Abstract
Heat shock protein (HSP) 72 is released by cells during stress and injury. HSP-72 also stimulates the release of cytokines in macrophages by binding to Toll-like receptors (TLR) 2 and 4. Circulating levels of HSP-72 increase during hepatic ischemia-reperfusion injury. The role of extracellular HSP-72 (eHSP-72) in the injury response to ischemia-reperfusion is unknown. Therefore, the objective of the present study was to determine whether eHSP-72 has any direct effects on hepatocytes. Primary mouse hepatocytes were treated with purified human recombinant HSP-72. Conditioned media were evaluated by ELISA for the cytokines, TNF-α, IL-6, and macrophage inflammatory protein 2 (MIP-2). Stimulation of hepatocytes with eHSP-72 did not induce production of TNFα or IL-6 but resulted in dose-dependent increases in MIP-2 production. To evaluate the pathway responsible for this response, expression of TLR2 and TLR4 was confirmed on hepatocytes by immunohistochemistry. Hepatocyte production of MIP-2 was significantly decreased in hepatocytes obtained from TLR2 or TLR4 knockout mice. MIP-2 production was found to be partially dependent on NF-κB because inhibition of NF-κB with Bay 11-7085 significantly decreased eHSP-72-induced MIP-2 production. Inhibitors of p38 mitogen-activated protein kinase or c-Jun NH2-terminal kinase had no effect on production of MIP-2 induced by eHSP-72. The data suggest that eHSP-72 binds to TLR2 and TLR4 on hepatocytes and signals through NF-κB to increase MIP-2 production. The fact that eHSP-72 did not increase TNF-α or IL-6 production may be indicative of a highly regulated signaling pathway downstream from TLR.
Keywords: liver, chemokines, Toll-like receptors, nuclear factor-κB, inflammation
the induction of heat shock proteins (HSP) represents an important response to cellular stress induced by environmental, metabolic, or pharmacologic sources (11, 19). Intracellularly, HSPs function to stabilize the cell and maintain cellular homeostasis (14). The family of HSPs at 70-kDa includes HSP-72, a highly stress-inducible 72-kDa protein. Expression of HSP-72 increases when heat shock factor 1 binds to the heat shock element in the promoter region of the HSP-70 gene, resulting in transcription of HSP-72 mRNA (19). HSP-72 is best known as an intracellular chaperone, assisting the cell in protein folding particularly in times of stress (24). During the response to stress or injury, HSP-72 may be released from dying cells that have lysed, as well as from live cells via receptor-mediated exocytosis (2). In many different models of injury, circulating levels of HSP-72 are increased (10, 12, 17, 25, 31, 35, 37). This extracellular HSP-72 (eHSP-72) is composed of an NH2-terminal ATPase domain and a COOH-terminal peptide binding domain and has the ability to interact with dendritic cells, monocytes, and macrophages. The COOH-terminal domain of HSP-72 is required for the stimulation of cytokines, binding to CD14, Toll-like receptor (TLR) 4, and CD40, and its many antiapoptotic effects (5, 16, 36). The interaction of eHSP-72 with immune cells has been shown to stimulate the production of proinflammatory cytokines (3). Furthermore, HSP-72 and gp96 peptide complexes can be taken up by antigen-presenting cells, and, via cross-presentation on major histocompatibility complex class 1 molecules, can initiate a CD-8-specific T-cell response (13, 32).
The signaling mechanisms used by eHSP-72 have been linked to TLR2 and TLR4 (3, 4). In general, there are two pathways of TLR activation, the first leading to NF-κB activation and the second leading to p38 and JNK activation. Both of these pathways use the adaptor protein MyD88 and IL-1 receptor-associated kinase (IRAK) in signal transduction. Beyond MyD88 and IRAK, adaptor proteins help determine the specific program of gene expression (29). In monocytes, eHSP-72 uses TLR2 and TLR4 to induce cytokine production via the MyD88/IRAK/NF-κB signal transduction pathway (4). Recently, our laboratories have demonstrated that HSP-72 also activates tissue parenchymal cells. In these studies, HSP-72 stimulated murine airway epithelial cells in a TLR4-dependent fashion (7).
