Abstract
We recently reported that megalin is subjected to regulated intramembrane proteolysis (RIP) and includes 1) protein kinase C (PKC)-regulated, metalloprotease-mediated ectodomain shedding producing a membrane-bound megalin COOH-terminal fragment (MCTF) and 2) γ-secretase-mediated cleavage of the MCTF producing a soluble megalin intracellular domain (MICD). Based on studies of RIP of other receptors, the MICD is predicted to target to the nucleus and regulate gene expression. To determine whether RIP of megalin regulates proximal tubule gene expression, we stably expressed the transfected MCTF (tMCTF) or transfected MICD (tMICD) in opossum kidney proximal tubule (OKP) cells and examined the resulting phenotype. Immunoblotting and immunocytochemical analysis of tMCTF cells showed the tMCTF was expressed and constitutively processed by γ-secretase. Analysis of specific protein expression in tMCTF- and tMICD-transfected cells using Western blot showed endogenous megalin and Na+/H+ exchanger 3 (NHE3) protein expression to be dramatically lower than that of control cells. Expression of other proteins including myosin VI, β-adaptin, and the Na-K-ATPase appeared unchanged. Analysis of specific mRNA expression using quantitative real-time PCR showed megalin and NHE3 mRNA levels were significantly lower in tMCTF- and tMICD-transfected cells compared with controls. Inhibition of γ-secretase activity in tMCTF cells resulted in an 8- to 10-fold recovery of megalin mRNA within 4 h. These data show that the COOH-terminal domain of megalin regulates expression of specific proteins in OKP cells and provides the first evidence that RIP of megalin may be part of a signaling pathway linking protein absorption and gene expression in proximal tubule.
Keywords: megalin intracellular domain, regulated intramembrane proteolysis, brush border
regulated intramembrane proteolysis (RIP) is an evolutionarily conserved process linking proteolysis of membrane proteins with the control of target gene expression (8). RIP includes protein kinase C (PKC)-regulated, metalloprotease-mediated ectodomain shedding of specific receptors producing a membrane-associated COOH-terminal fragment. The COOH-terminal fragment is in turn released from the membrane by γ-secretase activity acting at a cleavage site within the protein's membrane spanning domain. Once released, the COOH-terminal domain traffics to the nucleus where, through interaction with transcription factors, it controls expression of target genes. RIP was first discovered by Brown and Goldstein (3) during studies of the sterol regulatory element binding protein (SREBP) and was shown to regulate specific genes involved in both fatty acid and cholesterol metabolism. RIP is now known to process numerous receptors in many cell types where it seems to regulate a variety of cellular events (4). Among the more well-studied receptors subjected to RIP are members of the Notch and amyloid precursor protein (APP) families. Notch, a 300-kDa single membrane pass receptor, was first described in Drosophila melanogaster and shown to be necessary for neural development in that species. It is now clear that Notch is part of a signaling pathway that determines cell fate in many tissues in both vertebrates and invertebrates alike (16). Proteolytic processing of Notch appears to be similar to processing described for the APP. A detailed understanding of the proteolytic processing of APP has become important for understanding Alzheimer's disease. It has become increasingly clear that anomalies in APP processing by α-, β-, and γ-secretases result in the production of pathogenic fragments, known as the amyloid β peptides, that are linked to the onset and progression of Alzheimer's disease (4).
Megalin is a member of the low-density lipoprotein (LDL) receptor gene family and functions as the major scavenger receptor in the proximal tubule. Here, megalin mediates most of the protein absorbed in the kidney and is necessary for key steps in the vitamin D metabolic pathway (21). Based on studies in both kidney and the opossum kidney proximal tubule (OKP) cell line, we recently reported that megalin is subjected to RIP in a manner similar to that described for Notch and APP (29). Our published studies showed 1) high levels of γ-secretase expression in the brush border and endocytic pathway of the proximal tubule where it colocalizes with megalin, 2) megalin is subjected to PKC-regulated, metalloprotease-mediated ectodomain shedding that produces a 35- to 40-kDa megalin COOH-terminal fragment (MCTF), and 3) the MCTF is membrane bound and is constitutively processed by γ-secretase activity. Based on these data and on our knowledge of megalin's function as a scavenger receptor, we postulate that RIP of megalin may function to link protein absorption with gene regulation in the proximal tubule.
To test this model and to gain insight into the functional role of RIP of megalin, we stably expressed plasmids representing either the MCTF or the megalin intracellular domain (MICD), the predicted soluble product of γ-secretase activity, in OKP cells and examined the resulting phenotype. Our data support our model and show that the COOH terminus of megalin produced by RIP regulates specific mRNA and protein expression in cultured proximal tubule cells.
MATERIALS AND METHODS
Materials.
Compound E (γ-secretase inhibitor XVIII, no. 565764) and matrix metalloproteinases (MMP) inhibitor III were from Calbiochem (La Jolla, CA). Lactacystin was from A. G. Scientific (San Diego, CA). TNF-α processing inhibitor (TAPI)-1, TAPI-2, and GM 6001 were from BIOMOL Research Laboratories (Plymouth Meeting, PA). Reversible γ-secretase inhibitor (no. S2188) was from Sigma (St. Louis, MO). DMEM (high glucose), fetal bovine serum, penicillin/streptomycin, l-glutamine, sodium pyruvate, Lipofectamine 2000, hygromycin B, and Prolong Gold antifade reagent, secondary antibodies goat anti-rabbit AlexaFluor 594, goat anti-mouse AlexaFluor 488, and DAPI dilactate were purchased from Invitrogen (Carlsbad, CA). Sterile cloning disks were purchased from Bel-Art Products (Pequannock, NJ).
Primary antibodies.
