Abstract
Brain edema is an important factor leading to morbidity and mortality associated with primary brain tumors. Dexamethasone, a synthetic glucocorticoid, is routinely prescribed with antineoplastic agents to alleviate pain associated with chemotherapy and reduce intracranial pressure. We investigated whether dexamethasone treatment increased the expression and activity of multidrug resistance (MDR) transporters at the blood-brain barrier. Treatment of primary rat brain microvascular endothelial cells with submicromolar concentrations of dexamethasone induced significantly higher levels of drug efflux transporters such as breast cancer resistance protein (abcg2), P-glycoprotein (P-gp; abcb1a/abcb1b), and MDR protein 2 (Mrp2; abcc2) as indicted by protein and mRNA levels as well as by functional activity. The effect of dexamethasone on transporter function was significant within 6 h of treatment, was dose dependent, and was reversible. Dexamethasone-induced upregulation of Bcrp and P-gp expression and function was partially abrogated by the glucocorticoid receptor (GR) antagonist RU486. In contrast, RU486 had no effect on the dexamethasone-induced upregulation of Mrp2, suggesting a GR-independent regulation of Mrp2, and a GR-dependent regulation of P-gp and Bcrp. In addition to the dexamethasone-induced upregulation of MDR transporters, we measured a dose-dependent and reversible increase in the expression of the nuclear transcription factor pregnane xenobiotic receptor (PXR). Administering dexamethasone to rats caused increased expression of PXR in brain microvessels within 24 h. These results suggest that adjuvant therapy with corticosteroids such as dexamethasone in the treatment of brain tumors may increase the expression of MDR transporters at the blood-brain barrier through pathways involving GR and PXR.
Keywords: multidrug resistance proteins, pregnane xenobiotic receptor, breast cancer resistance protein
brain tumors account for approximately 2–3% of all cancers and approximately 25–30% of solid pediatric tumors (20). Treatment of brain tumors and other central nervous system diseases is limited by the ability of therapeutic drugs to cross the blood-brain barrier (BBB). Beyond the tight physical barrier provided by the endothelial cells, drug penetration is substantially reduced by the presence of multidrug resistance (MDR) efflux transporters of the ATP-binding cassette family (28). Previous in vitro studies have identified functionally active MDR efflux transporters such as P-glycoprotein (P-gp, or abcb1a/abcb1b), breast cancer resistance protein (Bcrp, or abcg2), MDR-associated protein 2 (Mrp2, or abcc2), and Mrp4 (or abcc4) in primary cultures of human, rat, and bovine cerebral endothelial cells (19, 26). These transporters are located at both the luminal and apical surfaces of the BBB and blood-cerebrospinal fluid barrier to protect the brain against xenobiotic substances (19, 24, 27). These efflux pumps are capable of transporting a wide variety of chemotherapeutic drugs such as topotecan, vinblastine, and doxorubicin (5, 36). Knockout mouse models have demonstrated the role of P-gp and Bcrp in preventing the entry of toxic substances, including chemotherapeutic agents, into the brain (2, 6, 36). For example, P-gp and Bcrp knockout mice show significantly higher accumulation of chemotherapeutic drugs such as imatinib in the brain in comparison with wild-type animals (5, 8). However, the factors that regulate the expression of these transporters at the BBB have not been well characterized.
Understanding the factors that regulate the expression and function of MDR transporters could lead to the development of improved therapeutic strategies for overcoming MDR and restricted drug delivery to the brain. Because of its rapid and beneficial clinical effects, the synthetic glucocorticoid dexamethasone is routinely used in the management of patients with intracranial tumors to reduce brain edema (15, 21). However, dexamethasone is known to differentially alter the expression of efflux proteins depending on the type of tissue or origin of the cells. Since dexamethasone therapy is often combined with chemotherapy for brain tumors, it is important to determine how dexamethasone affects the expression and function of MDR transporters in brain endothelial cells.
The purpose of the present study was to determine whether dexamethasone treatment regulates the expression and activity of relevant transporters, such as P-gp, Bcrp, and Mrp2, at the BBB. We examined both the expression and functional activity of MDR transporters in primary cultures of rat brain microvascular endothelial cells. In addition, since dexamethasone is known to activate nuclear transcription factors such as the glucocorticoid receptor (GR) and the pregnane xenobiotic receptor (PXR) in primary hepatocytes (31, 41), we also investigated their relative contributions in regulating the expression of these transporters in endothelial cells present at the BBB.
MATERIALS AND METHODS
Materials.
Unless otherwise specified, all reagents including dexamethasone, pregnenolone 16α-carbonitrile (PCN), and RU486 were of molecular biology grade and obtained from Sigma (St. Louis, MO). Endothelial cell growth supplement and fibronectin were obtained from Cell Sciences (Boston, MA). Specific BCRP inhibitor fumitremorgin C (FTC) was a kind gift from Dr. Douglas Ross (University of Maryland, Baltimore).
Isolation of microvessel endothelial cells from rodent brain.
Rats used for isolation of primary endothelial cells were euthanized in accordance with the procedures approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center and are in compliance with the guidelines of the American Association for Laboratory Animal Science.
Primary brain microvascular endothelial cells were prepared as described previously (17). Briefly, brains of 3-wk old male rats (30–40 g, Sprague-Dawley) were mechanically homogenized in dissecting medium (DM). The DM was composed of medium M199 containing 15 mM HEPES, 20 mM sodium bicarbonate, penicillin (50 IU/ml)-streptomycin (50 μg/ml) mixture in conjunction with amphotericin B (2.5 μg/ml) and 5% equine plasma-derived serum (Atlanta Biologicals, Atlanta, GA). The homogenate was subjected to primary incubation with 50 μg/ml Dispase I (Roche, Indianapolis, IN) in serum-free DM for 30 min at 37°C. The suspension was subjected to centrifugation with 15% dextran solution (in DM). The microvessels were subjected to secondary enzymatic treatment with 1 mg/ml collagenase/dispase (Roche) for 2 h at 37°C. The endothelial cell fragments were separated with a Percoll gradient. The primary brain microvascular endothelial cells were cultured in Dulbecco's modified essential medium (Invitrogen, Gray, CA) with 1 g/l glucose, 15% equine plasma-derived serum, 4% fetal bovine serum (FBS, Atlanta Biologicals), 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mg/ml endothelial cell growth supplement, 5 mg/ml heparin, and antibiotic/antimycotic solution composed of 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. The cell suspension was seeded onto fibronectin-coated (2–4 μg/cm2) T-25 cm2 flasks (Sarstedt, Newton, NC) or 12-well tissue culture plates (MidWest Scientific, St. Louis, MO). The cells attained confluence ∼4–6 days from the day of isolation. On the basis of preliminary assessments, approximately 95–98% of isolated endothelial cells stained positive for the cytoplasmic granules of von Willebrand factor. We assessed cell viability using a Live/Dead kit (Molecular Probes) in selected studies, and we routinely monitored viability using trypan blue exclusion. Cells maintained >95% viability under the conditions used in the present study.