The concept that eHSP-72 may function as a regulator of injury responses is supported by both in vitro and in vivo studies. The role of eHSP-72, however, remains elusive. Clinical reports have suggested that increased serum levels of HSP-72 in trauma patients correlate with survival, whereas in patients undergoing liver resection, serum HSP-72 is associated with liver dysfunction (17, 31). Focusing on the liver, we have previously shown that circulating levels of HSP-72 are elevated after hepatic ischemia-reperfusion (28). Subsequent studies suggested that induction of intracellular HSP-72 before ischemia-reperfusion is directly hepatoprotective (20). However, the manner in which eHSP-72 may affect hepatocyte function has not been studied. In the present study, we evaluated the effects of eHSP-72 on primary murine hepatocytes.
MATERIALS AND METHODS
Generation of recombinant human Hsp-72.
Recombinant Hsp-72 was synthesized as previously described (1). Endotoxin levels [110 endotoxin units (EU)/mg Hsp-72 protein or 11 ng/mg Hsp-72 protein] were independently measured at Charles River Laboratories.
Generation of truncated Hsp-72.
The 5′-HSP-72 (amino acids 1-430) was digested with BamHI and SmaI and cloned into pRSET (BamHI and PvuII). Clone was confirmed by sequencing analysis at the Cincinnati Children's Hospital DNA core. The Escherichia coli strain B21(DE3) pLysS transformed with the 5′-HSP-72 expression plasmid was induced for 16 h at 37°C in Luria-Bertani broth supplemented with 100 mg/ml ampicillin. These cultures were diluted 100-fold with fresh Luria-Bertani medium and cultured at 37°C for 3 h while shaking at 250 rpm. Protein expression was induced by the addition of 1 M isopropyl β-d-thiogalactoside to a final concentration of 1.0 mM for 3 h while shaking at 37°C. The induced cells were lysed in BugBuster lysis buffer (EMD Biosciences) supplemented with 1:1,000 benzonase nuclease. Cells were lysed for 30 min at room temperature with rocking. Cell debris was removed by centrifugation, and the cell extracts were then loaded into a His-Bind Ni-NTA resin column (EMD Biosciences). The column was washed, and the 5′-HSP-72 was eluted with elution buffer according to the manufacturer's instructions. The protein was further purified using Endotrap Blue resin (Cambrex), according to the manufacturer's instructions. The 3′-HSP-72 (amino acids 420-640) was digested with BglII and HindIII and cloned into pRSET (Bgl and HindIII). Clone was confirmed by sequencing analysis at the Cincinnati Children's Hospital DNA core. A single colony of the E. coli strain B21(DE3) pLysS transformed with the 5′-HSP-72 expression plasmid was grown in 8 ml of SOC broth supplemented with 200 μg/ml carbenicillin to an optical density (OD) of 0.2–0.6. The culture was centrifuged at 7,000 rpm for 20 min. The cell pellet was resuspended in 50 ml of SOC broth supplemented with 500 μg/ml carbenicillin and grown to an OD of 0.2–0.6. The culture was centrifuged at 7,000 rpm for 20 min. The cell pellet was resuspended in 100 ml of SOC broth supplemented with 500 μg/ml carbenicillin and grown to an OD of 0.2–0.6. The cell pellet was resuspended in 300 ml of SOC broth supplemented with 500 μg/ml carbenicillin and grown to an OD of 0.2–0.6. The culture was centrifuged at 7,000 rpm for 20 min. The cell pellet was resuspended in 300 ml of SOC broth supplemented with 500 μg/ml carbenicillin and 1 mM isopropyl β-d-thiogalactoside and grown at 30°C for 2 h. Isolation of 3′-HSP-72 was performed as for the 5′-HSP-72.
Hepatocyte isolation and treatment.
Hepatocytes were isolated from C57BL/6, Balb/C, C.C3-Tlr4Lps-d/J, and B6.129-Tlr2tm1Kir/J (Jackson Laboratory, Bar Harbor, ME) by nonrecirculating collagenase perfusion through the portal vein. This project was approved by the University of Cincinnati Animal Care and Use Committee and was in compliance with the National Institutes of Health guidelines. Livers were perfused in situ with 45 ml GIBCO Liver Perfusion Media (Invitrogen, Carlsbad, CA) followed by 45 ml of GIBCO Liver Digestion Media (Invitrogen). The liver was excised and minced and strained through a steel mesh. The dispersed hepatocytes were collected by centrifugation at 50 g for 2 min at 4°C. Cells were washed two times in Williams media. Hepatocytes were then isolated via Percoll separation as described elsewhere (18) and washed again two times in Williams media. Cells were counted and viability was checked by trypan blue exclusion. Cells were seeded in 24-well plates at 2 × 105. Twenty-four hours later, cells were treated with either 11 pg/ml LPS, HSP-72 boiled at 100°C for 10 min, or 1,000 ng/ml highly purified HSP-72 for 8 h. For inhibitor studies, hepatocytes were treated with the inhibitor for 1 h before the addition of 1,000 ng/ml HSP-72. Inhibitors used were Bay 11-7085 (Biomol, Plymouth Meeting, PA), SB-203580 (Calbiochem), and SP-600125 (Calbiochem). All were used at a final concentration of 20 μM. These concentrations have been shown to be effective for each of these inhibitors (6, 8, 30). Culture media were collected after 8 h and analyzed via ELISA for TNF-α, IL-6, and macrophage inflammatory protein 2 (MIP-2) as described elsewhere (22).
