Abstract
The SLC4A1/AE1 gene encodes the electroneutral Cl−/HCO3− exchanger of erythrocytes and renal type A intercalated cells. AE1 mutations cause familial spherocytic and stomatocytic anemias, ovalocytosis, and distal renal tubular acidosis. The mutant mouse Ae1 polypeptide E699Q expressed in Xenopus oocytes cannot mediate Cl−/HCO3− exchange or 36Cl− efflux but exhibits enhanced dual sulfate efflux mechanisms: electroneutral exchange of intracellular sulfate for extracellular sulfate (SO42−i/SO42−o exchange), and electrogenic exchange of intracellular sulfate for extracellular chloride (SO42−i/Cl−o exchange). Whereas wild-type AE1 mediates 1:1 H+/SO42− cotransport in exchange for either Cl− or for the H+/SO42− ion pair, mutant Ae1 E699Q transports sulfate without cotransport of protons, similar to human erythrocyte AE1 in which the corresponding E681 carboxylate has been chemically converted to the alcohol (hAE1 E681OH). We now show that in contrast to the normal cis-stimulation by protons of wild-type AE1-mediated SO42− transport, both SO42−i/Cl−o exchange and SO42−i/SO42−o exchange mediated by mutant Ae1 E699Q are inhibited by acidic pHo and activated by alkaline pHo. hAE1 E681OH displays a similarly altered pHo dependence of SO42−i/Cl−o exchange. Elevated [SO42−]i increases the K1/2 of Ae1 E699Q for both extracellular Cl− and SO42−, while reducing inhibition of both exchange mechanisms by acid pHo. The E699Q mutation also leads to increased potency of self-inhibition by extracellular SO42−. Study of the Ae1 E699Q mutation has revealed the existence of a novel pH-regulatory site of the Ae1 polypeptide and should continue to provide valuable paths toward understanding substrate selectivity and self-inhibition in SLC4 anion transporters.
Keywords: band 3; 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid; Xenopus oocyte; Woodward's reagent K
the slc4 bicarbonate transporter superfamily encodes Na+-dependent and Na+-independent anion carriers (3, 30, 32, 44). SLC4A1/AE1/band 3 is the most thoroughly studied among the Na+-independent, electroneutral Cl−/HCO3− exchangers. AE1 is the major intrinsic protein of the erythrocyte membrane, where it serves to increase the total CO2-carrying capacity of the blood (48). The kidney variant of AE1 is expressed in the basolateral membrane of the renal type A intercalated cell. AE1 mutations cause dominant hereditary spherocytosis, stomatocytosis, and ovalocytosis, and a distinct set of mutations cause dominant and recessive forms of distal renal tubular acidosis (2, 24).
In addition to 1:1 monovalent anion exchange, AE1 can mediate cotransport of H+/SO42− in exchange for Cl− or as a self-exchange reaction (27, 28). The binding site for the cotransported proton during H+/SO42− cotransport is believed to be E681 of human AE1 (19). Treatment of intact human erythrocytes with Woodward's reagent K (WRK) followed by borohydride (BH4) reduction converts AE1 E681 to the corresponding alcohol (hAE1 E681OH). This modification produces multiple alterations in anion transport. These changes include severely reduced rates of electroneutral Cl−/Cl− exchange rates and dramatically increased H+-independent rates of electrogenic SO42−/Cl− exchange and electroneutral SO42−/SO42− exchange (15, 17). Similar changes were observed in the function of recombinant mouse AE1 (mAe1) mutated at the corresponding E699 to the amide Gln. In particular, the high rate of Cl−/Cl− exchange characteristic of wild-type Ae1 became undetectable. In addition, the low wild-type rates of H+/SO42− cotransport were converted to greatly accelerated rates of H+-independent, electroneutral SO42−/SO42− exchange and electrogenic SO42−/Cl− exchange (7).
In the course of investigating the altered properties of mAe1 E699Q-mediated SO42− transport, we observed a novel pH dependence of SO42− transport. We show in the present study that, in contrast to activation of wild-type AE1-mediated SO42− transport by H+ consistent with H+/SO42− cotransport, SO42− transport by the mAE1 mutant E699Q was inhibited by acidic extracellular pH (pHo) and activated by alkaline pHo. This pattern of regulation, diametrically opposed to that of wild-type AE1, was shared by SO42−i/Cl−o and SO42−/SO42− exchanges mediated by mAE1 E699Q, and by SO42−i/Cl−o exchange mediated by hAE1 E681OH. The extracellular pHo(50) for 35SO42− efflux (the pHo at which efflux was half-maximal) was not significantly alkaline-shifted in the presence of acidic intracellular pH (pHi). Moreover, changing pHi at constant pHo had no effect on either SO42−i/Cl−o or SO42−/SO42− exchange. Elevation of intracellular [SO42−] ([SO42−]i) greatly attenuated inhibition of 35SO42− efflux by acidic pHo. Elevated [SO42−]i also increased K1/2 for extracellular SO42− ([SO42−]o) in SO42−/SO42− exchange and greatly increased K1/2 for extracellular Cl− ([Cl−]o) in SO42−i/Cl−o exchange. mAE1 E699Q mediated exchange of SO42−i with a wide range of extracellular oxyanions at rates comparable to those measured for SO42−i/Cl−o exchange and for SO42−/SO42− exchange.
METHODS
Reagents.
Na35SO42− was purchased from ICN Biomedicals (Costa Mesa, CA) or from MP Biomedicals (Irvine, CA). Other chemical reagents were of analytical grade and were obtained from Sigma-Aldrich (St. Louis, MO), Fluka (St. Louis, MO), or Calbiochem (San Diego, CA). Transcription plasmid cDNA encoding mouse AE1 mutant E699Q was described previously (7).
Solutions for Xenopus laevis oocytes.
ND-96 medium consisted of (in mM) 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, and 2.5 sodium pyruvate, pH 7.40. Flux media lacked sodium pyruvate. pH values of 7.0, 8.0, and 8.5 in flux media were achieved with 5 mM HEPES. 4-Morpholino-ethanesulfonic acid (MES; 5 mM) was used for flux media of pH values 5.0 and 6.0. In Cl−-free solutions, NaCl was replaced isosmotically with 96 mM sodium isethionate, and equimolar K, Ca, and Mg gluconate substituted for the corresponding Cl− salts. Addition to flux media of the weak acid salt sodium butyrate or of NH4Cl was in equimolar substitution for NaCl.
Expression of cRNA in Xenopus oocytes.
Transcription template was generated by linearizing plasmid cDNA with HindIII. cRNA transcription with T7 RNA polymerase was performed with Ambion's Megascript Kit (Austin, TX). Female Xenopus laevis were purchased from NASCO (Madison, WI). Manually defolliculated oocytes were prepared as previously described (13), in compliance with a protocol approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee, were microinjected with 10–20 ng cRNA encoding mAe1 E699Q, and were then incubated in ND-96 supplemented with gentamicin at 19°C for 2–7 days before use in efflux experiments (7).
35SO42− efflux experiments in Xenopus oocytes.
Oocytes were injected with 50 nl of a solution containing Na235SO4 (0.25–0.5 μCi, 3–6 μM) in 130 mM of either HEPES, pH 7.4, MES, pH 5.0, or occasionally, Bis-Tris, pH 5.0. When indicated, the injectate additionally contained 130 mM cold Na2SO4, yielding a final estimated [SO42−]i of ∼14 mM [including the endogenous [SO42−] of ∼1 mM (7)]. Following a 10-min recovery period in SO42−-free, Cl−-free medium containing Na isethionate, 35SO42− efflux was initiated by transfer of individual oocytes into 1 ml efflux medium containing 96 mM NaCl for assay of SO4i/Cl−o exchange, or containing 20 or 64 mM Na2SO4 for assay of SO42−i/SO42−o exchange. Experiments in which extracellular sulfate or chloride concentrations were varied used isethionate as substituting anion to maintain nominal isosmolarity. At regular intervals of 3 min, 950 μl of this medium was removed for scintillation counting and replaced with fresh medium. Experiments ended with a final efflux period in the presence of the AE1 inhibitor, 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS, 100 or 200 μM), before solubilization of the washed oocyte in 100 μl of 1% sodium dodecyl sulfate (SDS). All 35SO42− efflux experiments were conducted at room temperature.
Efflux cpm values for water-injected or cRNA-injected oocytes in the presence of DIDS were less than threefold above machine background values (∼20 cpm). Within each experiment, water-injected and cRNA-injected oocytes from the same frog were subjected to parallel measurements. With the exception of the SO42−/SO42− exchange experiment presented in Fig. 8B, each experiment was performed with oocytes obtained from at least two frogs.
