Abstract
Ca2+/calmodulin (CaM)-dependent phosphorylation of myosin regulatory light chain (RLC) in smooth muscle by myosin light chain kinase (MLCK) and dephosphorylation by myosin light chain phosphatase (MLCP) are subject to modulatory cascades that influence the sensitivity of RLC phosphorylation and hence contraction to intracellular Ca2+ concentration ([Ca2+]i). We designed a CaM-sensor MLCK containing smooth muscle MLCK fused to two fluorescent proteins linked by the MLCK CaM-binding sequence to measure kinase activation in vivo and expressed it specifically in mouse smooth muscle. In phasic bladder muscle, there was greater RLC phosphorylation and force relative to MLCK activation and [Ca2+]i with carbachol (CCh) compared with KCl treatment, consistent with agonist-dependent inhibition of MLCP. The dependence of force on MLCK activity was nonlinear such that at higher concentrations of CCh, force increased with no change in the net 20% activation of MLCK. A significant but smaller amount of MLCK activation was found during the sustained contractile phase. MLCP inhibition may occur through RhoA/Rho-kinase and/or PKC with phosphorylation of myosin phosphatase targeting subunit-1 (MYPT1) and PKC-potentiated phosphatase inhibitor (CPI-17), respectively. CCh treatment, but not KCl, resulted in MYPT1 and CPI-17 phosphorylation. Both Y27632 (Rho-kinase inhibitor) and calphostin C (PKC inhibitor) reduced CCh-dependent force, RLC phosphorylation, and phosphorylation of MYPT1 (Thr694) without changing MLCK activation. Calphostin C, but not Y27632, also reduced CCh-induced phosphorylation of CPI-17. CCh concentration responses showed that phosphorylation of CPI-17 was more sensitive than MYPT1. Thus the onset of agonist-induced contraction in phasic smooth muscle results from the rapid and coordinated activation of MLCK with hierarchical inhibition of MLCP by CPI-17 and MYPT1 phosphorylation.
Keywords: myosin regulatory light chain, calmodulin, bladder, Rho kinase, myosin light chain phosphatase
an increase in intracellular calcium concentration ([Ca2+]i) initiates smooth muscle contraction through phosphorylation of myosin regulatory light chain (RLC) at Ser19 by Ca2+/calmodulin (CaM)-dependent myosin light chain kinase (MLCK) (15, 16, 37). The phosphorylation of RLC allows actin to activate the myosin ATPase leading to the consequent contraction (34). The Ca2+-dependent activation of MLCK plays an important role in force development in smooth muscle, and RLC phosphorylation is in part regulated by changes in [Ca2+]i. Myosin light chain phosphatase (MLCP) dephosphorylates RLC, resulting in inactivation of actin-activated ATPase. Thus the extent of RLC phosphorylation results from a balance of MLCK and MLCP activities.
The dependence of agonist-induced force and RLC phosphorylation on [Ca2+]i is less than that observed for depolarization in smooth muscle (2, 11) due to Ca2+-sensitization mechanisms (33). It is generally assumed, but yet unmeasured, that force is similarly sensitized to MLCK activation. Mechanisms responsible for Ca2+ sensitization in smooth muscle involve primarily inhibition of MLCP activity by coupling among ligand receptors, G proteins, and guanine nucleotide-binding factors (33). Two major pathways have been identified for inhibition of MLCP activity in smooth muscle. One involves activation of RhoA and Rho kinase through receptor coupling with mainly G12/13 heterotrimeric G proteins (35). Rho-kinase phosphorylates myosin phosphatase targeting subunit-1 (MYPT1) at Thr694 and/or Thr850 (mouse sequence), leading to inhibition of MLCP activity in smooth muscle cells and tissues (14, 25, 27, 32, 33). This phosphorylation has been reported to be involved in the tonic phase of force development (6, 39); however, the phosphorylation of both sites is not invariably detectable in all tissues (18, 27). A second pathway involves activation of PKC through receptor coupling with Gq/11 and phospholipase Cβ (17). CPI-17 is a 17-kDa polypeptide and potential mediator of Ca2+ sensitization where PKC phosphorylates CPI-17 in smooth muscle (7, 17, 19). Phosphorylation of CPI-17 Thr38 enhances its potency for inhibiting MLCP activity.
To understand how signaling mechanisms involving MLCK activation are integrated with MLCP inhibition in smooth muscle contraction, we developed a genetically encoded sensor for activation of MLCK. The CaM-sensor MLCK contains short smooth muscle MLCK fused to two fluorophores, enhanced cyan fluorescent protein (ECFP) and enhanced yellow fluorescent protein (EYFP), linked by the MLCK calmodulin-binding sequence. Upon dimerization there is significant fluorescence resonance energy transfer (FRET) from the donor ECFP (480 nm emission) to the acceptor EYFP (525 nm emission) (8, 13). This CaM-sensor MLCK is capable of directly monitoring Ca2+/CaM binding and activation of the kinase, where Ca2+-dependent CaM binding increases kinase activity coincident with a decrease in FRET. The CaM-sensor MLCK is expressed specifically in smooth muscle tissue of transgenic mice to obtain real-time and quantitative information on MLCK activation in vivo in relation to [Ca2+]i, RLC phosphorylation, and isometric force.
