Abstract
After renal transplantation, immunosuppressive regimens associated with high short-term survival rates are not necessarily associated with high long-term survival rates, suggesting that regimens may need to be optimized over time. Calcineurin inhibitor (CNI) withdrawal from a sirolimus-based immunosuppressive regimen may maximize the likelihood of long-term graft and patient survival by minimizing CNI-associated nephrotoxicity. In this study, a lifetime Markov model was created to compare the cost-effectiveness of a sirolimus-based CNI withdrawl regimen (sirolimus plus steroids) with other common CNI-containing regimens in adult de novo renal transplantation patients. Long-term graft survival was estimated by renal function and data from published studies and the US transplant registry, including short- and long-term outcomes, utility weights, and health-state costs were incorporated. Drug costs were based on average daily consumption and wholesale acquisition costs. The model suggests that treatment with sirolimus plus steroids is more efficacious and less costly than regimens consisting of a CNI, mycophenolate mofetil, and steroids; therefore, CNI withdrawal not only shows potential for long-term clinical benefits but also is expected to be cost-saving over a patient's life compared with the most commonly prescribed CNI-containing regimens.
The primary focus of immunosuppressive therapy in renal transplant patients is optimal management of the renal allograft. In the first year after transplantation, the primary clinical goal is to prevent acute rejection and graft failure. In subsequent years, transplant recipients should receive ongoing surveillance of graft function as well as reevaluation of the efficacy, toxicity, and costs of immunosuppressive regimens.1
Long-term deterioration of renal function with consequent cardiovascular disease progression and ultimately graft loss or patient death2 is the current challenge in kidney transplantation. These cascading events have not only clinical consequences but also economic implications. Prolonged dialysis and subsequent retransplantation are associated with increased direct and indirect costs that affect both society and individual patients.
Regimens associated with high short-term survival rates are not necessarily associated with high long-term survival rates. Thus, treatment with immunosuppressive regimens needs to be adapted over time to optimize short- and long-term outcomes. Calcineurin inhibitor (CNI) withdrawal regimens have been tested in de novo adult renal allograft patients as a means to mitigate the long-term nephrotoxic effect of CNI.3–5 The Rapamune Maintenance Regimen study (RMR), which evaluated sirolimus (SRL) plus steroids after withdrawal of cyclosporine A (CsA) at 3 mo, reported long-term improvement in renal function for up to 5 yr.4–9 Currently, SRL is the only immunosuppressive agent that has an indication for CNI withdrawal10; however, the immunosuppressive regimen of SRL plus steroids (SRL+ST) may be associated with higher risk for acute rejection 1 yr after transplantation and elevated lipid levels but with lower blood pressure,5,6 better graft survival,7 and no difference in cumulative incidence of acute rejection.4–7 It is unclear, a priori, what impact these features of SRL after withdrawing a CNI have on costs and life expectancy.
With limited clinical and economic resources and the magnitude of the costs for dialysis and posttransplantation care, the relationship between cost and efficacy (short-term acute rejection and long-term graft survival) of alternative immunosuppressive regimens becomes even more important for decision makers to consider. In the absence of complete and perfect information, economic modeling techniques are widely used to calculate cost-effectiveness. Such models enable decision makers to examine the effects of various therapies and their potential impact on costs and quality of life in a cost-efficient manner. Economic models are useful in that they provide a method to synthesize and analyze data from the best information to date and various sources, such as clinical trials, claims databases, and many other sources, to estimate the impact of diseases and treatments over varying lengths of time for varying patient populations. Sensitivity analysis of existing models is particularly useful to assess the robustness of parameters obtained from those external data sources. This affords researchers the opportunity to assess the validity of the assumptions and impact of less certain data inherent in these economic models. In the presence of these issues, we constructed a decision-analytic model to compare the cost-effectiveness of commonly prescribed immunosuppressive therapies in renal transplantation with a SRL-based CNI withdrawal regimen.
RESULTS
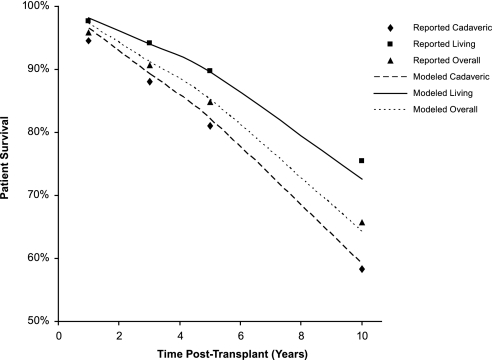
To ensure that the model accurately predicted real-world outcomes, we performed several validation analyses. Specifically, we compared the model's prediction of graft and patient survival with data reported in the published literature. For example, Figure 1 shows patient survival after transplantation over a 10-yr period, as reported in the Organ Procurement and Transplant Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) report,11 compared with patient survival after transplantation over a 10-yr period as estimated by the cost-effectiveness model. We observed that the model closely predicted patient survival. Graft survival may be underestimated by approximately 20% in the model. Graft survival probabilities in the model were estimated using the data from a study by Hariharan et al.12; however, because Hariharan et al. reported median graft half-life12 and a mean estimate was calculated in the model, the statistics may not be directly comparable. Median survival data were typically less than mean survival data, because the tail of the distribution generally pulled the mean to the right of the median. Thus, one might expect the mean estimate from the model shown here to be slightly greater than that of the data by Hariharan et al..12
Figure 1.
