Abstract
We describe here an important function of the novel calmodulin kinase I isoform, pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck). Pnck (also known as CaM kinase Iβ2) was previously shown to be differentially overexpressed in a subset of human primary breast cancers, compared with benign mammary epithelial tissue. In addition, during late pregnancy, Pnck mRNA was shown to be strongly upregulated in epithelial cells of the mouse mammary gland exhibiting decreased proliferation and terminal differentiation. Pnck mRNA is also significantly upregulated in confluent and serum-starved cells, compared with actively growing proliferating cells (Gardner HP, Seung HI, Reynolds C, Chodosh LA. Cancer Res 60: 5571–5577, 2000). Despite these suggestive data, the true physiological role(s) of, or the signaling mechanism(s) regulated by Pnck, remain unknown. We now report that epidermal growth factor receptor (EGFR) levels are significantly downregulated in a ligand-independent manner in human embryonic kidney-293 (HEK-293) cells overexpressing Pnck. MAP kinase activation was strongly inhibited by EGFR downregulation in the Pnck-overexpressing cells. The EGFR downregulation was not the result of reduced transcription of the EGFR gene but from protea-lysosomal degradation of EGFR protein. Knockdown of endogenous Pnck mRNA levels by small interfering RNA transfection in human breast cancer cells resulted in upregulation of unliganded EGFR, consistent with the effects observed in the overexpression model of Pnck-mediated ligand-independent EGFR downregulation. Pnck thus emerges as a new component of the poorly understood mechanism of ligand-independent EGFR degradation, and it may represent an attractive therapeutic target in EGFR-regulated oncogenesis.
Keywords: epidermal growth factor receptor
one of the ways by which calcium, acting as a second messenger, transduces intracellular signals is by binding to and activating the ubiquitous calcium-binding protein, calmodulin (6, 7, 33). Activated calmodulin, in turn, activates a group of serine/threonine kinases called calmodulin kinases (29). Four major calmodulin kinases have been described: calmodulin kinase I, II, III, and IV, with predominant expression and major roles in the nervous system (58). These enzymes catalyze a variety of biochemical reactions, with different outputs, such as altered gene expression, neurotransmitter release, proliferation, muscle contraction, and altered metabolism (25, 46). Calmodulin kinase kinase, the upstream activator of calmodulin kinase I and IV, is known to control cell survival processes by regulating the prosurvival kinase Akt (30, 64, 65, 69). Calmodulin kinase I was first purified from brain, based on its ability to phosphorylate synapsin I (10, 11, 45), and was subsequently cloned from rat, bovine (52), and human tissues (26). In addition to synapsin I and II, calmodulin kinase I phosphorylates a wide variety of substrates, such as cAMP response element-binding protein (57), cystic fibrosis transmembrane conductance regulator (51), and the Numb family proteins (Numb and Numb-1) (63). Over the years, two other isoforms of calmodulin kinase I, namely, β and γ, were reported (71), and, subsequently, a splice variant of calmodulin kinase Iβ, called calmodulin kinase Iβ2, was discovered (47), which is also known as pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck) (20, 21). Pnck is a unique member of the calmodulin kinase I family, being most homologous to calmodulin kinase I within the catalytic domain (21). Pnck is predominantly expressed in the central nervous system (39, 54, 66). During embryonic development, Pnck mRNA and protein are selectively expressed in the murine central nervous system throughout the midgestation period (21, 32), suggesting a developmental role for this protein. Of adult murine organs, besides the central nervous system, Pnck mRNA was detected in a variety of tissues including breast, uterus, brain, heart, and stomach (21). On the basis of studies of both endogenous and ectopic expression, Pnck is found both in the cytoplasm and nuclei of neurons, suggesting a role in cytoplasmic as well as nuclear signal transduction (54, 66). However, no functional study of Pnck/CaMKIβ2 protein in intact cells has been reported to date. A possible role for Pnck in human breast cancer was first identified in 2000, when Pnck mRNA expression was found to be three- to fivefold upregulated in a subset of human breast cancers as compared with benign mammary tissue (20). Pnck was also shown to be expressed in a c-Myc and int-2/Fgf3 oncogene-associated manner in transgenic mouse mammary tumors. However, in the normal mouse mammary gland, Pnck mRNA was also found to be upregulated during late pregnancy, associated with decreased proliferation and terminal differentiation. Furthermore, in a cell culture model, the Pnck mRNA level was shown to be significantly higher in confluent and serum-starved mammary epithelial cells (20), suggesting an inverse relationship between Pnck expression and cellular proliferation in normal cells and tissues and that this relationship might be deranged in cancer.
Epidermal growth factor receptor (EGFR/ErbB1/HER-1) and related members of the ErbB family (ErbB2/HER-2, ErbB3/HER-3, ErbB4/HER-4) are amplified, overexpressed, and activated in a variety of human cancers (40, 60, 70). ErbB family members possess tyrosine kinase activities (except ErbB3/HER-3), undergo homo- or heterodimerization, and initiate several intracellular signaling cascades that are responsible for many cellular fates, including survival, proliferation, and differentiation. Aberrant expression and activation of wild-type (WT) ErbB receptors, especially EGFR and ErbB2, are oncogenic, resulting in growth signal autonomy and limitless replicative potential, which are hallmarks of human cancer (24). Mutant versions of the growth factor receptors are expressed in some cancers that are constitutively active in the absence of ligand binding: for example, the EGFRvIII form in glioblastoma (49). In such human cancers, attenuating the origin and duration of signal at the receptor level holds enormous promise from the therapeutic standpoint. Activation of EGFR can be blocked by blocking antibodies (2, 53) and by small molecule tyrosine kinase inhibitors (18). Cell surface expression of EGFR is reduced by ligand-dependent internalization (31, 59), and subsequently by protea-lysosomal degradation (37), the latter mechanism primarily being mediated by a c-Cbl adapter protein ring finger domain-associated E-3 ubiquitin ligase activity (14, 22, 37). Although ligand-dependent EGFR degradation has been extensively studied, much less attention has been paid to ligand-independent EGFR downregulation or degradation, and the processes involved remain unclear. Recent studies have identified some components of this system (34, 48), but the mechanistic details are far from being completely understood.
Here, we have identified Pnck as a component of the poorly understood mechanism of ligand-independent EGFR degradation. Using Pnck overexpression and small interfering (si)RNA-mediated knockdown strategies, we have shown that ligand-independent degradation of EGFR is regulated by Pnck expression level. This observation, in the context of the previous report of differential overexpression of Pnck in a subset of human primary breast cancer (20), raises the possibility that Pnck could be an endogenous protein inhibitor of EGFR overexpression in different human cancers.
EXPERIMENTAL PROCEDURES
Materials.
Polylysine-coated (BD BioCoat) plates, panextracellular signal-regulated kinase MAb (pan-ERK), and mSos1 MAb were obtained from BD BioSciences. Pharmacological inhibitors AG-1478, bafilomycin A1, and MG-132 were purchased from Calbiochem. Anti-hemagglutinin (HA) MAb was obtained from Covance. Phospho-MAPK, P-Shc Y239/240 polyclonal antibody (PAb), P-Shc Y317 PAb were purchased from Cell Signaling Technology. H-Ras (C-20) PAb, Raf-1 (C-12) PAb, and MEK-1 (H-8) MAb were from Santa Cruz Biotechnology. Anti-β-actin MAb was from Sigma (St. Louis, MO). Anti-EGFR (Ab-15) MAb was from NeoMarkers. Anti-phosphotyrosine MAb (clone 4G10) and anti-Grb2 MAb were purchased from Upstate Biotechnology. Anti-Pnck PAb (catalog no. AP7097a) was purchased from Abgent. G-418 was obtained from GIBCO.
Plasmid constructs.
A human Pnck cDNA was cloned by PCR from an IMAGE clone from a cDNA library, enriched for full-length clones derived from a pool of six anonymous adult male brains (IMAGE clone no. 5194959; Resgen). The IMAGE clone was selected on the basis of its sequence similarity to the mouse Pnck cDNA (accession no. AF181984). The clone was obtained and sequenced in its entirety using one 5′-untranslated region primer and one internal primer generated from sequence information available in the expressed sequence tag database, and a full-length cDNA for Pnck was amplified by PCR, using the following primers containing EcoRI (5′-end) and BamHI (3′-end) restriction sites: 5′-primer, TCC CGA ATT CCC GGG ATG CTG; 3′-primer, TTG GAT CCC CAC TTG GGG GGC TGG CCA. The Pnck PCR product was double digested (EcoRI and BamHI) and subcloned into the same sites of the phCMV3 vector (Roche) in frame with a 3′-HA epitope to generate a COOH-terminal HA-tagged Pnck. The untagged, WT Pnck construct was generated by reintroducing a stop codon before the 3′-HA tag within the above mentioned vector by site-directed mutagenesis using the Stratagene Quick Change kit. An NH2-terminal HA-tagged Pnck was generated by cloning the WT Pnck cDNA into the BglII and EcoRI sites of the phCMV2 vector (Roche) containing an NH2-terminal HA epitope. All clones were verified by DNA sequencing.
Generation of polyclonal antisera against Pnck.
A polyclonal antibody against a COOH-terminal peptide of mouse Pnck protein was developed on contract basis by Spring Valley Laboratories (Woodbine, MD). The mouse peptide (326-342), with the sequence CMTRHSHPGLGTSQSPKW, was synthesized and conjugated with keyhole limpet hemocyanin and was used to inoculate two specific pathogen-free New Zealand White rabbits. A standard 52-day protocol was followed from primary immunization through boosting to final bleeding. Immunoglobulin was purified using AffinityPak Immobilized Protein A columns (Pierce).