We previously reported that membrane depolarization with KCl induced a greater maximal increase in [Ca2+]i and MLCK activation than that obtained with the agonist carbachol (CCh) in bladder smooth muscle from the transgenic mice (13). However, the force development and RLC phosphorylation were comparable. In addition, the Rho-kinase inhibitor Y27632 decreased CCh-induced force while not significantly affecting MLCK activation (13). These results were consistent with the hypothesis that Rho-kinase activation inhibits MLCP to enhance the small extent of agonist-induced MLCK activation. However, the mechanism of this apparent increase in sensitivity to [Ca2+]i with CCh was not fully elucidated. Although some reports showed that MLCP inhibition was induced slowly during tonic contraction in response to agonist treatment (35), MLCP inhibition appeared to occur rapidly during phasic contraction elicited by CCh in the bladder (13). Other evidence also suggests that Rho-kinase modulates contraction in phasic smooth muscle (9, 28, 31, 38). It is proposed for intestinal smooth muscle that there is an initial [Ca2+]i transient leading to MLCK activation and then inactivation followed by MLCP inhibition to sustain RLC phosphorylation and contraction (26). Thus activation of MLCK would be temporally distinct from inhibition of MLCP. In the present study, we test the hypothesis that agonist-induced contraction results from rapid Ca2+ sensitization with phosphorylation of CPI-17 and MYPT1 simultaneous with Ca2+/CaM-dependent activation of MLCK.
MATERIALS AND METHODS
Transgenic mice with CaM-sensor MLCK expression specifically in smooth muscle tissue were bred and screened as previously described (8, 13). Expressed amounts of the MLCK CaM-sensor were <40% the content of endogenous kinase, and stresses generated by bladder strips from wild-type and transgenic animals were comparable (13). Transgenic animals showed no obvious phenotypic differences from wild-type (13). All animal protocols were approved by the University of Texas Southwestern IACUC.
Simultaneous measurement of fluorescence and CaM-sensor MLCK FRET or [Ca2+].
Mouse bladder tissues were obtained from 8- to 12-wk-old transgenic mice expressing CaM-sensor MLCK where simultaneous measurements of force and FRET or [Ca2+]i were made as previously described (13). Bladder tissues were dissected into strips (0.5 × 0.5 × 8.0 mm), mounted, and stretched (1.2 × slack length) on a force transducer in a quartz cuvette (180 μl) for simultaneous force and fluorescence measurements in physiological salt solution (PSS, in mM: 118.5 NaCl, 4.74 KCl, 1.18 MgSO4, 1.18 KH2PO4, 24.9 NaHCO3, 1.6 CaCl2, and 10.0 d-glucose containing 10 μM indomethacin, pregassed with 95% O2-5% CO2 at 36°C). For FRET measurements the muscle strips were illuminated with an excitation wavelength of 430 nm, and emission intensity was measured at both 480 and 525 nm to derive the ratio of fluorescence values (R480/525) in strips contracted with 65 mM KCl (KCl replacing equivalent NaCl in PSS) or 10 μM CCh. After incubation in relaxing solution [in mM: 20 PIPES, 5 magnesium methanesulfonate, 90 potassium methanesulfonate, 4 ATP, 4 EGTA, 1 dithiothreitol, 0.1 diisopropylfluorophosphate; 0.05 trans-epoxysuccinyl-l-leucylamido-(4-guanidino)-butane], muscle strips were skinned with 100 μM β-escin in relaxing solution. The minimum ratio of fluorescence (Rmin) was obtained by superfusion with the relaxing solution, whereas the maximum ratio (Rmax) and maximum force (Fmax) were obtained by adding CaCl2 to a final concentration of pCa 3.7.
Intracellular calcium concentrations were measured with Indo-1 in intact bladder strips as described previously (13). Urothelium and mucosa layers were removed from intact bladder tissue. Muscle strips were stretched to 1.2 slack length and incubated in the dark with PSS containing 10 μM indo-1 AM, 0.01% pluronic F-127, and 0.02% cremophor for 4 h at room temperature. After being mounted and washed with fresh PSS for 30 min at 36°C, strips were illuminated at 365 nm (D365/10X), and emission intensities were measured at 405 nm (D405/30) and 485 nm (D485/25). The ratio of fluorescence (R405/485) was determined and used to calculate [Ca2+]i. Maximal fluorescence was obtained by superfusion with 50 μM ionomycin in the presence of 5 mM Ca2+; minimal fluorescence was obtained with Ca2+-free PSS containing 2 mM EGTA. After each experiment, autofluorescence was determined by superfusing with 20 mM Mn2+.
Sample preparation.
Muscle strips were mounted on isometric force transducers (Grass FT03.C), stretched to 1.2 slack length, and subjected to drug treatments as described. Strips were quick frozen by clamps prechilled in liquid nitrogen after specific treatments for measurements of the extent of phosphorylation of RLC, CPI-17, and MYPT1. Frozen muscles were processed as previously described (13) in 10% trichloroacetic acid followed by centrifugation. The insoluble protein pellets were resuspended in 8 M urea sample buffer containing 18.5 mM Tris (pH 8.6), 20.4 mM glycine, 10 mM dithiothreitol, 4 mM EDTA, 5% sucrose, and 0.004% bromophenol blue and stored at −80°C.