Fig. 8.
Elevated [SO42−]i attenuates inhibition by acidic pHo of mAe1 E699Q-mediated SO42−i/Cl−o exchange. A: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i 14 mM, injected with HEPES, pH 7.4) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces), during sequential exposure to pHo 5.0 and 7.4, then to 100 μM DIDS. B: mean normalized efflux rate constants for (n) oocytes at pHo 5.0 and pHo 7.4 measured as depicted in A. C: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i 14 mM, injected with MES, pH 5.0) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces), during sequential exposure to pHo 5.0 and 7.4, then to 100 μM DIDS. D: mean normalized efflux rate constants for (n) oocytes at pHo 5.0 and 7.4 measured as depicted in C.
Efflux time course data were plotted as ln (% cpm remaining in oocyte) vs. time. Efflux rate constants were measured from linear least squares regressions calculated from the final three time points of each experimental condition and were corrected by subtraction of the efflux rate constant exhibited by water-injected oocytes studied in the same experiment. Samples were counted for 3–5 min such that the magnitude of 2 SD was <5% of the sample mean; >98% of the originally injected cpm was accounted for in the sum of the combined efflux fractions and the cpm remaining in the oocyte at the end of the experiment. Oocyte water space was assumed to 450 nl (500 nl acutely following injection of the 50-nl volume) such that final estimated oocyte [SO42−]i was either ∼1 mM or ∼14 mM (7).
To describe the dependence of SO42−/anion exchange on pHo, efflux rate constants measured at each pHo value were fit (Ultrafit 3.0, Biosoft, Ferguson, MO; or Sigmaplot 8.0; Systat, San Jose, CA) by the following first-order logistic sigmoid equation:
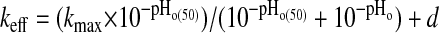 |
(1) |
where keff is the AE1 E699Q-mediated 35SO42− efflux rate constant (in units of min−1), kmax is the maximal AE1 E699Q-mediated 35SO42− efflux rate constant, pHo is the extracellular pH at which the rate constant was measured, pHo(50) is the pHo at which keff is half-maximal, and d = 0 or a nonzero value, as indicated in the text.
To describe the Cl−o dependence of SO42−i/Cl−o exchange and the SO42−o-dependence of SO42−/SO42− exchange, 35SO42− efflux rate constants plotted as functions of [anion]o were fit by the Michaelis-Menten equation modified to include an added constant, y0:
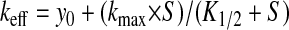 |
(2) |
where k and kmax are as above, K1/2 is the apparent Km for extracellular permeant substrate, S is the extracellular concentration of permeant anion substrate, and y0 is the efflux rate constant at S = 0 (in the presence of the nominally nonpermeant anion, isethionate). Fits were slightly inferior without addition of the y0 offset, but mean K1/2 values calculated with and without the y0 term did not significantly differ. The magnitude of the ratio y0/kmax was larger for SO42−/SO42− exchange experiments than for SO42−i/Cl−o exchange. The r2 values of the nine fits to Eq. 2 of SO42−/SO42− exchange at [SO42−]i ∼ 1 mM were 0.99 for seven individual oocytes and 0.96 and 0.92 for two additional oocytes (Fig. 7B).
Fig. 7.
[SO42−]i regulates K1/2 for SO42−o in mAe1 E699Q-mediated SO42−i/SO42−o exchange. A: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i = 1 mM) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces) during sequential exposures to progressively increased [SO42−]i, followed by 100 μM DIDS. B: plot of efflux rate constant vs. [SO42−]o up to 20 mM for an individual oocyte measured as depicted in A, with Michaelis-Menten fit to data. Inset: data for same oocyte including measurement at 64 mM SO42−o. C: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i = 14 mM) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces) during sequential exposures to progressively increased [SO42−]o. D: plot of efflux rate constant vs. [SO42−]o up to 20 mM for an individual oocyte containing 14 mM [SO42−]i measured as depicted in C, with Michaelis-Menten fit to data.
Measurement of oocyte pHi.
Oocytes loaded 30 min at room temperature with 5 μM BCECF-AM were set into a Leiden closed perfusion chamber of 1 ml internal volume and mounted on the stage of an Olympus BH-2 epifluorescence microscope. BCECF fluorescence emission at 530 nm was recorded at alternating excitation wavelengths of 440 and 490 nm by an Image-1 digital ratio imaging system (Universal Imaging, West Chester, PA). Fluorescence ratios in a crescent-shaped region of interest just inside the oocyte edge were measured before and 10 min after injection of 50 nl of 130 mM MES, pH 5.0. In situ calibration of the BCECF fluorescence ratio to pH was as previously described (13, 35, 47).
Treatment of human erythrocytes with Woodward's reagent K.
Red blood cells from healthy adults were obtained by a venipuncture protocol approved by the University of Arkansas for Medical Sciences Institutional Review Board, washed, and modified with Woodward's reagent K (WRK) and NaBH4 as described previously (15). Cells were washed three times and suspended at 5% hematocrit in 150 mM KCl and 10 mM MOPS, pH 7.0. After the suspension was chilled on ice for at least 20 min, solid WRK (Sigma-Aldrich, St. Louis, MO) was added to a final concentration of 2 mM. The suspension was mixed gently and incubated 10 min further on ice. NaBH4 (Sigma-Aldrich) was added from a freshly prepared 1 M stock (in 0.1 N NaOH) to a final concentration of 2 mM. After 5 min on ice, an additional 2 mM NaBH4 was added, and the suspension was incubated another 5 min. Between the NaBH4 additions, 10 mM MOPS acid was added to the suspension to keep the pH from rising above 7. This procedure converts ∼80% of the copies of wild-type hAE1 to hAE1 E681OH (15).
35SO42− efflux from erythrocytes.
After treatment with WRK and NaBH4, cells were washed three times in at least 20 volumes of 80 mM K2SO4 and 10 mM HEPES, pH 7.45, with a 10-min incubation at 37°C to allow SO42− to replace cellular Cl−. Cells were then loaded with 35SO42− by incubating 1 h, 37°C in the same medium containing 1 μCi Na35SO4/ml. Red blood cell [SO42−]i after this protocol of sulfate loading and labeling was ≥40 mM. To measure 35SO42−/Cl− exchange, loaded and labeled cells were washed twice at 0°C in K2SO4/HEPES medium containing no radioactivity and resuspended in media consisting of 120 mM KCl, or 60 mM KCl, 120 mM sucrose, in each case buffered with one of the following: 20 mM Na-HEPES (pH 7.5), 20 mM Na-MES, pH 6.4, 10 mM Na-MES/10 mM Na-glutamate, pH 5.7, or 20 mM Na-glutamate, pH 4.4. The suspension was incubated at 20°C, and aliquots were centrifuged at various times (4 time points per efflux) for determination of extracellular radioactivity. The rate constants for efflux were determined as previously described (15). Data plotting efflux rate constant vs. pHo data were fit using equation 1, with d = 0.
Statistical analysis.
Data are reported as means ± SE. Statistical significance of pairwise differences was assessed by Student's paired and unpaired t-tests. pHo(50) values were compared by Dunnett's two-way t-test. The level of significance was defined as P < 0.05.
RESULTS
Acidic pHo inhibits mAe1 E699Q-mediated SO42−/Cl− exchange.
