probed with matriptase mAb M32 or with HAI-1 MAb M19. Origin of human cell lines: T47D, human breast cancer (positive control); U937, myelogenous leukemia; THP-1, myelogenous leukemia; K562, myelogenous leukemia; Jurkat, T-cell leukemia; HuT78, T-cell leukemia; CCRF-HSB-2, T-cell leukemia; CCRF-SB, B-cell leukemia; RS4; 4, B-cell leukemia; Daudi, B-cell lymphoma (Burkitt's); ST486, B-cell lymphoma (Burkitt's).
DISCUSSION
The identification of matriptase complexed with secreted serpins, particularly ATIII and α1AT, in human milk provides evidence that free, active matriptase indeed exists extracellularly either on the cell surface or in the interstitial space of lactating mammary glands, where free, active matriptase encounters these secreted serpins. As a membrane-bound serine protease with a single-pass transmembrane domain, the bulk of the matriptase molecule, including its serine protease domain, has been believed to orient itself toward the extracellular side and to function as a protease activator on the cell surface to activate substrates extracellularly (17). However, the dual roles of HAI-1 as a conventional protease inhibitor for matriptase (1) and as a factor required for zymogen activation of the protease (27) would seem to dictate that free, active matriptase is only likely to exist for a very short time. Activated matriptase has been consistently detected in complexes with HAI-1 regardless of the cell source, such as breast cancer cells vs. immortal mammary epithelial cells (2), regardless of the mode of zymogen activation, such as the constitutive activation exhibited by breast cancer cells (2) compared with the induced zymogen activation in immortal mammary epithelial cells by sphingosine 1-phosphate (2), or in prostate cancer cells by androgen (13), or more broadly in other cell types by suramin (16). Although it has been challenging to determine the exact cellular locations where the activation of matriptase occurs, it seems likely that it takes place either at the plasma membrane or in the secretory pathway during its trafficking toward the plasma membrane because activated matriptase was previously detected at cell-cell junctions (2, 8) and in vesicle-like structures within cells (16). In either case, given that HAI-1 participates in the activation process and is therefore present and able to immediately bind to the newly activated enzyme, the accessibility of free, active matriptase to its potential extracellular substrates, or to protease inhibitors other than HAI-1, may be very limited. Therefore, in matriptase-expressing cells that also express a comparable amount of HAI-1, free, active matriptase may never reach the extracellular environment at meaningful levels. It is, therefore, paradoxical that, in the present study, we purified matriptase complexed with secreted serpins from human milk. The ATIII and α1AT are probably derived from blood or locally produced during lactation and deposited in the extracellular matrix (33). Furthermore, serpins are activated after secretion, and, therefore, it is unlikely that inhibition and formation of matriptase-serpin complexes occur in the secretory pathway. Therefore, free, active matriptase has to reach the extracellular environment, at least on the extracellular surface of the plasma membrane, for exposure to these secreted serpin-type serine protease inhibitors.
Mammary epithelial cells produce most milk proteins, and, since milk-derived, immortal mammary epithelial cells express both matriptase and HAI-1, the milk-derived matriptase-HAI-1 complexes are very likely produced by mammary epithelial cells (17). Robust zymogen activation of matriptase apparently takes places in these mammary epithelial cells during lactation since a large amount of matriptase-HAI-1 complexes are present in milk (Fig. 8). Since the activated matriptase produced by mammary epithelial cells is sequestered in HAI-1 complexes and in light of the stability of HAI-1/matriptase complexes, it seems very unlikely that the activated matriptase in serpin complexes is derived from matriptase liberated from HAI-1 complexes, being inhibited by and forming complexes with these three serpins. This possibility is made more unlikely by the facts that 1) the pH of milk does not favor the dissociation of matriptase-HAI-1 complexes, 2) no free HAI-1 was detected in milk as would be expected if the serpin-bound matriptase was derived from HAI-1 complexes (Figs. 1 and 8), and 3) free α2AP was undetectable in milk and is however found in complexes with matriptase (Fig. 5). Furthermore, there is probably much more free α1AT than free ATIII in human milk (20), yet the levels of matriptase-ATIII complexes are apparently higher than those of α1AT complexes in human milk. Had matriptase been dissociated from HAI-1 complexes in milk, one would expect α1AT to have a quantitative advantage in forming complexes with matriptase compared with ATIII due to the large amount of free α1AT. These observations suggest that there are complicated biological processes behind the formation of these matriptase-serpin complexes regarding the cell types that express matriptase and where the active matriptase encounters these serpins.