Total RNA purification and RT-PCR.
Total RNA was isolated from brain microvascular endothelial cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (5 μg) was reverse transcribed using avian myeloblastosis virus reverse transcriptase in a 20-μl volume containing 4 μl of 5× RT-buffer, 1 μl of 100 mM dNTP, 1 μl of random primers, and 1 μl of RNase inhibitor (SuperArray, Boston, MA) at 37°C for 60 min. PCR was performed with 2 μl of the RT reaction product in 25 μl volume with 100 mM dNTP, 50 mM MgCl2, 10 units Taq polymerase, and 1 μg of each primer. After an initial 15-min denaturation at 94°C, the reaction proceeded for 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C, followed by a final extension of 7 min at 72°C. Primers for Bcrp in primary endothelial cells were designed from the following rat sequence (accession number NM_181381): forward: 5′-AGT CCG GAA AAC AGC TGA GA-3′ and reverse: 5′-CCC ATC ACA ACG TCA TCT TG-3′. The expected PCR product was 234 bp. Primers for the Mrp2 transporter corresponded to sequences identical to the mouse receptor cDNA (accession number NM_012833), as follows: forward: 5′-GAC CAA CAT TGT GGC AGT TG-3′ and reverse: 5′-CAC TAC GCC GAC CTT CTC TC-3′. The expected PCR product was 211 bp. For the PXR, the primers were designed from the cDNA of the rat PXR (accession number AF151377), and the sequence of the primers for the PXR is as follows: forward: 5′-GAT GAT CAT GTC TGA TGC CGC TG-3′ and reverse: 5′-GAG GTT GGT AGT TCC AGA TGC TG-3′. The expected PCR product was 353 bp. The expression of β-actin was used for relative quantification (accession number NM_031144; forward: 5′-GTG ATG GTG GGT ATG GGT CAG-3′ and reverse: 5′-GGA CAA CAC AGC CTG GAT GG-3′). The expected PCR product was 298 bp. The amplicon spanned at least two exons for all primers. PCR products were visualized using ultraviolet illumination on a 1% agarose gel containing ethidium bromide.
Protein extraction and Western blot analysis.
The antibodies to the nuclear transcription factor PXR (sc-25381), GR (sc-1002), bcrp (M-70, sc-25822), and GAPDH (sc-32233) were procured from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against p-gp (C219, ALX-801-002) and mrp2 (clone M2III-6, ALX-801-016) were obtained from Allexis Biochemicals (San Diego, CA).
Primary rat brain microvascular endothelial cells were incubated with 250 nM dexamethasone for time periods as indicated. In other studies, the primary endothelial cells were treated with increasing concentrations of dexamethasone (0–500 nM) for 24 h. The cells were rapidly cooled in an ice bath and resuspended in 4°C lysis buffer containing 10 mM Tris·HCl (pH 7.4), 0.15 M NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM PMSF, and 25 μg/ml each of leupeptin, aprotinin, and chymostatin. Lysates were centrifuged for 10 min at 14,000 g at 4°C. In some cases, nuclear extracts and cytosolic fractions were isolated using the NE-PER kit (Pierce Biotechnologies, Rockford, IL). Total protein was determined using the bicinchoninic acid method (Pierce, CA). Lysates (100 μg for whole cell or 25 μg for nuclear fractions) were separated by SDS-PAGE using 4–12% gels and transferred to nitrocellulose membrane. Membranes were blocked overnight at 4°C in 5% nonfat milk. PXR and transporters (bcrp, p-gp, and mrp2) were visualized using primary antibodies at a concentration of 1:500 followed by using horseradish peroxidase-conjugated secondary antibody at 1:1,500. The primary antibody dilutions for the GR and for GAPDH were 1:1,500 and 1:2,000, respectively; the secondary antibody dilutions for GR and GAPDH were 1:2,500 and 1:3,500, respectively. The proteins were detected with chemiluminescence and autoradiography.
Separation of cytosolic and nuclear protein extracts.
Primary rat brain microvascular endothelial cells were lysed using a nuclear and cytoplasmic extraction reagent kit (NE-PER) to separate the cytoplasmic and nuclear components, according to the manufacturer's instructions. Samples were normalized for protein concentration using the bicinchoninic acid method. Nuclear extract (10 μg) was separated using 4–12% SDS-PAGE and was Western blotted using specific antibodies to PXR and GR.
Substrate accumulation studies.
To assess the combined functional activity of bcrp and p-gp in primary endothelial cells, we measured the accumulation of the fluorescent dye, Hoechst 33342 (Ho342; Molecular Probes, Eugene, OR). Confluent monolayers of primary endothelial cells were incubated at 37°C in 1 ml of transport buffer (120 mM NaCl, 5 mM KCl, 400 μM MgCl2, 40 μM Ca Cl2, 10 mM HEPES, 10 mM NaHCO3, 10 mM glucose, and 5 mM Na2HPO4) for 5 min and were further incubated with 5 μM Ho342 for 45 min. Fluorescence was measured continuously for the 45 min using a spectrophotometer at 350 nm (λexcitation) and 460 nm (λemission). Results are from at least three separate experiments performed in triplicate. Some studies were performed in different sized wells, and therefore the mean fluorescence values differed. However, for a given experimental comparison, the measurements were all done in the same sized wells.
In vivo dexamethasone and PXR immunohistochemistry.
Rats used in these experiments were euthanized in accordance with the procedures approved by the Animal Care and Use Committee of St. Jude Children's Hospital and are in compliance with the guidelines of the American Association for Laboratory Animal Science.
Male Sprague-Dawley rats approximately 4 wk old (Taconic, Germantown, NY), weighing 90 to 130 g, were divided into four treatment groups consisting of five rats in each group. Dexamethasone (50 mg/kg) or vehicle control (0.9% saline) was administered by intraperitoneal injection (dexamethasone sodium phosphate 4 mg/ml solution, American Pharmaceutical Partners, Schaumburg, IL). Groups 1 and 2 received a single dose of dexamethasone while groups 3 and 4 received a single intraperitoneal dose of dexamethasone or vehicle control daily for 3 days. Rats were euthanized by CO2 asphyxiation, and tissues (liver and brain) were harvested at 3 h after dexamethasone administration (group 1), 24 h after dexamethasone administration (group 2), 24 h after last dexamethasone administration receiving daily intraperitoneal dexamethasone (50 mg/kg) every 24 h for 3 consecutive days (group 3), and, finally, 24 h after last administration of vehicle control every 24 h for 3 consecutive days (group 4).