Measurement of RLC and CPI-17 phosphorylation in bladder smooth muscle tissue.
Protein samples solubilized in urea sample buffer were subjected to urea-glycerol-PAGE at 400 V for 100 min (RLC) or 150 min (CPI-17) to separate nonphoshorylated and monophosphorylated protein forms. The urea-glycerol-PAGE system separates proteins by mass as well as charge. Phosphorylation results in the addition of two extra negative charges that increases the mobility of the protein during electrophoresis, which is capable of separating nonphosphorylated, monophosphorylated, and diphosphorylated RLC (4, 23). Smooth muscle tissues contain almost exclusively nonphosphorylated and monophosphorylated RLC, in contrast to cells in culture that contain significant amounts of diphosphorylated RLC. Kitazawa and colleagues (19) previously showed for CPI-17 that Thr38 was predominantly phosphorylated by PKC. Thus CPI-17 would be expected to show primarily nonphosphorylated and monophosphorylated forms on urea-glycerol PAGE. If there were additional sites phosphorylated in vivo, distinct diphosphorylated and triphosphorylated proteins would be present.
After electrophoresis, proteins were transferred to polyvinyldifluoride and fixed with 0.4% glutaraldehyde for 30 min. RLC or CPI-17 was visualized by immunoblot using a monoclonal antibody against mouse RLC (generous gift from Kathy Trybus; 21) or a rabbit polyclonal antibody against mouse CPI-17. The polyclonal antibody was raised in rabbits by standard procedures to purified recombinant mouse CPI-17 expressed in Escherichia coli with cDNA kindly provided by Masumi Eto. The antibody binds by Western blot analysis to CPI-17 from mouse, rabbit, pig, and rat (data not shown). The ratio of phosphorylated RLC or CPI-17 to total (nonphosphorylated and monophosphorylated) was determined by quantitative densitometry and expressed as moles of phosphate per moles of protein.
Measurement of MYPT1 phosphorylation.
Protein samples prepared in urea sample buffer were added to 0.2 volume of SDS sample buffer containing 250 mM Tris (pH 6.8), 10% SDS, 50 mM dithiothreitol, 40% glycerol, and 0.01% bromophenol blue, and then boiled and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose and visualized by immunoblot using antibodies to either total MYPT1 or phospho-MYPT1 at Thr694 or Thr850 (mouse sequence) (Upstate, Waltham, MA). The phosphorylation of MYPT1 is expressed as the ratio relative to that obtained with tissues treated for 1 min with 10 μM CCh in the presence of 5 μM okadaic acid (a protein phosphatase type 1 and type 2a inhibitor).
Statistics.
Statistical comparisons were performed by paired Student's t-test for [Ca2+]i and MLCK activation, independent t-test for force development, and phosphorylation of RLC, CPI-17, and MYPT1. Two-tailed values were used and P values <0.05 were considered significant.
RESULTS
Time course of responses to depolarization and agonist.
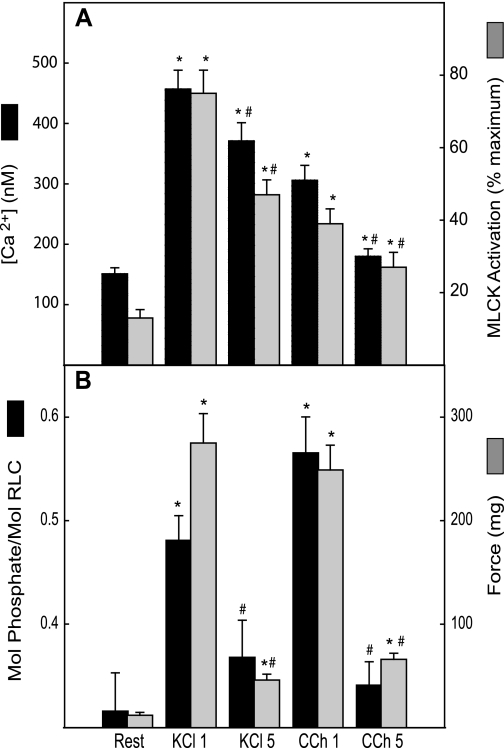
Both KCl and CCh elicit transient force development in the phasic bladder smooth muscle. Bladder strips were stimulated for 1 or 5 min with KCl (65 mM) or CCh (10 μM) and force, RLC phosphorylation, intracellular calcium, and MLCK activation were measured (Fig. 1). Rates of force development were similar for KCl and CCh with times to peak of 41 ± 5 s and 31 ± 7 s, respectively; forces at 1 min were near maximal at 87 ± 6% and 87 ± 3% (n = 8, 7), respectively. Agonist-dependent sensitization of force to [Ca2+]i and MLCK activation was well manifested at 1 min of stimulation where forces comparable to KCl were obtained by CCh at one-half the value of MLCK activation (Fig. 1). At 5 min of stimulation, force was reduced to low but significantly elevated values following reductions in [Ca2+]i and MLCK activation (Fig. 1). However, both [Ca2+]i and MLCK activation remained significantly elevated above resting values at 5 min. Agonist-dependent sensitization of force was also observed at 5 min where comparable forces were achieved with CCh compared with KCl at about one-half the value of MLCK activation.