For megalin, a polyclonal Ab (anti-MC220) and monoclonal Ab (6E10) both raised to the COOH terminus of megalin (29) were used. A polyclonal antibody to myosin VI (11) was provided by Dr. M. Mooseker, Yale University. A mAb to NHE3 (3H3) was used as described previously (13). A mAb to the α-subunit of the Na-K-ATPase (12) was provided by Dr. M. Caplan, Yale University. Rabbit anti-Rab 11 was purchased from Abcam (Cambridge, MA). MAbs to GM130 (Golgi matrix protein of 130 kDa) and β-adaptin were purchased from BD Transduction Laboratories (San Jose, CA).
Cell culture.
OKP cells were grown in media containing DMEM, 10% fetal bovine serum, 1% penicillin-streptomycin, 1% l-glutamine, and 1% sodium pyruvate. Lipofectamine 2000 was used for the transfection of plasmids into OKP cells. The tMICD-, tMCTF-, and empty vector (EV)-transfected OKP cells were cultured in regular OKP media with 0.5 mg/ml hygromycin B.
Construction and expression of plasmids representing MCTF and MICD in OKP cells.
Plasmids representing the MICD and MCTF were constructed based on the partial megalin sequence of Didelphis virginiana published previously (29). Details of each plasmid are shown in Fig. 1. The 684-bp fragment of the opossum megalin gene encoding the entire intracellular domain (hereafter referred to as tMICD) was PCR amplified using forward primer 5′-AGGCCTATGGTGATTGGAGGATTTTTTAAC-3′ and reverse primer 5′-GGATCCTACCTCAGAGTCTTCTTTAACAAG-3′. The 918-bp fragment encoding the entire cytosolic domain, the transmembrane region, and 62 amino acids of the ectodomain (hereafter referred to as tMCTF) was PCR amplified using forward primer 5′-AGGCCTATGGGGGGTTCTCATCATCATCATCATCATGGGAGCAGTACGCTATGTGATG-3′ and reverse primer 5′-GGATCCAGCGTAATCTGGAACATCGTATGGGTATACCTCAGAGTCTTCTTTAAC-3′ (His and HA tags incorporated into sense and antisense primers, respectively, are underlined). The PCR products were ligated into TOPO TA cloning vector (Invitrogen) and digested with StuI and BamHI. The digested PCR products were subcloned into the pIREShyg3 vector (Clonetech Laboratories, Mountain View, CA) and used for both transient and stable expression. The tMCTF plasmid contained the 75-bp signal peptide of rat megalin (22) fused to the NH2 terminus of the expressed protein. The signal peptide of rat megalin was PCR amplified using forward primer 5′-TGTACAATGGAGCGCGGGGCCGCA-3′ and reverse primer 5′-AGGCCTGCTACTGACTGGCTCCAG-3′.
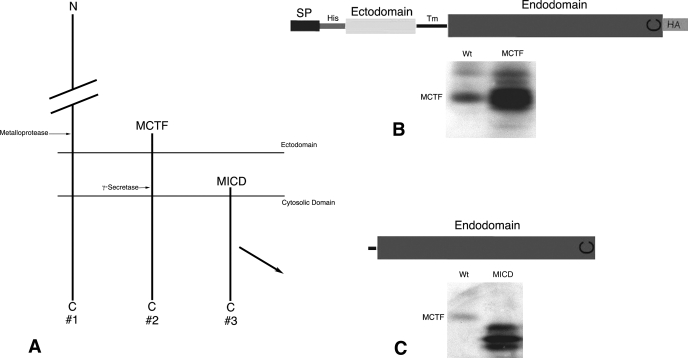
Fig. 1.
Design and transient expression of plasmids representing megalin COOH-terminal domains in opossum kidney proximal tubule (OKP) cells. A: key steps in regulated intramembrane proteolysis (RIP) of megalin. Metalloprotease-mediated ectodomain shedding of megalin (#1) produces a membrane-bound COOH-terminal fragment (MCTF; #2). The MCTF becomes the substrate for γ-secretase activity and the resulting intracellular domain (MICD; #3) is released into the cytosol. B and C: details of the expressed transfected (t)MCTF (B) and tMICD (C) plasmids. Also shown in B and C are Western blots of transiently expressed plasmids compared with the ∼40-kDa endogenous MCTF shown in wild-type (Wt) OKP cells. Blots were probed with anti-MC220 diluted 1:5,000. It should be noted that when we expressed tMCTF, the NH2-terminal His tag was not detectable and the COOH-terminal HA tag was only poorly detectable using both Western blot and immunocytochemical methods. Therefore, we monitored expression and localization of the megalin COOH-terminal proteins with an anti-megalin COOH-terminal antibody (anti-MC220). See discussion for details. SP, signal peptide; Tm, transmembrane.
For transient expression, the OKP cells were plated on day 1 at a density of 0.5–2 × 105 cells in 500 μl of medium without antibiotics in each well of a 24-well plate. This plating density ensured that the cells were 90–95% confluent on day 2. On day 2 Lipofectamine 2000 was used to transfect 0.8 μg/well of plasmid cDNA according to the manufacturer's protocol. Cells were grown for 24–48 h at which point they were assayed for plasmid expression by Western blot.
For stable cell lines, OKP cells were transfected as above. Twenty-four hours after transfection, the cells were split and serial dilutions (1:10, 1:100, 1:1,000, 1:10,000) were prepared in selection media (OKP cell media with 0.5 mg/ml hygromycin B). The cells were grown for 2–3 wk and the selection media changed every 5 days. Individual clones were picked using sterile cloning disks. Multiple clones expressing each plasmid were maintained and the phenotype was determined using biochemical and immunocytochemical methods. The data presented below represent cell lines at passage 10 or less.
Quantitative real-time reverse-transcriptase PCR.