Tissues were fixed in 10% neutral buffered formalin and were embedded in paraffin blocks for sectioning. Immunohistochemistry was performed as previously described, with minor modifications (42). Briefly, paraffin sections were heated for 1–2 min using a microwave oven. The specimens were deparaffinized in a series of CitriSolv clearing agents (Fisher Scientific) and rehydrated through graded alcohols to water. Endogenous peroxidases were blocked by incubation with 3% H2O2 in methanol (1:10; 30% H2O2, Sigma-Aldrich), followed by blocking of endogenous avidin/biotin (Vector Labs, Burlingame, CA). A nonspecific protein blocking step was incorporated using a commercially available protein blocking solution (DAKO, Carpinteria, CA). Primary polyclonal antibody against PXR.1 clone A-20, (Santa Cruz Biotechnology) was diluted 1:100 (2 μg/ml) in a commercially available antibody diluent (Zymed, South San Francisco, CA). Negative controls were isotope matched for caprine IgG at a concentration of 2 μg/ml (Vector Labs). Primary antibodies were incubated overnight at 4°C in a humid chamber. The following day, specimens were treated with biotinylated secondary antibody at a concentration of 4 μg/ml in antibody diluent (Vector Labs) and were treated subsequently with undiluted streptavidin-horseradish peroxidase solution (DAKO) in phosphate-buffered saline (pH 7.4). Sections were visualized with diaminobenzidine (DAKO) and counterstained with hematoxylin (DAKO). Specimens were mounted in permanent medium (Fisher Scientific, Suwannee, GA). Data were recorded by light microscope examination by a blinded reviewer, and grading of intensity was assessed. Staining was scored as nonexistent, light, intermediate, or intense staining.
Data analysis.
All experiments were replicated at least three times. Where applicable, data are presented as means ± SE. Densitometric values were determined from digitized images of autoradiographs using Quantity One software (Bio-Rad). Values were then corrected for background and normalized to controls. Statistical analysis of data obtained was carried out using the GraphPad software (San Diego, CA). Statistically significant differences among treatment groups were evaluated by ANOVA followed by the Student-Newman-Keuls posttest for multiple comparisons. Differences were considered statistically significant at P < 0.05.
RESULTS
Dexamethasone increased transcription of Bcrp and Mrp2.
Previous studies demonstrated that treatment with dexamethasone caused increased expression of P-gp in rat brain microvessels (4, 32). To determine whether dexamethasone treatment altered the expression of other drug efflux transporters at the BBB, freshly isolated, primary cultures of rat brain endothelial cells were cultured with increasing concentrations of dexamethasone (0 to 500 nM) for 24 h. As shown in Fig. 1 dexamethasone significantly increased transcript levels of Bcrp and Mrp2 in a concentration-dependent manner. The transcripts of Bcrp and Mrp2 were significantly induced by treatment with 250 nM dexamethasone, and maximal induction was attained with 500 nM dexamethasone. No further increase was observed with higher dexamethasone concentrations (up to 10 μM; data not shown).
Fig. 1.
Dexamethasone-induced transcripts of breast cancer resistance protein (Bcrp) and multidrug resistance associated protein 2 (Mrp2) in brain endothelial cells. RT-PCR shows that dexamethasone treatment (24 h) increased transcription of Bcrp and Mrp2 in primary rat brain endothelial cells in a concentration-dependent manner. Thirty cycles were used for gene amplification. Quantitative analysis by densitometry of three different experiments is also shown. **P < 0.01 vs. untreated cells.
Dexamethasone increased expression of bcrp, p-gp, and mrp2 protein.
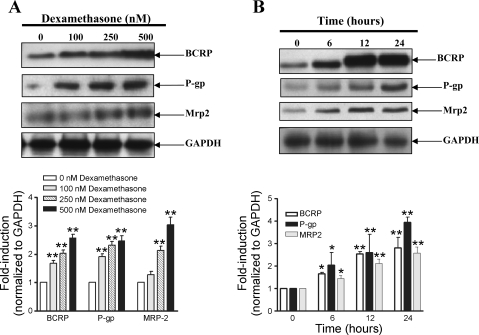
To determine whether the increase in mRNA expression resulted in changes in protein expression, endothelial cells were cultured with increasing concentrations of dexamethasone for 24 h. Fig. 2A demonstrates a significant (2- to 3-fold) upregulation of protein expression of bcrp, p-gp, and mrp2 transporters that was concentration dependent. To assess the kinetics of induction of protein expression, primary cultures were incubated with 250 nM dexamethasone for varying time periods up to 24 h. As shown in Fig. 2B, significant induction of the bcrp, p-gp, and mrp2 protein was observed as early as 6 h and was elevated for as long as 24 h.
Fig. 2.
Dexamethasone induced bcrp, p-glycoprotein (p-gp), and mrp2 protein expression in brain endothelial cells. A: representative Western blots demonstrating the concentration-dependent increase in protein expression of bcrp, p-gp, and mrp2 in primary endothelial cells when cultured with dexamethasone for 24 h. Quantitative analysis by densitometry of three different experiments is also shown. B: dexamethasone-induced increase in bcrp, p-gp, and mrp2 expression after incubation of endothelial cells with 250 nM dexamethasone was dependent on the time of exposure. Quantitative analysis by densitometry of three different experiments is also shown. *P < 0.05 vs. untreated cells, **P < 0.01 vs. untreated cells.
Dexamethasone increased the functional activity of bcrp and p-gp.
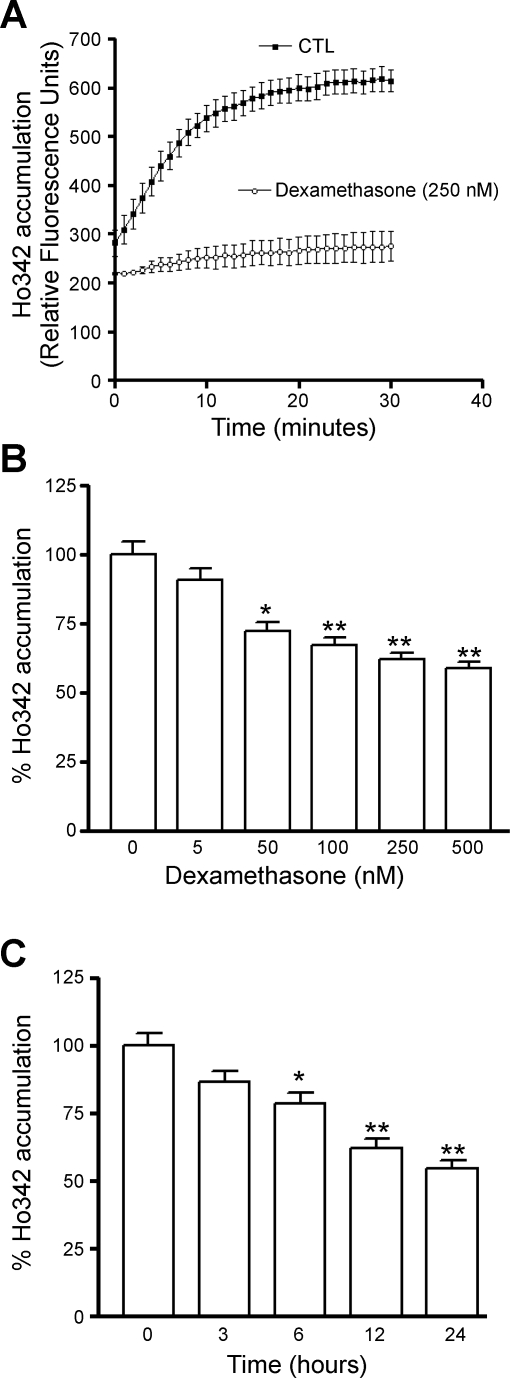
To investigate whether the increase in protein expression resulted in greater functional activity of bcrp and p-gp, we measured Ho342 accumulation in cells after dexamethasone treatment. Previous studies have shown that in addition to being a substrate for bcrp, Ho342 is also recognized by p-glycoprotein (p-gp) as one of its substrates (38). Moreover, consistent with our observations, a report by Bauer et al. (4) showed that in vivo treatment of rats with dexamethasone caused increased expression and activity of p-gp in rat brain microvessels. Therefore, our functional studies, shown in Fig. 3A, represent the activity of both transporters. Fig. 3A shows accumulation of Ho342 in endothelial cells treated with or without dexamethasone (250 nM) for 24 h. Dexamethasone treatment resulted in significantly less Ho342 accumulation. To evaluate whether the effect of dexamethasone was concentration dependent, endothelial cells were treated with increasing concentrations of dexamethasone (up to 500 nM) for a period of 24 h. Endothelial cells treated with increasing concentrations of dexamethasone accumulated correspondingly lower levels of fluorescent dye. The steady-state accumulation of Ho342 was significantly decreased by treatment with as little as 50 nM dexamethasone (Fig. 3B). In time-course experiments, endothelial cells were treated with dexamethasone (250 nM) for specified time periods, up to a maximum of 24 h. Consistent with the kinetics of upregulation of mRNA and protein, the increase in functional activity was also dependent on the time of treatment with dexamethasone (Fig. 3C). The accumulation of Ho342 dye was significantly reduced by 6 h of dexamethasone treatment and continued to decrease with increasing duration of dexamethasone treatment up to 24 h.