Transient and stable expression of human Pnck cDNA in HEK-293 cells.
Human embryonic kidney-293 epithelial cells (HEK-293), HEK-293T cells (ATCC, Rockville, MD), were transiently transfected with expression plasmids harboring either control or WT/HA-Pnck cDNAs, using Fugene-6 (Roche, Indianapolis, IN) following the manufacturer's protocol. Briefly, subconfluent, actively proliferating cells were transfected in the presence of serum at a 1:3 plasmid: Fugene-6 ratio. Cells were allowed to recover for 24 h and were serum starved for another 24 h before ligand stimulation. For stable cell line development, HEK-293 cells were selected with 800 μg/ml G-418 for 3 wk following transfection. Clonal populations were developed from pooled clones by limiting dilution and were always maintained as subconfluent proliferative cells in 500 μg/ml G-418.
Cell culture, pharmacological inhibitor treatment, and lysis.
Stable, clonal HEK-293 cell lines were plated on BD BioCoat plates at a density of 100 × 103/cm2 and grown in DMEM (GIBCO; catalog no. 11965-092) containing 10% heat-inactivated fetal bovine serum (FBS). Cells were grown for 16 h and were then serum starved for another 24 h. Pharmacological inhibitors were added either in the serum-containing or serum-starved medium depending on the experiment. Cells were stimulated with 10 nM EGF for 3 min or for the indicated time period, 20% FBS for 10 min, 50 ng/ml each of IGF-I and II for 10 min, and 1 μg/ml insulin for 10 min at 37°C and were lysed in lysis buffer (10 mM Tris-base, pH 7.4, 1% Triton X-100, 5 mM EDTA, 50 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/ml each of pepstatin, leupeptin, and aprotinin). Lysates were vortexed and centrifuged at 15,000 g for 15 min at 4°C. Lysate protein concentrations were measured using a bicinchoninic acid protein assay kit (Pierce) and the Ultramark Microplate Imaging System (Bio-Rad).
siRNA transfection.
Human SK-BR-3 breast cancer cells were transfected using Oligofectamine (Invitrogen) with siRNAs directed against luciferase (control) and human Pnck gene. Pnck siRNA (Dharmacon) was based on sequence AGAACGAGATCGCAGTGCT (accession no. NM_198452). Both siRNAs were transfected in the presence of serum at 50% cellular confluence and allowed to grow for 48 h. Cells were serum starved for another 24 h, stimulated without or with EGF or FBS, and lysed. An identical set was processed for total RNA extraction for real-time RT-PCR analysis.
RNA preparation and real-time RT-PCR.
Total RNA was prepared from cells using Tri-Reagent (Sigma), following the manufacturer's recommendations. The quality and concentration of RNA were checked by spectrophotometer, and 1-μg aliquots were treated with RQ1 RNase-free DNase (Promega) to remove any contaminating genomic DNA and then used to generate cDNA using the avian myeloblastosis virus reverse transcriptase system (Promega), according to the manufacturer's instructions. The resultant cDNA was subjected to real-time PCR using Assays-on-Demand gene expression products (20× mix of unlabeled PCR primers and FAM or VIC dye-labeled TaqMan MGB probe) specific for EGFR, Pnck, or GAPDH (Applied Biosystems) according to the manufacturer's instructions. The relative standard curve method was used to quantitate the expression levels of each gene. GAPDH was used as the endogenous reference to normalize samples, and results are expressed relative to the level in control cells. The mean and standard deviation of mean of triplicate determinations are presented. Statistical analysis of results was conducted using the two-tailed paired Student's t-test as described in the ABI technical manual.
Immunoprecipitation and Western blot analysis.
Immunoprecipitation and Western blot analysis were performed as previously described (8). Briefly, 1 μg of antibody was added to 500 μg of clarified, whole cell lysates and incubated overnight at 4°C. Protein G-agarose (5 μl; Amersham BioSciences) beads were added, and lysates were further incubated for 1 h at 4°C. Beads were precipitated by centrifugation at 15,000 g for 2 min and were washed three times in lysis buffer. Bound proteins were released by boiling in SDS-PAGE sample buffer for 3 min. Lysate proteins and immunoprecipitates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride (Immobilon-P, Millipore) membranes. For phospho-MAPK detection, membranes were incubated in primary antibody for 2 h, followed by biotinylated secondary antibody for 1 h, and were detected by Vectastain ABC Elite kit (Vector Labs) and enhanced chemiluminescence (PerkinElmer). All other immunoblots were incubated with either horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies, following primary antibody, and were detected by enhanced chemiluminescence. Protein bands were scanned using Scion image software.
Immunokinase assay.
Immunokinase assay was performed according to Deb et al. (9) with modifications. Briefly, lysates from both Neo and HA-Pnck HEK-293 cells were subjected to immunoprecipitation as described above. After 2 h of immunoprecipitation, protein G beads were washed twice with lysis buffer and once with kinase assay buffer (25 mM HEPES, pH 7.0, 10 mM MgCl2, 2.5 mM MnCl2, and 50 μM sodium orthovanadate). Washed beads were incubated in kinase assay buffer containing 10 μM ATP and 15 μCi [γ-32P] ATP for 30 min at 30°C. Reactions were stopped by SDS-PAGE sample buffer and boiled for 4 min. Supernatants were resolved on a 12% SDS-PAGE and autoradiographed.
RESULTS
Pnck inhibits EGF-induced MAP kinase activation.
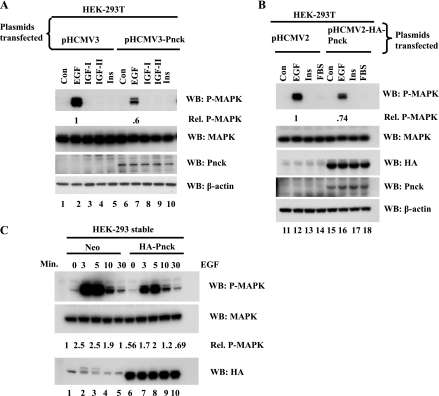
Previously, Pnck expression was found to be upregulated in serum-starved and confluent cells and in a subset of epithelial cells in the mammary gland associated with decreased proliferation and terminal differentiation, during late pregnancy (20). We hypothesized that Pnck might inhibit a proliferative signaling mechanism, which could be the reason for decreased proliferation in the mammary gland, or might support a cell cycle arresting role. To this end, we opted to examine Pnck's role in regulating MAP kinase activation, which is a major signaling axis in many cells. Accordingly, human Pnck cDNA was cloned and found to be 95.3% identical to mouse Pnck (accession no. AF181984) at the amino acid level (21). The greatest dissimilarity between the mouse and human Pnck protein was seen at the COOH-terminal end. Control and expression plasmids encoding WT or NH2-terminally HA-tagged human Pnck protein were transiently transfected in HEK-293T cells. Upon stimulation of serum-starved, subconfluent control cells with a panel of ligands (EGF, IGF-I, IGF-II, and insulin), we observed that EGF was the only ligand able to stimulate MAP kinase activation in HEK-293T cells. Interestingly, EGF-induced MAP kinase activation was inhibited in cells transfected with the WT Pnck expression construct, compared with the cells transfected with the control vector (Fig. 1A, lanes 2 and 7). Similarly, EGF-induced MAP kinase activation was also inhibited in HA-Pnck expressing cells, compared with control cells (Fig. 1B, lanes 12 and 16), implying no functional defect in the HA-tagged Pnck construct. MAP kinase inhibition was not due to reduced expression of MAP kinase protein in WT Pnck or HA-Pnck cDNA-expressing cells, as demonstrated by anti-MAPK immunoblots (Fig. 1, A and B; WB: MAPK). Both ERK-1 and ERK-2 components of MAP kinase were inhibited in WT and HA-Pnck-expressing cells. Expression of WT and HA-Pnck was detected by our COOH-terminally derived anti-Pnck PAb (Fig. 1, A and B; WB: Pnck) and anti-HA MAb (Fig. 1B; WB: HA), indicating that full-length, functional constructs were expressed. The mouse antigenic peptide used to develop the polyclonal Pnck antibody possesses ∼67% homology with the equivalent region of the human Pnck protein. Despite this moderate homology, the antibody detected both recombinant human WT and HA-tagged Pnck protein (Fig. 1, A and B; WB: Pnck). Endogenous Pnck was not detected using this polyclonal Pnck antibody, which is probably due to very low levels of Pnck expression in these cells. This may relate to the transformation state of the cells since expression was previously shown to be upregulated in human breast cancer cell lines and in primary human breast cancer (20). Thus Pnck, for the first time, was shown to be a negative regulator of EGF-induced MAP kinase activation.
Fig. 1.
Pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck) inhibits EGF-induced MAP kinase activation in human embryonic kidney (HEK)-293T cells. A: inhibition of EGF-induced MAP kinase activity by wild-type (WT) Pnck. Subconfluent HEK-293T cells were transiently transfected with control plasmid phCMV3 (lanes 1–5) or WT Pnck cDNA in phCMV3 (lanes 6–10). Following transfection, cells were allowed to grow for 24 h and were then serum starved for another 24 h in DMEM containing 10 mM HEPES, pH 7.4. Plates were stimulated in serum-free medium at room temperature either without any ligand [control (Con), lanes 1 and 6] or with the following ligands: 10 nM EGF for 3 min (lanes 2 and 7), 50 ng/ml IGF-I for 5 min (lanes 3 and 8), 50 ng/ml IGF-II for 5 min (lanes 4 and 9), and 100 μM insulin (Ins) for 5 min (lanes 5 and 10). Lysates were prepared, protein concentration was determined, and equal amounts of protein were resolved on a 12% SDS-PAGE gel and transferred onto a polyvinylidene difluoride (PVDF) membrane. The polyclonal antibody (PAb) membrane was immunoblotted with anti-phospho-MAP kinase (Thr202/Tyr204) PAb [Western blot (WB): P-MAPK], anti-pan-ERK monoclonal antibody (MAb; WB: MAPK), or polyclonal Pnck antibodies (WB: Pnck) and anti-β-actin antibodies (WB: β-actin). EGF-induced phospho-MAPK band densities were normalized to MAPK, and EGF-induced phospho-MAPK density in Pnck-overexpressing cells is presented relative (Rel) to identical treatment in control vector-transfected cells. Experiment was repeated three times with identical results. B: EGF-induced MAP kinase inhibition by hemagglutinin (HA)-Pnck. HEK-293T cells were transiently transfected with control plasmid phCMV2 (lanes 11–14) or HA-Pnck cDNA expressing phCMV2 plasmid (lanes 15–18). Cells were processed as described in A and were stimulated either without any ligand (Con, lanes 11 and 15) or by EGF (lanes 12 and 16), insulin (lanes 13 and 17), or by 20% fetal bovine serum (FBS) for 10 min (lanes 14 and 18) at room temperature. Lysates were immunoblotted for phospho-MAP kinase (WB: P-MAPK), MAP kinase (WB: MAPK), HA-tagged Pnck (WB: HA), Pnck (WB: Pnck), and for β-actin (WB: β-actin). EGF-induced phospho-MAPK band densities were normalized to MAPK, and EGF-induced phospho-MAPK density in Pnck-overexpressing cells is presented relative to identical treatment in control vector-transfected cells. The experiment was repeated four times with essentially identical results, and a representative experiment is presented. C: time course of MAP kinase inhibition. Stable clonal Neo (control) (lanes 1–5) and HA-Pnck protein-expressing (lanes 6–10) HEK-293 cells were serum starved and stimulated with 10 nM EGF for the indicated time periods. Lysates were immunoblotted with anti-phospho-MAP kinase PAb (WB: P-MAPK), anti-pan-ERK MAb (WB: MAPK), or with anti-HA MAb (WB: HA). Phospho-MAPK band densities were normalized to MAPK and are presented relative to nonstimulated Neo-HEK-293 cells (lane 1).
Ligand-independent EGFR downregulation by Pnck inhibits MAP kinase signaling.
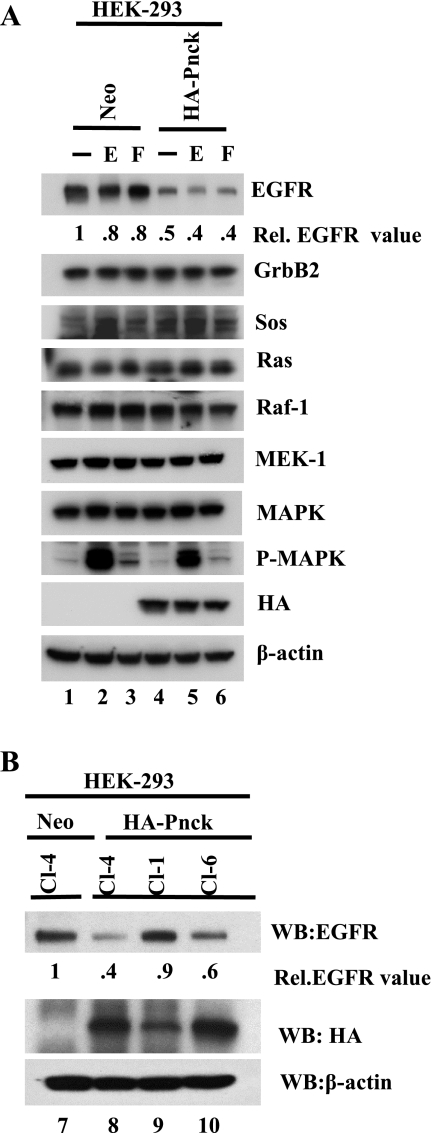
To dissect the mechanism of Pnck-mediated MAP kinase inhibition, several stable HEK-293 mass transfectant populations expressing the HA-Pnck plasmid (HA-Pnck-HEK-293) and corresponding control vector (Neo-HEK-293) were developed by G-418 selection. We switched to HEK-293 cells from HEK-293T cells for stable populations development because HEK-293T cells are already G-418 resistant and Pnck-expressing construct possesses a G-418 resistance gene. Each of these HA-Pnck-HEK-293 transfectant populations was found to express comparable levels of HA-Pnck protein, as determined by immunoblotting (data not shown), and all exhibited suppression of EGF-induced MAP kinase activation (data not shown). One of these populations (P2) and a control were subjected to limiting dilution, and several clones with different HA-Pnck expression levels (and control clones) were selected. A representative clone (clone 4) from each group was used for the rest of the study. Both Neo and HA-Pnck clones were examined in a time course experiment, and EGF-induced MAP kinase activation was inhibited in all the time points tested, thereby confirming the result of transient transfection experiments (Fig. 1C, WB: P-MAPK). Since the MAP kinase inhibition might be the result of either downregulation and/or inactivation of one or multiple components of this signaling axis, we analyzed the expression pattern of each major component, downstream of EGFR from GrbB2 to MAPK. Triplicate plates of the Neo-HEK-293 and HA-Pnck HEK-293 clones were serum starved and stimulated without or with EGF and FBS. It should be noted that the stable HEK-293 mass and clonal populations (Neo and HA-Pnck) are responsive to serum stimulation in the context of MAP kinase activation, although HEK-293T cells did not respond to FBS, following transient transfection of a HA-Pnck cDNA expressing plasmid (Fig. 1B, lanes 14 and 18). Immunoblotting of lysates, using an activation-specific antibody, revealed that both EGF- and FBS-induced MAP kinase activity was inhibited in HA-Pnck-expressing cells, relative to their control counterparts as expected (Fig. 2A, WB: P-MAPK, lanes 2 and 5 and lanes 3 and 6). The levels of the majority of the signaling molecules studied were essentially unaltered comparing the control and HA-Pnck clones (Fig. 2A, WB: GrbB2, WB: Sos, WB: Ras, WB: Raf-1, WB: MEK-1, WB: MAPK); however, strikingly, the levels of EGFR were greatly reduced in the HA-Pnck cells, irrespective of treatment, when compared with the control cells (Fig. 2A, WB: EGFR, lanes 1 and 4) and were further slightly downregulated in EGF-stimulated HA-Pnck cells (Fig. 2A, WB: EGFR, lanes 4 and 5). To extend this observation, we extended the study to additional clones that express different levels of HA-Pnck. As can be seen in Fig. 2B, among HA-Pnck-expressing clones, EGFR levels are lowest in clone 4, highest in clone 1, and at an intermediate EGFR level in clone 6 (lanes 8–10). EGFR levels were found to be approximately inversely proportional to HA-Pnck expression among HA-Pnck clones. Thus, depending on the HA-Pnck expression level, each of the HA-Pnck clones was able to downregulate EGFR in a ligand-independent manner.
Fig. 2.
Ligand-independent EGFR downregulation by Pnck and dissection of MAP kinase signaling in Pnck-expressing HEK-293 cells. A: EGFR undergoes ligand-independent downregulation in HA-Pnck-expressing stable HEK-293 cells. Three dishes each of Neo and HA-Pnck HEK-293 cells [clone 4 (Cl-4)] were grown in complete medium overnight and then serum starved for 24 h in DMEM containing 10 mM HEPES, pH 7.4. Cells were left either without treatment (lanes 1 and 4) or were stimulated with 10 nM EGF for 3 min (E, lanes 2 and 5) or with 20% FBS (F, lanes 3 and 6) for 10 min. Lysates were immunoblotted for EGFR, GrbB2, Sos, Ras, Raf-1, MEK-1, MAPK, phospho-MAP kinase, HA-Pnck-, and β-actin. EGFR band density was normalized to β-actin and is presented relative to nonstimulated Neo-HEK-293 cells (lane 1). The experiment was repeated three times, and a representative experiment is presented. B: clonal HA-Pnck expression is approximately inversely proportional to EGFR expression. Stable Neo (Cl-4, lane 7) and HA-Pnck HEK-293 clones (Cl-4, lane 8; Cl-1, lane 9; and Cl-6, lane 10) were grown in complete medium and lysed. Equal amounts of total proteins were probed for EGFR (WB: EGFR), HA-Pnck (WB: HA), and β-actin (WB: β-actin) by Western blotting. EGFR band densities were normalized to β-actin and are presented relative to EGFR in Neo-HEK-293 Cl-4 (lane 7).
EGF-induced total tyrosine and Shc phosphorylation and FBS-induced Shc phosphorylation are downregulated in Pnck-expressing cells.