Immunocytochemical labeling.
Liver samples were fixed in 10% neutral buffered formalin, processed, mounted in paraffin, and sectioned onto positively charged slides. Following deparaffinization, heat-induced epitope retrieval was performed by placing the slides in 300 ml of working citrate buffer solution (pH 6.0, 1:10 dilution in distilled water, Dako) and boiling for 10 min. Slides were cooled, rinsed in PBS, placed in a solution of 3.0% hydrogen peroxide in PBS for 15 min at room temperature to block endogenous peroxidase, and then rinsed in PBS. Biotin block (Dako) was applied, and slides were incubated for 10 min each (A&B) followed by a gentle PBS rinse. Next, a serum block (1% BSA, 15% goat, 1% mouse) was added to the slides, and the slides were incubated for 30 min at room temperature. After removal of serum, slides were incubated in rabbit polyclonal anti-TLR2 or anti-TLR4 antibodies (Santa Cruz Biotechnology) overnight at 4°C. The sections were gently rinsed with PBS and were then incubated with a goat anti-rabbit secondary antibody (Vector Labs) for 30 min at room temperature. After the sections were washed, color was developed by the addition of 3,3′-diaminobenzidine substrate-chromagen solution, followed by incubation for 15 min. Slides were then rinsed with water and mounted.
Statistical analysis.
All data are expressed as means ± SE. Data were analyzed with a one-way ANOVA with subsequent Student-Newman-Keuls test. Differences were considered significant at P < 0.05.
RESULTS
HSP-72 selectively induces CXC chemokine expression in hepatocytes.
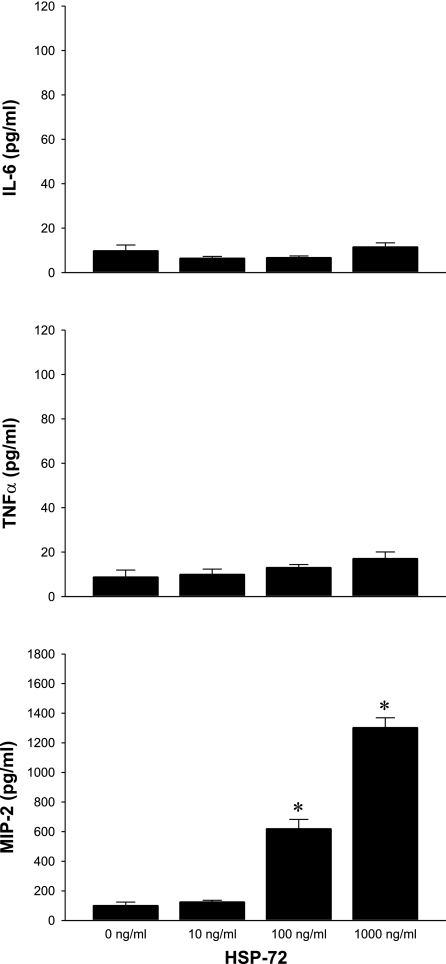
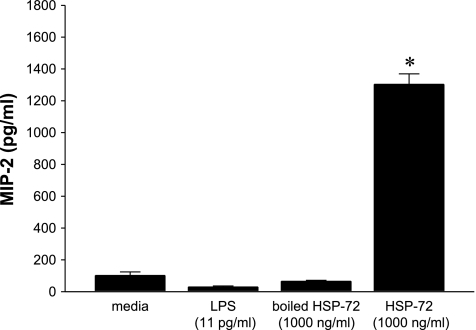
To determine whether hepatocytes were responsive to HSP-72, we cultured primary hepatocytes from C57BL/6 mice and treated them with a dose range of HSP-72 for 8 h. Media were collected, and IL-6, TNF-α, and MIP-2 were measured by ELISA. Neither IL-6 nor TNF-α was induced by HSP-72 at any dose tested (Fig. 1). In contrast, release of MIP-2 increased in a dose-dependent fashion, with 1,000 ng/ml of HSP-72 resulting in a nearly 18-fold increase in MIP-2 compared with no treatment (Fig. 1). We also examined the effect of 1,000 ng/ml of HSP-72 on expression of TNF-α, IL-6, and MIP-2 at 2, 4, and 8 h. No expression of TNF-α or IL-6 was observed at any of these time points, and MIP-2 expression peaked at 8 h (data not shown). The endotoxin contamination of our recombinant HSP-72 was determined to be 11 ng/mg by an