Fig. 3.
Transport function of bcrp and p-gp in primary rat brain endothelial cell cultures after exposure to dexamethasone. A: time course of accumulation of Hoechst 33342 dye (Ho342) in endothelial cells cultured with or without 250 nM dexamethasone. CTL, control. B: concentration-dependent decrease in steady-state Ho342 accumulation (after 45 min) in rat brain microvascular endothelial cells that were exposed to dexamethasone for 24 h. C: time-dependent decrease in Ho342 accumulation at steady state in rat brain microvascular endothelial cells that were exposed to 250 nM dexamethasone for the indicated time periods. Each bar represents the mean value (relative fluorescence units) of endothelial cells from at least three independent isolations; error bars indicate SE. *P < 0.05 vs. untreated cells, **P < 0.01 vs. untreated cells.
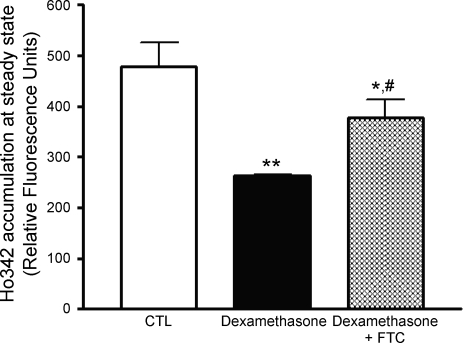
To distinguish between the contributions made by dexamethasone-induced increase in p-gp and bcrp activity, we used a selective and potent inhibitor of bcrp function, FTC (16). Endothelial cells were cultured with dexamethasone (250 nM) for 24 h. Endothelial cells were then incubated with FTC (5 μM) for 15 min before and then during the functional assay. FTC partially restored accumulation of the dye in dexamethasone-treated endothelial cells as shown in Fig. 4. However, the restoration of function was still significantly less than untreated endothelial cells, suggesting that both p-gp and bcrp participate in effluxing the dye.
Fig. 4.
Both p-gp and bcrp contribute to the dexamethasone-induced increase in Ho342 efflux. Primary rat brain endothelial cells were cultured with 250 nM dexamethasone for 24 h. Before the drug accumulation assay was conducted, the endothelial cells were preincubated with 5 μM of the specific BCRP inhibitor fumitremorgin C (FTC) for 15 min and subsequently for the entire duration of the drug accumulation assay. **P < 0.01 vs. untreated cells, *P < 0.05 vs. dexamethasone-treated cells, #P < 0.05 vs. untreated cells.
Inductive effects of dexamethasone were reversible.
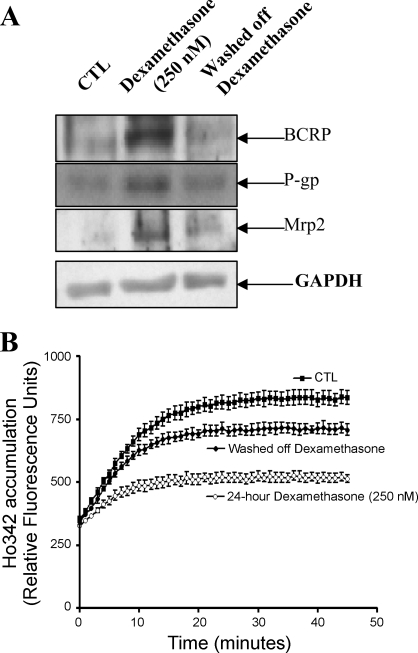
To assess whether the stimulatory effects of dexamethasone were reversible, endothelial cells were initially cultured in media containing dexamethasone (250 nM) for 24 h followed by a 24-h incubation without dexamethasone. As shown in Fig. 5A, treatment of endothelial cells with dexamethasone significantly induced the expression of drug efflux transporters, bcrp, p-gp, and mrp-2. However, the endothelial cells that were further cultured in the absence of dexamethasone exhibited protein levels similar to that of control, untreated cells. Partial recovery of function also occurred (Fig. 5B). Endothelial cells cultured in the presence of dexamethasone accumulated significantly less Ho342 dye in comparison with untreated cells, but Ho342 dye accumulation increased after dexamethasone was washed off. However, the accumulation of Ho342 dye did not fully return to the level of untreated endothelial cells.
Fig. 5.
Reversibility of dexamethasone-induced effects. Primary rat brain endothelial cells were cultured with 250 nM dexamethasone for 24 h. Dexamethasone was washed off, and cells were then cultured in media without dexamethasone for 24 h. A: representative Western blot demonstrating reversible effect of dexamethasone-induced upregulation of bcrp, p-gp, and mrp2. B: functional activity of bcrp and p-gp was determined by measuring the accumulation of Ho342 dye in primary endothelial cells after initial 24-h treatment with 250 nM dexamethasone, removal of dexamethasone, and subsequent 24 h in medium without dexamethasone.
Dexamethasone-induced upregulation of bcrp: role of the GR.
