Abstract
The Na+/Ca2+ exchanger is the major Ca2+ extrusion mechanism in cardiac myocytes. The activity of the cardiac Na+/Ca2+ exchanger is dynamically regulated by intracellular Ca2+. Previous studies indicate that Ca2+ binding to a high-affinity Ca2+-binding domain (CBD1) in the large intracellular loop is involved in regulation. We generated transgenic zebrafish with cardiac-specific expression of CBD1 linked to yellow and cyan fluorescent protein. Ca2+ binding to CBD1 induces conformational changes, as detected by fluorescence resonance energy transfer. With this transgenic fish model, we were able to monitor conformational changes of the Ca2+ regulatory domain of Na+/Ca2+ exchanger in intact hearts. Treatment with the positive inotropic agents ouabain and isoproterenol increased both Ca2+ transients and Ca2+-induced changes in fluorescence resonance energy transfer. The results indicate that Ca2+ regulation of the Na+/Ca2+ exchanger domain CBD1 changes with inotropic state. The transgenic fish models will be useful to further characterize the regulatory properties of the Na+/Ca2+ exchanger in vivo.
Keywords: Ca2+-binding domain, sodium/calcium exchange, zebrafish, fluorescence resonance energy transfer
the na+/ca2+ exchanger (NCX) is a sarcolemmal protein that catalyzes the electrogenic countertransport of Na+ and Ca2+ (transport ratio is about 3 Na+:1 Ca2+) (19). Depending on the gradients of Na+ and Ca2+ and membrane potential, the exchanger can function in either the Ca2+-efflux (forward) or Ca2+-influx (reverse) mode. NCX is the primary Ca2+ efflux mechanism of cardiac myocytes and removes Ca2+ brought into cells by the L-type Ca2+ channel on a beat-to-beat basis. By extruding Ca2+ from the cell, NCX plays an important role in the regulation of cardiac contractility. Abnormal Ca2+ cycling caused by altered NCX expression and activity has been associated with cardiac pathophysiology, including ischemia-reperfusion injury, heart failure, and arrhythmias (7, 17, 18).
The transport activity of the exchanger is allosterically regulated by cytosolic Ca2+ (25). Ca2+ binds to two Ca2+-binding domains (CBD1 and CBD2) located in the large intracellular loop and activates the exchanger (1, 4, 9, 10, 13). The function of CBD1 has been analyzed in most detail. Mutations of acidic residues (e.g., D447V or D498I) within CBD1 decrease Ca2+ affinity (11). A double mutation (D447V/D498I) of CBD1 further decreases Ca2+ affinity (15). Different apparent Ca2+ affinities have been reported for Ca2+ regulation. For example, half-maximal concentration values obtained with giant excised patches range from 100 to 400 nM (5), whereas much lower half-maximal concentration values (20–80 nM) have been suggested from experiments using intact cells (12). Previous studies from our laboratory applied the noninvasive fluorescence resonance energy transfer (FRET) technique to monitor Ca2+-induced conformational changes of CBD1. We demonstrated that this Ca2+ regulatory site of the exchanger can sense changes in intracellular Ca2+ in cultured neonatal cardiac myocytes during excitation-contraction (EC) coupling (15).
Here, we present the use of the zebrafish Danio rerio as an expression system for studying the Ca2+ regulation of the cardiac NCX in vivo. We generated transgenic zebrafish with cardiac-specific expression of the CBD1 linked to yellow (YFP) and cyan fluorescent protein (CFP). Using FRET, we monitored conformational changes of the Ca2+ regulatory domain of NCX during EC coupling in the myocardium of intact zebrafish. With this transgenic fish model, we demonstrate that the increase in Ca2+ transients of intact hearts induced by the positive inotropic agents ouabain and isoproterenol leads to an increase in the state of activation of CBD1 and, therefore, most likely of the intact NCX.
METHODS
Zebrafish maintenance and generation of transgenic lines.