Total RNA was isolated from transfected OKP cells with RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer′s protocol. Total RNA was treated with DNase (Ambion, Austin, TX) to digest and remove DNA. Reverse transcription reactions were performed using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA) according to standard protocols. Quantitative PCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems) and the product was detected using a 7300 Real-Time PCR System (Applied Biosystems). PCR primers were designed using Primer Express 3.0 (Applied Biosystems). Real-time PCR was performed using the following primers: megalin forward primer 5′-TGCCCCACCCGTTATCCTA-3′ and reverse primer 5′-ACAGACATGGTTCTTACACTCAAACAT-3′; NHE3 forward primer 5′-ATCAAACGAAGATGAAAAACAAGACAAAGA-3′ and reverse primer 5′-TGGTTTGGTTTATTCCAAGTTTGGTAGA-3′; cyclophilin forward primer 5′-CGGGTCACTTTCGAGCTCTTT-3′ and reverse primer 5′-CTCAGAGCCCGGAAGTTTTCT-3′. The megalin primers were designed to detect only endogenous megalin. The specificity of each primer pair was confirmed by dissociation curve analysis and agarose gel electrophoresis. The quantity of mRNA was calculated using the ΔΔCt method (15) when PCR efficiency was close to 100%. All PCR reactions were performed in triplicate. The data were analyzed using Sequence Detection Software V.1.3.1 (Applied Biosystems). Results were calculated and normalized relative to an endogenous control gene (cyclophilin) and relative to a calibrator (EV-transfected OKP cells).
Immunocytochemistry.
For immunocytochemistry, transfected OKP cells were grown to confluence on glass coverslips. The cells were fixed in periodate-lysine-paraformaldehyde (PLP) fixative (18) for 30 min at 4°C, washed in PBS (2 × 10 min), and incubated for 15 min at room temperature in blocking buffer (10% goat serum, 0.05% Triton X-100 in PBS, pH 7.4). All antibodies were diluted as described in figure legends in blocking buffer. For staining, cells were incubated in primary antibodies for 1 h, washed 3 × 10 min in PBS, and incubated in secondary antibodies for 1 h. All cells were counterstained with DAPI to visualize nuclei. For double-label experiments, each secondary antibody was tested to ensure that it did not cross-react with the inappropriate primary antibody. Labeled cells were examined and photographed using the Imaging Workstation in the Section of Nephrology at Yale University. The Workstation is equipped with a Nikon TE2000U inverted epifluorescence microscope, Sutter Lambda 10–3 shutter controller, Sutter Lamda LS xenon arc lamp, Roper Scientific CoolSnap HQ CCD camera, and Prior Focus Z-drive system. The entire image analysis workstation is controlled by MetaMorhph/MetaFluor imaging acquisition software (Universal Imaging) complemented by AutoQuant Deconvolution Software. Final digital images were prepared using Photoshop.
SDS-PAGE and immunoblotting.
SDS-PAGE and immunoblotting were performed exactly as described (29) previously using either 7.5 or 3.5–8% gradient polyacrylamide gels.
RESULTS
Design and expression of plasmids representing megalin COOH-terminal domains produced by RIP.
Our hypothesis predicts that RIP of megalin activates cellular signaling through the proteolytic release of COOH-terminal peptides. To test this, we made expression plasmids designed to bypass regulated steps of this process and to mimic, in a constitutive manner, the proteolytic release of megalin's COOH terminus. (Fig. 1A). The regulated step in RIP of megalin and other receptors including Notch and APP is metalloprotease-mediated ectodomain shedding. Ectodomain shedding of megalin produces both soluble extracellular fragments as well as the membrane-bound COOH terminus referred to previously (29) as the MCTF. Although the exact location of the metalloprotease cleavage site in megalin is unknown, we designed plasmids lacking most of megalin's ectodomain. The transfected MCTF (tMCTF) included 62 amino acids of megalin's ectodomain and the entire transmembrane and cytosolic domains (Fig. 1B). γ-Secretase activity constitutively cleaves the MCTF (29) and is predicted to release MICD into the cytosol. The design of the transfected MICD (tMICD) was based on the conserved γ-secretase cleavage site within the membrane spanning domain described for Notch and APP (23) and predicted to be conserved in megalin (22). The tMICD plasmid contains six amino acids of the transmembrane domain and the entire cytosolic endodomain of megalin (Fig. 1C). To assess the size of the expressed tMICD and tMCTF, we transiently expressed each plasmid in OKP cells and compared the size of each with endogenous MCTF (29). As shown in Fig. 1, expressed tMCTF had approximately the same size as endogenous MCTF (35–40 kDa) while the tMICD appeared, as predicted, to be smaller than endogenous MCTF. These data show that the plasmids express COOH-terminal domains of megalin whose sizes are consistent with the MCTF and MICD produced by RIP and which are based on our model shown in Fig. 1A. We therefore developed OKP cell lines stably expressing the tMCTF, tMICD, or the EV as control to look for megalin-dependent changes in the OKP cell phenotype. The data presented below are representative of multiple clones of each cell line.
Characterization of tMICD- and tMCTF-transfected OKP cells.
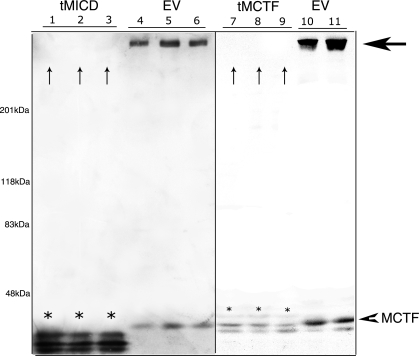
Stable cell lines expressing the tMCTF or tMICD grew normally and had the same epithelial appearance as did wild-type cells and cells expressing the EV (data not shown). To characterize the phenotype of each cell line, we first measured the expressed level of tMCTF and tMICD proteins (Fig. 2). Compared with the tMICD, we found very low levels of tMCTF in all clones examined. Surprisingly, we also noticed that the expression of endogenous megalin was dramatically reduced in all cells expressing either the tMICD or tMCTF.
Fig. 2.