Dexamethasone has been demonstrated to transcriptionally modulate the expression of a multitude of genes involved in drug metabolism and disposition via the GR pathway. Ligand-activated GR is translocated from the cytoplasm to the nucleus, wherein it binds at specific glucocorticoid response elements with promoters (25). Therefore, we investigated whether the GR pathway regulated expression of bcrp, p-gp, and mrp-2.
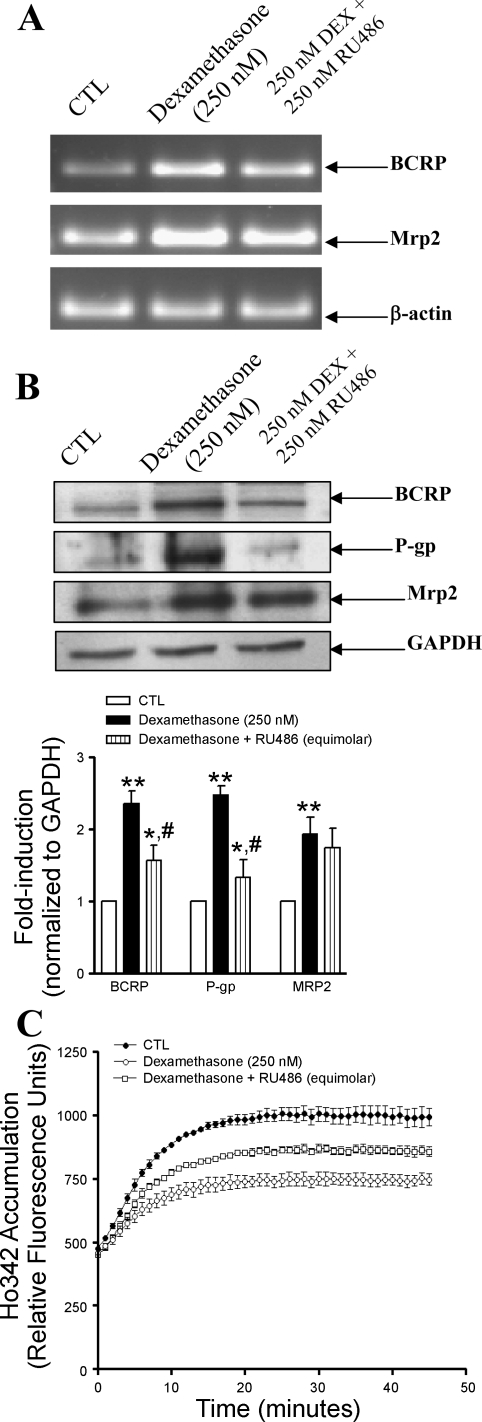
Primary endothelial cells treated with dexamethasone (250 nM) exhibited significant accumulation of GR in the nuclear compartment in comparison with untreated cells, where the GR was mainly concentrated in the cytoplasmic extract (data not shown). To investigate the potential contribution of GR in modulating transporter expression, cells were incubated with either dexamethasone alone (250 nM) or dexamethasone in conjunction with the GR antagonist, RU486 (250 nM) for 24 h. As shown in Fig. 6A, endothelial cells treated with dexamethasone exhibited enhanced transcription of Bcrp and Mrp2, but transcript levels of Bcrp were reduced in the presence of RU486. In contrast, RU486 did not influence the dexamethasone-induced transcription of Mrp-2. These findings were further reflected in the protein expression and functional studies. As shown in Fig. 6B, dexamethasone significantly induced protein expression of bcrp, p-gp, and mrp-2, but treatment with dexamethasone and RU486 resulted in decreased bcrp and p-gp protein expression and no change in mrp2 expression. Fig. 6C depicts the functional activity of bcrp and p-gp in endothelial cells cultured in the presence of dexamethasone (250 nM) alone or equimolar concentrations of dexamethasone in conjunction with RU486 for 24 h. Dexamethasone-treated endothelial cells accumulated significantly less Ho342 dye, whereas endothelial cells cultured with dexamethasone and RU486 accumulated significantly higher Ho342 dye compared with cells treated with dexamethasone alone. RU486 by itself had no effect on expression or function of any of the transporters investigated (data not shown).
Fig. 6.
Glucocorticoid receptor antagonist RU486 partially inhibited the dexamethasone (Dex)-induced stimulation of Bcrp, P-gp, and pregnane xenobiotic receptor (PXR) but had no effect on Mrp-2. Primary endothelial cells were untreated (CTL) or treated with dexamethasone (250 nM) alone or in combination with RU486 (250 nM) for 24 h. A: transcript levels of Bcrp and Mrp-2 were evaluated by RT-PCR, using total cellular RNA as described in materials and methods. B: protein levels of bcrp, p-gp, and mrp-2 from whole cells were evaluated by immunoblotting using 100 μg protein loaded per lane. Representative blots from an experiment performed 3 times are quantified by densitometry in the bar graph. **P < 0.01 vs. untreated (CTL) cells, *P < 0.05 vs. dexamethasone-treated cells, #P < 0.05 vs. untreated (CTL) cells. C: functional activity of BCRP and P-gp was determined by measuring the Ho342 dye accumulation in primary endothelial cells as described as materials and methods.
Specific PXR ligand pregnenolone PCN increased expression of bcrp protein.
Since blockade of the GR pathway only partially restored bcrp and p-gp expression and function and had no effect on mrp-2, and since the nuclear transcription factor PXR has been shown to regulate the expression of p-gp, we investigated whether the well-established activator of PXR, PCN, increased the expression of bcrp. As shown in Fig. 7A, there was an increase in bcrp protein expression in endothelial cells treated with 10 μM of PCN for 24 h. To investigate whether the increase in protein expression resulted in greater functional activity, we measured Ho342 accumulation in cells following PCN treatment. Fig. 7B shows the time course of accumulation of Ho342 in endothelial cells treated with or without PCN (10 μM) for 24 h. PCN treatment resulted in significantly less Ho342 accumulation.
Fig. 7.
Specific PXR ligand, pregnenolone 16α-carbonitrile (PCN), induced bcrp expression and function in brain endothelial cells. A: representative Western blot (from 3 experiments) demonstrating increase in expression of bcrp in primary endothelial cells when cultured with 10 μM PCN for 24 h. B: time course of accumulation of Ho342 dye in endothelial cells cultured with or without 10 μM PCN for 24 h (n = 3 experiments performed in triplicate).
Rat brain microvascular endothelial cells express PXR, and dexamethasone increased PXR expression.