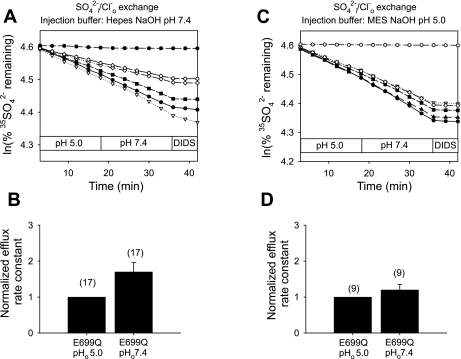
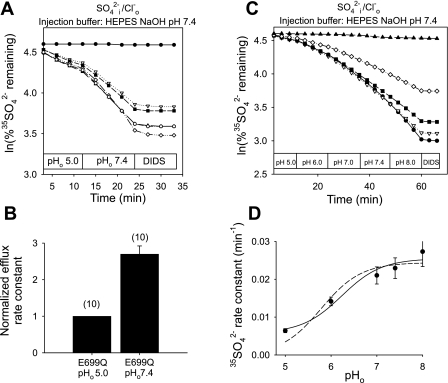
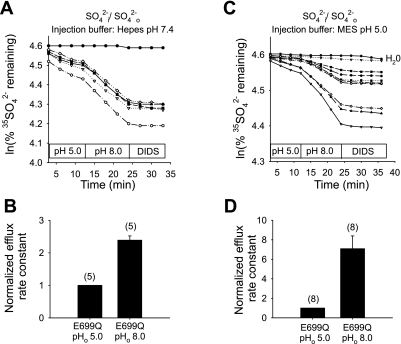
mAe1 E699Q-expressing oocytes injected with HEPES, pH 7.4 (final estimated intracellular concentration 13 mM, to minimize changes in pHi) showed a 2.7 ± 0.2-fold stimulation in the rate of 35SO42−i/Cl−o exchange upon transition from pHo 5.0 to pHo 7.4 (Fig. 1, A and B). In a separate set of oocytes not injected with buffer, transition from pHo 5.0 to 8.0 stimulated the rate of 35SO42−i/Cl−o exchange by 7.5-fold (n = 15, not shown). This inhibition by acidic pHo is diametrically opposed to the acceleration of human red blood cell AE1-mediated 35SO42−i/Cl−o exchange by acidic pHo (27, 28). E699Q-mediated 35SO42−i/Cl−o exchange was nearly completely inhibited by 100 μM DIDS (Fig. 1, A and C) and so did not represent a nonspecific divalent anion leak. 35SO42−i/Cl−o exchange was also inhibited 35% in a rapidly reversible manner by 1 mM Zn2+ (n = 8, not shown). More detailed analysis of the pHo dependence of mAe1 E699Q-mediated 35SO42−i/Cl−o exchange (Fig. 1C) revealed a pHo(50) value (Fig. 1D) of 6.20 ± 0.18 (n = 7, r2 = 0.97) calculated with parameter d of Eq. 1 as a variable (solid line), and 5.68 ± 0.14 (r2 = 0.92) for d = 0 (dashed line).
Fig. 1.
Mouse Ae1 (mAe1) E699Q-mediated SO42−i/Cl−o exchange is inhibited by acidic extracellular pH (pHo). A: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (bottom traces). After oocytes were injected with 50 nl 130 mM HEPES pH 7.4 containing carrier-free 35SO42− [estimated oocyte [SO42−]i 1 mM (7)], they were exposed sequentially to Cl−-containing solutions of pHo 5.0 and 7.4, and then to the inhibitor, DIDS (100 μM). B: mean normalized efflux rate constants for (n) oocytes studied as depicted in A. C: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (bottom traces), injected with 35SO42− as in A, and exposed sequentially to solutions of the indicated pH values, followed by DIDS (100 μM). D: plot of efflux rate constants vs. pHo (n = 7) fit to Eq. 1 with parameter d = 0 (dashed line) or treated as a variable (solid line).
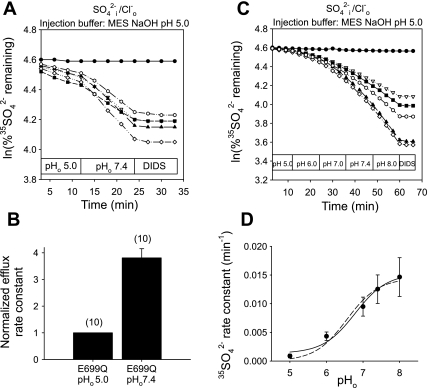
Acidic pHi does not significantly shift pHo sensitivity of mAe1 E699Q-mediated SO42−/Cl− exchange.
Since altering pHo modestly changes pHi in Xenopus oocytes (41), we investigated directly the effects of acidic pHi on the pHo sensitivity of mAe1 E699Q-mediated 35SO42−i/Cl−o exchange. Figure 2, A and B, shows that oocytes injected with MES, pH 5.0 (final estimated intracellular concentration 13 mM) exhibited a 3.8 ± 0.3-fold acceleration of 35SO42−i/Cl−o exchange (n = 10) upon transition from pHo 5.0 to 7.4. This concentration of MES reduced oocyte pHi (as measured at pHo 7.4 by BCECF ratio fluorimetry) by 0.44 + 0.13 units (n = 6). More detailed analysis of the pHo dependence of mAe1 E699Q-mediated 35SO42−i/Cl−o exchange in the setting of acidic pHi (Fig. 2C) revealed a pHo(50) value (Fig. 2D) of 6.53 ± 0.13 (n = 10, r2 = 0.99) with parameter d of Eq. 1 as a variable, and 6.43 ± 0.09 (r2 = 0.96) with parameter d = 0. The alkaline-shift in pHo(50) value associated with the more acidic pHi of MES-injected oocytes (Fig. 2D) was significant compared with that in HEPES-injected oocytes only when modeled with parameter d = 0 (Fig. 1D, dashed line, P < 0.0005), but not when d was allowed to vary (the better fit solid line of Fig. 1D, P = 0.15). Thus, the present data suggest that decreasing pHi ∼0.45 units does not significantly alter pHo dependence of mAe1 E699Q-mediated 35SO42−i/Cl−o exchange.
Fig. 2.
Inhibition of mAe1 E699Q-mediated SO42−i/Cl−o exchange by acidic pHo seems insensitive to modulation by intracellular pH (pHi). A: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (lower traces). After oocytes were injected with 50 nl 130 mM MES pH 5.0 containing carrier-free 35SO42−, they were exposed sequentially to Cl−-containing solutions of pHo 5.0 and 7.4, and then to 100 μM DIDS. B: mean normalized efflux rate constants for (n) oocytes studied as depicted in A. C: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (bottom traces), injected with 35SO42− as in A, and exposed sequentially to solutions of the indicated pH values, followed by 100 μM DIDS. D: plot of efflux rate constant vs. pHo (n = 10) fit to Eq. 1 with parameter d = 0 (dashed line) or treated as a variable (solid line).
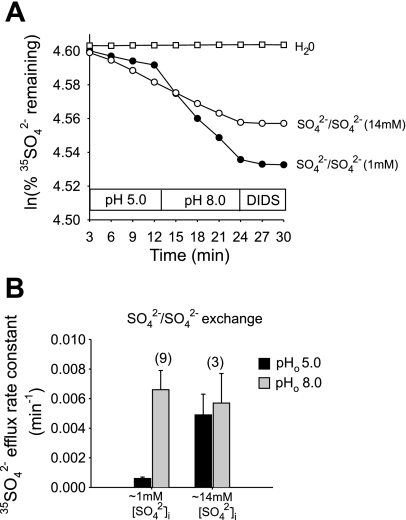
Acidic pHo inhibits mAe1 E699Q-mediated SO42−/SO42− exchange.
Since mAe1 E699Q-mediated SO42−i/Cl−o exchange is electrogenic but SO42−/SO42− exchange is electroneutral (7, 15), we investigated the effects of changing pHo and pHi on SO42−/SO42− exchange rates. As shown in Fig. 3, A and B, upon injection of HEPES, pH 7.4, transition from pHo 5.0 to 8.0 led to 2.4 ± 0.1-fold stimulation of SO42−/SO42− exchange. This stimulation was completely reversible upon changing pHo from 8.0 to 5.0 (n = 6, not shown). SO42−/SO42− exchange by oocytes injected with MES, pH 5.0 (estimated intracellular concentration 13 mM) was also stimulated upon pHo alkalinization from 5.0 to 8.0 (Fig. 3, C and D). Oocytes injected with unbuffered carrier-free 35SO42− similarly exhibited an eightfold stimulation of SO42−/SO42− exchange upon pHo transition from 5.0 to 8.0 (n = 9, not shown).
Fig. 3.
mAe1 E699Q-mediated SO42−i/SO42−o exchange is inhibited by acidic pHo. A: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (bottom traces). After oocytes were injected with 50 nl 130 mM HEPES pH 7.4 containing carrier-free 35SO42−, each was exposed sequentially to Cl−-free solutions containing 20 mM SO42− at pHo 5.0 and 8.0, followed by DIDS (100 μM). B: mean normalized efflux rate constants for the experiments in A. C: 35SO42− efflux time courses of representative individual oocytes previously injected with water (top trace) or with cRNA encoding mAe1 E699Q (bottom traces). After oocytes were injected with 50 nl 130 mM MES pH 5.0 containing carrier-free 35SO42−, each was exposed sequentially to Cl−-free solutions containing 20 mM SO42− at pHo 5.0 and 8.0, followed by 100 μM DIDS. D: mean normalized efflux rate constants for the experiments in C.