Fig. 1.
Time course of responses to KCl and carbachol (CCh). Bladder strips were stimulated by 65 mM KCl for 1 or 5 min (KCl 1, KCl 5) or by 10 μM CCh for 1 or 5 min (CCh 1, CCh 5). Intracellular Ca2+ concentration ([Ca2+]i), myosin light chain kinase (MLCK) activation (A), regulatory light chain (RLC) phosphorylation, and force (B) were plotted. Values are means ± SE; n = 5–7 animals. *P < 0.05 when compared with value at rest; #P < 0.05 when compared with same treatment at 1 min.
Dependence of force on MLCK activation and RLC phosphorylation.
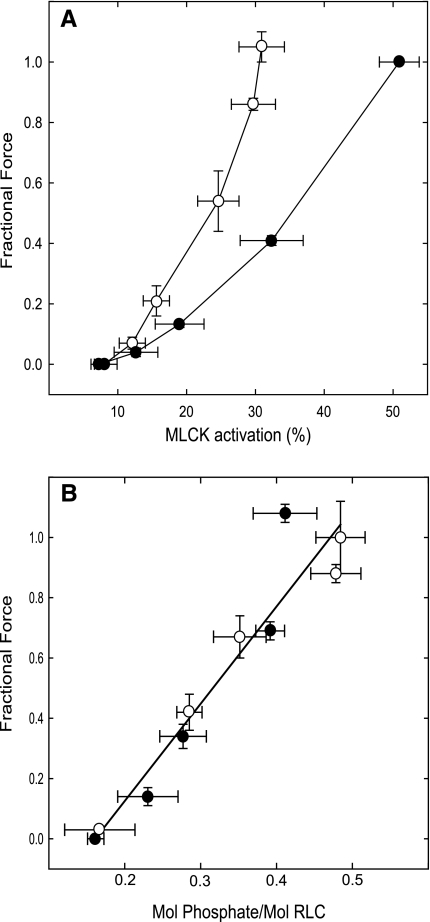
To more clearly define the sensitization of force to MLCK activation, bladder muscle strips from transgenic mice were stimulated for 1 min by different concentrations of KCl or CCh. After each stimulus, strips were washed with PSS and equilibrated for 15 min. The results illustrate agonist-dependent sensitization of force to MLCK activation, with the CCh relation lying to the left of that for KCl (Fig. 2A). In a similar set of experiments, strips stimulated for 1 min were frozen for measurement of RLC phosphorylation. Isometric force was generally linearly dependent on RLC phosphorylation for both CCh and KCl (Fig. 2B). This result is consistent with the hypothesis that the sensitization of force to MLCK activation arises from inhibition of phosphatase activity. Interestingly, the relationship between MLCK activation and force was not linear for either KCl or CCh, with higher concentrations eliciting proportionally greater force relative to MLCK activation. This was more pronounced for CCh and suggests that additional sensitization factors may come into play at higher agonist concentrations.
Fig. 2.
Dependence of force on MLCK activation and RLC phosphorylation in bladder smooth muscle stimulated by KCl or CCh. A: MLCK activation and force were measured in bladder muscle strips from transgenic mice stimulated with 30, 40, 50, and 65 mM KCl (•, n = 5) or with 0.3, 1, 3, 10, and 30 μM CCh (○, n = 8). Measurements were obtained at 1 min. Contraction was normalized by the 65 mM KCl-induced contraction for each strip. B: force was measured in bladder muscle strips stimulated with concentrations of KCl (•, n = 5) or CCh (○, n = 5) as described in A. Strips were frozen at 1 min and processed for measurement of RLC phosphorylation. Values are means ± SE.
Effects of depolarization and agonist on MYPT1 and CPI-17 phosphorylation.
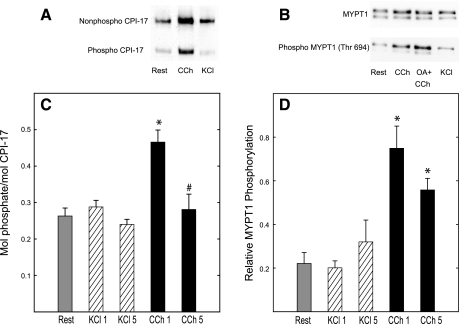
Myosin phosphatase activity is regulated by signaling pathways that target CPI-17 or MYPT1 for phosphorylation. Phosphorylation of these proteins was measured at 65 mM KCl and 10 μM CCh. The stoichiometry of CPI-17 phosphorylation was about 0.25 mol phosphate per mole at rest. KCl stimulation did not significantly change the extent of phosphorylation of CPI-17 or MYPT1 at either 1 or 5 min (Fig. 3), consistent with the action of KCl to depolarize smooth muscle cells without activation of cell surface receptors. CCh treatment significantly increased phosphorylation of CPI-17 at 1 min, whereas MYPT1 phosphorylation was increased at both at 1 and 5 min (Fig. 3). Western blot analyses show the two isoforms of MYPT1 with masses of 130 kDa and 110 kDa with both containing the phosphorylation site Thr694 (20, 33).
Fig. 3.