independent source, Charles River Laboratories. To determine whether LPS contamination was responsible for the hepatocyte response to HSP-72, we stimulated hepatocytes with 11 pg/ml of LPS (the amount of contamination present in 1,000 ng/ml HSP-72 treatment) and measured the production of MIP-2 after 8 h. This amount of LPS had no effect on the production of MIP-2 (Fig. 2). To further demonstrate that the observed effects of HSP-72 were due to the protein and not contaminants, we treated cells with HSP-72 that had been denatured by boiling at 100°C for 10 min. This treatment also showed no increase in MIP-2 production from baseline (Fig. 2).
Fig. 1.
Extracellular heat shock protein 72 (HSP-72) increases macrophage inflammatory protein 2 (MIP-2) production in hepatocytes. Primary C57BL/6 hepatocytes were treated with 0-1,000 ng/ml HSP-72 for 8 h. Supernatants were harvested and analyzed by ELISA for IL-6, TNF-α, and MIP-2. Data represent means ± SE of 3 experiments. *P < 0.05 compared with all other groups.
Fig. 2.
Hepatocyte production of MIP-2 is stimulated by HSP-72 and not contaminants. Primary C57BL/6 hepatocytes were treated with media, 11 pg/ml LPS, 1,000 ng/ml heat-denatured (boiled) HSP-72, or 1,000 ng/ml HSP-72 for 8 h. Supernatant was harvested and MIP-2 production was analyzed by ELISA. Data represent means ± SE of 4 experiments. *P < 0.05 compared with all other groups.
Full-length HSP-72 is required for maximal MIP-2 production.
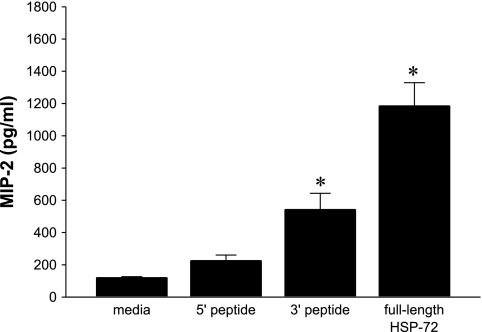
To determine whether a particular portion of HSP-72 is responsible for signal transduction leading to expression of MIP-2, we used truncated forms of HSP-72. The HSP-72 protein was separated into amino acids 1-430 (5′ peptide) and amino acids 420-640 (3′ peptide). Primary hepatocytes were stimulated with 1,000 ng/ml of 5′ peptide, 3′ peptide, or full-length HSP-72 for 8 h. Treatment with 5′ peptide did not significantly increase MIP-2 production (Fig. 3). However, treatment with 3′ peptide increased MIP-2 production, but not to the extent of that of the full-length HSP-72 (Fig. 3).
Fig. 3.
Full-length HSP-72 is required for complete induction of MIP-2. Primary C57BL/6 hepatocytes were treated with media or 1,000 ng/ml of truncated 5′-HSP-72, 3′-HSP-72, or full-length HSP-72 for 8 h. Supernatant was harvested and analyzed by ELISA for MIP-2. Data represent means ± SE of 4 experiments. *P < 0.05 compared with all other groups.
HSP-72 signals through TLR2 and TLR4.