Zebrafish were maintained under standard conditions (26). The AB strain was used as the wild-type line. To generate transgenic zebrafish expressing canine YFP-CBD1-CFP, the YFP-CBD1-CFP DNA (15), excised with NheI/NotI, was subcloned into the T2K αkss MCS vector (a kind gift of Dr. K Kawakami) (8) downstream of the cardiac myosin light-chain promoter to create the T2K YFP-CBD1-CFP construct. Transposase mRNA was synthesized in vitro from pCS-TP using the mMESSAGE mMACHINE SP6 kit. The T2K YFP-CBD1-CFP plasmid and transposase mRNA were mixed at final concentrations of 25 ng/μl in RNase-free water. Approximately 1 nl of a DNA/RNA solution was microinjected into zebrafish embryos at the one-cell stage by microinjection. Injected embryos were examined for YFP expression under a Zeiss SV-11 epifluorescence microscope at 2 days postfertilization (dpf), and only embryos with YFP expression were raised to adulthood. F0 founder fish were identified by crossing with wild-type zebrafish and examining YFP expression of 2-day-old F1 embryos. A similar strategy was utilized to obtain transgenic zebrafish expressing a YFP-CBD1-CFP mutant (D447V/D498I).
Animal experiments were carried out in accordance with protocols and guidelines established by the National Institutes of Health and were approved by the University of California Los Angeles Animal Research Committee.
Microinjection of morpholino oligonucleotide.
An antisense morpholino oligonucleotide (20), 5′-CATGTTTGCTCTGATCTGACACGCA-3′, targeting the cardiac troponin T translation start codon and flanking 5′ sequence, was synthesized by Gene Tools (Philomath, OR). Approximately 4 ng oligonucleotide were injected into one- to two-cell stage YFP-CBD1-CFP transgenic embryos. Cardiac phenotypes of the injected embryos were examined at 30 and 48 h postfertilization.
FRET measurements.
Hearts of 2-day-old transgenic zebrafish embryos were dissected out in Tyrode solution supplemented with 1.8 mM CaCl2. Cytochalasin D (20 μM) was included in the solution to suppress contraction-related artifactual signals. Fluorescent imaging was performed with a Nikon Eclipse TE300 microscope equipped with a ×40 oil objective (numerical aperture 1.2) and excitation and dichroic filters appropriate for CFP excitation, as described previously (15). The samples were excited at wavelengths for CFP absorption (royal blue LED, Lumileds; San Jose, CA). YFP and CFP emission were monitored simultaneously using the Dual View (Optical Insights, Tucson, AZ) image splitter, equipped with a 505-nm long-pass dichroic filter to separate the CFP and YFP signals, a CFP emission filter (480/30), and a YFP emission filter (535/40) (15, 16). YFP and CFP images were captured with a Cascade 512B digital camera (Photometrics, Tucson, AZ). YFP and CFP emissions were measured online in real time, and the ratio between YFP and CFP emission was calculated as an indicator of FRET. The FRET ratio was corrected by subtracting the background signal (measured from areas without fluorescent heart sample). Exposure times were optimized for each experiment, but varied between 80 and 200 ms and were recorded at a rate between 2 and 5 Hz. LED illumination, camera exposure, and data acquisition were controlled by MetaFluor Imaging software (Molecular Devices, Sunnyvale, CA). All experiments were performed at room temperature.
Ca2+ imaging.
Wild-type embryos were injected with 1 nl of 10 mg/ml calcium green-1 dextran (70,000 molecular weight, Molecular Probes, Eugene, OR) at the one-cell stage. Hearts of 2-day-old embryos were dissected out in Tyrode solution, supplemented with 1.8 mM CaCl2 and 20 μM cytochalasin D. Calcium green fluorescence was detected with the same microscope as described above with YFP cube (excitation 500/20 nm, emission 535/30 nm; dichroic long wave pass 515). Images were acquired every 200 ms with a Cascade 512B digital camera and analyzed with MetaFluor Imaging 6.1 software.
RESULTS
Ca2+ handling in zebrafish heart.
Intracellular Ca2+ regulation during cardiac EC coupling has been studied in great detail in a variety of species. However, very little is known about Ca2+ handling during EC coupling in embryonic zebrafish myocardium. Before developing the use of transgenic zebrafish to monitor Ca2+ regulation of NCX, we performed initial experiments to begin to assess the contributions of various pathways to cytoplasmic Ca2+ regulation in wild-type zebrafish heart.