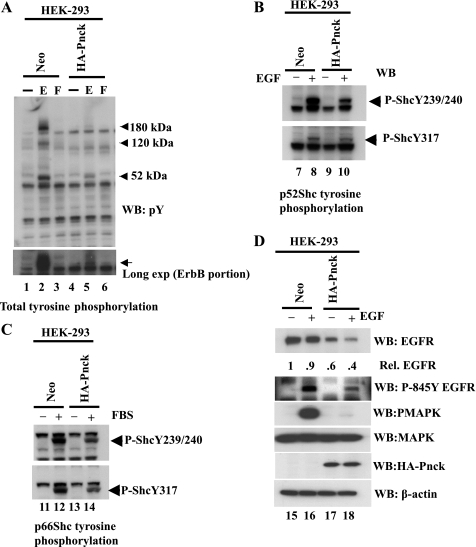
To examine the signaling events upstream of MAP kinase but downstream of EGFR, we measured EGF-and FBS-induced total tyrosine phosphorylation and Shc phosphorylation in HA-Pnck-expressing stable HEK-293 cells. Tyrosine phosphorylation pattern analysis of the lysates revealed that EGF-induced tyrosine phosphorylation of three proteins was strongly inhibited in HA-Pnck-expressing cells, as compared with Neo cells (Fig. 3A, WB: pY, lanes 2 and 5). The approximate molecular mass of these proteins is 180 kDa, 120 kDa, and 52 kDa. Immunoblotting with a tyrosine phosphorylation-specific Shc antibody confirmed that EGF-induced tyrosine phosphorylation (Y239/240) of p52Shc was inhibited in HA-Pnck-expressing HEK-293 cells (Fig. 3B, WB: P-ShcY239/240, lanes 8 and 10). p52ShcY317 tyrosine phosphorylation was also inhibited in HA-Pnck HEK-293 cells, but not as strongly as the Y239/240 site (Fig. 3B, WB: P-ShcY317, lanes 8 and 10). No EGF-induced phosphorylation was observed at these tyrosines on p66Shc or p46Shc proteins (data not shown). In contrast with EGF stimulation, both tyrosines (Y239/240 and Y317) were strongly phosphorylated on p66 Shc protein in FBS-stimulated Neo cells, whereas they were inhibited in HA-Pnck cells (Fig. 3C, WB: P-ShcY239/240, WB: P-Shc Y317, lanes 12 and 14). No FBS-induced tyrosine phosphorylation was observed at these sites on p52 Shc and p46 Shc in either of the cell lines (data not shown). Thus, depending on the ligand, different Shc isoforms that were specifically tyrosine phosphorylated in HEK-293-Neo cells remain unphosphorylated in HA-Pnck HEK-293 cells. To determine how ligand-independent suppression of EGFR influences EGF-dependent EGFR tyrosine phosphorylation, we serum starved and stimulated both Neo and HA-Pnck stable HEK-293 cells (clone 4) with EGF and immunoblotted the lysates with a phosphospecific anti-EGFR (Y845) antibody. As expected, EGF-induced tyrosine phosphorylation at this site was significantly reduced in HA-Pnck-expressing cells (Fig. 3D, WB: P-845 Y EGFR, lanes 16 and 18). Thus, downregulation of EGFR in an EGF-independent manner in HA-Pnck cells reduced total EGFR protein, leading to inhibition of subsequent EGF-induced EGFR tyrosine kinase activity and Shc phosphorylation, which ultimately resulted in MAP kinase inhibition. It is not exactly known how FBS-induced Shc and MAP kinase activations are inhibited in HA-Pnck-expressing cells. Within the detection limits of our assays, we have not observed any tyrosine phosphorylation of ErbB (EGFR or ErbB2) by FBS stimulation in Neo cells or inhibition of ErbB phosphotyrosine in HA-Pnck HEK-293 cells (Fig. 3A, lanes 3 and 6). Furthermore, tyrosine phosphorylation of different Shc isoforms by EGF (p52Shc) and FBS (p66Shc) supports the notion that the FBS-induced Shc-MAP kinase signaling axis does not include activated EGFR as upstream component. However, we do not rule out downregulation of any FBS signaling component(s) such as a different receptor, upstream of Shc and MAPK by HA-Pnck, which may suggest that Pnck also targets signaling components other than EGFR.
Fig. 3.
Inhibition of total tyrosine, EGFR, and Shc tyrosine phosphorylation by Pnck. A: inhibition of EGF-induced tyrosine phosphorylation in HA-Pnck expressing HEK-293 cells (lanes 1–6). Neo (lanes 1–3) and HA-Pnck HEK-293 (lanes 4–6) lysates were immunoblotted with anti-phosphotyrosine MAb (clone 4G-10). EGF-induced tyrosine-phosphorylated proteins at ∼180 kDa, 120 kDa, and 52 kDa that were strongly inhibited in HA-Pnck HEK-293 cells (lanes 2 and 5) are marked with arrowheads. Longer exposure (long exp) of ErbB portion of the blot is presented at bottom. B and C: inhibition of EGF-and FBS-induced Shc tyrosine phosphorylation in HA-Pnck HEK-293 cells. Two dishes of confluent Neo (lanes 7 and 8) and HA-Pnck HEK-293 (lanes 9 and 10) cells were serum starved overnight and stimulated without 10 nM EGF (lanes 7 and 9) or with 10 nM EGF (lanes 8 and 10) for 3 min at room temperature. Equal amounts of total lysates were immunoblotted with anti-phospho-Shc (Tyr239/240) PAb (WB: P-Shc Y239/240), or anti-phospho-Shc (Tyr317) PAb (WB: P-Shc Y317). EGF-induced tyrosine phosphorylation was observed only with the p52 Shc isoform, but not with p66Shc and p46Shc isoforms (data not shown). C: in this experiment, serum-starved, confluent Neo and HA-Pnck HEK-293 cells were stimulated without 20% FBS (lanes 11 and 13) or with 20% FBS (lanes 12 and 14) for 10 min at 37°C. Lysates were immunoblotted for Shc tyrosine phosphorylation (WB: P-Shc Y239/240 and WB: P-Shc Y317) as previously described. FBS-induced tyrosine phosphorylation was observed only in p66 Shc isoform but not in p52Shc and p46Shc isoforms (data not shown). The experiment was repeated three times with essentially identical results. D: inhibition of EGF induced EGFR Y845 phosphorylation by Pnck. Two dishes each of Neo and HA-Pnck HEK-293 (stable clone 4) were serum starved overnight and stimulated with EGF (lanes 16 and 18) or without EGF (lanes 15 and 17). Equal amounts of lysates were probed for EGFR (WB: EGFR), P-845Y EGFR (WB: P-845Y EGFR), phospho-MAPK (WB: PMAPK), MAPK (WB: MAPK), HA-Pnck (WB: HA-Pnck) and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to EGFR in nonstimulated Neo-HEK-293 cells (lane 15).
In vitro phosphorylation of Pnck.
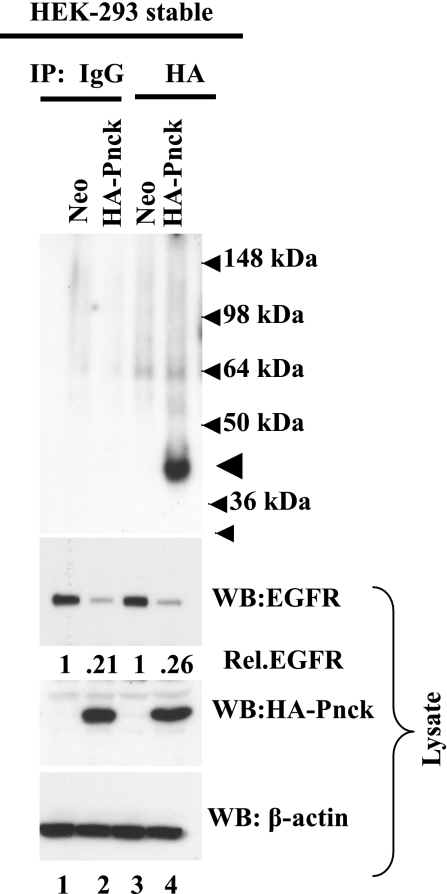
To examine whether the Pnck itself is an active kinase in this setting and undergoes phosphorylation during EGFR downregulation, as might be expected of a serine/threonine and calmodulin kinase, immunokinase assays were performed. HA-Pnck immunoprecipitated from stable HEK-293 cells was phosphorylated in the presence of [32P] γ-ATP, indicating that the Pnck is kinase active in cells undergoing EGFR degradation (Fig. 4, lane 4). As expected, immunoprecipitates from both Neo and HA-Pnck HEK-293 cell lysates with control antibodies (lanes 1 and 2) and Neo cell lysates with anti-HA antibodies (lane 3) did not produce any radioactive bands corresponding to the Pnck position, confirming the identity of the band seen. It is not currently known whether Pnck kinase activity or phosphorylation is required for EGFR downregulation, and if kinase activity is required, how the enzymatic activity of Pnck is regulated or the identity of its key substrates.
Fig. 4.
Immunokinase assay. Lysates of HEK-293 stable Neo (lanes 1 and 3) and HA-Pnck clones (lanes 2 and 4) were immunoprecipitated (IP) with control antibodies (lanes 1 and 2) or anti-HA monoclonal antibodies (lanes 3 and 4). Immunoprecipitates were subjected to immunokinase assay as described in experimental procedures, resolved by SDS-PAGE, transferred to PVDF membrane, and autoradiographed. The phosphorylated HA-Pnck band is indicated by an arrowhead. EGFR band densities (lysate input) are presented relative to that in Neo cells in each set of immunoprecipitations. EGFR band densities were normalized to β-actin and are presented relative to EGFR in Neo-HEK-293 cells in each set of immunoprecipitations (lanes 1 and 3, respectively). The experiment was repeated twice with identical results.