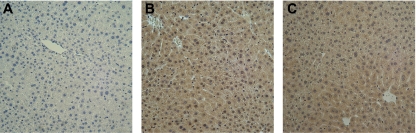
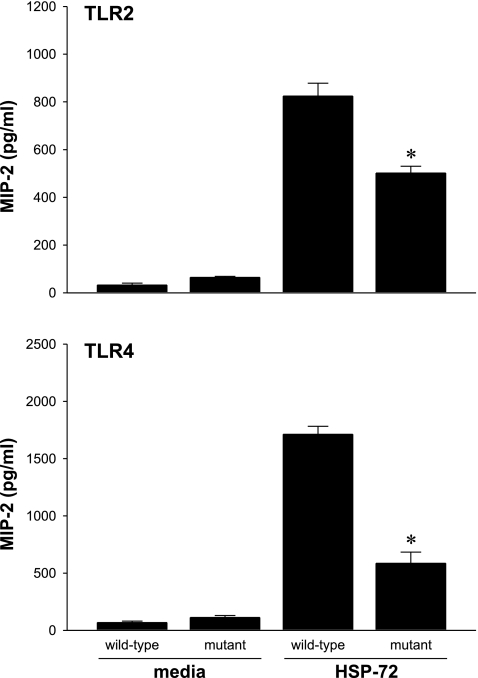
It has been previously shown that HSP-72 signals via TLR2 and TLR4 (3, 4). In hepatocytes, most of the studies on TLR2 and TLR4 expression have used mRNA as the primary measurement. To assess the expression of TLR2 and TLR4 proteins on hepatocytes, liver sections of unmanipulated C57BL/6 mice were immunostained for TLR2 and TLR4. We observed positive staining for both receptors (Fig. 4). Next, using TLR2-mutant and TLR4-mutant mice and their appropriate controls (C57BL/6 and Balb/C, respectively), we isolated and cultured primary hepatocytes and treated them with media or 1,000 ng/ml HSP-72. Treatment with HSP-72 resulted in the expected increase in MIP-2 production in wild-type hepatocytes (Fig. 5). However, hepatocytes from both TLR2- and TLR4-mutant mice had significantly decreased production of MIP-2 compared with the respective wild-type controls (Fig. 5).
Fig. 4.
Toll-like receptors (TLR) 2 and 4 are expressed in normal murine hepatocytes. Livers were harvested from C57BL/6 mice and processed for immunohistochemistry. Livers were stained without primary antibody (A) or with primary antibodies to TLR2 (B) or TLR4 (C). Original magnification is ×50.
Fig. 5.
Mutation of TLR2 or TLR4 disrupts HSP-72 induction of MIP-2 in hepatocytes. Primary hepatocytes from wild-type mice or mice with mutations in TLR2 or TLR4 that rendered these receptors nonfunctional were treated with media or 1,000 ng/ml HSP-72 for 8 h. Supernatant was harvested and analyzed by ELISA for MIP-2. Data represent means ± SE of 4 experiments. *P < 0.05 compared with wild-type.
NF-κB drives MIP-2 expression in hepatocytes.
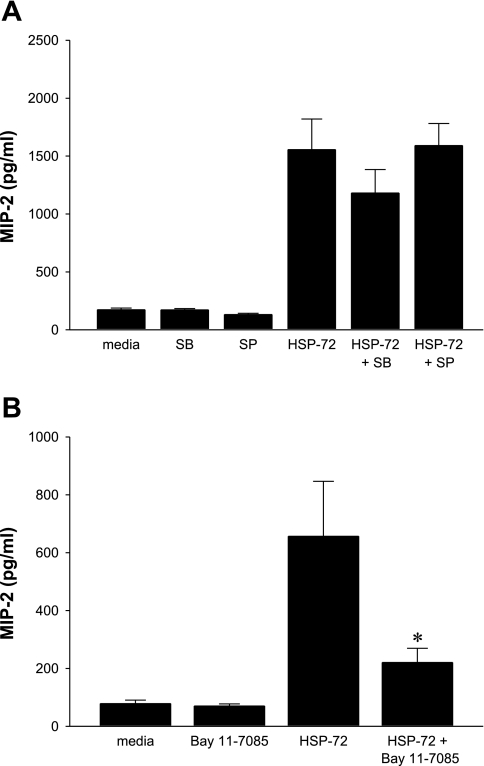
TLR2 and TLR4 have been shown to use p38 MAPK, JNK, and NF-κB pathways for intracellular signaling. To investigate whether one or all of these pathways were activated after stimulation with HSP-72, hepatocytes were incubated with inhibitors for 1 h before treatment with 1,000 ng/ml HSP-72. No significant decreases in MIP-2 production were noted with SB-203580, an inhibitor of p38 MAPK (Fig. 6A). Similarly, SP-600125, a JNK inhibitor, had no effect on HSP-72-induced production of MIP-2 (Fig. 6A). To test whether NF-κB is used in HSP-72 signaling, Bay 11-7085, a selective and irreversible inhibitor of NF-κB, was used. Pretreatment of hepatocytes with Bay 11-7085 significantly decreased the production of MIP-2 induced by HSP-72 (Fig. 6B).