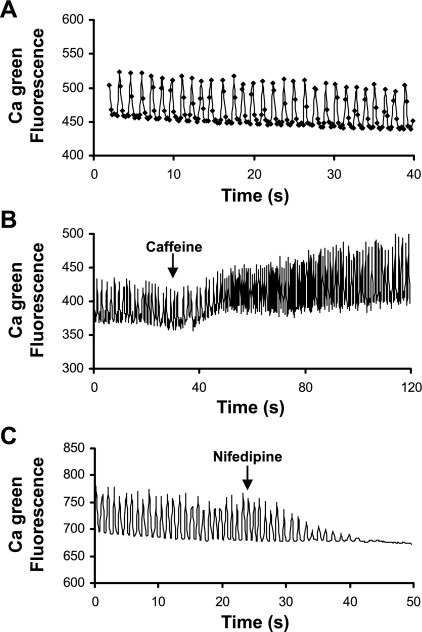
To measure Ca2+ transients, we injected calcium green dextran into wild-type zebrafish embryos at the one-cell stage and imaged calcium green fluorescence from isolated zebrafish hearts at 2 dpf. By visual inspection, we observed repetitive Ca2+ fluorescent waves traveling from the atrium to the ventricle (not shown). Ca2+ transients accompanied spontaneous contractions (Fig. 1A). To assess sarcoplasmic reticulum (SR) function, we recorded Ca2+ transients before and after the addition of caffeine (5 mM). Caffeine increased both diastolic and peak systolic intracellular Ca2+ concentration, as well as the amplitude of Ca2+ transients (Fig. 1B). The data indicate the presence of a functional SR, although the effects of caffeine were relatively modest. Compared with mammalian adult myocardium, the SR in fish myocardium is underdeveloped, has lesser ability to store and release Ca2+, and has lesser importance in EC coupling (3). We also tested the contribution of Ca2+ influx through L-type Ca2+ channels to EC coupling. As shown in Fig. 1C, the dihydropyridine, nifedipine (10 μM), resulted in cessation of Ca2+ transients and beating. These results indicate that the L-type Ca2+ channel has an essential role in EC coupling in the 2-day-old embryonic zebrafish heart.
Fig. 1.
Ca2+ transients in wild-type zebrafish hearts. A: Ca2+ transients recorded from isolated wild-type zebrafish hearts during spontaneous contractions. Ca2+ signals are shown as calcium green fluorescence intensity. B: time course of Ca2+ transients before and after application of 5 mM caffeine. C: time course of Ca2+ transients before and after application of 10 μM nifedipine. Note the use of different time scales. Fluorescence intensity is expressed in arbitrary units.
FRET monitoring of conformational changes of CBD1.
As previously reported (15), we have expressed CBD1 (amino acids 371–508) of the NCX with the fluorescent probes YFP and CFP on the NH2- and COOH-termini, respectively. This construct, YFP-CBD1-CFP, undergoes conformational changes upon binding Ca2+ (15). The conformational motions of the CBD can be monitored by changes of FRET measured as the YFP-to-CFP fluorescence ratio. To study the Ca2+-dependent regulation of the exchanger in intact hearts and in vivo, we made transgenic zebrafish with cardiac-specific expression of YFP-CBD1-CFP. We also created transgenic zebrafish expressing YFP-CBD1-CFP with two mutations, D447V and D498I. These mutations have been shown to induce a decreased Ca2+-binding affinity in the intact NCX and abolish Ca2+-induced FRET in YFP-CBD1-CFP (11, 15).