EGFR undergoes protea-lysosomal degradation in Pnck-expressing cells, independent of EGFR tyrosine kinase activity.
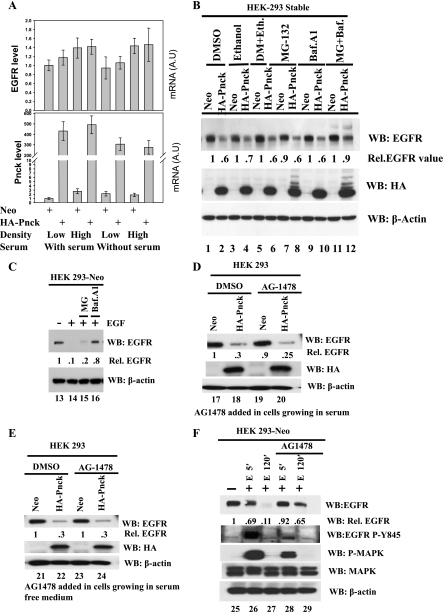
Downregulation of cell surface expression of EGFR has been reported to result from multiple factors, such as high cell density (23, 61), ligand-independent downregulation by viral infection (28), ligand-independent degradation (34, 48), endocytosis, and ligand-dependent ubiquitination and degradation (3, 19, 41, 43). We have already observed that overexpression of HA-Pnck resulted in increased ligand-independent EGFR downregulation, leading to inhibition of EGF-induced MAP kinase activity. To examine whether EGFR protein downregulation resulted from reduced transcription of the EGFR gene, we conducted real-time PCR analysis of the human EGFR and Pnck gene products. We conducted this analysis under several different culture conditions since Pnck mRNA expression has previously been reported to be regulated by serum starvation and cell density (20). Both Neo and HA-Pnck HEK-293 cells were plated at low (25 × 103/cm2) and at high (100 × 103/cm2) density and were allowed to grow for 48 h. A parallel set of Neo and HA-Pnck HEK-293 cells plated at both low and high density were placed in serum-free medium after 24 h of plating and were further allowed to grow for another 24 h. Total RNA extracted from each plate was subjected to real-time quantitative RT-PCR analysis for human EGFR, Pnck, and GAPDH transcripts. As shown in Fig. 5A, the relative EGFR message in HA-Pnck HEK-293 cells was not lower than Neo cells under any of the four different growth conditions tested (low density/with serum, high density/with serum, low density/without serum, and high density/without serum) and were not statistically significantly different from each other (P > 0.2 in each condition). This implies that EGFR was not downregulated in HA-Pnck HEK-293 cells at the transcriptional level. To test whether EGFR is posttranslationally degraded in HA-Pnck HEK-293 cells, we incubated each set of Neo and HA-Pnck HEK-293 cells in the presence of DMSO, ethanol, or DMSO+ethanol (vehicles), the proteasomal inhibitor MG-132, the lysosomal inhibitor bafilomycin A1, or a combination of MG-132 and bafilomycin A1 for 6 h in complete medium. Immunoblotting of lysates revealed that combined treatment (but not individual treatment) of cells with MG-132 and bafilomycin A1 partially restored EGFR protein levels in HA-Pnck HEK-293 cells (Fig. 5B, WB: EGFR, lanes 11 and 12) compared with control (DMSO+ethanol) treatment (Fig. 5B, WB: EGFR, lanes 5 and 6). This suggests that EGFR undergoes protea-lysosomal degradation in HA-Pnck HEK-293 cells. Interestingly, the HA-Pnck protein exhibits a laddering pattern in MG-13-treated cells, suggesting the accumulation of ubiquitinated forms of HA-Pnck protein and implying that HA-Pnck itself undergoes proteasomal degradation (Fig. 5B, WB: HA, lanes 8 and 12). In contrast with the ligand-independent EGFR degradation, EGF-induced EGFR degradation was significantly restored by pretreatment of Neo cells with bafilomycin A1 (Fig. 5C, WB: EGFR, lanes 16) but not significantly by MG-132 (lane 15), indicating that EGFR undergoes predominantly lysosomal degradation in an EGF-dependent manner.
Fig. 5.
Ligand-independent EGFR downregulation by Pnck occurs by protea-lysosomal degradation, independent of EGFR tyrosine kinase activity. A: ligand-independent EGFR downregulation does not occur by transcriptional downregulation of the EGFR gene. Two sets of dishes, each containing a pair of either stable Neo- and HA-Pnck-expressing HEK-293 cells, plated at low density (25 × 103 cells/cm2) or high density (100 × 103 cells/cm2), were allowed to grow for 48 h in complete medium (DMEM containing 10% heat-inactivated FBS). Another two identical sets of dishes were allowed to grow for 24 h in complete medium and were then switched to serum-free medium (DMEM containing 10 mM HEPES, pH 7.4) for another 24 h. Total RNA was extracted from each dish, and relative levels of endogenous EGFR and Pnck (endogenous plus HA-Pnck) transcripts were determined by quantitative real-time RT-PCR. Values are means ± SD of RNA levels in each sample. A representative experiment of two experiments in which each point was assayed in triplicate is presented here. EGFR mRNA levels were not statistically significantly different as determined by two-tailed paired Student's t-test (P > 0.05). B: ligand-independent EGFR downregulation occurs by protea-lysosomal degradation of EGFR protein. Neo and HA-Pnck HEK-293 stable cells were incubated in serum with DMSO (lanes 1 and 2), ethanol (lanes 3 and 4), DMSO+ethanol (lanes 5 and 6), 10 μM MG-132 (MG; lanes 7 and 8), 300 nM bafilomycin A1 (Baf.A1; lanes 9 and 10), or a combination of 10 μM MG-132 and 300 nM bafilomycin A1 (lanes 11 and 12) for 6 h. After 6 h, lysates were prepared from the cells, normalized for total protein concentration, resolved by SDS-PAGE, and transferred to PVDF membranes. Membranes were probed for EGFR (WB: EGFR), HA-Pnck (WB: HA), and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to levels in Neo cells in each treatment group. A representative example of three experiments is presented. C: lysosomal degradation of EGFR in an EGF-induced manner. HEK-293 Neo cells were serum starved either without any drug treatment (lanes 13 and 14) or in the presence of 10 μM MG-132 (lane 15) or 300 nM bafilomycin A1 (lane 16) for 3 h. Cells were stimulated without 10 nM EGF (lane 13) or with 10 nM EGF (lanes 14–16) for another 2 h in the same serum-free medium with or without the indicated drugs. Lysates were immunoblotted for EGFR (WB: EGFR) and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to EGFR in non-EGF-stimulated, non-drug-pretreated Neo-HEK-293 cells (lane 13). D and E: EGF-independent EGFR degradation by Pnck is EGFR tyrosine kinase independent. D: two pairs of dishes, each containing HEK-293 Neo (lanes 17 and 19) or HA-Pnck (lanes 18 and 20) stable cells were incubated in the presence of either DMSO (lanes 17 and 18) or 1 μM AG-1478 (lanes 19 and 20) in serum for 1 h. Cells were lysed, and the lysates were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA), and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to EGFR in DMSO-pretreated Neo-HEK-293 cells (lane 17). E: this experiment is identical to that presented in D except that each set of HEK-293 Neo and HA-Pnck stable cells was incubated in the presence of either DMSO (lanes 21 and 22) or 1 μM AG-1478 (lanes 23 and 24) in serum-free medium for 1 h. Cells were lysed, and the lysates were immunoblotted for EGFR (WB: EGFR), HA-Pnck (WB: HA), and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to EGFR in DMSO-pretreated Neo-HEK-293 cells (lane 21). The experiments in D and E were repeated four times with consistent results, and a representative example is presented. F: EGF-dependent EGFR degradation requires EGFR tyrosine kinase activity. HEK-293 Neo stable cells were serum starved overnight and were either left untreated (lanes 25–27) or were treated with 1 μM AG-1478 (lanes 28 and 29) for 1 h. Following drug treatment, plates were either left unstimulated (lane 25) or were treated with 10 nM EGF for either 5 min (lanes 26 and 28) or 2 h (lanes 27 and 29). Cells lysates were immunoblotted for EGFR (WB: EGFR), phospho-EGFR (Y845) (WB: EGFR P-Y845), phospho-MAPK (WB: P-MAPK), MAPK (WB: MAPK), and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are expressed relative to levels EGFR in non-EGF-stimulated, non-drug-pretreated Neo-HEK-293 cells (lane 25).