Fig. 6.
Extracellular HSP-72 signals through NF-κB and not p38 MAPK or JNK. Hepatocytes from C57BL/6 mice were harvested and cultured. A: cells were pretreated for 1 h with media, 20 μM SB-203580 (a specific inhibitor of p38 MAPK), or 20-μM SP-600125 (a specific inhibitor of JNK) before treatment with media or 1,000 ng/ml HSP-72. Supernatant was harvested and analyzed by ELISA for MIP-2. Data represent means ± SE of 4 experiments. B: cells were pretreated for 1 h with media or Bay 11-7085 (a specific inhibitor of NF-κB) before treatment with media or 1,000 ng/ml HSP-72. Supernatant was harvested and analyzed by ELISA for MIP-2. Data represent means ± SE of 4 experiments. *P < 0.05 compared with HSP-72.
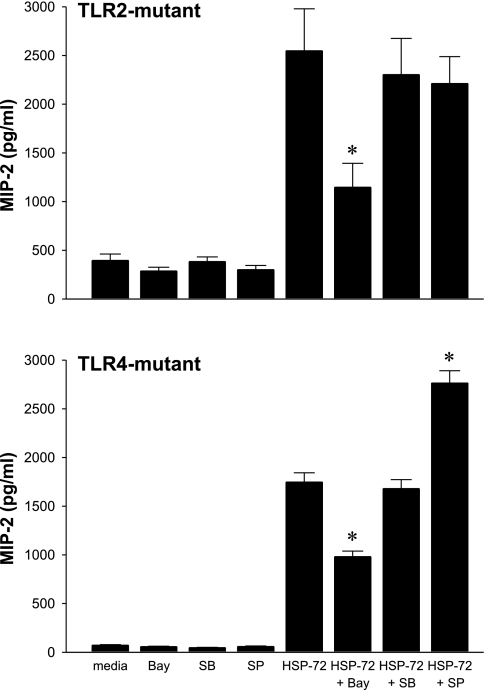
To determine the specificity of these signaling intermediates for TLR2 or TLR4, inhibitor studies were completed on hepatocytes from TLR2- and TLR4-mutant mice. Inhibition of NF-κB with Bay 11-7085 significantly decreased HSP-72-induced MIP-2 production in both TLR2- and TLR4-mutant hepatocytes (Fig. 7). Blockade of p38 with SB-203580 had no effect on MIP-2 production in TLR2- or TLR4-mutant hepatocytes. Blockade of JNK with SP-600125 had no effect in TLR2-mutant hepatocytes. Interestingly, pretreatment of TLR4-mutant hepatocytes with SP-600125 resulted in an increase in HSP-72-induced MIP-2 production, potentially indicating the blocking of an inhibitory pathway involving JNK.
Fig. 7.
Both TLR2 and TLR4 transduce extracellular HSP-72 signals via NF-κB. Primary hepatocytes from TLR2- and TLR4-mutant mice were cultured and treated with Bay 11-7085, SB-203580, and SP-600125 at concentrations of 20 μM for 1 h before treatment with 1,000 ng/ml HSP-72 for 8 h. Supernatant was harvested and analyzed by ELISA for MIP-2. Data represent means ± SE of 4 experiments. *P < 0.05 compared with HSP-72.
DISCUSSION
Extracellular HSP-72 has been proposed to modulate a number of injury responses, including trauma, traumatic brain injury, sepsis, cardiac surgery, and preeclampsia (10, 12, 25, 31, 35, 37). In addition, after liver resection, circulating levels of HSP-72 have been correlated with increased organ dysfunction (17). A primary limitation to all of these studies is the correlative nature of the conclusions. Whether eHSP-72 is a causative factor in poor outcome after these insults has not been established. Using a mouse model of hepatic ischemia-reperfusion, we have shown that this insult increases serum levels of HSP-72 (20, 28). Our previous attempts to dissect the manner in which HSP-72 modulates liver ischemia-reperfusion injury have been limited by an inability to distinguish the roles of intracellular versus extracellular HSP-72 (20). To the best of our knowledge, the present study is the first to demonstrate a direct effect of eHSP-72 on hepatocytes. Here we show that eHSP-72 activates hepatocytes via both TLR2 and TLR4. This activation resulted in selective production of the chemokine MIP-2 by a mechanism dependent on NF-κB.