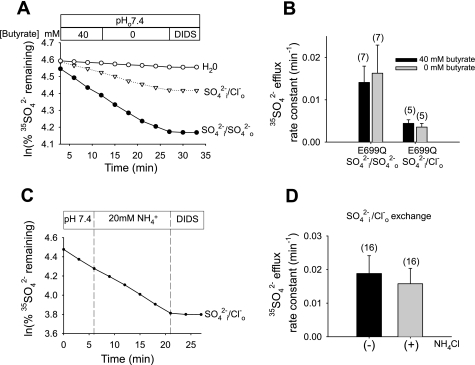
As shown in Fig. 4 in oocytes containing only endogenous sulfate (∼1 mM), intracellular acidification by ∼0.5 pH units at constant pHo 7.4 did not alter rates of mAe1 E699Q-mediated SO42−/SO42− exchange or of SO42−i/Cl−o exchange, whether acidification was imposed by exposure to 40 mM sodium butyrate or to 20 mM NH4Cl (12, 40). Exposure to 20 mM KCl to assess the effect of moderate membrane depolarization without acidification also had no effect (n = 4, not shown).
Fig. 4.
At constant pHo 7.4, mAe1 E699Q-mediated SO42−i/Cl−o exchange and SO42−i/SO42−o exchange are not inhibited by acidic pHi. A: 35SO42− efflux time courses of representative individual oocytes previously injected with water (○, Cl− bath) or with mAe1 E699Q cRNA (bottom two traces). Oocytes preequilibrated with 40 mM butyrate in Cl−-free conditions were exposed to baths containing either Cl− (ND-96, ▿) or SO42− (20 mM, •), and subjected to subsequent butyrate removal and 200 μM DIDS exposure. B: mean efflux rate constants for SO42−/Cl− exchange and SO42−/SO42− exchange in the presence (black bars) and absence (gray bars) of butyrate, measured as depicted in A. C: 35SO42− efflux time course of a representative individual mAe1 E699Q-expressing oocyte exposed to the absence and subsequent presence of NH4Cl, followed by 200 μM DIDS. D: mean efflux rate constants for SO42−/Cl− exchange in the absence (black bars) and presence (gray bars) of NH4Cl, measured as depicted in C.
Thus, we have shown that both SO42−/SO42− exchange and SO42−i/Cl−o exchange mediated by mAe1 E699Q were acutely regulated by changes in pHo at near constant pHi, but not by changes in pHi at constant pHo.
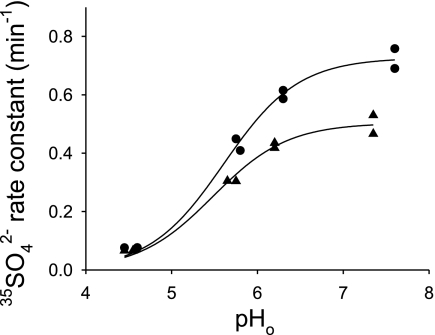
hAE1 E681OH-mediated SO42−/Cl− exchange is also inhibited by acidic pHo.
To test the possibility that the reversed pH dependence of mAe1 E699Q-mediated SO42− transport might be a cell type-specific property of the Xenopus oocyte expression system, we tested pHo dependence of hAE1 E681OH-mediated 35SO42− efflux from intact erythrocytes. As shown in Fig. 5, acidic pHo inhibited 35SO42− efflux from WRK-BH4-treated human red blood cells, with pHo(50) value ∼5.6 tested at [Cl−]o of 60 and 120 mM. In these conditions, red blood cell pHi is expected to be close to neutral at neutral pHo. Thus hAE1 E681OH-mediated SO42−i/Cl−o exchange is also inhibited by extracellular H+, a pattern opposite to that of wild-type hAE1 in erythrocytes (27).
Fig. 5.
Inhibition of hAE1 E681OH-mediated SO42−i/Cl−o exchange by acidic pHo in human erythrocytes. Human erythrocytes treated with Woodward's reagent K (WRK) and BH4− undergo ∼80% conversion of hAE1 E681 (equivalent to mouse AE1 E699) to the corresponding alcohol (AE1 E681OH). 35SO42− efflux rate constants from WRK/BH4-treated human erythrocytes were measured in media buffered at the indicated pHo containing 120 mM KCl (•) or 60 mM KCl, 120 mM sucrose (▴). Indicated pHo values were as measured in the efflux supernatants immediately after the final efflux time point. The curves represent fits with Eq. 1 with d = 0.
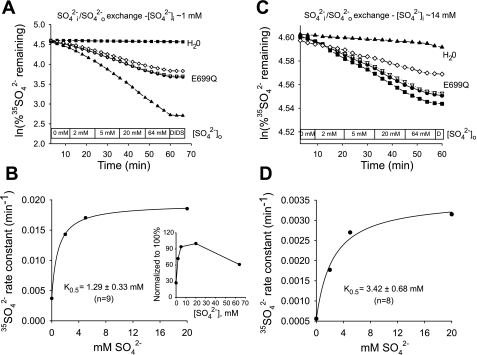
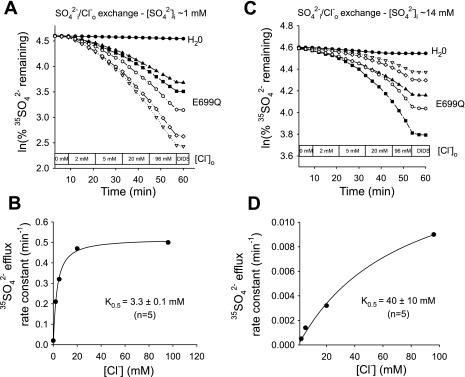
Elevated [SO42−]i greatly increases the K1/2 for Cl−o of mAe1 E699Q-mediated SO42−i/Cl−o exchange.
Some aspects of hAE1 E681OH kinetics do not conform to the predictions of ping-pong kinetics (14). We therefore tested the effect of [SO42−]i on the apparent K1/2 for the extracellular substrate Cl− of mAe1 E699Q. At 1 mM nominal [SO42−]i (Fig. 6, A and B), 35SO42− efflux from oocytes injected with HEPES pH 7.4 exhibited a hyperbolic Cl−o dependence of SO42−i/Cl−o exchange, with a K1/2 for Cl−o of 3.3 ± 0.1 mM (n = 5). Oocytes with nominal [SO42−]i of 14 mM (Fig. 6, C and D) exhibited a similarly hyperbolic Cl−o dependence of SO42−i/Cl− exchange, but with a ∼10-fold higher K1/2 for Cl−o of 40 ± 10 mM (n = 5). This increase of extracellular K1/2 in response to elevation of intracellular substrate concentration is consistent with a ping-pong mechanism of SO42−i/Cl−o exchange (11).
Fig. 6.
[SO42−]i regulates K1/2 for Cl−o in mAe1 E699Q-mediated SO42−i/Cl−o exchange. A: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i 1 mM) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces) during sequential exposures to progressively increased [Cl−]o, followed by 100 μM DIDS. B: plot of efflux rate constant vs. [Cl−]o for an individual oocyte measured as depicted in A, with Michaelis-Menten fit to data. C: 35SO42− efflux time courses of representative individual oocytes (estimated [SO42−]i = 14 mM) previously injected with water (top trace) or with mAe1 E699Q cRNA (bottom traces) during sequential exposure to progressively increased [Cl−]o. D: plot of efflux rate constant vs. [Cl−]o for an individual oocyte measured as depicted in C, with Michaelis-Menten fit to data.
Elevated [SO42−]i increases the K1/2 for SO42−o of mAe1 E699Q-mediated SO42−/SO42− exchange, but it does not greatly modify the enhanced affinity of the autoinhibitory “site” for SO42−o.
The extent of similarity between mechanisms of electrogenic SO42−i/Cl−o exchange and electroneutral SO42−/SO42− exchange mediated by mAe1 E699Q remains unknown. We therefore tested the effect of [SO42−]i on the apparent K1/2 for extracellular SO42−o. At 1 mM nominal [SO42−]i (Fig. 7, A and B), 35SO42− efflux from oocytes injected with HEPES pH 7.4 exhibited a hyperbolic [SO42−]0 dependence out to a concentration of 20 mM, but it was inhibited by further elevation of [SO42−]o to 64 mM (Fig. 7B, inset). A hyperbolic fit excluding the 64 mM efflux data indicated a SO42−/SO42− exchange K1/2 of 1.29 ± 0.33 mM for SO42−o (n = 9). This value is somewhat larger than that of 0.3 mM observed for SO42−/SO42− exchange in ghosts from E681OH human red blood cells (14). The SO42−/SO42− exchange rate measured at 64 mM [SO42−]o was 44 ± 7% (at [SO42−]i ∼1 mM, n = 4) lower than the rates at 20 mM [SO42−]o. Such apparent autoinhibition was not evident for SO42−i/Cl−o exchange for [Cl−]o as high as 103 mM.