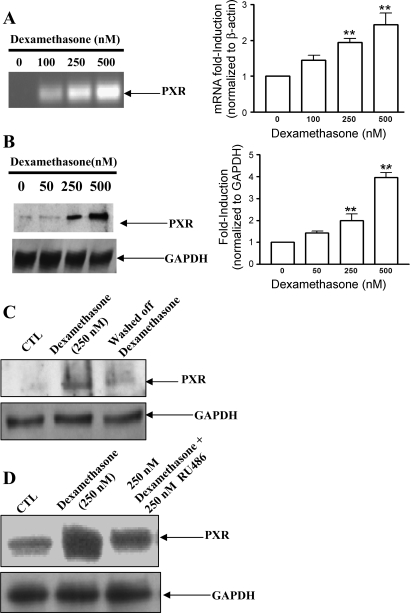
Using RT-PCR, we observed PXR mRNA in rat brain microvascular endothelial cells. Treatment of rat brain microvascular endothelial cells with increasing concentrations of dexamethasone (0 to 500 nM) for 24 h caused increased levels of PXR mRNA (Fig. 8A). We next evaluated whether dexamethasone influenced the protein levels of PXR. Western blot analysis of nuclear extract proteins revealed significantly higher levels of PXR in the nucleus of dexamethasone-treated cultures in comparison with control, untreated cultures (Fig. 8B). Similar to the results obtained with bcrp, p-gp, and mrp-2, expression of PXR returned to control levels 24 h after removal of dexamethasone (Fig. 8C).
Fig. 8.
Dexamethasone stimulated increased transcript levels and protein expression of PXR in rat brain endothelial cells. Primary endothelial cells were treated with increasing concentrations of dexamethasone for 24 h. A: transcript levels of PXR were evaluated by RT-PCR, using total cellular RNA as described in materials and methods. **P < 0.01 vs. untreated cells. B: protein levels of PXR in nuclear extract were evaluated by immunoblotting using 25 μg protein loaded per lane. Representative autoradiograph from an experiment performed at least 3 times is shown. **P < 0.01 vs. untreated cells. C: representative Western blot demonstrating increased PXR expression in the presence of dexamethasone and reduced expression after 24 h without dexamethasone. D: representative Western blot demonstrating that glucocorticoid receptor antagonist RU486 partially inhibited the dexamethasone-induced stimulation of PXR.
To investigate whether the dexamethasone-induced increase in p-gp and expression of PXR were related to the GR pathway, endothelial cells were cultured with 250 nM dexamethasone or equimolar concentrations of dexamethasone and RU486. As shown in Fig. 8D, cells cultured in the presence of dexamethasone expressed significantly higher levels of PXR, but treatment with RU486 significantly reduced the dexamethasone-induced increase. Taken together, these results suggest that the GR pathway contributed to dexamethasone-mediated induction of PXR and bcrp, but the full response was not GR dependent.
Dexamethasone increased PXR expression in vivo.
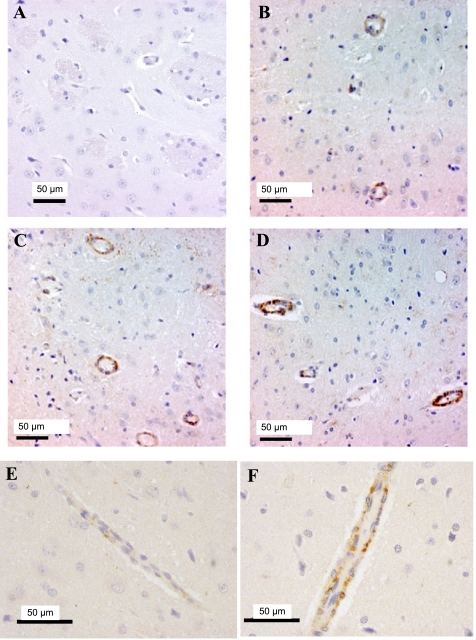
To determine whether the increase in PXR expression that we observed in response to dexamethasone occurred in vivo, we injected rats with dexamethasone (50 mg/kg) every 24 h for 3 days and evaluated the expression of PXR in rat brains by immunohistochemistry. Fig. 9 shows basal expression of PXR in endothelial cells of brain microvessels from control, saline-treated rats (Fig. 9, B and E) compared with rats treated with dexamethasone for 24 h (Fig. 9C) and 72 h (Fig. 9D). Figure 9, E and F, shows higher-magnification fields that include axial views of microvessels from rats treated with either saline control (Fig. 9E) or dexamethasone for 72 h (Fig. 9F). When slides were scored by a blinded observer, PXR expression was increased by both 24-h and 72-h treatment with dexamethasone compared with controls. The observer found that in saline-treated rats, four out of five sections exhibited light staining and one out of five exhibited moderate staining. In rats treated with dexamethasone for 24 h, four out of five exhibited moderate staining and one out of five exhibited dark staining. After 72 h of treatment, four out of four sections exhibited dark staining.
Fig. 9.
Dexamethasone stimulated protein expression of PXR in vivo. Representative sections of brain microvessels of rats treated with dexamethasone (50 mg/kg) are shown. Increased exposure to dexamethasone demonstrated increased levels of PXR as evaluated by immunohistochemistry. A: a negative control showing staining following incubation with an isotope-matched IgG instead of the primary antibody to PXR. All other panels were stained following incubation with the PXR antibody. B: a section from a saline-treated rat exhibiting minimal staining. C and D: microvessel sections from rats treated with dexamethasone for 24 h (C, moderate staining) and 72 h (D, intense staining). E and F: higher-magnification views from rats treated with either saline (E) or dexamethasone (F) for 72 h. Axial views of microvessels are shown.
DISCUSSION
The use of chemotherapy to treat brain tumors is limited by the ability of the antitumor compounds to cross the BBB, and the expression of MDR proteins in endothelial cells of the BBB contributes significantly to the limited drug penetration. Our results demonstrate that dexamethasone, commonly used to reduce edema in the treatment of brain tumors, significantly increased the expression of three important drug efflux proteins in primary cultures of rat brain endothelial cells: bcrp, p-gp, and mrp2. The increase in expression and functional activity of the transporters was dose dependent and reversible, and both the GR and PXR were involved in the upregulation. We also found that treatment with dexamethasone, both in vitro and in vivo, increased the endothelial cell expression of PXR, a nuclear receptor that plays a prominent role in the regulation of expression of drug efflux pumps and metabolizing enzymes.
Although dexamethasone is a recognized inducer of drug-metabolizing enzymes such as members of the cytochrome P450 (CYP450) family (31, 41), the effect of dexamethasone on the expression of drug efflux proteins appears to be tissue- and cell-type specific. For example, while treatment of rats with dexamethasone caused increased expression of p-gp in brain microvessels (4, 32), liver and lung (9), and small intestine (29), other results from these studies demonstrated no change or decreased p-gp expression in the brain, kidney, and liver (9, 29). Mrp2 expression was also increased in brain microvessels in the studies by Bauer and colleagues (4). In primary cultures of rat hepatocytes, dexamethasone treatment stimulated decreased expression of P-gp (12, 37) and increased expression of Mrp2 (7). In other cell culture studies, dexamethasone was shown to increase transcription of MRP3 in human lung carcinoma A549 cells, whereas it had no effect on transcription of the MRP2 gene (33). A 1-h treatment of the rat brain endothelial cell line GPNT caused decreased uptake of vincristine, a p-gp substrate, but there was no increase in p-gp expression (34). Our results with primary cultures of rat brain endothelial cells demonstrate that dexamethasone increased the transcription and expression of both p-gp and mrp2. These results are consistent with those of Bauer et al. (4), but studies by Mei et al. (29) did not demonstrate an effect of dexamethasone on p-gp expression in the rat brain. However, the studies by Mei and collaborators used lower doses of dexamethasone (<20 mg/kg), and this may account for the lack of an effect in their studies.