Stable expression of megalin COOH-terminal plasmids in OKP cells. Immunoblots of lysates (10 μg total protein) from individual clones transfected with tMICD (lanes 1–3), tMCTF (lanes 7–9), or empty vector (EV) control (lanes 4–6, 10, and 11). Lysates were separated by SDS-PAGE using 3.5–8% gradient gels and the resulting blots were probed with anti-MC220 (1:5,000). Expressed tMICD and tMCTF are indicated by *. Large horizontal arrow indicates intact endogenous megalin seen in EV control lanes. The small arrowhead denotes the endogenous MCTF. The vertical arrows (lanes 1–3 and 7–9) indicate the lack of staining for endogenous megalin in clones expressing tMICD and tMCTF.
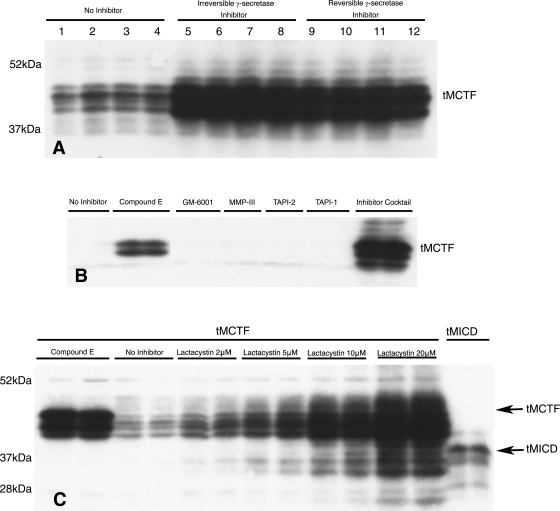
Our experimental model predicts that removal of the megalin ectodomain should allow the tMCTF to be constitutively processed by γ-secretase and thus may explain the low levels of tMCTF seen in Fig. 2. This is important because it suggests that the downstream events of the postulated signaling pathway are continuously activated. To test this idea, we incubated tMCTF cells with two specific, membrane-permeable γ-secretase inhibitors and examined the levels of tMCTF by Western blot. As predicted, Fig. 3A shows that inhibiting γ-secretase activity with either inhibitor resulted in a significant increase in the tMCTF protein. We also examined the effect of several broad-spectrum metalloprotease inhibitors on the appearance of the tMCTF. As seen in Fig. 3B, only the γ-secretase inhibitor and a very general protease inhibitor cocktail prevented the degradation of the tMCTF. These data indicate that newly synthesized tMCTF traffics to a cellular compartment containing γ-secretase where its activity constitutively cleaves the megalin COOH terminus.
Fig. 3.
Effect of protease inhibitors on expression of tMCTF. In all experiments, OKP cells stably transfected with tMCTF (except where noted in C) were grown in 24-well tissue culture plates to confluence. The cells were then incubated in the presence of protease inhibitors overnight. The cells were lysed in solubilization buffer and samples were prepared for immunoblotting using 10% polyacrylamide gels. All blots were probed with anti-MC220 diluted 1:5,000. A: effect of inhibiting γ-secretase activity; no γ-secretase inhibitor (lanes 1–4), an irreversible γ-secretase inhibitor (compound E, 500 nM; lanes 5–8), or a reversible γ-secretase inhibitor (Sigma no. S2188, 50 mM; lanes 9–12). For this experiment, 20 μg of cell lysate were used for each lane. Each condition is shown in quadruplicate. B: effect and specificity of various protease inhibitors on tMCTF expression. Each condition is shown in duplicate: no inhibitor, compound E (500 nM), GM-6001 (25 mM), MMP-III (20 mM), TAPI-2 (20 mM), TAPI-1 (20 mM), or Complete protease inhibitor cocktail tablet (7× according to manufacturer's protocol). In all lanes, 10 μg of cell lysate were used. C: effect of lactacystin on tMCTF expression. Each condition is shown in duplicate. Here, cells were incubated in compound E (500 nM), no inhibitor, or in varying amounts of lactacystin as indicated. In the tMCTF lanes 10 μg and in the tMICD lanes 2 μg of cell lysate were used. Molecular weights, expressed as ×10−3 Mr, are on the left.
As with other receptors subjected to RIP (2), we had difficulty visualizing the cytosolic domain of megalin (MICD) produced by γ-secretase activity (29). Since the intracellular domains of both Notch (NICD) and APP (AICD) are rapidly degraded by the proteasome (7, 23), we studied the effect of the proteasomal inhibitor, lactacystin, on the appearance of the COOH terminus of megalin in the transfected cells. Lactacystin is a cell-permeable inhibitor that blocks 26S proteasome activity by targeting an active threonine in the catalytic β-subunit and is thus very specific. Figure 3C shows that in the presence of lactacystin we were able to visualize both the larger tMCTF as well as smaller COOH-terminal fragments of the tMCTF. The smaller fragments of the tMCTF were similar in size to the tMICD. Importantly, the lower molecular weight fragments of the tMCTF were not seen when γ-secretase inhibitors alone were used indicating that these products were probably degraded in the proteasome. Taken together, the data show that the tMCTF is cleaved by γ-secretase and the resulting COOH-terminal fragments are degraded by the proteasome.