Oocytes with nominal [SO42−]i of 14 mM (Fig. 7, C and D) exhibited a similarly hyperbolic Cl−o dependence of SO42−/SO42− exchange, but with a ∼2.7-fold higher K1/2 for SO42−o of 3.4 ± 0.68 mM (n = 8). Thus elevation of [SO42−]i increases the K1/2 of mAe1 E699Q for extracellular SO42− (P < 0.02), but to a lesser degree than for extracellular Cl−. But the increases in both values are qualitatively consistent with a ping-pong mechanism for both electrogenic and electroneutral anion exchange by mAE1 E699Q. Measurements of SO42−/SO42− exchange from similar oocytes with nominal [SO42−]i of 14 mM during the transition from 20 to 64 mM [SO42−]o showed apparent autoinhibition of 39 ± 7% (n = 12). Thus, changes in [SO42−]i did not detectably alter the apparent affinity of the autoinhibitory site for SO42−o.
Elevated [SO42−]i severely attenuates inhibition by pHo of both SO42−i/Cl−o exchange and SO42−/SO42− exchange mediated by mAe1 E699Q.
In mAe1 E699Q-expressing oocytes containing 14 mM [SO42−]i and 13 mM HEPES, pH 7.4, stimulation of SO42−i/Cl−o exchange by the pHo transition from 5.0 to 7.4 (Fig. 8A) was reduced to 1.7 ± 0.3-fold (n = 17; Fig. 8B). In mAe1 E699Q-expressing oocytes containing 14 mM [SO42−]i and 13 mM MES, pH 5.0, stimulation of SO42−i/Cl−o exchange by the pHo 5.0–7.4 transition (Fig. 8C) was reduced further to 1.2 ± 0.2-fold (n = 9, Fig. 8D). In mAe1 E699Q-expressing oocytes containing 1 mM [SO42−]i without injected buffer, SO42−/SO42− exchange was stimulated eightfold by the pHo 5.0-to-8.0 transition, but only 1.2 ± 0.4-fold in oocytes containing 14 mM [SO42−]i (Fig. 9). Thus, elevated [SO42−]i attenuated the pHo sensitivity of both SO42−/SO42− exchange and SO42−i/Cl−o exchange. In separate experiments, elevation of oocyte [SO42−]i to ∼26 mM similarly abolished inhibition of both SO42−/SO42− and SO42−i/Cl−o exchange by acidic pHo (n = 5–7, not shown).
Fig. 9.
Elevated [SO42−]i attenuates inhibition by acidic pHo of mAe1 E699Q-mediated SO42−/SO42− exchange. A: 35SO42− efflux time courses of SO42−/SO42− exchange in representative individual oocytes previously injected with water (□, top trace) or with mAe1 E699Q cRNA [estimated [SO42−]i = 1 mM (•) or 14 mM (○)], during sequential exposure to baths of pHo 5.0 and 8.0, then to 200 μM DIDS. B: mean normalized efflux rate constants at pHo 5.0 (black bars) and 8.0 (gray bars) for oocytes with estimated [SO42−]i of 1 or 14 mM.
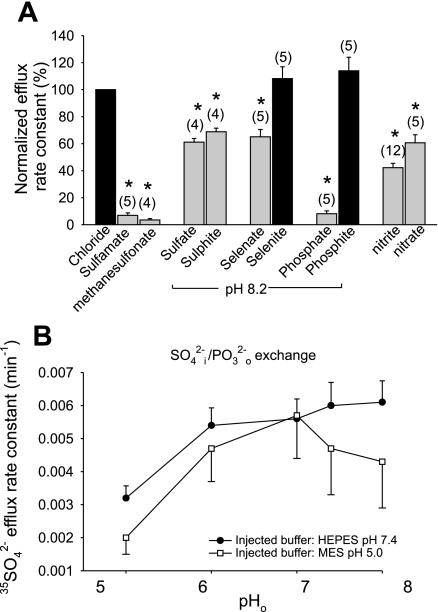
Extracellular oxyanion selectivity of mAe1 E699Q.
Human red blood cell anion exchange is characterized by more rapid transport of planar than of nonplanar anions. Red blood cell Cl−/anion exchange rates correlate with the vertical atomic dimensions of trigonal and pyramidal anions (8). Because oxyanion selectivity can reveal information about transporter binding pockets, and E699 is believed to constitute the H+ binding site for wild-type H+/SO42− cotransport, we assessed oxyanion selectivity of mAe1 E699Q in the presence of 1 mM SO42−i. Figure 10A shows that, as true for wild-type mAE1, sulfamate and methanesulfonate are transported at very slow rates, such as to be operationally impermeant in Xenopus oocyte assays. Rates of SO42−/SO42− and SO42−i/SO32− o exchange were nearly equivalent at ∼65–70% the rate of SO42−i/Cl−o exchange. SO42−i/SeO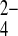 o was 65% the rate of SO42−i/Cl−o exchange, but SO42−i/SeO
o was 65% the rate of SO42−i/Cl−o exchange, but SO42−i/SeO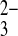 o exchange was roughly comparable in rate to SO42−i/Cl−o exchange. As true for wild-type hAE1 in red blood cells (8), SO42−i/PO
o exchange was roughly comparable in rate to SO42−i/Cl−o exchange. As true for wild-type hAE1 in red blood cells (8), SO42−i/PO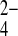 o exchange was much slower than SO42−i/PO
o exchange was much slower than SO42−i/PO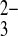 o exchange. SO42− exchange rates for extracellular NO3− or NO2− were nearly equivalent to those for SO42−i/Cl−o exchange.
o exchange. SO42− exchange rates for extracellular NO3− or NO2− were nearly equivalent to those for SO42−i/Cl−o exchange.
Fig. 10.
Oxyanion specificity of mAe1 E699Q. A: relative 35SO42− efflux rate constants (normalized to SO42−i/Cl−o exchange) into baths containing the listed anions at pHo 7.4 or (as indicated) pHo 8.2. *P < 0.05 compared with Cl− bath at same pHo (Student's paired two-tailed t-test; gray bars). B: pHo dependence of 35SO42−i/PO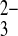 o exchange in oocytes containing ∼1 mM [SO42−]i injected with HEPES, pH 7.4, or with MES, pH 5.0.
o exchange in oocytes containing ∼1 mM [SO42−]i injected with HEPES, pH 7.4, or with MES, pH 5.0.
SO42−i/PO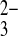 o exchange was activated approximately twofold by extracellular alkalinization of oocytes injected with HEPES, pH 7.4, and nearly threefold in oocytes injected with MES, pH 5.0 (Fig. 10B). Thus, acute inhibition of mAe1 E699Q-mediated 35SO42− efflux by pHo is also a property of exchange with other oxyanions recognized by the extracellular substrate site of this mutant polypeptide.
o exchange was activated approximately twofold by extracellular alkalinization of oocytes injected with HEPES, pH 7.4, and nearly threefold in oocytes injected with MES, pH 5.0 (Fig. 10B). Thus, acute inhibition of mAe1 E699Q-mediated 35SO42− efflux by pHo is also a property of exchange with other oxyanions recognized by the extracellular substrate site of this mutant polypeptide.
DISCUSSION
Mouse Ae1 (mAe1) mutant E699Q expressed in Xenopus oocytes exhibits undetectable 36Cl− efflux and greatly accelerated 35SO42− efflux, mediated either by electrogenic SO42−i/Cl−o exchange or electroneutral SO42−/SO42− exchange (7). These properties resemble characteristics of human red blood cell hAE1 E681OH, produced by chemical modification with WRK and BH4 reduction (15). Both mAe1 E699Q and hAE1 E681OH mediate transport of SO42− without cotransport of H+, in contrast to the H+/SO42− cotransport characteristic of wild-type AE1. Here we report that the AE1 E699Q mutation of mouse AE1 reverses the wild-type pHo dependence of SO42− transport. In contrast to acid pHo-activated SO42− transport by wild-type AE1, SO42− efflux from AE1 E699Q-expressing oocytes is inhibited by acid pHo and stimulated by alkaline pHo in a manner consistent with a single titratable “site.” This novel pattern of pHo sensitivity is exhibited by mAe1 E699Q-mediated electrogenic SO42−i/Cl−o exchange as well as by electroneutral SO42−/SO42− exchange and is also a property of hAE1 E681OH in chemically modified human red blood cells. Inhibition of SO42− efflux from Xenopus oocytes by acid pHo is severely attenuated by increased [SO42−]i.