To further validate our hypothesis that ligand-independent EGFR degradation by Pnck is EGFR tyrosine kinase independent, we treated both Neo and HA-Pnck HEK-293 cells with the specific EGFR tyrosine kinase inhibitor, AG-1478 (12, 13), under both serum-free and serum-supplemented conditions. Pnck mRNA was previously shown to be upregulated under serum-free conditions (20), and this stress condition could be a physiological regulator of Pnck function. Pretreatment with AG-1478 failed to inhibit EGFR degradation in HA-Pnck HEK-293 cells in any of the above mentioned conditions (Fig. 5D, lanes 17–20; Fig. 5E, lanes 21–24), indicating that EGFR tyrosine kinase activity is not required for EGF-independent EGFR degradation in HA-Pnck-expressing cells. Stimulation with EGF led to the partial degradation of EGFR at 5 min and completely degraded EGFR at 2 h in Neo cells (Fig. 5F, lanes 25–27). In contrast with Pnck-mediated ligand-independent EGFR degradation (Fig. 5, D and E), the EGF-induced EGFR degradation in Neo cells was significantly inhibited by pretreatment with AG-1478, confirming the specific requirement of EGFR tyrosine kinase activity for EGF-induced EGFR degradation (Fig. 5F, lanes 28 and 29). As previously mentioned, EGFR tyrosine phosphorylation at Y845 site, which is an indication of EGFR tyrosine kinase activity (WB: EGFR P-Y845, lane 29) was completely inhibited, confirming the specific activity of AG-1478.
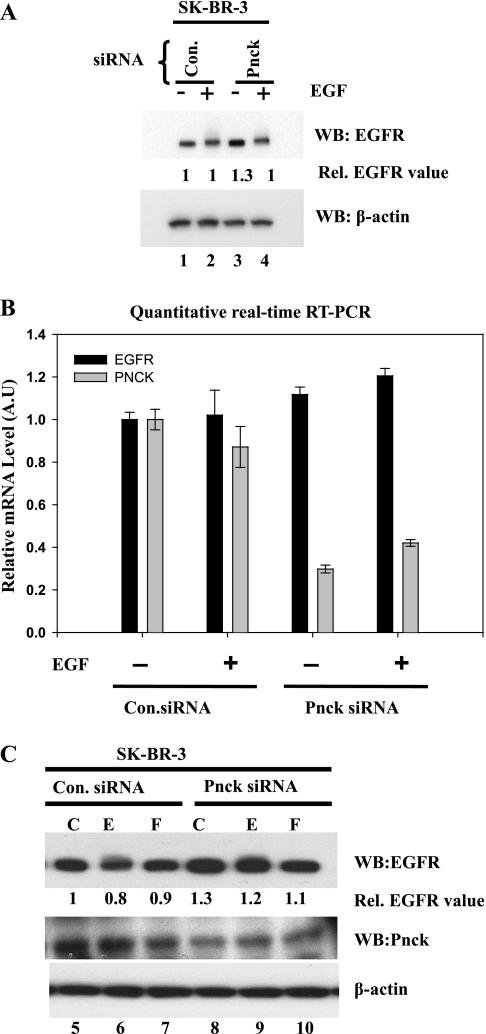
Knockdown of endogenous human Pnck message by siRNA upregulates unliganded EGFR protein in SK-BR-3 breast cancer cells.
Since stable overexpression of HA-Pnck protein in HEK-293 cells efficiently degraded endogenous EGFR, we reasoned that inhibition of endogenous Pnck expression should upregulate or stabilize EGFR in cells endogenously expressing Pnck. We chose to downregulate the Pnck message by transfection of a siRNA oligo directed against human Pnck to test this hypothesis. Human SK-BR-3 human breast cancer cells were selected for siRNA transfection on the basis of a previous report of detectable expression of Pnck mRNA in these cells (20). Cells were grown to confluence and serum starved following transfection with Pnck or control siRNA. Knockdown of Pnck, in the absence of EGF stimulation, resulted in elevated levels of EGFR compared with the level in cells transfected with the control siRNA (Fig. 6A, WB: EGFR, lane 3). This elevated EGFR level was suppressed by subsequent EGF-induced degradation (Fig. 6A, WB: EGFR, lane 4). Measurement of EGFR and Pnck mRNA levels by real-time PCR confirmed that the Pnck transcript level was approximately 60–70% lower in the Pnck siRNA-transfected cells, compared with control siRNA-transfected cells (Fig. 6B). Changes in EGFR transcript levels were found to be very modest, implying that upregulation of unliganded EGFR protein was not the result of altered EGFR transcription. In a complementary experiment, we immunoblotted the lysates with anti-Pnck antibodies, which also revealed significant Pnck protein downregulation by Pnck siRNA (Fig. 6C, WB: Pnck) with concomitant ligand-independent EGFR upregulation (Fig. 6C, WB: EGFR, lanes 5 and 8). These data complement the overexpression studies and provide further evidence that Pnck regulates EGFR protein levels by a nontranscriptional mechanism and probably by inducing a protea-lysosomal degradation machinery.
Fig. 6.
Upregulation of unliganded EGFR by small interfering (si)RNA-mediated knockdown of endogenous human Pnck in SK-BR-3 cells. A: upregulation of endogenous EGFR by Pnck siRNA. Subconfluent SK-BR-3 breast cancer cells were transfected with either control siRNA (luciferase) (lanes 1 and 2) or with siRNA directed against human Pnck (lanes 3 and 4). Cells were allowed to grow for the next 48 h following transfection and were then serum starved for another 24 h. Cells were stimulated with or without 10 nM EGF for 5 min and lysed. The lysates were normalized for total protein, and equal amounts of total protein were resolved by SDS-PAGE, transferred to PVDF membrane, and immunoblotted for EGFR (WB: EGFR), and β-actin (WB: β-actin). EGFR band densities were normalized to β-actin and are presented relative to EGFR in non-EGF-stimulated, control siRNA-transfected cells (lane 1). B: real-time quantitative RT-PCR for endogenous EGFR and Pnck in SK-BR-3 cells. Total RNA was extracted from an identical set of plates (described in A), and the relative levels of endogenous EGFR and endogenous Pnck were determined by quantitative RT-PCR. Samples were analyzed from each RNA sample, and values are means ± SD [in arbitrary units (AU)] of each sample. A representative example of two experiments conducted in triplicate is presented here. Statistical analysis was by the two-tailed paired Student's t-test as described in results. C: SK-BR-3 cells were transfected with either control siRNA (lanes 5–7) or Pnck siRNA (lanes 8–10) as described in A. Cells were serum starved and left nonstimulated (lanes 5 and 8) or were stimulated with either 10 nM EGF for 5 min (lanes 6 and 9) or 20% FBS for 10 min (lanes 7 and 10). Equal amounts of lysates were immunoblotted for EGFR (WB: EGFR), endogenous Pnck (WB: Pnck), or β-actin (WB: β-actin). EGFR band density was normalized to β-actin and is presented relative to EGFR in control siRNA-transfected nonstimulated cells (lane 5).
DISCUSSION
Overexpression of both WT and NH2-terminally HA-tagged human Pnck cDNAs in HEK-293 and HEK-293T cells resulted in inhibition of EGF-induced MAP kinase activation. Dissection of the MAP kinase signaling cascade revealed that ligand-independent downregulation of endogenous EGFR (P < 0.02) followed by inhibition of Shc tyrosine phosphorylation is primarily responsible for this observed inhibition in EGF-induced MAP kinase activity in HA-Pnck-expressing cells. However, Pnck's effect is not specific to EGFR, and unidentified other Pnck targets probably remain, since FBS-induced Shc and MAP kinase activation was also downregulated, probably through effects on cellular component(s) other than EGFR. Ligand-independent downregulation of EGFR resulted from protea-lysosomal degradation of EGFR protein and not from downregulation of EGFR mRNA levels. Suppression of endogenous Pnck levels by siRNA in a human breast cancer cell line resulted in upregulation of unliganded EGFR, corroborating the effect seen on EGFR in the overexpression model. Thus, Pnck may act as an in-built regulator of EGFR expression in EGFR-expressing cells. Pnck, a recently discovered unique calmodulin kinase I isoform, was previously described as being upregulated in normal mammary gland during late pregnancy (20). This temporal expression was shown to be associated with decreased proliferation and terminal differentiation of mammary epithelial cells. At present, the mechanism(s) responsible for the decreased proliferation of Pnck-expressing mouse mammary glands during late pregnancy is not known, but it is possible that MAP kinase inhibition caused by Pnck-mediated EGFR degradation is a contributory mechanism. Pnck mRNA was also shown to be upregulated in serum-starved and confluent cells and downregulated by serum addition. In addition to Pnck's expression in normal mammary gland, which suggests an antiproliferative role, Pnck mRNA was also shown to be three- to fivefold upregulated in primary human breast cancer compared with benign tissues (20).
In our study, ligand-independent EGFR downregulation resulted from protea-lysosomal degradation of EGFR protein, but not from transcriptional downregulation of the EGFR gene. HA-Pnck was observed to be undergoing proteasomal but not lysosomal degradation, which was inhibited by MG-132 and not by bafilomycin A1. Previous reports indicated downregulation of EGFR cell surface expression in association with increasing cell density (61), which was attributed to a decrease in EGFR mRNA (23). A significant, negative correlation between EGFR surface expression and cell density was also observed in normal cervical epithelium and endometrial cancer (50). Since cell density and the absence of serum are two governing factors in endogenous Pnck upregulation, we conducted real-time PCR analysis for human EGFR gene in Neo and HA-Pnck HEK-293 cells, grown in low or high density and in the presence or absence of serum. Regardless of the growth conditions mentioned above, we did not observe any transcriptional downregulation of the EGFR gene in HA-Pnck HEK-293 cells, which prompted us to hypothesize that the EGFR protein downregulation in HA-Pnck-expressing cells is posttranscriptional. Combined treatment with MG-132 and bafilomycin A1 relieved EGFR downregulation in HA-Pnck HEK-293 cells, which implies that EGFR undergoes a combination of two pathways, i.e., protea-lysosomal degradation in HA-Pnck-expressing HEK-293 cells. Previous reports described a requirement for both EGFR tyrosine kinase activity, lysosome and proteasome activity for ligand-induced EGFR degradation (1, 38). In HEK-293-Neo cells, EGF-induced EGFR degradation was blocked by the lysosomal inhibitor bafilomycin A1, but not significantly by MG-132, indicating that EGF-induced EGFR degradation is predominantly lysosomal in HEK-293 cells. AG-1478, a specific EGFR tyrosine kinase inhibitor, also blocked a significant amount of EGF-degraded EGFR in Neo cells. In contrast, EGF-independent EGFR degradation in HA-Pnck HEK-293 was dependent on both lysosome and proteasome activity but not on EGFR tyrosine kinase activity, implying a mechanistic difference between EGF-dependent and -independent (mediated by Pnck) EGFR degradation in HEK-293 cells.