Phosphorylation of PKC-potentiated phosphatase inhibitor (CPI-17) and myosin phosphatase targeting subunit-1 (MYPT1) in bladder strips in response to 65 mM KCl or 10 μM CCh. A: Western blot analysis of tissue extracts subjected to urea-glycerol PAGE and detected with anti-CPI-17 antibody. Top band, nonphosphorylated CPI-17; bottom band, phosphorylated CPI-17. B: Western blot analysis of tissue extracts subjected to SDS PAGE and detected with anti-MYPT1 or anti-phospho-MYPT1 (Thr694) antibodies. C: phosphorylation of CPI-17 with KCl treatment at 1 and 5 min (KCl 1, KCl 5) or CCh treatment at 1 and 5 min (CCh 1, CCh 5), (n = 10–13). Ratio of phosphorylated CPI-17 to total (unphosphorylated and phosphorylated) is expressed as moles of phosphate per mole of protein. D: phosphorylation of MYPT1 with KCl or CCh treatment for times as indicated in C (n = 7–9). Phosphorylation of MYPT1 is expressed as the ratio relative to that stimulated with CCh in the presence of 5 μM okadaic acid (OA). Values are means ± SE. *P < 0.05 when compared with value at rest; #P < 0.05 when compared with same treatment at 1 min.
Effects of Rho-kinase and PKC inhibitors on CCh signaling to myosin regulatory proteins.
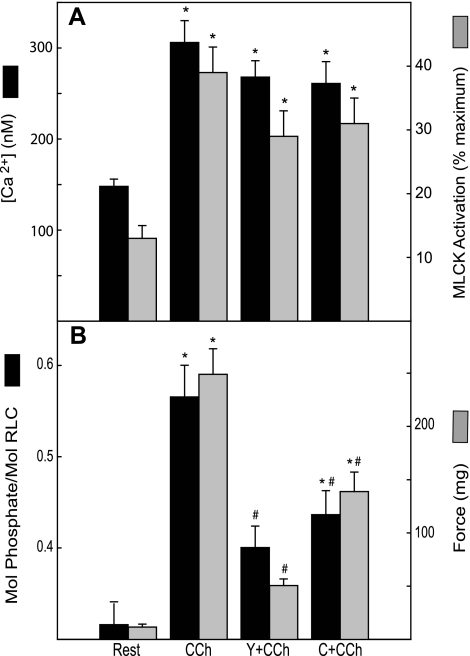
As shown above, CCh led to significant increases in [Ca2+]i, MLCK activation, RLC phosphorylation, and force after 1-min treatment in bladder strips. Pretreatment with Rho-kinase inhibitor Y27632 or the PKC inhibitor calphostin C significantly inhibited RLC phosphorylation and force responses to CCh without significantly affecting the values of intracellular calcium or the extent of MLCK activation (Fig. 4).
Fig. 4.
Effects of Y27632 or calphostin C on CCh-induced responses of bladder strips. [Ca2+]i and MLCK activation (n = 7) (A) and RLC phosphorylation and force (n = 5–7) (B) are plotted. Strips were preincubated 15 min with 10 μM Y27632 (Y+CCh) or 1 μM calphostin C (C+CCh) followed by 1 min with 10 μM CCh (CCh) as indicated. Values are means ± SE; n = 14. *P < 0.05 when compared with value at rest; #P < 0.05 when compared with CCh alone.
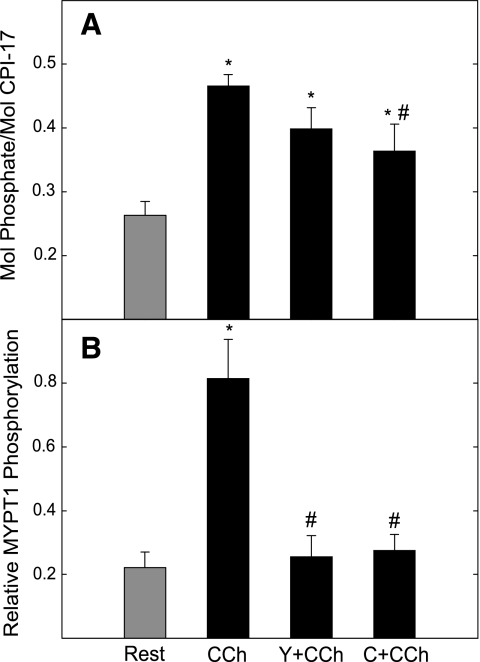
CCh-induced phosphorylation of CPI-17 was inhibited by the PKC inhibitor calphostin C but not by the Rho-kinase inhibitor Y27632 (Fig. 5). Both Y27632 and calphostin C reduced CCh-stimulated phosphorylation of MYPT1. Phosphorylation of neither CPI-17 nor MYPT1 was reduced below resting values by the inhibitors. Thus basal phosphorylation of these two proteins does not appear to be mediated by PKC or Rho-kinase.
Fig. 5.
Effects of Y27632 and calphostin C on phosphorylation of CPI-17 and MYPT1 in bladder strips in response to CCh. Strips were preincubated for 15 min with 10 μM Y27632 (Y+CCh) or 1 μM calphostin C (C+CCh) followed by 1 min with 10 μM CCh as indicated. Phosphorylations of CPI-17 (A) and MYPT1 (Thr694) (B) were assessed as described in Fig. 3. Values are means ± SE; n = 7–9. *P < 0.05 when compared with value at rest; #P < 0.05 when compared with CCh alone.