Thus, the E699Q mutation unmasks a novel pHo-regulatory site on mAE1 that can be transregulated by intracellular substrate SO42−. We also show for mAe1 E699Q that elevated [SO42−]i increases apparent K1/2 ∼10-fold for Cl−o and ∼2.5-fold for SO42−o, consistent with sequential (ping-pong) mechanisms for both SO42−i/Cl−o exchange and for SO42−/SO42− exchange. In addition, we document in mAe1 E699Q enhanced SO42− affinity of an autoinhibitory site for SO42−.
Opposite pH sensitivities of SO42−/anion exchange by wild-type AE1 and mAE1 E699Q or hAE1 E681OH.
Cotransport of H+ and SO42−o in exchange for Cl−i by wild-type hAE1 in human red blood cells is activated by pH, with pKa values of 5.5 (27, 28). However, whereas SO42−/SO42− exchange in human (27, 36) and in mouse red blood cells (31) peaked at pHo values between 5.9 and 6.5, with progressive inhibition upon further extracellular acidification, Cl−i/SO42−o exchange continued to increase upon further acidification until peaking at pH 4.5–4.0, with transport rates 20-fold higher than peak rates of SO42−/SO42− exchange (26). In contrast, both SO42−i/Cl−o exchange and SO42−/SO42− exchange mediated by mAe1 E699Q are inhibited by extracellular H+, with peak transport rates observed at values ∼≥pHo 8. Sulfate transport by hAE1 E681OH in WRK/BH4-treated human red blood cells exhibited similar inhibition by extracellular protons, with pHo(50) ∼5.6 for SO42−i/Cl− exchange (Fig. 5) similar to the pHo(50) value for mouse AE1 E699Q-mediated SO42−i/Cl−o exchange (Fig. 1D) as fit by Eq. 1 with parameter d = 0, but lower than the pHo(50) value of 6.2 predicted from the better curve fit (Fig. 1D). Thus, the reversed pH dependence of mAe1 E699Q is not a function of the expression system or of the E-to-Q mutation, but rather results from replacement of the catalytically important carboxylate of E699/E681 with polar amide or alcohol residues,
The H+ binding site for H+/SO42− cotransport in wild-type AE1 is likely E681 in the human protein (15) and E699 in the mouse protein (7). The identity of the protonation site mediating inhibition of SO42− transport by mAe1 E699Q and by hAE1 E681OH is unknown, but it may be the inhibitory protonation site for wild-type AE1 SO42−/SO42− exchange, apparently inactive or inaccessible to protonation in the presence of Cl−o, and unmasked by mutation of E699/E681. Existence of the site was predicted from reversal of carrier asymmetry in hAE1 E681OH (15) and may relate to the inhibitory titratable side chain carboxylate modifiable by carbodiimides (5, 6). Similarly uncertain is a possible relationship to any among the conserved amino acid residues of mAe2 whose titration contribute to its H+-sensitive inhibition of monovalent anion transport (40–43, 45, 46). Intramolecular cross-linking of hAE1 with the cross-linker bis(sulfosuccinimidyl)-suberate (BS3) applied to intact erythrocytes also reveals an inhibitory protonation site coextant with the wild-type activating protonation site. The inhibitory site may reflect BS3's acid-shifting the pHo dependence of monovalent anion exchange by 5 pH units such that Cl−/Br− exchange, very slow at pHo >8, is activated to maximal rates at pH 5–6 (18).
The ability of ∼14 mM SO42−i to attenuate or prevent inhibition by acidic pHo of mouse AE1 E699Q-mediated exchange of SO42−i for either Cl−o or SO42−o might represent a transmembrane conformational effect of either an internal sulfate modifier site or, alternatively, of saturation of the intracellular substrate binding site. In either case, the effect must reflect the increased outward asymmetry of the carrier induced by elevated intracellular substrate. The ability of elevated [SO42−]i to attenuate inhibition by acidic pHo of hAE1 E681OH-mediated SO42−i/Cl−o exchange may be less than for mouse AE1 E699Q in oocytes, since red blood cell [SO42−]i at the onset of the efflux period approximated 40 mM. This apparent difference between AE1 E681OH and mouse AE1 E699Q may reflect in part the more extensive acid pHo range tested for WRK-BH4 red blood cells (Fig. 5), likely accompanied by much larger pHi excursions in red blood cells than in Xenopus oocytes (13, 41). Alternatively, the difference may reflect distinct SO42−i affinities at the intracellular substrate site and/or the putative internal modifier site (21) in the mouse and human proteins.
Proton-mediated inhibition of SO42−/anion exchange among SLC4 polypeptides.
The Glu residue corresponding to mAe1 E699 and hAE1 E681 is conserved in the Na+-independent anion exchangers SLC4A2/AE2 and SLC4A3/AE3, and in the borate transporter, SLC4A11/BTR1. Preliminary results indicate that mutation to Gln of the corresponding mAe2 residue E1007 similarly abolishes Cl−/Cl− and Cl−i/HCO3−o exchange activities, and greatly accelerates activities of SO42−i/Cl−o and SO42−/SO42− exchange. mAe2 E1007Q-mediated SO42−/SO42− exchange is also inhibited by acidic pHo, exhibiting eightfold activation upon pHo shift from 5.0 to 8.0 (n = 4; Stewart AK and Alper SL, unpublished observations). These results differ from an earlier report that 35SO42− uptake by proteoliposomes reconstituted from microsomes of HEK-293 cells transiently transfected with mouse AE2 E1007Q was no slower at pHo 5.5 than at pHo 7.5 (38).
A conservative Asp substitution is present in all other SLC4 polypeptides, but sequence polymorphisms at this codon of SLC4 genes have not yet been described in the Single Nucleotide Polymorphism database. Mutation of the corresponding D754 to Cys in rat NBCe1/Slc4a4 abolished Na/HCO3− cotransport current despite normal oocyte surface expression (25). Mutation of the same Asp residue in human NBCe1 to Glu, Asn, and Arg resulted in Na+-dependent base flux at 23%, 9%, and 0% of wild-type rates (1). Possible alteration in substrate selectivity was not evaluated in either study.
Evidence favoring a ping-pong transport mechanism for mAe1 E699Q.
Many characteristics of anion exchange by wild-type human red blood cell AE1 and by hAE1 E681OH can be described by simple ping-pong kinetics with translocation as the rate-limiting step (15, 22). Preservation of ping-pong kinetics in settings of mAe1 E699Q's altered substrate selectivities and substrate-conditional electrogenic anion exchange had not been tested. The 10-fold increase in mAe1 E699Q K1/2 for Cl−o (Fig. 6) and the smaller increase in K1/2 for SO42−o produced by elevation of [SO42−]i from ∼1 mM to ∼14 mM (Fig. 7) are both compatible with a substantial degree of intracellular substrate-driven polypeptide reorientation from inward to outward facing conformations of substrate binding sites. The smaller increase in K1/2 for SO42−o than for Cl−o may reflect the predominance of outward facing sites in SO42− medium, the large difference in magnitude of charge transfer during the inward anion translocation step (15), or intrinsic changes in carrier conformation resulting from occupancy of the outward facing substrate site by SO42−o or Cl−o. This proposed reorientation from inward to outward conformation was not very much influenced by pHi (Fig. 9). Unlike hAE1 E681OH red blood cells, the [Cl−]o dependence of 35SO42− efflux from oocytes by mAe1 E699Q was not sigmoidal (15) but hyperbolic. This difference may reflect different intrinsic consequences of mutation of the critical Glu residue to the alcohol as opposed to the amide. Alternatively, the difference may relate to the larger fractional contribution of electrogenic SO42−i/Cl−o exchange by hAE1 E681OH to total red blood cell conductance than that of mAe1 E699Q to total oocyte conductance.
Substrate selectivity of mAe1 E699Q.