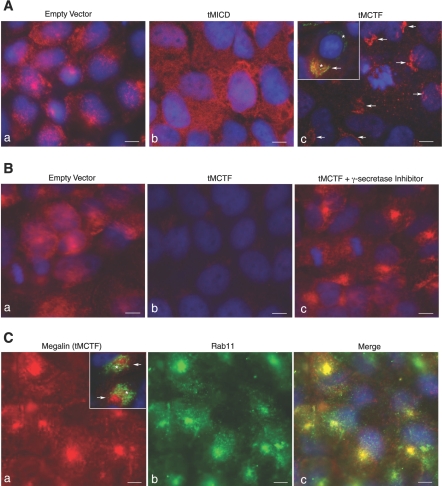
To complement our biochemical data and to gain insight into the localization of the megalin COOH-terminal domains, we used indirect immunofluorescence microscopy and compared the expression and subcellular distribution of megalin, tMICD, and tMCTF in the transfected cells. In mock-transfected cells, we found endogenous megalin associated with vesicular, largely intracellular structures that were distributed throughout the cells (Fig. 4, Aa and Ba). By contrast, in tMICD cells we found no membranous or vesicular staining, but rather very strong diffuse staining seen within the cytoplasm (Fig. 4Ab). The staining pattern seen in tMICD cells is consistent with the fact that there is little if any endogenous megalin in these cells (as determined by Western blot in Fig. 2) and that the tMICD was designed to be a soluble protein lacking megalin's transmembrane domain. Finally, when we labeled the tMCTF cells we found very weak staining that appeared to be localized to the Golgi (Fig. 4, Ac and Bb). The weak staining for megalin seen in the tMCTF cells is again consistent with the Western blotting data provided in Fig. 2 showing little if any endogenous megalin and low levels of tMCTF protein in these cells. Golgi localization of the tMCTF was confirmed in these cells by double-labeling with an antibody to the Golgi matrix protein GM130 (Fig. 4Ac, inset).
Fig. 4.
Localization of megalin and the megalin COOH-terminal peptides in transfected OKP cells. A: OKP cells transfected with EV (a), tMICD (b), or tMCTF (c) were grown on glass coverslips and fixed with periodate-lysine-paraformaldehyde (PLP). The cells were stained for the megalin COOH terminus using rabbit anti-MC220. Ac, inset: tMCTF cells were double-labeled for the megalin COOH terminus (red) and the Golgi matrix protein GM130 (green). The arrows indicate staining for megalin (red) while the * denotes staining of the Golgi (green). Note that the 3 images (a, b, and c) shown in A were individually optimized such that exposure times and processing in Photoshop were used that provided the clearest details for each image. B: relative expression of megalin and tMCTF and the effect of γ-secretase inhibitors. EV (a) and tMCTF cells (b and c) were grown as in A. The cells in c were incubated overnight with the γ-secretase inhibitor, compound E (500 nM). The cells were fixed in PLP and stained with rabbit anti-MC220 (1:500). Note that in B all images (a, b, and c) were taken at the same exposure and processed in an identical manner in Photoshop. C: tMCTF cells were grown on coverslips and incubated overnight with compound E. After fixation, the cells were double-labeled with mAb 6E10 (anti-megalin COOH terminus) and rabbit anti-Rab 11 (1:100). Images a and b show single channel for megalin (red) and for Rab11 (green), respectively. Cc: channels are merged to show colocalization (yellow). Ca, inset: cells were double-labeled for megalin (red) and GM130 (green). The arrows indicate staining for megalin (red) while the * denotes staining of the Golgi (green). All cells were counterstained with DAPI which labels the nuclei (blue). In all images, the micron marker represents 5 μm.
The subcellular location whereby γ-secretase cleaves megalin's COOH terminus is unknown. We postulate that in tMCTF cells under conditions in which γ-secretase activity is inhibited the tMCTF will accumulate at or near to the site of γ-secretase cleavage. As predicted from our Western blotting data (Fig. 3), when we inhibited γ-secretase activity and immunolabeled the cells for the megalin COOH terminus, we found a dramatic increase in staining (compare Fig. 4, Bb with Bc). The increase in staining when γ-secretase activity is inhibited with compound E is consistent with the increase in the tMCTF seen by Western blots in Fig. 3. In cells incubated in compound E, the anti-megalin antibody labeled a dense pool of vesicles (Fig. 4Bc) located close to the nucleus and that appeared similar to staining described for recycling endosomes (20). To confirm this localization, we double-labeled tMCTF cells that were incubated in compound E with a monoclonal antibody to the COOH terminus of megalin and with a polyclonal antibody to Rab 11, a marker for recycling endosomes. As seen in Fig. 4C staining for the tMCTF colocalized with Rab 11, we also compared the localization of the tMCTF with GM130. In the absence of the γ-secretase inhibitor, the anti-megalin antibody colocalized with GM130 (Fig. 4Ac, inset). In contrast, in the presence of a γ-secretase inhibitor staining for GM130 and the tMCTF did not coincide (Fig. 4Ca, inset). These data, together with our Western blotting data, show 1) in both tMICD and tMCTF cells, endogenous megalin expression is reduced to very low levels and 2) the tMCTF is constitutively cleaved by γ-secretase probably within recycling endosomes.
Megalin COOH terminus regulates expression of specific brush-border proteins.
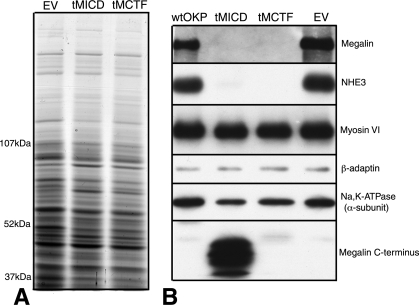
As shown in previous figures, endogenous megalin expression was dramatically reduced in cells overexpressing either tMICD or tMCTF. To determine the specificity of this effect, we examined both total and specific protein expression in each cell line. Figure 5A shows that total protein expression appeared the same in each cell type. However, when we used immunoblotting to examine expression of specific proximal tubule proteins, we found, in addition to megalin, that the brush-border Na+/H+ exchanger NHE3 was also greatly reduced (Fig. 5B). The expressions of other proteins including: 1) the clathrin adaptor protein, β-adaptin, 2) an unconventional myosin, myosin VI, and 3) the α-subunit of the Na-K-ATPase were all unchanged.
Fig. 5.
Total and specific protein expression in transfected OKP cells. Transfected cells were analyzed by SDS-PAGE and immunoblotting to assess total and specific protein expression. A: 100 μg of cell lysate were separated on a 3.5–8% gradient gel and stained with Coomassie blue. B: Western blots using lysates (10 μg) from Wt-, tMICD-, tMCTF-, and EV-transfected OKP cells. Blots were probed with antibodies to indicated proteins.