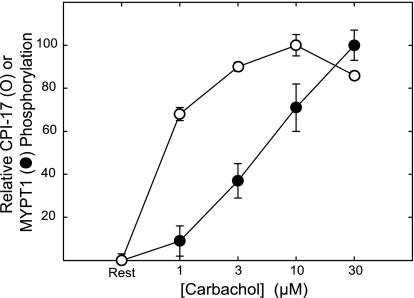
To test the hypothesis that increased sensitivity of force to MLCK activation with higher concentrations of CCh depends on recruitment of additional phosphatase inhibition, the dependence of CPI-17 and MYPT1 phosphorylation on CCh concentration was measured in a protocol similar to that described for Fig. 2. Results were normalized to compare the CCh sensitivities of CPI-17 and MYPT1 phosphorylation (Fig. 6). CPI-17 was significantly phosphorylated in response to 1 μM CCh, reaching a maximal value at 10 μM. In contrast, phosphorylation of MYPT1 (Thr694) increased significantly at greater concentrations of CCh. Estimated EC50 values were 0.7 and 5.0 μM for CPI-17 and MYPT1 phosphorylation, respectively. This differential recruitment may in part contribute to the increased sensitization seen at high concentrations of agonist.
Fig. 6.
Dependence of CPI-17 and MYPT1 phosphorylation on CCh concentration. Strips were incubated 1 min at indicated concentrations. The percent change in phosphorylation of CPI-17 (○) or MYPT1 (Thr694) (•) was calculated as the difference between resting and stimulated values and normalized by the maximal change observed. CPI-17 and MYPT1 phosphorylation values were determined as described in Fig. 3. Values are means ± SE; n = 5–7.
DISCUSSION
Previously we reported that CCh induced smaller increases in [Ca2+]i and MLCK activation than those induced with membrane depolarization by KCl in bladder smooth muscle, whereas force development and RLC phosphorylation were comparable. These results suggest that CCh enhanced the effect of MLCK activation through Ca2+-sensitization mechanisms (13). One general view is that MLCK activation occurs in the initial phase of a contraction with the sustained phase maintained by inhibition of MLCP (26, 33). In phasic intestinal smooth muscle it is proposed that a brief [Ca2+]i transient results in MLCK activation, but [Ca2+]i is quickly dissipated leading to MLCK inactivation while RLC phosphorylation and contractile force are maintained (26). By measuring MLCK activation directly in bladder tissue strips, we could evaluate the temporal relationships among these signaling processes. Similar to previous results (13) we find that CCh stimulation results in a fractional activation of MLCK that is smaller than results with KCl. However, in both cases, significant activation is maintained during the sustained phase of contraction.
Agonist-induced Ca2+ sensitization in smooth muscle is known to result from inhibition of MLCP activity primarily through signaling pathways that target CPI-17 and/or MYPT1 for phosphorylation (33). In this study, the Rho-kinase inhibitor Y27632 and PKC inhibitor calphostin C diminished RLC phosphorylation and force development without affecting MLCK activation, indicating that agonist-induced RLC phosphorylation and force result from not only MLCK activation but also involve Rho kinase and PKC activation. Elevated phosphorylation of MYPT1 and CPI-17 with CCh at 1 min supports the hypothesis that MLCP activity is inhibited by both MYPT1 and CPI-17 phosphorylation. CPI-17 phosphorylation was attenuated by calphostin C but not by Y27632, which is consistent with PKC-mediated phosphorylation. Interestingly, calphostin C did not reduce CPI-17 phosphorylation below that found in resting muscle, which was a significant amount (∼0.25 mol/mol). It has been reported that protein kinases other than PKC can phosphorylate CPI-17 in vitro: ZIP-like kinase (22), protein kinase N (10), integrin-linked kinase (5), p21-activated kinase (40), and Rho kinase (20). However, Y27632 had no effect on CPI-17 phosphorylation, suggesting that the transient phosphorylation induced by CCh and the extent of phosphorylation in resting muscle do not result from Rho-kinase activity. Furthermore, although CPI-17 phosphorylation occurred rapidly, it was not sustained. Thus CPI-17 phosphorylation would not be involved in Ca2+ sensitization during the latter phase of the contraction.
In contrast, MYPT1 phosphorylation at the inhibitory site Thr694 induced by CCh treatment was abolished by both calphostin C and Y27632, which were associated with attenuation of RLC phosphorylation and force. These results support a role for MYPT1 phosphorylation in inhibiting MLCP activity, although the calphostin C results are difficult to interpret mechanistically because PKC has not been reported to directly phosphorylate MYPT1. Our results with the bladder are consistent with those in another phasic smooth muscle, the ileum, where PKC inhibitors diminished MYPT1 phosphorylation (12). It is possible that PKC may affect some upstream signaling target that leads to MYPT1 phosphorylation by either Rho kinase or integrin-linked kinase (12). Recent studies reported direct or indirect interactions of PKC with RhoA in different cell types (1, 28, 30, 32), although the exact mechanism by which Rho kinase is activated by PKC remains elusive. It is possible that the decrease of MYPT1 phosphorylation by calphostin C results in part from inhibition of RhoA/Rho-kinase pathway via PKC activation.