The oxyanion substrate selectivity of mAe1 E699Q shows substantial differences from that of wild-type hAE1. Thus, whereas wild-type hAE1 exchanges Cl−i for SO42−o 100-fold more slowly than SO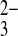 o at neutral pHo (8), mAe1 E699Q exchanges SO42−i for SO42−o and for SO
o at neutral pHo (8), mAe1 E699Q exchanges SO42−i for SO42−o and for SO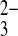 o at equivalent rates (Fig. 10). Whereas wild-type hAE1 exchanges Cl−i for SeO
o at equivalent rates (Fig. 10). Whereas wild-type hAE1 exchanges Cl−i for SeO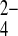 o 100-fold more slowly than for SeO
o 100-fold more slowly than for SeO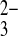 o (8), mAe1 E699Q exchanges SO42−i for SeO
o (8), mAe1 E699Q exchanges SO42−i for SeO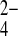 o only 35% more slowly than SeO
o only 35% more slowly than SeO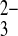 o. Wild-type hAE1 exchanges Cl−i for PO
o. Wild-type hAE1 exchanges Cl−i for PO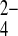 o 100-fold more slowly than for PO
o 100-fold more slowly than for PO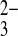 o, mAe1 exchanges SO42−i for PO
o, mAe1 exchanges SO42−i for PO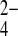 o only 10-fold more slowly than for PO
o only 10-fold more slowly than for PO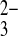 o. These differences suggest that the transition state in the E699Q mutant can accommodate certain oxyanion substrates larger in the z dimension (8) than tolerated by the wild-type protein, and that this flexibility can be modified by pHo. The smaller monovalent anions NO2− and NO3− are transported by wild-type and mutant proteins at similar rates (50–70% those of Cl−o), whereas the bulky monovalent anions sulfamate and methanesulfonate are transported at negligible rates by both proteins (Fig. 10).
o. These differences suggest that the transition state in the E699Q mutant can accommodate certain oxyanion substrates larger in the z dimension (8) than tolerated by the wild-type protein, and that this flexibility can be modified by pHo. The smaller monovalent anions NO2− and NO3− are transported by wild-type and mutant proteins at similar rates (50–70% those of Cl−o), whereas the bulky monovalent anions sulfamate and methanesulfonate are transported at negligible rates by both proteins (Fig. 10).
Wild-type SLC4 polypeptides also transport CO2 equivalents as the divalent oxyanion, CO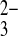 . Wild-type AE1 in the human red blood cell mediates slow exchange of Cl−i for the extracellular ion pairs lithium carbonate (Li:CO3)− and lithium sulfite (Li:SO3)−. The even slower exchange of Cl−i for the extracellular Li/oxalate ion pair is more rapid at pHo 7.5 than at pHo 6.0 (4), likely reflecting competition for H+/oxalate cotransport, with its opposite pHo dependence (16). Electrogenic Na+/HCO3− cotransporter activity measured in basolateral membrane vesicles from rabbit kidney proximal tubule was postulated to cotransport Na+, HCO3−, and CO
. Wild-type AE1 in the human red blood cell mediates slow exchange of Cl−i for the extracellular ion pairs lithium carbonate (Li:CO3)− and lithium sulfite (Li:SO3)−. The even slower exchange of Cl−i for the extracellular Li/oxalate ion pair is more rapid at pHo 7.5 than at pHo 6.0 (4), likely reflecting competition for H+/oxalate cotransport, with its opposite pHo dependence (16). Electrogenic Na+/HCO3− cotransporter activity measured in basolateral membrane vesicles from rabbit kidney proximal tubule was postulated to cotransport Na+, HCO3−, and CO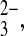 functioning with 3:1 charge stoichiometry as in the native proximal tubule. SO
functioning with 3:1 charge stoichiometry as in the native proximal tubule. SO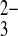 was found to stimulate HCO3−-dependent Na+ uptake, with characteristics suggesting occupancy of a proposed CO
was found to stimulate HCO3−-dependent Na+ uptake, with characteristics suggesting occupancy of a proposed CO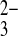 site distinct from the HCO3− site (39). However, recombinant rat kidney NBCe1 expressed in Xenopus oocytes, with its 2:1 charge stoichiometry attributed at first to Na+/2HCO3− cotransport (37) and later reinterpreted as Na/CO
site distinct from the HCO3− site (39). However, recombinant rat kidney NBCe1 expressed in Xenopus oocytes, with its 2:1 charge stoichiometry attributed at first to Na+/2HCO3− cotransport (37) and later reinterpreted as Na/CO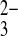 cotransport (10), revealed no evidence for either binding or transport of SO
cotransport (10), revealed no evidence for either binding or transport of SO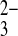 (9). Gln substitution of hNBCe1, the residue corresponding to hAE1 E681/mAe1 E699, has not been reported (1, 25), and the hNBCe1 mutant D754N was not tested for upregulation of SO
(9). Gln substitution of hNBCe1, the residue corresponding to hAE1 E681/mAe1 E699, has not been reported (1, 25), and the hNBCe1 mutant D754N was not tested for upregulation of SO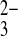 transport (1).
transport (1).
Extracellular modifier site of mAe1 E699Q.
The extracellular modifier site of erythroid hAE1 is believed not to block access to the substrate site, but rather to slow the rate of the anion transporting conformational change. The modifier site likely has no physiological significance in the red blood cell, since Cl− and HCO3− at their physiological systemic plasma concentrations interact with it only weakly. Model-dependent estimates of modifier site K1/2 for Cl−o range from 270 to 375 mM (20). However, the modifier site on kidney AE1 of the type A intercalated cell might gain importance under antidiuretic conditions, in which renal medullary interstitial [Cl−] can be sustained at 300 mM in the human and at 600–1,200 mM or more in rodents. The modifier site on erythroid AE1 might thus exert a significant effect also on red blood cells retarded in their passage through the antidiuretic renal medulla. An internal inhibitory modifier site requiring high concentrations of Cl−i (21) may also become meaningful in these high ionic strength conditions.
The wild-type hAE1 extracellular modifier site exhibits highest affinity for I− [although K1/2 estimates range between 8 and 120 mM (18, 23)], with lesser affinity for Br−. The binding sites of the noncompetitive anion transport inhibitors NAP-taurine and NIP-taurine overlap the extracellular modifier site (23), and BS3 treatment attenuates or abolishes binding of I− or NAP-taurine to the modifier site (18). SO42− interaction with the modifier site has been little studied. However, no self-inhibition by SO42− of human red blood cell anion exchange has been noted at concentrations up to 100 mM. Thus, the ∼40% inhibition of mAe1 E699Q-mediated SO42−/SO42− exchange upon increasing [SO42−]o from 20 to 64 mM represents a change in both the anion selectivity as well as apparent affinity of the external modifier site, if not the emergence of a novel modifier site. The existence of a similar modifier site in mAe1 E681OH, preferring occupancy with SO42− over Cl−, was proposed to explain the transacceleration of SO42−i efflux by Cl−o to a degree higher than explicable by simple ping-pong kinetics (15). The interaction of SO42− with a similar modifier site in hAE1 E681OH may mediate the cis-stimulation of SO42−o uptake into WRK-BH4-modified human red blood cells by 10–20 mM Cl−o, possibly via displacement of SO42−o from the modifier site by noninhibitory Cl−o, but more likely via Cl−o-accelerated post-outward translocation release of SO42− from hAE1 E681OH (14).
The relationship of the inhibitory substrate modifier site of wild-type hAE1 to the inhibitory protonation site of mAe1 E699Q is not clear. Similarly unknown is the modifier site's relationship with either the second Cl− binding site of hAE1 E681OH (14, 34), the second stilbene binding site of hAE1 E681OH (33), or the distinct binding sites for HCO3− and CO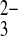 postulated in some models of NBCe1 activity (10, 29, 39). Nonetheless, the presumed structural similarities among homologous SLC4 polypeptides, most prominent in their transmembrane domains, encourages consideration and testing of these possibilities.
postulated in some models of NBCe1 activity (10, 29, 39). Nonetheless, the presumed structural similarities among homologous SLC4 polypeptides, most prominent in their transmembrane domains, encourages consideration and testing of these possibilities.