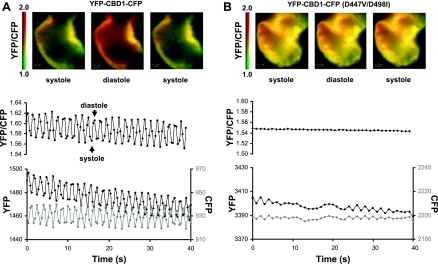
We isolated hearts from zebrafish embryos at 2 dpf. Spontaneous contractions of the hearts were monitored, and changes of FRET were measured as changes in the YFP-to-CFP ratio. As shown in Fig. 2A, YFP and CFP emissions oscillated in opposite directions as embryonic hearts expressing YFP-CBD1-CFP contracted. The YFP-to-CFP fluorescence ratio was maximal during diastole when cytoplasmic Ca2+ was decreased and minimal during systole when cytoplasmic Ca2+ was elevated. The data indicate that the Ca2+ regulatory site of NCX undergoes conformational changes on a beat-to-beat basis during EC coupling. Ca2+-induced FRET changes were not observed in hearts expressing mutant YFP-CBD1-CFP (D447V/D498I) (Fig. 2B). This confirms that the FRET signals from beating hearts expressing YFP-CBD1-CFP were not artifactual. The magnitude of cardiac contraction was reduced by the presence of cytochalasin D (20 μM) to minimize possible motion artifacts.
Fig. 2.
Fluorescence resonance energy transfer (FRET) studies in transgenic zebrafish hearts expressing yellow fluorescent protein (YFP)-Ca2+-binding domain 1 (CBD1)-cyan fluorescent protein (CFP) (A) or YFP-CBD1-CFP mutant (D447V/D498I) (B). Shown are representative FRET (YFP/CFP) images of transgenic zebrafish hearts (ventricle, shown in pseudocolor) and the corresponding changes in the YFP-to-CFP ratio (black, top traces), YFP (black), and CFP (gray) emissions (bottom traces) during spontaneous contractions. The decrease in the ratio of YFP/CFP emissions correlates with cardiac contraction (not shown). Pseudocolor scale bars are shown to the left of the images. Dissected zebrafish hearts were kept in Tyrode solution at room temperature. Data were collected at 2 frames/s with a ×40 oil immersion lens. Data are representative of several experiments.
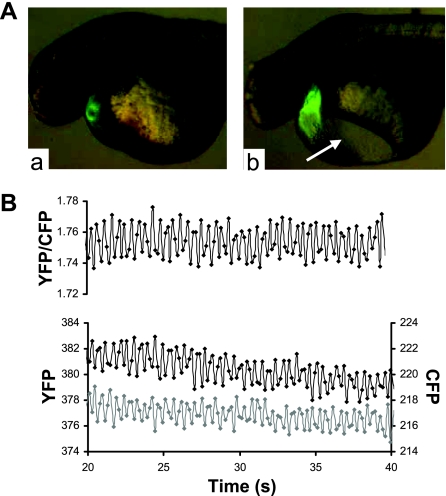
To monitor conformational changes of YFP-CBD1-CFP in the myocardium of intact zebrafish, we injected cardiac troponin T (tnnt2) morpholino at the one-cell stage to uncouple EC coupling and silence the heart (20). This allowed Ca2+ transients to occur in the absence of contraction. In vivo images were obtained at 2 dpf. The tnnt2 morpholino-injected fish exhibit some pericardial edema (arrow in Fig. 3A, right) and an absence of cardiac contraction and blood flow. We detected rapid changes in the FRET signal at an average rate of about 120/min (Fig. 3B), consistent with the average heart rate of wild-type zebrafish at 2 dpf.
Fig. 3.
FRET measurements from intact transgenic zebrafish embryos. A: epifluorescent images of control (a) and tnnt2 morpholino-injected (b) transgenic zebrafish embryos expressing YFP-CBD1-CFP at 2 days postfertilization. Arrow indicates the presence of pericardial edema in morpholino-injected fish. B: representative recording of changes in YFP (black) and CFP (gray) emission and the YFP-to-CFP ratio in an intact tnnt2 morpholino-injected YFP-CBD1-CFP transgenic zebrafish. Data were collected at 6 frames/s with a ×20 oil immersion lens.
The conformation of CBD1 is affected by inotropic state.