The activated matriptase found in these serpin-complexes could be produced by cell types other than mammary epithelial cells. There are multiple cell types other than epithelial cells present in the stroma of a lactating mammary tissues, including fibroblasts, adipocytes, endothelial cells in the blood vessels, and migrating leukocytes, such as macrophages, plasma cells, and eosinophils to name a few. Among all these cell types, migrating leukocytes are a potential source of free active matriptase for what could subsequently be inhibited by ATIII and α1AT. Peritoneal macrophages have been reported to express matriptase but not HAI-1 (4). THP-1 human monocytic cells express matriptase and very low levels of HAI-1 (Fig. 6). In the absence of HAI-1, migrating macrophages may secret free active matriptase. ATIII and α1AT are produced by the liver, are found at high levels in the blood, and may be transported and deposited in the interstitial spaces of the lactating mammary tissues, thus finding their way into milk, with their free forms detected in milk. ATIII has been detected in the basement membrane bound to heparin sulfate proteosaminoglycan underneath endothelial cells in blood vessels from a variety of tissues (33). The mRNA of α1AT has also been detected in the lactating mammary tissues, suggesting that local expression of the serpin may also contribute to its presence in milk (6). Therefore, an appealing hypothesis for the presence of both free and matriptase-complexed ATIII and α1AT in human milk is that matriptase is activated in migrating leukocytes and/or plasma cells when these cells are extravasating and migrating through the basement membrane of capillaries in the lactating mammary gland, where both serpins are deposited.
The biology of the inhibition of matriptase by α2AP may be different from the other two secreted serpins since free α2AP was not detected in human milk by immunoblot analysis (Fig. 8). Transcytosis of α2AP into milk apparently does not occur to any significant extent compared with the levels of ATIII and α1AT (Fig. 5). The presence of matriptase-α2AP complexes in the absence of free α2AP in human milk suggests that α2AP may be expressed by the same cells that are expressing and activating matriptase, resulting in the formation of α2AP complexes before shedding into the extracellular milieu, in a fashion similar to HAI-1. In contrast to ATIII for which expression is restricted to hepatocytes, α2AP has been detected in several other tissues, including kidney, intestine, striated muscle, testis, hippocampus, and placenta (22). It is of interest to determine whether and what cell types might produce α2AP in mammary tissues in future studies.
Unlike Kunitz-type protease inhibitors, such as HAI-1, which inhibit proteases by forming very tight but reversible complexes, the mode of protease inhibition by serpin-type serine protease inhibitors has been considered to be a “suicide” or “single-use” approach due to its distinctive conformational alteration for inhibiting serine proteases. Serpin-type serine protease inhibitors inactivate their target proteases by binding to them covalently in a 1:1 stoichiometry. Protease inactivation by serpin inhibitors involves the attack of the target protease on the P1-P1′ bond in the reactive center loop of the serpins that results in the trapping of the protease in complexes with serpin inhibitors and deforms the protease catalytic triad (9, 34). In the case of the 110-kDa matriptase-serpin complexes, the covalent linkage between the reactive centers of the serpins and active site triad serine of matriptase held both proteins together under boiled and nonreduced conditions (Fig. 2). The binding, inhibition, and trapping of serine proteases by serpins likely significantly disrupts the active site triad and substrate binding pocket of proteases since the activation-associated epitope recognized by the mAb M69 is no longer present on the 110-kDa complexes (Figs. 2 and 5).
In summary, in this study, we have purified three novel matriptase complexes containing secreted serpins. These secreted serpins may provide the long-sought answer for the question as to how the proteolytic activity of matriptase is controlled in those cells that express no or very low levels of HAI-1. In addition, the presence of free, active matriptase in the extracellular milieu and the formation of matriptase-serpin complexes suggest that these serpins could be useful tools to detect active matriptase in those cells that express very low levels of or no HAI-1. Despite their common ability to inhibit matriptase, secreted serpins, at least for ATIII and α1AT, are likely to be responsible to the control of active matriptase possibly produced by migrating leukocytes in contrast to HAI-1, which regulates matriptase in mammary epithelial cells. Therefore, in the lactating mammary tissues, there appears to be two sources of matriptases: epithelial matriptase and stromal matriptase. The major difference between these two matriptase forms is their inhibitions by different inhibitor systems.