Other studies have shown that HSP-72 activates monocytes via TLR2 and TLR4, resulting in NF-κB activation and production of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 (3, 4). Interestingly, hepatocytes stimulated with HSP-72 did not have increased production of TNF-α and IL-6. The selective nature of the response to HSP-72 in hepatocytes may represent an important difference in the regulation of the signal transduction pathway in these cells, downstream of TLR activation. This selective transcription, while not directly investigated in the present study, could be a result of differential availability of transcriptional coactivators or transcription factors that collaborate with NF-κB for gene transcription (26, 27, 38, 39). Alternatively, it could be a result of various heterodimers of NF-κB, which have been shown to affect the specificity of gene expression in a number of different cell types (21). Previous studies by a member of our research group have documented a similar ability of eHSP-72 to stimulate chemokine production in airway epithelial cells (7). In those cells, activation was found to involve TLR4, but TLR2 was not investigated.
The current study provides direct evidence that eHSP-72 signals in hepatocytes via TLR2 and TLR4 and that these receptors transduce the eHSP-72 signal via NF-κB. Data from mice with a mutant, defective TLR2 demonstrated that MIP-2 production induced by eHSP-72 was reduced by ∼45%. Similar studies in TLR4-mutant mice suggest more than 70% reduction in eHSP-72-induced MIP-2. Not tested in these studies was whether double TLR2/TLR4-mutant mice have a completely ablated response. Our subsequent experiments showed that blockade of NF-κB reduced MIP-2 production by ∼50% in both TLR2- and TLR4-mutant hepatocytes, suggesting that this is a major transcription factor responsible for eHSP-72-induced production of MIP-2 in hepatocytes. Interestingly, we found that blockade of JNKs with SP-600125 resulted in a nearly 40% increase in MIP-2 production in TLR4-mutant hepatocytes. This response was not observed in wild-type hepatocytes, but it may suggest that, in the setting of deficient TLR4 signaling, JNK actually may function to suppress production of MIP-2.
So what might be the biological significance of eHSP-72 in enhancing the production of chemokines like MIP-2 by hepatocytes? Previous studies by us and others have demonstrated that CXC chemokines, including MIP-2, mediate neutrophil infiltration during hepatic ischemia-reperfusion injury (9, 23). These studies demonstrated that blockade of MIP-2, or related chemokines, prevents neutrophil infiltration and attenuates hepatic ischemia-reperfusion injury. Thus, elaboration of MIP-2 by hepatocytes in response to eHSP-72 would be expected to promote the inflammatory response, leading to increased liver injury. Furthermore, there is evidence that CXC chemokines such as MIP-2 may have direct effects on hepatocytes. However, these effects of CXC chemokines on hepatocytes may depend on the context of liver injury. In a model of liver injury induced by CCl4, treatment with the CXC chemokine, keratinocyte chemokine, resulted in exacerbated injury that was independent of inflammation (34). In contrast, in models of partial hepatectomy or acetaminophen toxicity, MIP-2 has been shown to promote hepatocyte proliferation and liver regeneration (15, 33). Thus, the time at which eHSP-72 is available to the hepatocyte may determine what overall effect it may have on the injury response. An early presence of eHSP-72, as we have previously documented during ischemia-reperfusion injury (20, 28), may facilitate the inflammatory response through the recruitment of neutrophils. More prolonged availability of eHSP-72 to hepatocytes, as may occur following liver resection (17), may be detrimental to liver function.
In summary, our data show that eHSP-72 activates hepatocytes via TLR2 and TLR4. This activation is accomplished in a manner dependent upon activation of NF-κB and results in selective expression of MIP-2 over other proinflammatory cytokines, including TNF-α and IL-6. These data provide new insights into the mechanisms by which eHSP-72 may influence cell and tissue responses to injury.