Our results extend the findings from the previous studies because we have demonstrated for the first time that dexamethasone increased the expression and functional activity of bcrp in rat brain endothelial cells. We detected changes in protein expression with dexamethasone concentrations as low as 100 nM (Fig. 2), and both protein expression and functional activity were increased within 6 h of treatment with 250 nM (Figs. 2 and 3). Changes in functional activity were apparent with dexamethasone doses as low as 50 nM (Fig. 3). Forster and colleagues (13) found that dexamethasone induced the expression of the metalloproteinase inhibitor TIMP-1 in a cerebral endothelial cell line, and that the induction was most prominent at 100 nM (13). Because we did not determine mRNA expression levels at earlier times and since we did not observe significant changes in functional activity at times as early as 3 h, we cannot speculate on whether the effects of dexamethasone were strictly genomic in nature. We also found that the effect of dexamethasone was partially reversible; while protein expression appeared to return to pretreatment levels 24 h after removal of dexamethasone, the functional activity did not recover completely (Fig. 5). Using the bcrp inhibitor, FTC, we demonstrated that the increase in functional activity (Ho342 efflux) was due to both p-gp and bcrp (Fig. 4). We did not specifically examine the functional activity of mrp2.
The expression of drug efflux transporters is generally thought to be regulated at the transcriptional level, and while the transcriptional regulation of P-gp expression has been well studied, the regulation of Bcrp expression is not as extensively studied (3, 35, 40). Furthermore, the role of nuclear receptors such as constitutive androstane and GR has been investigated in the regulation of xenobiotic metabolizing and transporter systems in intestinal cells and hepatocytes (30), but the roles of these and other transcription factors in regulating MDR transporters at the BBB have not been well characterized. Using the GR antagonist RU486, we partially blocked the dexamethasone-stimulated increase in expression of p-gp and bcrp (Fig. 6), suggesting that the GR pathway is involved in the regulation of these transporters. Consistent with the findings of others in hepatocytes (7, 11), Mrp2 expression appeared to be independent of GR in brain endothelial cells. Mrp2 expression has previously been reported to be regulated by PXR in rat hepatocytes (22) and in rat brain microvessels (4).
Bauer and collaborators (4) were the first to report that PXR was expressed in BBB microvessels in the rat and that both dexamethasone and a specific PXR ligand, PCN, stimulated expression of P-gp and Mrp2. On the basis of their findings, we treated primary cultures of brain endothelial cells with PCN and demonstrated a significant increase in the expression and functional activity of bcrp (Fig. 7). To our knowledge, this is the first report of PXR-mediated regulation of Bcrp expression at the BBB. A recent report demonstrated induction of Bcrp mRNA in the livers of PXR+/+ but not in PXR−/− mice following treatment with 2-acetylaminofluorene (2), while others have reported no induction of Bcrp in the livers or intestines of mice following treatment with PCN (18). Both of these studies examined mRNA isolated from whole organ homogenates, while our study examined primary cultures of brain endothelial cells. Another study found a correlation between the expression of mRNA of four ABC transporters (P-gp, MRP1, MRP2, and BCRP) and PXR expression in liver and peripheral blood mononuclear cells in humans (1). With limited information about the promoter region of the Bcrp gene (3), we can only speculate that there may be a PXR-response element in the promoter region of the Bcrp gene.
In addition to demonstrating that PXR can regulate the expression of Bcrp, our results also show that dexamethasone increased the expression of PXR both in vitro (Fig. 8) and in vivo (Fig. 9). Down and colleagues (10) recently reported an increase in PXR mRNA expression in the livers of mice following dexamethasone treatment, but to our knowledge, our results are the first to demonstrate the increase in protein expression at the BBB. This finding is significant since PXR is known to be an important regulator of transcription for many genes that are important in the regulation of xenobiotic metabolism (23). The expression of PXR protein in isolated cells paralleled that of BCRP protein in its dose dependence (Figs. 1, 2, and 8). In addition, the effect of dexamethasone on PXR expression was reversible (Fig. 8), similar to the reversibility for each of the transporters investigated (Fig. 5). Supporting the in vitro data, the PXR-inductive effects of dexamethasone were also observed in brain slices harvested from dexamethasone-treated rats. The effects of dexamethasone were time dependent, with visibly increased staining for PXR observed at 24 h and increased staining observed at 72 h. Although we did not demonstrate changes in bcrp expression in vivo following dexamethasone treatment, our in vitro studies suggest that this may occur.
As discussed above, our results suggest that the GR pathway in part regulates expression of p-gp and bcrp, but we also demonstrated that the dexamethasone-induced increase in PXR expression was partially inhibited by treatment with RU486. This suggests that dexamethasone may be capable of inducing MDR transporter expression through direct interactions with GR and PXR, but also through GR-mediated upregulation of PXR. Pascussi et al. (31) proposed a similar regulatory pathway for the expression of CYP3A4 in human hepatocytes. They suggested that low concentrations (submicromolar) of glucocorticoids activated the expression of PXR through the GR pathway, but that higher concentrations directly activated PXR. Our results do not confirm this possibility, but they do support the possibility of a glucocorticoid response element in the promoter region of the PXR gene. It should also be pointed out that RU486 is a known activator of PXR in hepatocytes (39). However, RU486 did not induce PXR or any of the transporters in our studies (data not shown). However, the concentrations used in our studies were significantly lower than those used in the study by Teng and colleagues [25–50 μM (39)]. We observed cytotoxicity in our endothelial cells when we used micromolar concentrations of RU486.
The concentrations of dexamethasone used in our in vitro studies are comparable to those observed in patients treated with glucocorticoids (12). Moreover, the results appear consistent with the fact that dexamethasone typically becomes effective within 24–72 h of treatment. These results may have significant clinical implications. Dexamethasone-induced alterations in transporter levels may lead to altered pharmacokinetics and variable drug disposition of concomitant chemotherapeutic agents. More specifically, overexpression of transporters at the BBB may further restrict the penetration of therapeutic agents targeted to specific areas of the brain. These findings may also explain the clinical finding that dexamethasone increased the clearance of irinotecan, a substrate of BCRP (14). In addition, a wide spectrum of proteins belonging to the cytochrome P450 family is expressed by brain endothelial cells, and these may in turn be regulated by PXR. Dexamethasone may then induce both drug efflux transporters and drug-metabolizing enzymes, acting through both GR and PXR. Such effects of dexamethasone treatment on brain endothelial cells would reduce the effectiveness of chemotherapeutic treatments for patients with malignant brain tumors. As the blood-cerebrospinal fluid barrier provides another significant interface between the central nervous system and blood, and transporters expressed by the choroid plexus epithelial cells regulate entry of substances into the brain ventricles, it would also be of interest to assess the influence of dexamethasone at this barrier.