GRANTS
This work was supported by NIH Grants DK-43495 (to S. L. Alper) and GM-026861 (to M. L. Jennings).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abuladze N, Azimov R, Newman D, Sassani P, Liu W, Tatishchev S, Pushkin A, Kurtz I. Critical amino acid residues involved in the electrogenic sodium-bicarbonate cotransporter kNBC1-mediated transport. J Physiol 565: 717–730, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL Genetic diseases of acid-base transporters. Annu Rev Physiol 64: 899–923, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Alper SL Molecular physiology of SLC4 anion exchangers. Exp Physiol 91: 153–161, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Becker BF, Duhm J. Evidence for anionic cation transport of lithium, sodium and potassium across the human erythrocyte membrane induced by divalent anions. J Physiol 282: 149–168, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghout A, Raida M, Legrum B, Passow H. The effects of dansylation on the pH dependence of SO42− and Cl− equilibrium exchange and on the H+/SO42− cotransport across the red blood cell membrane. Biochim Biophys Acta 986: 75–82, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Bjerrum PJ, Andersen OS, Borders CL Jr, Wieth JO. Functional carboxyl groups in the red cell anion exchange protein Modification with an impermeant carbodiimide. J Gen Physiol 93: 813–839, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernova MN, Jiang L, Crest M, Hand M, Vandorpe DH, Strange K, Alper SL. Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. J Gen Physiol 109: 345–360, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galanter WL, Hakimian M, Labotka RJ. Structural determinants of substrate specificity of the erythrocyte anion transporter. Am J Physiol Cell Physiol 265: C918–C926, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Grichtchenko II, Romero MF, Boron WF. Extracellular HCO(3)(−) dependence of electrogenic Na/HCO(3) cotransporters cloned from salamander and rat kidney. J Gen Physiol 115: 533–546, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grichtchenko II, Boron WF. Surface pH measurements in voltage-clamped Xenopus oocytes co-expressing NBCe1 and CAIV: evidence for carbonate transport (Abstract). FASEB J 16: A795, 2002. [Google Scholar]
- 11.Gunn RB, Frohlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. Evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol 74: 351–374, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphreys BD, Chernova MN, Jiang L, Zhang Y, Alper SL. NH4Cl activates AE2 anion exchanger in Xenopus oocytes at acidic pHi. Am J Physiol Cell Physiol 272: C1232–C1240, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys BD, Jiang L, Chernova MN, Alper SL. Functional characterization and regulation by pH of murine AE2 anion exchanger expressed in Xenopus oocytes. Am J Physiol Cell Physiol 267: C1295–C1307, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Jennings ML Evidence for a second binding/transport site for chloride in erythrocyte anion transporter Ae1 modified at glutamate 681. Biophys J 88: 2681–2691, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings ML Rapid electrogenic sulfate-chloride exchange mediated by chemically modified band 3 in human erythrocytes. J Gen Physiol 105: 21–47, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings ML, Adame MF. Characterization of oxalate transport by the human erythrocyte band 3 protein. J Gen Physiol 107: 145–159, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennings ML, Al-Rhaiyel S. Modification of a carboxyl group that appears to cross the permeability barrier in the red blood cell anion transporter. J Gen Physiol 92: 161–178, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings ML, Monaghan R, Douglas SM, Nicknish JS. Functions of extracellular lysine residues in the human erythrocyte anion transport protein. J Gen Physiol 86: 653–669, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. Role of glutamate 68. J Biol Chem 267: 13964–13971, 1992. [PubMed] [Google Scholar]
- 20.Knauf PA, Gasbjerg PK, Brahm J. The asymmetry of chloride transport at 38 degrees C in human red blood cell membranes. J Gen Physiol 108: 577–589, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauf PA, Mann NA, Kalwas JE, Spinelli LJ, Ramjeesingh M. Interactions of NIP-taurine, NAP-taurine, and Cl− with the human erythrocyte anion exchange system. Am J Physiol Cell Physiol 253: C652–C661, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Knauf PA, Pal P. Band 3 mediated transport. In: Red Cell Membrane Transport in Health and Disease, edited by Bernhardt I and Ellory JC. New York: Springer, 2003, p. 253–301.
- 23.Knauf PA, Spinelli LJ. NIP- and NAP-taurine bind to external modifier site of AE1 (band 3), at which iodide inhibits anion exchange. Am J Physiol Cell Physiol 269: C410–C416, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Kurschat CE, Alper SL. Hereditary renal tubular acidosis. In: Molecular and Genetic Basis of Renal Disease: A Companion to Brenner and Rector's The Kidney, edited by Mount DB and Pollak MR. Philadephia, PA: Elsevier, 2007, chapt. 17, p. 269–294.
- 25.McAlear SD, Bevensee MO. A cysteine-scanning mutagenesis study of transmembrane domain 8 of the electrogenic sodium/bicarbonate cotransporter NBCe1. J Biol Chem 281: 32417–32427, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Milanick MA Kinetics of the Human Erythrocyte Anion Transporter: Proton, Sulfate, and Chloride Interactions at the External Membrane Surface (PhD thesis). Chicago, IL: Univ. of Chicago, 1981, p. 0–189.
- 27.Milanick MA, Gunn RB. Proton-sulfate co-transport: mechanism of H+ and sulfate addition to the chloride transporter of human red blood cells. J Gen Physiol 79: 87–113, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milanick MA, Gunn RB. Proton-sulfate cotransport: external proton activation of sulfate influx into human red blood cells. Am J Physiol Cell Physiol 247: C247–C259, 1984. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Berger S, Ducoudret O, Diakov A, Fromter E. The renal Na-HCO3− cotransporter expressed in Xenopus laevis oocytes: change in stoichiometry in response to elevation of cytosolic Ca2+ concentration. Pflügers Arch 442: 718–728, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Pushkin A, Kurtz I. SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am J Physiol Renal Physiol 290: F580–F599, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Romano L Comparative aspects of sulfate transport in the erythrocytes of different mammalian species. Cell Biol Int Rep 13: 851–855, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflügers Arch 447: 495–509, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Salhany JM, Cordes KS, Sloan RL. Band 3 (AE1, SLC4A1)-mediated transport of stilbenedisulfonates. II: Evidence for transmembrane allosteric interactions between the “primary” stilbenedisulfonate binding site and the stilbenedisulfonate efflux site. Blood Cells Mol Dis 37: 149–154, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Salhany JM, Sloan RL, Cordes KS. The carboxyl side chain of glutamate 681 interacts with a chloride binding modifier site that allosterically modulates the dimeric conformational state of band 3 (AE1). Implications for the mechanism of anion/proton cotransport. Biochemistry 42: 1589–1602, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki S, Ishibashi K, Nagai T, Marumo F. Regulation mechanisms of intracellular pH of Xenopus laevis oocyte. Biochim Biophys Acta 1137: 45–51, 1992. [DOI] [PubMed] [Google Scholar]
- 36.Schnell KF, Gerhardt S, Schoppe-Fredenburg A. Kinetic characteristics of the sulfate self-exchange in human red blood cells and red blood cell ghosts. J Membr Biol 30: 319–350, 1977. [DOI] [PubMed] [Google Scholar]
- 37.Sciortino CM, Romero MF. Cation and voltage dependence of rat kidney electrogenic Na+-HCO3− cotransporter, rkNBC, expressed in oocytes. Am J Physiol Renal Physiol 277: F611–F623, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Sekler I, Lo RS, Kopito RR. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J Biol Chem 270: 28751–28758, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Soleimani M, Aronson PS. Ionic mechanism of Na+-HCO3− cotransport in rabbit renal basolateral membrane vesicles. J Biol Chem 264: 18302–18308, 1989. [PubMed] [Google Scholar]
- 40.Stewart AK, Chernova MN, Kunes YZ, Alper SL. Regulation of AE2 anion exchanger by intracellular pH: critical regions of the NH2-terminal cytoplasmic domain. Am J Physiol Cell Physiol 281: C1344–C1354, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Stewart AK, Chernova MN, Shmukler BE, Wilhelm S, Alper SL. Regulation of AE2-mediated Cl− transport by intracellular or by extracellular pH requires highly conserved amino acid residues of the AE2 NH2-terminal cytoplasmic domain. J Gen Physiol 120: 707–722, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart AK, Kerr N, Chernova MN, Alper SL, Vaughan-Jones RD. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem 279: 52664–52676, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Stewart AK, Kurschat CE, Alper SL. Role of nonconserved charged residues of the AE2 transmembrane domain in regulation of anion exchange by pH. Pflügers Arch 454: 373–384, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Stewart AK, Kurschat CE, Alper SL. The SLC4 anion exchanger gene family. In: The Kidney: Physiology and Pathophysiology (4th ed.), edited by Alpern RJ and Hebert SC. New York: Elsevier, 2007, chapt. 53, p. 1499–1538.
- 45.Stewart AK, Kurschat CE, Burns D, Banger N, Vaughan-Jones RD, Alper SL. Transmembrane domain histidines contribute to regulation of AE2-mediated anion exchange by pH. Am J Physiol Cell Physiol 292: C909–C918, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Stewart AK, Kurschat CE, Vaughan-Jones RD, Shmukler BE, Alper SL. Acute regulation of mouse AE2 anion exchanger requires isoform-specific amino acid residues from most of the transmembrane domain. J Physiol 584: 59–73, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218, 1979. [DOI] [PubMed] [Google Scholar]
- 48.Wieth JO, Andersen OS, Brahm J, Bjerrum PJ, Borders CL Jr. Chloride-bicarbonate exchange in red blood cells: physiology of transport and chemical modification of binding sites. Philos Trans R Soc Lond B Biol Sci 299: 383–399, 1982. [DOI] [PubMed] [Google Scholar]