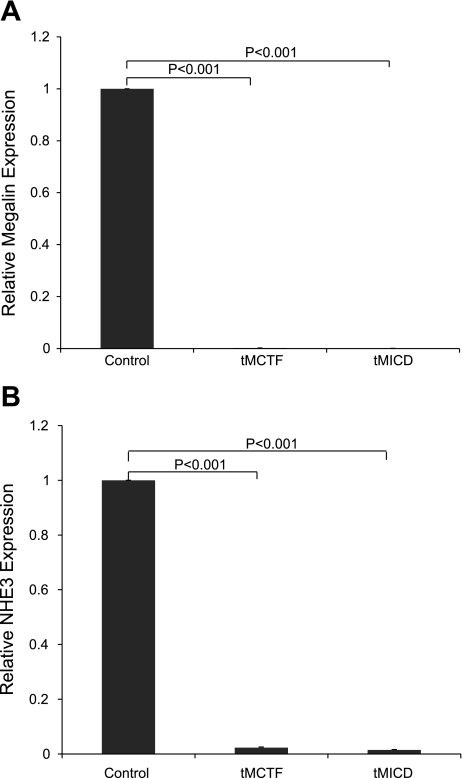
Since we postulate that RIP of megalin regulates gene expression, we performed experiments to determine whether the observed changes in specific protein expression in cells expressing the megalin COOH-terminal peptides result from regulation at the mRNA level. As shown in Fig. 6, we used quantitative real-time RT-PCR to measure megalin and NHE3 mRNA levels in transfected OKP cells. We found that overexpression of either the tMICD or tMCTF coincided with a reduction of both megalin and NHE3 mRNA by >100-fold and 50- to 70-fold, respectively.
Fig. 6.
Expression of megalin (A) and NHE3 (B) mRNA in control (EV)-, tMCTF-, and tMICD-transfected OKP cells. Expression of megalin and NHE3 mRNA in tMCTF- and tMICD-transfected OKP cells compared with EV-transfected OKP cells was quantified using real-time RT-PCR. Data were normalized to cyclophilin gene expression, n = 6, P < 0.001.
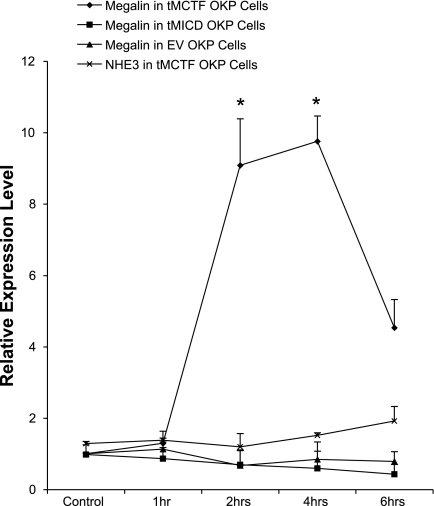
Finally, if our model is correct and constitutive processing of the tMCTF by γ-secretase produces soluble COOH-terminal fragments that in turn inhibit megalin gene expression, we predict that blocking γ-secretase activity in the tMCTF cells should block this effect and allow endogenous megalin mRNA levels to increase. In contrast, since the tMICD is soluble and lacks the γ-secretase cleavage site, we should not expect inhibition of γ-secretase activity in tMICD cells to change endogenous megalin expression. Figure 7 summarizes experiments where we measured megalin gene expression in the presence of a γ-secretase inhibitor as a function of time. As predicted, we found that when we blocked γ-secretase activity in tMCTF cells, we routinely measured an 8- to 10-fold increase in megalin mRNA expression. This increase peaked at 4 h and began to decline thereafter for reasons that are not clear. In contrast, the inhibitor had no effect on megalin expression in the tMICD or EV control cells. In contrast to megalin, we found NHE3 mRNA did not increase in tMCTF cells when we inhibited γ-secretase activity suggesting that changes in NHE3 expression are not a direct effect of the production of megalin COOH-terminal peptides.
Fig. 7.
Effect of γ-secretase inhibitors on megalin gene expression in tMCTF-, tMICD-, and EV-transfected OKP cells. tMCTF-, tMICD-, and EV-transfected OKP cells were incubated with reversible γ-secretase inhibitor at a concentration of 50 μM. At the indicated times, the cells were collected, mRNA isolated, and quantitative real-time RT-PCR performed as described in materials and methods. Megalin mRNA levels increased 8- to 10-fold in tMCTF OKP cells (n = 8) at 2 and 4 h of γ-secretase inhibitor treatment. *P < 0.001 vs. no γ-secretase inhibitor control. NHE3 mRNA did not increase when examined under the same conditions. Megalin mRNA did not change in γ-secretase inhibitor-treated tMICD (n = 3) or EV-transfected OKP cells (n = 3) at 2, 4, and 6 h compared with no inhibitor control.
DISCUSSION
In this study, we directly tested the hypothesis that COOH-terminal peptides produced by RIP of megalin can regulate gene expression in cultured proximal tubule cells. We previously showed that RIP of megalin includes metalloprotease-mediated ectodomain shedding producing a membrane-bound MCTF (29). The MCTF in turn undergoes additional, and apparently unregulated, proteolytic cleavage by γ-secretase releasing a predicted soluble COOH-terminal fragment, called the MICD, into the cytosol. The processing steps for megalin are similar, if not identical, to those described for Notch. Studies of Notch showed that regulated ectodomain shedding must take place before γ-secretase-mediated release of the intracellular domain (NICD) and the subsequent signaling events can occur (reviewed in Ref. 25). Therefore, a common approach in Notch studies has been to express plasmids representing specific regions of the COOH terminus lacking the ectodomain (14, 24). Here, we used a similar approach to study megalin. We found that by removing most or all of megalin's ectodomain and stably overexpressing either the tMCTF or tMICD in OKP cells, we were able to achieve specific and dramatic changes in megalin and NHE3 expression. These data are the first evidence that the COOH terminus of megalin is capable of regulating protein and mRNA expression and provide a novel molecular mechanism possibly linking the uptake of urinary protein and regulation of gene expression in the proximal tubule.