The role of drug efflux proteins at the BBB to protect the brain from xenobiotic compounds is well established. The consequences of adjuvant therapy such as the use of corticosteroids have not been extensively investigated to determine whether or not such treatment would be detrimental for the penetration of other drugs. Our findings suggest that corticosteroids such as dexamethasone may diminish the effectiveness of drugs that are required to penetrate the BBB.
GRANTS
This study was supported by National Institutes of Health Grant GM-071321.
Acknowledgments
Present address of N. Kumar: Dept. of Pharmaceutical Sciences, Texas A&M University, College Station, TX 77843.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albermann N, Schmitz-Winnenthal FH, Z'Graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol 70: 949–958, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Anapolsky A, Teng S, Dixit S, Piquette-Miller M. The role of pregnane X receptor in 2-acetylaminofluorene-mediated induction of drug transport and -metabolizing enzymes in mice. Drug Metab Dispos 34: 405–409, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim Biophys Acta 1520: 234–241, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bauer B, Hartz AM, Fricker G, Miller DS. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66: 413–419, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci 27: 17–24, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65: 2577–2582, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Courtois A, Payen L, Guillouzo A, Fardel O. Up-regulation of multidrug resistance-associated protein 2 (MRP2) expression in rat hepatocytes by dexamethasone. FEBS Lett 459: 381–385, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther 304: 1085–1092, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Demeule M, Jodoin J, Beaulieu E, Brossard M, Beliveau R. Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett 442: 208–214, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Down MJ, Arkle S, Mills JJ. Regulation and induction of CYP3A11, CYP3A13 and CYP3A25 in C57BL/6J mouse liver. Arch Biochem Biophys 457: 105–110, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Duret C, Daujat-Chavanieu M, Pascussi JM, Pichard-Garcia L, Balaguer P, Fabre JM, Vilarem MJ, Maurel P, Gerbal-Chaloin S. Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol 70: 329–339, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Fardel O, Lecureur V, Guillouzo A. Regulation by dexamethasone of P-glycoprotein expression in cultured rat hepatocytes. FEBS Lett 327: 189–193, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Forster C, Kahles T, Kietz S, Drenckhahn D. Dexamethasone induces the expression of metalloproteinase inhibitor TIMP-1 in the murine cerebral vascular endothelial cell line cEND. J Physiol 580: 937–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J, Parry M, Houghton PJ, Lovell S, Rasheed K, Cloughsey T, Stewart ES, Colvin OM, Provenzale JM, McLendon RE, Bigner DD, Cokgor I, Haglund M, Rich J, Ashley D, Malczyn J, Elfring GL, Miller LL. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17: 1516–1525, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Galicich JH, French LA. Use of dexamethasone in the treatment of cerebral edema resulting from brain tumors and brain surgery. Am Pract Dig Treat 12: 169–174, 1961. [PubMed] [Google Scholar]
- 16.Garimella TS, Ross DD, Eiseman JL, Mondick JT, Joseph E, Nakanishi T, Bates SE, Bauer KS. Plasma pharmacokinetics and tissue distribution of the breast cancer resistance protein (BCRP/ABCG2) inhibitor fumitremorgin C in SCID mice bearing T8 tumors. Cancer Chemother Pharmacol 55: 101–109, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gordon EL, Danielsson PE, Nguyen TS, Winn HR. A comparison of primary cultures of rat cerebral microvascular endothelial cells to rat aortic endothelial cells. In Vitro Cell Dev Biol 27A: 312–326, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Sugiyama Y. Expression and regulation of breast cancer resistance protein and multidrug resistance associated protein 2 in BALB/c mice. Biol Pharm Bull 29: 1032–1035, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s). J Neurochem 90: 526–536, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin 53: 5–26, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol 16: 593–600, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277: 2908–2915, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev 23: 687–702, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kubota H, Ishihara H, Langmann T, Schmitz G, Stieger B, Wieser HG, Yonekawa Y, Frei K. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res 68: 213–228, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol 94: 383–394, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain Res 1064: 21–31, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, Mercer KE, Zhuang Y, Panetta JC, Johnston B, Scheper RJ, Stewart CF, Schuetz JD. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol 24: 7612–7621, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2: 86–98, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mei Q, Richards K, Strong-Basalyga K, Fauty SE, Taylor A, Yamazaki M, Prueksaritanont T, Lin JH, Hochman J. Using real-time quantitative TaqMan RT-PCR to evaluate the role of dexamethasone in gene regulation of rat P-glycoproteins mdr1a/1b and cytochrome P450 3A1/2. J Pharm Sci 93: 2488–2496, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58: 361–372, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Pascussi JM, Drocourt L, Gerbal-Chaloin S, Fabre JM, Maurel P, Vilarem MJ. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes. Sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem 268: 6346–6358, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Perloff MD, von Moltke LL, Greenblatt DJ. Ritonavir and dexamethasone induce expression of CYP3A and P-glycoprotein in rats. Xenobiotica 34: 133–150, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Pulaski L, Kania K, Ratajewski M, Uchiumi T, Kuwano M, Bartosz G. Differential regulation of the human MRP2 and MRP3 gene expression by glucocorticoids. J Steroid Biochem Mol Biol 96: 229–234, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Regina A, Romero IA, Greenwood J, Adamson P, Bourre JM, Couraud PO, Roux F. Dexamethasone regulation of P-glycoprotein activity in an immortalized rat brain endothelial cell line, GPNT. J Neurochem 73: 1954–1963, 1999. [PubMed] [Google Scholar]
- 35.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86: 1179–1236, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96: 1698–1705, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuetz JD, Silverman JA, Thottassery JV, Furuya KN, Schuetz EG. Divergent regulation of the class II P-glycoprotein gene in primary cultures of hepatocytes versus H35 hepatoma by glucocorticoids. Cell Growth Differ 6: 1321–1332, 1995. [PubMed] [Google Scholar]
- 38.Shapiro AB, Corder AB, Ling V. P-glycoprotein-mediated Hoechst 33342 transport out of the lipid bilayer. Eur J Biochem 250: 115–121, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Teng S, Jekerle V, Piquette-Miller M. Induction of ABCC3 (MRP3) by pregnane X receptor activators. Drug Metab Dispos 31: 1296–1299, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci 94: 1169–1186, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Faucette SR, Gilbert D, Jolley SL, Sueyoshi T, Negishi M, LeCluyse EL. Glucocorticoid receptor enhancement of pregnane X receptor-mediated CYP2B6 regulation in primary human hepatocytes. Drug Metab Dispos 31: 620–630, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Wilson MW, Fraga CH, Fuller CE, Rodriguez-Galindo C, Mancini J, Hagedorn N, Leggas ML, Stewart CF. Immunohistochemical detection of multidrug-resistant protein expression in retinoblastoma treated by primary enucleation. Invest Ophthalmol Vis Sci 47: 1269–1273, 2006. [DOI] [PubMed] [Google Scholar]