GRANTS
This work was supported by National Institutes of Health Grants AG-025881 and DK-56029 (to A. B. Lentsch) and GM-064619 (to H. R. Wong).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol 177: 7184–7192, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Asea A Mechanisms of HSP72 release. J Biosci 32: 579–584, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 6: 435–442, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Beere HM Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Invest 115: 2633–2639, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chase MA, Wheeler DS, Lierl KM, Hughes VS, Wong HR, Page K. Hsp72 induces inflammation and regulates cytokine production in airway epithelium through a TLR4- and NF-κB-dependent mechanism. J Immunol 179: 6318–6324, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerk A, Sugden PH. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs). FEBS Lett 426: 93–96, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat. The role of epithelial neutrophil activating protein. J Clin Invest 95: 134–141, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Rocha AB, Zanoni C, de Freitas GR, Andre C, Himelfarb S, Schneider RF, Grivicich I, Borges L, Schwartsmann G, Kaufmann M, Regner A. Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma 22: 966–977, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett 581: 3702–3710, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through Toll-like receptor-4. Circulation 105: 685–690, 2002. [DOI] [PubMed] [Google Scholar]
- 13.El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett 111: 36–44, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartl FU Molecular chaperones in cellular protein folding. Nature 381: 571–579, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Lukacs NW, Colletti LM, Simpson KJ, Strieter RM, Kunkel SL. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J 13: 1565–1574, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 79: 425–434, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kimura F, Itoh H, Ambiru S, Shimizu H, Togawa A, Yoshidome H, Ohtsuka M, Shimamura F, Kato A, Nukui Y, Miyazaki M. Circulating heat-shock protein 70 is associated with postoperative infection and organ dysfunction after liver resection. Am J Surg 187: 777–784, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol 22: 201–211, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Kregel KC Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 92: 2177–2186, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 292: G1141–G1149, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kunsch C, Rosen CA. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13: 6137–6146, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lentsch AB, Kato A, Davis B, Wang W, Chao C, Edwards MJ. STAT4 and STAT6 regulate systemic inflammation and protect against lethal endotoxemia. J Clin Invest 108: 1475–1482, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology 27: 1172–1177, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62: 670–684, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molvarec A, Prohaszka Z, Nagy B, Szalay J, Fust G, Karadi I, Rigo J Jr. Association of elevated serum heat-shock protein 70 concentration with transient hypertension of pregnancy, preeclampsia and superimposed preeclampsia: a case-control study. J Hum Hypertens 20: 780–786, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem 265: 21128–21133, 1990. [PubMed] [Google Scholar]
- 27.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol 56: 554–558, 1994. [PubMed] [Google Scholar]
- 28.Okaya T, Blanchard J, Schuster R, Kuboki S, Husted T, Caldwell CC, Zingarelli B, Wong H, Solomkin JS, Lentsch AB. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock 24: 421–427, 2005. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill LA How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol 18: 3–9, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272: 21096–21103, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Pittet JF, Lee H, Morabito D, Howard MB, Welch WJ, Mackersie RC. Serum levels of Hsp 72 measured early after trauma correlate with survival. J Trauma 52: 611–617, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Radons J, Multhoff G. Immunostimulatory functions of membrane-bound and exported heat shock protein 70. Exerc Immunol Rev 11: 17–33, 2005. [PubMed] [Google Scholar]
- 33.Ren X, Carpenter A, Hogaboam C, Colletti L. Mitogenic properties of endogenous and pharmacological doses of macrophage inflammatory protein-2 after 70% hepatectomy in the mouse. Am J Pathol 163: 563–570, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanovic L, Brenner DA, Stefanovic B. Direct hepatotoxic effect of KC chemokine in the liver without infiltration of neutrophils. Exp Biol Med (Maywood) 230: 573–586, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Terry DF, Wyszynski DF, Nolan VG, Atzmon G, Schoenhofen EA, Pennington JY, Andersen SL, Wilcox MA, Farrer LA, Barzilai N, Baldwin CT, Asea A. Serum heat shock protein 70 level as a biomarker of exceptional longevity. Mech Ageing Dev 127: 862–868, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Kelly CG, Karttunen JT, Whittall T, Lehner PJ, Duncan L, MacAry P, Younson JS, Singh M, Oehlmann W, Cheng G, Bergmeier L, Lehner T. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15: 971–983, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler DS, Fisher LE Jr, Catravas JD, Jacobs BR, Carcillo JA, Wong HR. Extracellular hsp70 levels in children with septic shock. Pediatr Crit Care Med 6: 308–311, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Wood LD, Richmond A. Constitutive and cytokine-induced expression of the melanoma growth stimulatory activity/GRO alpha gene requires both NF-kappa B and novel constitutive factors. J Biol Chem 270: 30619–30626, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem 272: 2396–2403, 1997. [PubMed] [Google Scholar]