To be valid, these experiments assume that the tMICD and tMCTF target properly within the cell and interact with critical, and at this point largely unknown, elements of the proposed signaling pathway. Our studies support the notion that the tMCTF does target properly and helps validate a key element of our model which predicts that by removing megalin's ectodomain, we can constitutively activate downstream elements of this pathway. Our studies using γ-secretase inhibitors show that the low level of tMCTF found in the cells is a direct result of constitutive processing by γ-secretase and imply that the tMCTF must traffic to an intracellular compartment that expresses the protease. In addition, we found that by inhibiting γ-secretase activity, we not only increased the tMCTF protein level but increased endogenous megalin mRNA expression by 10-fold within 2–4 h. We also report that NHE3 gene expression is regulated by the megalin COOH terminus. However, this appears to be an indirect effect since inhibiting γ-secretase cleavage of the tMCTF did not restore NHE3 mRNA expression. Taken together, these data show that the expressed tMCTF is constitutively processed by γ-secretase and that the resulting COOH-terminal fragments inhibit megalin gene expression.
Our immunocytochemical studies suggest that the γ-secretase cleavage of the tMCTF probably occurs in recycling endosomes. When we inhibited γ-secretase activity, the tMCTF accumulated in a cellular compartment that had the same appearance as that expressing Rab11, a marker for recycling endosomes (20). This conclusion is consistent with our previous studies in kidney showing presenilin-1, an active part of the γ-secretase protein complex, largely localizes to endocytic compartments of the proximal tubule (29). The idea that γ-secretase cleavage of megalin occurs in endosomes is also supported by studies of Notch. Gupta-Rossi and co-workers (10) reported that Notch requires monoubiquitination on the plasma membrane followed by endocytosis in order for γ-secretase cleavage to occur.
We were surprised to find the tMCTF, in the absence of γ-secretase inhibitors, was detected at low levels largely in the Golgi. Although this might reflect a targeting artifact in the transfected cells, recent studies from the Farquhar laboratory show the apical plasma membrane targeting sequence of megalin is localized to the COOH-terminal domain (27) and thus is present within the tMCTF. Although details of the biogenesis of megalin are incomplete, it is known to assemble with the soluble chaperone protein, receptor-associated protein (RAP), and occurs cotranslationally in the endoplasmic reticulum (1). The megalin-RAP complex is thought to remain associated until the complex traffics to endosomes after reaching the plasma membrane (6). The interaction with RAP may be important for efficient trafficking of megalin to the brush border. Since RAP interacts with megalin at ectodomain sites (9) not found in the tMCTF, trafficking of the tMCTF may be less efficient and it may have a longer residence time in the Golgi thus making it more easily detected by immunocytochemistry.
Megalin is a multifunctional cell surface receptor that, our data suggest, is downregulated by RIP. It seems likely that RIP of megalin is part of a more complex molecular pathway that ensures megalin expression is maintained at some necessary physiologic level. RIP of megalin would tend to not only reduce the cellular levels of megalin but would have an inhibitory effect on the uptake of its ligands and thus the metabolic/signaling pathways they serve as well. A full appreciation of the significance of RIP of megalin depends on understanding the physiologic importance of the cellular uptake of specific ligands and the molecular relationship of this receptor function with RIP-mediated signaling. It has become clear that megalin is more than a scavenger receptor whose only function is to clear macromolecules from the urine. Megalin is important for renal vitamin homeostasis and plays a key role in signaling pathways during development of the mammalian brain. Studies of megalin knockout mice showed that megalin mediates uptake of circulating, inactive 25-OH-vitamin D3 into the proximal tubule where it is converted to active 1,25-(OH)2 vitamin D3 (21). Megalin−/− mice exhibit vitamin D deficiency and defects of bone mineralization. In a similar vein, megalin-dependent endocytosis of vitamin A (5) and vitamin B12 (19), along with their respective binding proteins, has been well documented. Megalin−/− mice also exhibit holoprosencephaly, a neurodevelopmental defect in which the prosencephalon fails to separate into discrete hemispheres (28). More recent attempts to define megalin's role in neural development showed that megalin is part of the sonic hedgehog signaling pathway (17) and that it mediates the uptake and degradation of bone morphogenic protein 4, a signaling molecule and central regulator of neural tube patterning (26). Taken together, these data show megalin to be one of the more functionally complex members of the lipoprotein receptor family and predict the need for cell type-specific regulation of megalin expression.
The initial regulated step of RIP of megalin is metalloprotease-mediated ectodomain shedding of the receptor. However, neither the stimulus that activates the metalloprotease nor the identity of the protease itself is known. It is important to emphasize that ∼80% of the ectodomain shedding of megalin we reported previously appeared to be ligand independent (29). This raises the possibility that unknown factors, other than ligand binding, may have a more important role in regulating the initial steps of megalin's processing. It must also be emphasized that we have not studied the efficiency of all of megalin's known ligands to activate shedding. It's possible that only some ligands activate shedding or that different ligands activate this pathway with different efficiencies.