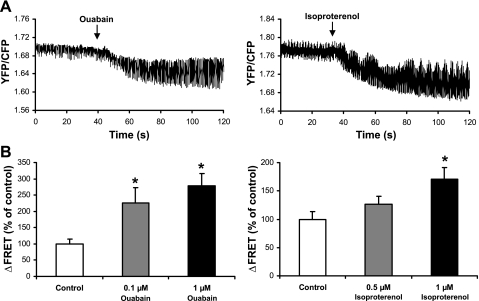
Using FRET, we assessed whether Ca2+ regulation of NCX is altered by the inotropic state of the heart. Positive inotropic agents (ouabain or isoproterenol) were added to isolated transgenic zebrafish hearts expressing YFP-CBD1-CFP to monitor effects on FRET. Sample traces and summary data are shown in Fig. 4. Both ouabain and isoproterenol increased the changes in FRET between contraction and relaxation in a concentration-dependent manor. Ouabain (0.1 and 1 μM) significantly increased the change (Δ) in FRET to 230 ± 50% (n = 5, P < 0.05) and 280 ± 40% (n = 4, P < 0.05) of control values, respectively. Treatment of the heart with 0.5 μM isoproterenol had a modest effect on ΔFRET (130 ± 10% of control values, n = 4, P = 0.15). A higher concentration of isoproterenol (1 μM) led to a significant stimulation (170 ± 20% of control values, n = 4, P < 0.05).
Fig. 4.
Effects of ouabain and isoproterenol on changes (Δ) of FRET from YFP-CBD1-CFP during spontaneous contractions of transgenic zebrafish hearts. A: time course of ΔFRET (YFP-to-CFP ratio) before and after application of 1 μM ouabain or isoproterenol. B: summary data comparing ΔFRET in the absence and presence of ouabain or isoproterenol. ΔFRET is calculated as the difference of the YFP-to-CFP ratio between contraction and relaxation. Data are normalized against control. Values for n are 5 (0.1 μM ouabain), 4 (1 μM ouabain), 4 (0.5 μM isoproterenol), and 4 (1 μM isoproterenol). *P < 0.05.
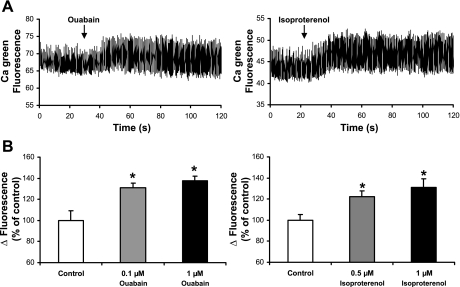
We also examined the effects of these concentrations of ouabain and isoproterenol on Ca2+ transients in wild-type hearts (Fig. 5). As shown in Fig. 5A, ouabain and isoproterenol increased the magnitudes of the Ca2+ transients. Ca2+ transient amplitudes were significantly increased by 0.1 μM (130 ± 10% of control, n = 4, P < 0.01) and 1 μM (140 ± 10% of control, n = 4, P < 0.01) ouabain, as well as by 0.5 μM (120 ± 10% of control, n = 4, P < 0.05) and 1 μM isoproterenol (130 ± 10% of control, n = 4, P < 0.05) (Fig. 5B). The effects of the inotropic agents on Ca2+ transients correlated with the changes of FRET. These data suggest that the positive inotropic agents ouabain and isoproterenol raised intracellular Ca2+ and activated NCX. There are no previous studies indicating that the activation state of the NCX is controlled by regulatory Ca2+ during changes in inotropic state.
Fig. 5.
Effects of ouabain and isoproterenol on Ca2+ transients during spontaneous contractions of wild-type zebrafish hearts. A: time course of ΔCa2+ (calcium green fluorescence intensity) before and after application of 1 μM ouabain or isoproterenol. B: summary data comparing ΔCa2+ transients in the absence and presence of ouabain or isoproterenol. ΔCalcium green fluorescence intensity is calculated as the difference of calcium green fluorescence intensity between contraction and relaxation. Data are normalized against control. Values for n are 4 (0.1 μM ouabain), 4 (1 μM ouabain), 4 (0.5 μM isoproterenol), and 4 (1 μM isoproterenol). *P < 0.05.