The phosphorylation site of MYPT1 in smooth muscle stimulated with agonist needs to be considered. There are two major phosphorylation sites on MYPT1, Thr694 and Thr850, and several minor sites mediated by Rho-kinase activation (33). Thr694 phosphorylation by Rho kinase has been detected in a variety of cells including smooth muscle (14, 36, 38), and this phosphorylation is inhibited by Y27632. Previous studies show a positive correlation between Thr694 phosphorylation and force in smooth muscle tissue with agonist treatment (14, 29, 32). However, it is not invariably detectable in intact tissues showing Ca2+ sensitization (18, 27, 42). The reasons for this inconsistency are not clear. They may depend partly on the type of cells/tissues, methods of stimulation, or developmental regulation of MYPT1 (3). Additional analysis of data in different types of smooth muscle tissue stimulated with agonists that activate different signaling pathways may provide insights.
Depolarization by KCl did not lead to changes in phosphorylation of either Thr694 on MYPT1 or CPI-17 in the mouse bladder. However, KCl-dependent phosphorylation of MYPT1 or CPI-17 has been observed in vascular smooth muscle (6, 41). Preliminary data with the bladder indicate that, like Thr694, phosphorylation of Thr850 on MYPT1 was not changed with KCl treatment (0.25 ± 0.03 and 0.31 ± 0.05, control and stimulated 1 min, respectively). The dependence of force on MLCK activation with KCl is not entirely linear (Fig. 2A), suggesting that sensitization may occur at higher concentrations. Unlike the case for CCh, this effect does not appear to arise from inhibition of myosin phosphatase activity as neither MYPT1 nor CPI-17 phosphorylations are affected, and force is significantly elevated with no change in RLC phosphorylation between 50 and 65 mM KCl (Fig. 2B). Whereas this observation is tangential to the present study, it warrants speculation as to whether the effect reveals minor forms of regulation possibly mediated through actin-binding proteins caldesmon or calponin, though the physiological importance of thin filament regulation remains unclear (33). It is also worth noting a second aspect of the phasic contraction in mouse bladder, which is the apparent desensitization of contraction to calcium, as defined by the greater reduction in force than [Ca2+]i between 1 and 5 min, particularly evident with KCl. This phenomenon has been observed in skinned fibers of phasic smooth muscles as well and is shown to be calcium dependent, although the mechanism remains to be defined (24).
It is evident that the relative contributions of CPI-17 and MYPT1 in regulating MLCP activity differ depending on the time, type, and intensity of stimulation for a specific smooth muscle. In this study of phasic urinary bladder muscle, results suggest that both CPI-17 and MYPT1 participate in the early phase of sensitization, whereas only MYPT1 participates in the latter phase. This contrasts with tonic muscle, where only CPI-17 is phosphorylated in the early phase, and both CPI-17 and MYPT1 are phosphorylated in the sustained phase of contraction (6). Tonic, compared with phasic, smooth muscle contains relatively high ratios of CPI-17 to MYPT1, shows greater potentiation in response to activators of PKC, and thus utilizes CPI-17 extensively in regulating contraction (5, 43). Whereas phasic muscles may rely less on signaling to CPI-17, classification by tissue origin in rabbit revealed a gradation in PKC-dependent calcium sensitization where airway>bladder>vas deferens, with bladder using this pathway for nearly half the total response (43). We found at low agonist concentrations, only CPI-17 was phosphorylated, with MYPT1 phosphorylation recruited as CCh concentration was raised to higher values (Fig. 6). This may suggest that muscarinic receptors in the bladder signal more effectively through the Gq/11-phospholipase Cβ pathway than through the G12/13-RhoGEF pathway to inhibit myosin phosphatase. It is interesting to speculate that the accelerated sensitization seen at high CCh concentrations (Fig. 2) may result from the recruitment of phospho-MYPT1. The respective contributions of CPI-17 and MYPT1 to Ca2+ sensitization will depend on their expression levels as well as extents of phosphorylation, which varies in different types of smooth muscle tissues.
In conclusion, our genetically encoded CaM-sensor MLCK provides opportunities for investigations on physiological processes involved in smooth muscle contraction. Results from these studies in bladder smooth muscle suggest that agonist-induced contraction results from the rapid and coordinated activation of Ca2+/CaM-dependent MLCK that is significantly sustained as well as inhibition of MLCP through phosphorylation of CPI-17 and MYPT1.
GRANTS
This study was supported by a grant from the National Heart, Lung, Blood Institute (HL-26043), the Moss Heart Fund, and the Fouad A. and Val Imm Bashour Distinguished Chair in Physiology (to J. T. Stull).