Using Notch signaling as paradigm, our model predicts that γ-secretase activity cleaves the membrane-bound MCTF releasing the MICD into the cytosol. The soluble MICD, either directly or indirectly, acts as a transcriptional regulator. However, we and others found that the predicted intracellular domains of receptors subjected to RIP including megalin, Notch, APP, and SorLA are not usually detectable (2, 7, 23, 29). It has been assumed, and discussed extensively in the literature, that the half-life of the “free” intracellular domains of these receptors is very short. Our data presented here support this notion and suggest that the cytosolic domains of megalin, produced by γ-secretase, are further degraded in the proteasome. Lactacystin is a cell-permeable, irreversible, and specific inhibitor of the 26S multicatalytic protease complex. When tMCTF cells were incubated with lactacystin, we found a dramatic increase in the appearance of a series 25- to 40-kDa peptides. The fact that the 25- to 40-kDa peptides were not identical to the 35-kDa tMICD implies that the γ-secretase products are subjected to additional posttranslational modifications. We were surprised to see an increase in the 35- to 40-kDa tMCTF band(s) in the presence of lactacystin. This observation suggests that either lactacystin or the production of the 25- to 40-kDa fragments of the tMCTF has an inhibitory effect on γ-secretase.
Stably and transiently expressed tMICD and tMCTF always appear as multiple bands by Western blot. The possible explanations for this observation include 1) multiple translation start sites, 2) proteolytic events, or 3) other posttranslational modifications. While there may be multiple start sites in the tMICD, we think it unlikely for the tMCTF since it has a signal peptide (and thus an ATG start site) and would not be expected to enter the membrane if the signal peptide were bypassed. Proteolysis may also explain why the epitope tags we added to the plasmid expressing the tMCTF were not detectable. We constructed the tMCTF plasmid with NH2-terminal His and COOH-terminal HA epitope tags. This was done not only to distinguish transfected tMCTF from endogenous megalin but also so that we could follow the proteolytic products of tMCTF cleavage through the cell. Neither tag was detectable by Western blot or by immunocytochemistry even though careful sequencing of the plasmid showed it to be present.
Members of the lipoprotein receptor gene family play important roles in diverse signaling pathways. Our present study showing RIP of megalin regulates specific gene expression in OKP cells helps to define the molecular mechanism of megalin's signaling function and begins to describe megalin as a molecular link between urinary protein and gene regulation in the proximal tubule. Future goals must include transgenic studies in a murine model that will allow proteomic and genomic analysis and provide a more complete description of the genes regulated by this pathway.
GRANTS
This work was supported by grants from the National Institutes of Health (R01-DK-054933 to D. Biemesderfer) and the American Society of Nephrology (Scherbenske Grant to D. Biemesderfer).
Portions of this work were presented in abstract form at the 39th annual meeting of the American Society of Nephrology, November 14–19, 2006, San Diego, CA.
Acknowledgments
The authors thank Dr. P. Preisig for help with the quantitative real-time PCR experiments, Dr. M. Caplan for critical reading of this manuscript, and the members of the Cantley, Preisig and Aronson laboratories for helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Biemesderfer D, Dekan G, Aronson PS, Farquhar MG. Biosynthesis of the gp330/44-kDa Heymann nephritis antigenic complex: assembly takes place in the ER. Am J Physiol Renal Fluid Electrolyte Physiol 264: F1011–F1020, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Bohm C, Seibel NM, Henkel B, Steiner H, Haass C, Hampe W. SorLA signaling by regulated intramembrane proteolysis. J Biol Chem 281: 14547–14553, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89: 331–340, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100: 391–398, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Christensen EI, Willnow TE. Essential role of megalin in renal proximal tubule for vitamin homeostasis. J Am Soc Nephrol 10: 2224–2236, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Czekay RP, Orlando RA, Woodward L, Lundstrom M, Farquhar MG. Endocytic trafficking of megalin/RAP complexes: dissociation of the complexes in late endosomes. Mol Biol Cell 8: 517–532, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Ebinu JO, Yankner BA. A RIP tide in neuronal signal transduction. Neuron 34: 499–502, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Farquhar MG, Saito A, Kerjaschki D, Orlando RA. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J Am Soc Nephrol 6: 35–47, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, Israel A, Brou C. Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor. J Cell Biol 166: 73–83, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasson T, Mooseker MS. Porcine myosin-VI: characterization of a new mammalian unconventional myosin. J Cell Biol 127: 425–440, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashgarian M, Biemesderfer D, Caplan M, Forbush B 3rd. Monoclonal antibody to Na, K-ATPase: immunocytochemical localization along nephron segments. Kidney Int 28: 899–913, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Kopan R, Schroeter EH, Weintraub H, Nye JS. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc Natl Acad Sci USA 93: 1683–1688, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci 7: 93–102, 2006. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy RA, Barth JL, Chintalapudi MR, Knaak C, Argraves WS. Megalin functions as an endocytic sonic hedgehog receptor. J Biol Chem 277: 25660–25667, 2002. [DOI] [PubMed] [Google Scholar]
- 18.McClean W, Nakane PF. Periodate-lysine-paraformaldehyde fixative a new fixative for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. [DOI] [PubMed] [Google Scholar]
- 19.Moestrup SK, Birn H, Fischer PB, Petersen CM, Verroust PJ, Sim RB, Christensen EI, Nex E. Megalin-mediated endocytosis of transcobalamin-vitamin-B12 complexes suggests a role of the receptor in vitamin-B12 homeostasis. Proc Natl Acad Sci USA 93: 8612–8617, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray RZ, Kay JG, Sangermani DG, Stow JL. A role for the phagosome in cytokine secretion. Science 310: 1492–1495, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507–515, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci USA 91: 9725–9729, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron MM, Teplow DB, Haass C. Presenilin-dependent gamma-secretase processing of beta-amyloid precursor protein at a site corresponding to the S3 cleavage of Notch. EMBO Rep 2: 835–841, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393: 382–386, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26: 565–597, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Spoelgen R, Hammes A, Anzenberger U, Zechner D, Andersen OM, Jerchow B, Willnow TE. LRP2/megalin is required for patterning of the ventral telencephalon. Development 132: 405–414, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Takeda T, Yamazaki H, Farquhar MG. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol 284: C1105–C1113, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA 93: 8460–8464, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279: 34302–34310, 2004. [DOI] [PubMed] [Google Scholar]