DISCUSSION
We describe the use of zebrafish myocardium for FRET-based detection of conformation changes of the CBD of the NCX expressed in the cytoplasm. The zebrafish offers several distinct advantages as a model system, including external fertilization, rapid development, and the ability to easily up- and downregulate gene expression. The optical clarity of the zebrafish embryo provides a unique opportunity to monitor physiological processes in excised hearts and in vivo by fluorescence. Here we generated transgenic zebrafish expressing CBD1 of NCX with the fluorophores YFP and CFP linked to its NH2- and COOH-termini. Using noninvasive FRET, we monitored Ca2+-induced conformation changes of CBD1 in isolated, spontaneously contracting zebrafish heart and in intact zebrafish in vivo.
We did initial assessment of the contributions of Ca2+ influx and SR Ca2+ release to intracellular Ca2+ regulation during EC coupling in zebrafish embryonic myocardium. Unlike mammals in which SR Ca2+ release is prominent in EC coupling, in lower vertebrates, such as the fish, the SR is generally a poorly developed organelle of lesser importance in EC coupling (2, 3, 6). Depletion of SR Ca2+ in zebrafish myocardium with caffeine increased diastolic Ca2+, as well as Ca2+ transients. However, caffeine did not stop the repetitive Ca2+ transients, implying that Ca2+ entry across the sarcolemmal membrane is sufficient for a relatively synchronous and uniform rise in whole cell intracellular Ca2+ concentration. The relative importance of SR Ca2+ release to EC coupling is subject to temperature and adrenergic regulation, and prominent species-specific differences exist among fish (21–24). Ca2+ transients were completely abolished by the L-type Ca2+ channel blocker, nifedipine, confirming that transsarcolemmal Ca2+ influx through the dihydropyridine receptor is essential to EC coupling in cardiomyocytes from embryonic zebrafish.
To monitor the conformational changes of the CBD of NCX in intact myocardium, we developed the use of zebrafish as an expression system for CBD1 tagged with CFP and YFP. This fusion protein has previously been shown to change conformation upon binding Ca2+, as indicated by FRET changes in human embryonic kidney cells and in rat neonatal cardiac myocytes (15). Our zebrafish model allows us to monitor changes in conformation of CBD1 during EC coupling in living zebrafish myocardium and in excised hearts. The FRET changes were monitored at a much higher frequency than occurred in the more slowly contracting neonatal myocytes (15). The FRET signal correlated well with cardiac contraction: maximal and minimal FRET occurred during relaxation and contraction, respectively. The changes in FRET were not motion artifacts, as we uncoupled EC coupling with cytochalasin D in isolated heart and with cardiac troponin T morpholino in living zebrafish. Furthermore, FRET changes were not observed in control experiments using YFP-CBD1-CFP mutant (D447V/D498I) transgenic fish, which has decreased Ca2+ affinity (11).
Combining FRET with intracellular Ca2+ imaging, we examined whether the inotropic agents ouabain and isoproterenol modulate Ca2+ regulation of NCX in zebrafish heart. We found that the increased Ca2+ transients induced by ouabain and isoproterenol increased the ΔFRET signals from YFP-CBD1-CFP. The strong implication is that increased Ca2+ transients directly lead to enhanced activation of the NCX through the increased binding of regulatory Ca2+. Increased exchange activity could augment both the upstroke and relaxation of Ca2+ transients through reverse and forward mode exchange, respectively. There has not previously been experimental evidence directly linking Ca2+ regulation of the exchanger to inotropic state. Our experiments suggest that direct Ca2+-dependent regulation of NCX may be involved in an inotropic stimulation of NCX. The data are also inconsistent with reports suggesting that the apparent affinity of NCX is 20–80 nM (12, 14). If this were the case, Ca2+ binding would likely be saturated during diastole, and FRET changes would not be observed in these experiments.
In summary, we have developed the use of zebrafish as a novel expression system to monitor conformations of a CBD of the NCX in living myocardium. We hope to expand these studies to include the use of full-length NCX constructs. We expect this model will help us better understand the physiological function of the exchanger and the roles of cytoplasmic factors in the beat-to-beat regulation of NCX activity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL49101 (to K. D. Philipson) and a postdoctoral fellowship (0725026Y) from the American Heart Association (to Y. Xie).