Inhibition of EGFR tyrosine kinase activity due to ligand-independent EGFR degradation resulted in inhibition of subsequent EGF-induced tyrosine phosphorylation in HA-Pnck-expressing HEK-293 cells. Three proteins with approximate molecular masses of 180 kDa, 120 kDa, and 52 kDa were strongly affected. The identity of the 120-kDa protein is not known but could be c-Cbl protein, which is known to be tyrosine phosphorylated in an EGF-dependent manner. The 180-kDa tyrosine phosphorylated protein(s) is likely to be predominantly EGFR, since HEK-293 cells do not express detectable levels of ErbB3 or ErbB4, as determined by immunoblotting and heregulin-induced cell signaling (data not shown). Although HEK-293 cells express ErbB2, no constitutive or EGF-induced EGFR-ErbB2 heterodimer was detected in either Neo or HA-Pnck HEK-293 cells (data not shown). P52 kDa protein is likely to be p52Shc, EGF-induced tyrosine phosphorylation of which was shown to be inhibited by Pnck. Sodium orthovanadate pretreatment of HA-Pnck-expressing cells was not able to restore tyrosine phosphorylation of 180-kDa protein (data not shown), implying that downregulation of EGFR tyrosine kinase activity (but not upregulation of any tyrosine phosphatase) resulting from EGFR protein downregulation occurred in HA-Pnck-expressing cells. This could explain the observed inhibition of EGF-induced MAP kinase activation downstream of EGFR.
The mechanism of ligand-dependent EGFR degradation has been studied in detail and is largely mediated by the c-Cbl proto-oncogene. Upon EGF or other EGFR ligand stimulation and consequent EGFR tyrosine kinase activation, c-Cbl is tyrosine phosphorylated and associates with activated EGFR (3, 19, 43). The c-Cbl-EGFR interaction may be direct, between the c-Cbl phosphotyrosine binding and EGFR tyrosine phosphorylated at 1045 (Y1045) (37). Alternatively, c-Cbl in complex with GrbB2 might be recruited to the GrbB2-specific docking site of EGFR (68). Either way, the E3 ubiquitin ligase activity of the c-Cbl ring finger domain is activated, which results in EGFR ubiquitination and subsequent proteasomal and lysosomal degradation (38). In contrast, very little is known about ligand-independent EGFR downregulation and degradation. EGFR was shown to be downregulated in human epithelial carcinoma-derived A431 cells, during adenovirus infection, by an EGF-independent mechanism (5, 28), and the E3 transcription unit of adenovirus was identified as sufficient for this effect (27). Upon expression of the Drosophila homologue of human suppressors of cytokine signaling-5 (SOCS-5) (called SOCS36E) in transgenic flies, an EGFR signaling defect, due to genetic interaction between SOCS36E and Drosophila-EGF-R, was observed (4). Subsequent studies confirmed a role of SOCS associated E3 ubiquitin ligase activation in EGFR degradation (34, 48).
Calmodulin kinases mediate numerous biological functions by catalyzing different cell signaling mechanisms. Calmodulin kinase II was previously reported to downregulate EGFR tyrosine kinase activity by phosphorylating EGFR at COOH-terminal sites (16). MAP kinase was shown to be activated by calmodulin kinase I in depolarization-induced neuroblastoma cells (55). In most cases, the observed effect of calmodulin kinase at the protein activation/inactivation level involves posttranslational modification (phosphorylation) and is not due to alteration in protein levels, as observed in the case of Pnck. Although studies of the role of calmodulin kinase in maintaining protein stability have not been addressed in detail, a recent study demonstrated oxidative stress-induced proteasomal degradation of cyclin D1 by calmodulin kinase II (15). In this context, it remains to be determined whether the EGFR-degrading ability is restricted to Pnck alone or is a more general function of other calmodulin kinase I isoforms (α, β1, or γ) or other members of calmodulin kinase family. Three laboratories have recently reported stress-activated p38 MAP kinase activation as a central event in both EGF-dependent EGFR degradation as well as ligand-independent EGFR internalization and subsequent degradation (17, 67, 72). It is not known whether Pnck is linked to the p38 MAP kinase signaling axis. Another mechanistic possibility is that Pnck regulates the chaperoning ability of heat shock protein 90 (HSP90) or ATPase-independent chaperone HSP27, thus dissociating EGFR from chaperones, leading to protea-lysosomal degradation. Pharmacologic HSP90 inhibitor geldanamycin has been previously used to study ligand-independent EGFR degradation (62). HSP90 is known to be inhibited by several posttranslational modifications, including phosphorylation (36, 44), acetylation (35, 56), and S-nitrosylation (42). Pnck being a serine/threonine and calmodulin kinase could thus modulate HSP90 or HSP27 or other co-chaperone(s) by direct phosphorylation or indirectly through the above mentioned posttranslational modification by regulating intermediates. The current focus of the laboratory is to understand this and other possible mechanisms.
In conclusion, our data present, for the first time, a functional characterization of a novel protein, termed Pnck. The present study demonstrates Pnck to be a regulator of the poorly understood ligand-independent EGFR degradation pathway. Pnck is differentially expressed in primary human breast cancer compared with normal breast epithelium. A protein with differential expression and/or activation in cancer cells with a prominent role in pro- or anti-oncogenesis is potentially an excellent target from a therapeutic standpoint. Overexpression and activation of EGFR are considered as causal events in a variety of human cancers, including glioblastoma, non-small cell lung carcinoma, and renal cancer. Thus, exploring the combined-expression profile of EGFR and Pnck in different human cancers and examining whether Pnck functions as an EGFR downregulator in cancer cells could be significant. In addition, the detailed biochemical mechanism of Pnck-mediated EGFR degradation warrants further study.
GRANTS
This work was supported in part by an award from the Susan G. Komen for the Cure Foundation (to T. B. Deb; BCTR0707114) and by National Institutes of Health (NIH) Grant 2RO1-AG-014963 (to M. D. Johnson) and Department of Defense Grant DAMD 17-01-1-0251 (to R. B. Dickson). T. B. Deb was also partly supported by an NIH Specialized Program of Research Excellence (SPORE) grant in breast cancer of the Lombardi Comprehensive Cancer Center (2P5OCA58185). C. M. Coticchia was supported by a predoctoral training grant from the Department of Defense (DAMD 17-01-1-0246). Assistance from Lombardi Comprehensive Cancer Center core facilities for DNA sequencing is gratefully acknowledged (NIH 4P30CA51008).