GRANTS
This study was supported by NIH R01CA096851 and R01CA104944 (to Lin, C.-Y.) and by the Maryland Cigarette Restitution Fund Program.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem 268: 1439–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem 277: 10539–10546, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis 19: 639–649, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt AS, Welm A, Farady CJ, Vasquez M, Wilson K, Craik CS. Coordinate expression and functional profiling identify an extracellular proteolytic signaling pathway. Proc Natl Acad Sci USA 104: 5771–5776, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carney TJ, von der HS, Sonntag C, Amsterdam A, Topczewski J, Hopkins N, Hammerschmidt M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development 134: 3461–3471, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Chowanadisai W, Lonnerdal B. Alpha(1)-antitrypsin and antichymotrypsin in human milk: origin, concentrations, and stability. Am J Clin Nutr 76: 828–833, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Fan B, Brennan J, Grant D, Peale F, Rangell L, Kirchhofer D. Hepatocyte growth factor activator inhibitor-1 (HAI-1) is essential for the integrity of basement membranes in the developing placental labyrinth. Dev Biol 303: 222–230, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Hung RJ, Hsu I, Dreiling JL, Lee MJ, Williams CA, Oberst MD, Dickson RB, Lin CY. Assembly of adherens junctions is required for sphingosine 1-phosphate-induced matriptase accumulation and activation at mammary epithelial cell-cell contacts. Am J Physiol Cell Physiol 286: C1159–C1169, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Huntington JA Shape-shifting serpins—advantages of a mobile mechanism. Trends Biochem Sci 31: 427–435, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka H, Suganuma T, Shimomura T, Itoh H, Kitamura N, Nabeshima K, Koono M. Distribution of hepatocyte growth factor activator inhibitor type 1 (HAI-1) in human tissues. Cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem 47: 673–682, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A, Ellis V. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood 108: 2616–2623, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim MG, Chen C, Lyu MS, Cho EG, Park D, Kozak C, Schwartz RH. Cloning and chromosomal mapping of a gene isolated from thymic stromal cells encoding a new mouse type II membrane serine protease, epithin, containing four LDL receptor modules and two CUB domains. Immunogenetics 49: 420–428, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and subsequent shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol Cell Physiol 291: C40–C49, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol 293: C95–C105, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and HAI-1-mediated inhibition of matriptase induced at activation foci in immortal human mammary epithelial cells. Am J Physiol Cell Physiol 288: C932–C941, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem 274: 18237–18242, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Lin CY, Anders J, Johnson M, Sang QA, Dickson RB. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem 274: 18231–18236, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Lin CY, Wang JK, Torri J, Dou L, Sang QA, Dickson RB. Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem 272: 9147–9152, 1997. [PubMed] [Google Scholar]
- 20.Lindberg T, Ohlsson K, Westrom B. Protease inhibitors and their relation to protease activity in human milk. Pediatr Res 16: 479–483, 1982. [DOI] [PubMed] [Google Scholar]
- 21.List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev 19: 1934–1950, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menoud PA, Sappino N, Boudal-Khoshbeen M, Vassalli JD, Sappino AP. The kidney is a major site of alpha(2)-antiplasmin production. J Clin Invest 97: 2478–2484, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberst M, Anders J, Xie B, Singh B, Ossandon M, Johnson M, Dickson RB, Lin CY. Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol 158: 1301–1311, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberst MD, Chen LY, Kiyomiya KI, Williams CA, Lee MS, Johnson MD, Dickson RB, Lin CY. Hepatocyte growth factor activator inhibitor 1 (HAI-1) regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol 289: C462–C470, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Oberst MD, Johnson MD, Dickson RB, Lin CY, Singh B, Stewart M, Williams A, al Nafussi A, Smyth JF, Gabra H, Sellar GC. Expression of the serine protease matriptase and its inhibitor HAI-1 in epithelial ovarian cancer: correlation with clinical outcome and tumor clinicopathological parameters. Clin Cancer Res 8: 1101–1107, 2002. [PubMed] [Google Scholar]
- 26.Oberst MD, Singh B, Ossandon M, Dickson RB, Johnson MD, Lin CY. Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51: 1017–1025, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem 278: 26773–26779, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Saleem M, Adhami VM, Zhong W, Longley BJ, Lin CY, Dickson RB, Reagan-Shaw S, Jarrard DF, Mukhtar H. A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomarkers Prev 15: 217–227, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene 26: 1546–1556, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi T, Shuman MA, Craik CS. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci USA 96: 11054–11061, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H, Nagaike K, Takeda N, Itoh H, Kohama K, Fukushima T, Miyata S, Uchiyama S, Uchinokura S, Shimomura T, Miyazawa K, Kitamura N, Yamada G, Kataoka H. Hepatocyte growth factor activator inhibitor type 1 (HAI-1) is required for branching morphogenesis in the chorioallantoic placenta. Mol Cell Biol 25: 5687–5698, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimoto H, Underwood LJ, Wang Y, Shigemasa K, Parmley TH, O'Brien TJ. Ovarian tumor cells express a transmembrane serine protease: a potential candidate for early diagnosis and therapeutic intervention. Tumour Biol 22: 104–114, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Slayter HS. Immunocytochemical localization of endogenous anti-thrombin III in the vasculature of rat tissues reveals locations of anticoagulantly active heparan sulfate proteoglycans. J Histochem Cytochem 42: 1365–1376, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Ye S, Goldsmith EJ. Serpins and other covalent protease inhibitors. Curr Opin Struct Biol 11: 740–745, 2001. [DOI] [PubMed] [Google Scholar]