Acknowledgments
Some of the data have been presented previously in abstract form (Annual Meeting of the Biophysical Society, 2008).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Besserer GM, Ottolia M, Nicoll DA, Chaptal V, Cascio D, Philipson KD, Abramson J. The second Ca2+-binding domain of the Na+ Ca2+ exchanger is essential for regulation: crystal structures and mutational analysis. Proc Natl Acad Sci USA 104: 18467–18472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugun A, Oyamada T, Temma K, Hara Y, Kondo H. Intracellular Ca2+ storage sites in the carp heart: comparison with the rat heart. Comp Biochem Physiol A Mol Integr Physiol 123: 61–67, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Driedzic WR, Gesser H. Energy metabolism and contractility in ectothermic vertebrate hearts: hypoxia, acidosis, and low temperature. Physiol Rev 74: 221–258, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Hilge M, Aelen J, Vuister GW. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell 22: 15–25, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Hilgemann DW, Collins A, Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol 100: 933–961, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hove-Madsen L, Llach A, Tort L. Quantification of Ca2+ uptake in the sarcoplasmic reticulum of trout ventricular myocytes. Am J Physiol Regul Integr Comp Physiol 275: R2070–R2080, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E. Cardiac-specific ablation of the Na+-Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res 97: 916–921, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami K Transposon tools and methods in zebrafish. Dev Dyn 234: 244–254, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Levitsky DO, Fraysse B, Leoty C, Nicoll DA, Philipson KD. Cooperative interaction between Ca2+ binding sites in the hydrophilic loop of the Na+-Ca2+ exchanger. Mol Cell Biochem 160–161: 27–32, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Levitsky DO, Nicoll DA, Philipson KD. Identification of the high affinity Ca2+-binding domain of the cardiac Na+-Ca2+ exchanger. J Biol Chem 269: 22847–22852, 1994. [PubMed] [Google Scholar]
- 11.Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD. Regulation of the cardiac Na+-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca2+-binding domain. J Gen Physiol 105: 403–420, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miura Y, Kimura J. Sodium-calcium exchange current. Dependence on internal Ca and Na and competitive binding of external Na and Ca. J Gen Physiol 93: 1129–1145, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicoll DA, Sawaya MR, Kwon S, Cascio D, Philipson KD, Abramson J. The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J Biol Chem 281: 21577–21581, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Noda M, Shepherd RN, Gadsby DC. Activation by [Ca]i, and block by 3′,4′-dichlorobenzamil, of outward Na/Ca exchange current in Guinea-pig ventricular myocytes (Abstract). Biophys J 53: 342a, 1988. [Google Scholar]
- 15.Ottolia M, Philipson KD, John S. Conformational changes of the Ca2+ regulatory site of the Na+-Ca2+ exchanger detected by FRET. Biophys J 87: 899–906, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottolia M, Philipson KD, John S. Xenopus oocyte plasma membrane sheets for FRET analysis. Am J Physiol Cell Physiol 292: C1519–C1522, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Pogwizd SM Increased Na+-Ca2+ exchanger in the failing heart. Circ Res 87: 641–643, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85: 1009–1019, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Reeves JP, Hale CC. The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem 259: 7733–7739, 1984. [PubMed] [Google Scholar]
- 20.Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31: 106–110, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Shiels H, Farrell A. The effect of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200: 1607–1621, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Thomas MJ, Hamman BN, Tibbits GF. Dihydropyridine and ryanodine binding in ventricles from rat, trout, dogfish and hagfish. J Exp Biol 199: 1999–2009, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Tiitu V, Vornanen M. Ryanodine and dihydropyridine receptor binding in ventricular cardiac muscle of fish with different temperature preferences. J Comp Physiol [B] 173: 285–291, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Vornanen M, Shiels HA, Farrell AP. Plasticity of excitation-contraction coupling in fish cardiac myocytes. Comp Biochem Physiol A Mol Integr Physiol 132: 827–846, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Weber CR, Ginsburg KS, Philipson KD, Shannon TR, Bers DM. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J Gen Physiol 117: 119–131, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerfield M The Zebrafish book: a Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). Eugene, OR: University of Oregon Press, 1993, p. 1.