Acknowledgments
This work is dedicated to the memory of Prof. Robert B. Dickson, who passed away suddenly during the final stages of the preparation of this article.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem 278: 35781–35790, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J, Mendelsohn J. The epidermal growth factor receptor as a target for therapy in breast carcinoma. Breast Cancer Res Treat 29: 127–138, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Bowtell DD, Langdon WY. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene 11: 1561–1567, 1995. [PubMed] [Google Scholar]
- 4.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene 21: 4812–4821, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Carlin CR, Tollefson AE, Brady HA, Hoffman BL, Wold WS. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell 57: 135–144, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Cheung WY Calmodulin plays a pivotal role in cellular regulation. Science 207: 19–27, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10: 322–328, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem 279: 38903–38911, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Deb TB, Wong L, Salomon DS, Zhou G, Dixon JE, Gutkind JS, Thompson SA, Johnson GR. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J Biol Chem 273: 16643–16646, 1998. [DOI] [PubMed] [Google Scholar]
- 10.DeRemer MF, Saeli RJ, Brautigan DL, Edelman AM. Ca2+-calmodulin-dependent protein kinases Ia and Ib from rat brain. II. Enzymatic characteristics and regulation of activities by phosphorylation and dephosphorylation. J Biol Chem 267: 13466–13471, 1992. [PubMed] [Google Scholar]
- 11.DeRemer MF, Saeli RJ, Edelman AM. Ca2+-calmodulin-dependent protein kinases Ia and Ib from rat brain I. Identification, purification, and structural comparisons. J Biol Chem 267: 13460–13465, 1992. [PubMed] [Google Scholar]
- 12.Ellis AG, Doherty MM, Walker F, Weinstock J, Nerrie M, Vitali A, Murphy R, Johns TG, Scott AM, Levitzki A, McLachlan G, Webster LK, Burgess AW, Nice EC. Preclinical analysis of the analinoquinazoline AG1478, a specific small molecule inhibitor of EGF receptor tyrosine kinase. Biochem Pharmacol 71: 1422–1434, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Ellis AG, Nice EC, Weinstock J, Levitzki A, Burgess AW, Webster LK. High-performance liquid chromatographic analysis of the tyrphostin AG1478, a specific inhibitor of the epidermal growth factor receptor tyrosine kinase, in mouse plasma. J Chromatogr B Biomed Sci Appl 754: 193–199, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Ettenberg SA, Magnifico A, Cuello M, Nau MM, Rubinstein YR, Yarden Y, Weissman AM, Lipkowitz S. Cbl-b-dependent coordinated degradation of the epidermal growth factor receptor signaling complex. J Biol Chem 276: 27677–27684, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Fasanaro P, Magenta A, Zaccagnini G, Cicchillitti L, Fucile S, Eusebi F, Biglioli P, Capogrossi MC, Martelli F. Cyclin D1 degradation enhances endothelial cell survival upon oxidative stress. FASEB J 2006. [DOI] [PubMed]
- 16.Feinmesser RL, Wicks SJ, Taverner CJ, Chantry A. Ca2+/calmodulin-dependent kinase II phosphorylates the epidermal growth factor receptor on multiple sites in the cytoplasmic tail and serine 744 within the kinase domain to regulate signal generation. J Biol Chem 274: 16168–16173, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25: 5683–5692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fry DW, Kraker AJ, McMichael A, Ambroso LA, Nelson JM, Leopold WR, Connors RW, Bridges AJ. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 265: 1093–1095, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Galisteo ML, Dikic I, Batzer AG, Langdon WY, Schlessinger J. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem 270: 20242–20245, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Gardner HP, Ha SI, Reynolds C, Chodosh LA. The CaM kinase, Pnck, is spatially and temporally regulated during murine mammary gland development and may identify an epithelial cell subtype involved in breast cancer. Cancer Res 60: 5571–5577, 2000. [PubMed] [Google Scholar]
- 21.Gardner HP, Rajan JV, Ha SI, Copeland NG, Gilbert DJ, Jenkins NA, Marquis ST, Chodosh LA. Cloning, characterization, and chromosomal localization of Pnck, a Ca2+/calmodulin-dependent protein kinase. Genomics 63: 279–288, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res 300: 388–395, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hamburger AW, Mehta D, Pinnamaneni G, Chen LC, Reid Y. Density-dependent regulation of epidermal growth factor receptor expression. Pathobiology 59: 329–334, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 100: 57–70, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Hanson PI, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinases. Annu Rev Biochem 61: 559–601, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Haribabu B, Hook SS, Selbert MA, Goldstein EG, Tomhave ED, Edelman AM, Snyderman R, Means AR. Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. EMBO J 14: 3679–3686, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman BL, Ullrich A, Wold WS, Carlin CR. Retrovirus-mediated transfer of an adenovirus gene encoding an integral membrane protein is sufficient to down regulate the receptor for epidermal growth factor. Mol Cell Biol 10: 5521–5524, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman P, Rajakumar P, Hoffman B, Heuertz R, Wold WS, Carlin CR. Evidence for intracellular down-regulation of the epidermal growth factor (EGF) receptor during adenovirus infection by an EGF-independent mechanism. J Virol 66: 197–203, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hook SS, Means AR. Ca2+/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41: 471–505, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Inoue S, Mizutani A, Sugita R, Sugita K, Hidaka H. Purification and characterization of a novel protein activator of Ca2+/calmodulin-dependent protein kinase I. Biochem Biophys Res Commun 215: 861–867, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol Cell Biol 26: 389–401, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jusuf AA, Rina S, Sakagami H, Terashima T. Expression of Ca2+/calmodulin-dependent protein kinase (CaMK) Ibeta2 in developing rat CNS. Neuroscience 109: 407–420, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev 24: 719–736, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Kario E, Marmor MD, Adamsky K, Citri A, Amit I, Amariglio N, Rechavi G, Yarden Y. Suppressors of cytokine signaling 4 and 5 regulate epidermal growth factor receptor signaling. J Biol Chem 280: 7038–7048, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18: 601–607, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Lees-Miller SP, Anderson CW. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem 264: 17275–17280, 1989. [PubMed] [Google Scholar]
- 37.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell 4: 1029–1040, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev 12: 3663–3674, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loseth OP, de Lecea L, Calbet M, Danielson PE, Gautvik V, Hovring PI, Walaas SI, Gautvik KM. Developmental regulation of two isoforms of Ca2+/calmodulin-dependent protein kinase I beta in rat brain. Brain Res 869: 137–145, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 58: 903–913, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene 23: 2057–2070, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Ruiz A, Villanueva L, González de Orduña C, Lopez-Ferrer D, Higueras MA, Tarin C, Rodriguez-Crespo I, Vazquez J, Lamas S. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA 102: 8525–8530, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meisner H, Czech MP. Coupling of the proto-oncogene product c-Cbl to the epidermal growth factor receptor. J Biol Chem 270: 25332–25335, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Mimnaugh EG, Worland PJ, Whitesell L, Neckers LM. Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase. J Biol Chem 270: 28654–28659, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Nairn AC, Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J Biol Chem 262: 7273–7281, 1987. [PubMed] [Google Scholar]
- 46.Nairn AC, Picciotto MR. Calcium/calmodulin-dependent protein kinases. Semin Cancer Biol 5: 295–303, 1994. [PubMed] [Google Scholar]
- 47.Naito Y, Watanabe Y, Yokokura H, Sugita R, Nishio M, Hidaka H. Isoform-specific activation and structural diversity of calmodulin kinase I. J Biol Chem 272: 32704–32708, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Nicholson SE, Metcalf D, Sprigg NS, Columbus R, Walker F, Silva A, Cary D, Willson TA, Zhang JG, Hilton DJ, Alexander WS, Nicola NA. Suppressor of cytokine signaling (SOCS)-5 is a potential negative regulator of epidermal growth factor signaling. Proc Natl Acad Sci USA 102: 2328–2333, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 91: 7727–7731, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiffer D, Kimmig R, Herrmann J, Ruge M, Fisseler-Eckhoff A, Scheidel P, Jensen A, Schatz H, Pfeiffer A. Epidermal-growth-factor receptor correlates negatively with cell density in cervical squamous epithelium and is down-regulated in cancers of the human uterus. Int J Cancer 79: 49–55, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Picciotto MR, Cohn JA, Bertuzzi G, Greengard P, Nairn AC. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 267: 12742–12752, 1992. [PubMed] [Google Scholar]
- 52.Picciotto MR, Czernik AJ, Nairn AC. Calcium/calmodulin-dependent protein kinase I. cDNA cloning and identification of autophosphorylation site. J Biol Chem 268: 26512–26521, 1993. [PubMed] [Google Scholar]
- 53.Prewett M, Rockwell P, Rockwell RF, Giorgio NA, Mendelsohn J, Scher HI, Goldstein NI. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol 19: 419–427, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Rina S, Jusuf AA, Sakagami H, Kikkawa S, Kondo H, Minami Y, Terashima T. Distribution of Ca2+/calmodulin-dependent protein kinase I beta 2 in the central nervous system of the rat. Brain Res 911: 1–11, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Schmitt JM, Wayman GA, Nozaki N, Soderling TR. Calcium activation of ERK mediated by calmodulin kinase I. J Biol Chem 279: 24064–24072, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of hsp90 regulates chaperone function. Mol Cell 25: 151–159, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430, 1991. [DOI] [PubMed] [Google Scholar]
- 58.Soderling TR CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol 10: 375–380, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol Biol Cell 15: 3591–3604, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stern DF Tyrosine kinase signalling in breast cancer: ErbB family receptor tyrosine kinases. Breast Cancer Res 2: 176–183, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suarez-Quian CA, Byers SW. Redistribution of epidermal growth factor receptor as a function of cell density, cell-cell adhesion and calcium in human (A-431) cells. Tissue Cell 25: 1–17, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Supino-Rosin L, Yoshimura A, Yarden Y, Elazar Z, Neumann D. Intracellular retention and degradation of the epidermal growth factor receptor, two distinct processes mediated by benzoquinone ansamycins. J Biol Chem 275: 21850–21855, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Tokumitsu H, Hatano N, Inuzuka H, Sueyoshi Y, Yokokura S, Ichimura T, Nozaki N, Kobayashi R. Phosphorylation of Numb family proteins. Possible involvement of Ca2+/calmodulin-dependent protein kinases. J Biol Chem 280: 35108–35118, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Tokumitsu H, Soderling TR. Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J Biol Chem 271: 5617–5622, 1996. [DOI] [PubMed] [Google Scholar]
- 65.Tokumitsu H, Wayman GA, Muramatsu M, Soderling TR. Calcium/calmodulin-dependent protein kinase kinase: identification of regulatory domains. Biochemistry 36: 12823–12827, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Ueda T, Sakagami H, Abe K, Oishi I, Maruo A, Kondo H, Terashima T, Ichihashi M, Yamamura H, Minami Y. Distribution and intracellular localization of a mouse homologue of Ca2+/calmodulin-dependent protein kinase Ibeta2 in the nervous system. J Neurochem 73: 2119–2129, 1999. [PubMed] [Google Scholar]
- 67.Vergarajauregui S, San Miguel A, Puertollano R. Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7: 686–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J 21: 303–313, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396: 584–587, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Yokokura H, Terada O, Naito Y, Hidaka H. Isolation and comparison of rat cDNAs encoding Ca2+/calmodulin-dependent protein kinase I isoforms. Biochim Biophys Acta 1338: 8–12, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J 25: 4195–4206, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]