DISCLOSURES
Current address of E. Isotani: Dept. of Neurosurgery, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyoku, Tokyo, 113-8519.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barandier C, Ming XF, Rusconi S, Yang Z. PKC is required for activation of ROCK by RhoA in human endothelial cells. Biochem Biophys Res Commun 304: 714–719, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Bradley AB, Morgan KG. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J Physiol 385: 437–448, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brozovich FV Myosin light chain phosphatase: it gets around. Circ Res 90: 500–502, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Colburn JC, Michnoff CH, Hsu LC, Slaughter CA, Kamm KE, Stull JT. Sites phosphorylated in myosin light chain in contacting smooth muscle. J Biol Chem 263: 19166–19173, 1988. [PubMed] [Google Scholar]
- 5.Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J 367: 517–524, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res 100: 121–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem (Tokyo) 118: 1104–1107, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Geguchadze R, Zhi G, Lau KS, Isotani E, Persechini A, Kamm KE, Stull JT. Quantitative measurements of Ca2+/calmodulin binding and activation of myosin light chain kinase in cells. FEBS Lett 557: 121–124, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Pinilla PJ, Gomez MF, Swärd K, Hedlund P, Hellstrand P, Camello PJ, Andersson KE, Pozo MJ. Melatonin restores impaired contractility in aged guinea pig urinary bladder. J Pineal Res 44: 416–425, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi T, Ito M, Feng J, Seko T, Koyama M, Machida H, Takase K, Amano M, Kaibuchi K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by protein kinase N. Biochem Biophys Res Commun 274: 825–830, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Himpens B, Casteels R. Different effects of depolarization and muscarinic stimulation on the Ca2+/force relationship during the contraction-relaxation cycle in the guinea pig ileum. Pflügers Arch 416: 28–35, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Ihara E, Moffat L, Ostrander J, Walsh MP, MacDonald JA. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 293: G699–G710, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci USA 101: 6279–6284, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Shimomura E, Iwanaga T, Shiraishi M, Shindo K, Nakamura J, Nagumo H, Seto M, Sasaki Y, Takuwa Y. Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F(2alpha)-induced contraction of rabbit aortae. J Physiol 546: 823–836, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25: 593–620, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem 275: 9897–9900, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546: 879–889, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in Triton X-100-demembranated rabbit arterial smooth muscle. J Physiol 520: 139–152, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koyama M, Ito M, Feng J, Seko T, Shiraki K, Takase K, Hartshorne DJ, Nakano T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett 475: 197–200, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Lau KS, Grange RW, Chang WJ, Kamm KE, Sarelius I, Stull JT. Skeletal muscle contractions stimulate cGMP formation and attenuate vascular smooth muscle myosin phosphorylation via nitric oxide. FEBS Lett 431: 71–74, 1998. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald JA, Eto M, Borman MA, Brautigan DL, Haystead TAJ. Dual Ser and Thr phosphorylation of CPI-17, an inhibotor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett 493: 91–94, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Hance WC, Miller JR, Wells JN, Stull JT, Kamm KE. Biochemical events associated with activation of smooth muscle contraction. J Biol Chem 263: 13979–13982, 1988. [PubMed] [Google Scholar]
- 24.Murahashi T, Fujita A, Kitazawa T. Ca2+-induced Ca2+ desensitization of myosin light chain phosphorylation and contraction in phasic smooth muscle. Mol Cell Biochem 190:91–98, 1999. [PubMed] [Google Scholar]
- 25.Muranyi A, Derkach D, Erdodi F, Kiss A, Ito M, Hartshorne DJ. Phosphorylation of Thr695 and Thr850 on the myosin phosphatase target subunit: inhibitory effects and occurrence in A7r5 cells. FEBS Lett 579: 6611–6615, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Murthy KS Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin-binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J 369: 117–128, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nozu F, Tsunoda Y, Ibitayo AI, Bitar KN, Owyang C. Involvement of RhoA and its interaction with protein kinase C and Src in CCK-stimulated pancreatic acini. Am J Physiol Gastrointest Liver Physiol 276: G915–G923, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Ohama T, Hori M, Sato K, Ozaki H, Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J Biol Chem 278: 48794–48804, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Pang H, Bitar KN. Direct association of RhoA with specific domains of PKC-α. Am J Physiol Cell Physiol 289: C982–C993, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Shabir S, Borisova L, Wray S, Burdyga T. Rho-kinase inhibition and electromechanical coupling in phasic smooth muscle; Ca2+-dependent and independent mechanisms. J Physiol 560: 839–855, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin HM, Je HD, Gallant C, Tao TC, Hartshorne DJ, Ito M, Morgan KG. Differential association and localization of myosin phosphatase subunits during agonist-induced signal transduction in smooth muscle. Circ Res 90: 546–553, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522: 177–185, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somlyo AV, Phelps C, Dipierro C, Eto M, Read P, Barrett M, Gibson JJ, Burnitz MC, Myers C, Somlyo AP. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J 17: 223–234, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: functional domains and structural motifs. Acta Physiol Scand 164: 471–482, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Swärd K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol 522: 33–49, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swärd K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep 5: 66–72, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Takizawa N, Koga Y, Ikebe M. Phosphorylation of CPI17 and myosin binding subunit of type 1 protein phosphatase by p21-activated kinase. Biochem Biophys Res Commun 297: 773–778, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Yoshioka K, Azam MA, Takuwa N, Sakurada S, Kayaba Y, Sugimoto N, Inoki I, Kimura T, Kuwaki T, Takuwa Y. Class II phosphoinositide 3-kinase α-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J 394: 581–592, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J 389: 763–774, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contractin in rabbit smooth muscle. J Physiol 535: 553–564, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]