Reported patient survival compared with patient survival as estimated in the cost-effectiveness model. ♦, Reported cadaveric-donor patient survival; ▪, reported living-donor patient survival; ▴, reported patient survival for all donor types; dashed lines, modeled cadaveric-donor patient survival; solid lines, modeled living-donor patient survival; dotted lines, modeled patient survival for all donor types. Figure shows patient survival after transplantation for patients receiving grafts from various donor types as reported by the cost-effectiveness model. These data are compared with the patient survival after transplantation for patients receiving grafts from various donor types, as reported by the OPTN/SRTR, as an example of model predictability.
The overall results of the model showed that the CNI withdrawal regimen considered in the model, SRL+ST, increased patient survival (life-years), reduced graft loss, and, therefore, increased quality-adjusted life-years (QALY) when compared with CNI-containing regimens (Table 1). Total costs were lower for SRL+ST than for mycophenolate mofetil plus CsA plus ST (MMF+CsA+ST) or for MMF plus tacrolimus plus steroids (MMF+Tac+ST). Thus, SRL+ST was shown to be dominant (more efficacious and less costly) compared with MMF+Tac+ST (see Table 1).
Table 1.
Lifetime outcomes, costs, and incremental cost-effectiveness ratios versus MMF+Tac+ST for all renal transplant patients
| Parameter | SRL+ST | MMF+CsA+ST | MMF+Tac+ST |
|---|---|---|---|
| Outcomes per patient | |||
| graft loss | 0.90 | 0.94 | 0.92 |
| life-years | 11.43 | 11.37 | 11.13 |
| QALYa | 8.21 | 8.09 | 7.91 |
| Costs per patient | |||
| drug costsa | $68,678 | $69,300 | $88,752 |
| medical costsa | $404,121 | $414,720 | $416,668 |
| total costsa | $472,799 | $484,020 | $505,420 |
| incremental cost per QALY gained | Cost savingb | Cost savingb | – |
Discounted at 3% per annum.
Cost saving means that the regimen is more efficacious and less costly than MMF+Tac+ST.
Donor Type Subanalysis
In a subanalysis based on donor type, results were similar to those observed in all renal transplant patients. Patients who were treated with SRL+ST accrued greater QALYs than patients who were treated with CNI-containing regimens in grafts from living donors receiving CNI-containing regimens (SRL+ST = 9.74; MMF+CsA+ST = 9.54; and MMF+Tac+ST = 9.28), patients with grafts from deceased expanded-criteria donors (ECD; SRL+ST = 6.03; MMF+CsA+ST = 5.97; and MMF+Tac+ST = 5.82), or patients with grafts from deceased non-ECD (SRL+ST = 7.76; MMF+CsA+ST = 7.66; and MMF+Tac+ST = 7.51). These outcomes were gained at an overall lower total lifetime cost for living donors (SRL+ST = $497,940; MMF+CsA+ST = $510,000; and MMF+Tac+ST = $542,741), deceased ECD (SRL+ST = $419,548; MMF+CsA+ST = $430,976; and MMF+Tac+ST = $437,767), and deceased non-ECD (SRL+ST = $474,004; MMF+CsA+ST = $484,269; and MMF+Tac+ST = $501,166).
Sensitivity Analyses
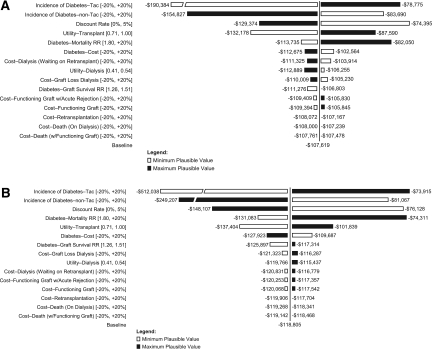
Results for one-way sensitivity analyses for all parameters, except serum creatinine concentrations, are shown in the form of tornado diagrams in Figure 2. Specifically, we examined the impact of the change in parameters on results in patients on SRL+ST compared with MMF+Tac+ST (Figure 2A) and MMF+CsA+ST compared with MMF+Tac+ST (Figure 2B). In this analysis, we observed that when the incidence of diabetes for patients on Tac was 20% lower than the assumed baseline estimate used in the model, the incremental cost per QALY gained was still cost saving for SRL+ST at −$78,775 and for MMF+CsA+ST at −$73,915 when compared with MMF+Tac+ST. Overall, the results were most sensitive to the chosen discount rate and diabetes-related parameters: Excess death as a result of diabetes, the incidence of diabetes, and diabetes costs.
Figure 2.
One-way sensitivity analysis of changes in the incremental cost per QALY. (A) Cost-effectiveness of SRL+ST versus MMF+Tac+ST. (B) Cost-effectiveness of MMF+CsA+ST versus MMF+Tac+ST. Tornado diagrams examine the changes in cost-effectiveness across the range of plausible values for each input.
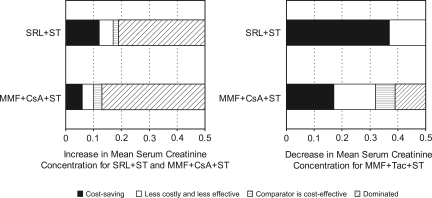
The results were found to be very sensitive to changes in serum creatinine level. These values were examined in greater detail. In this analysis, serum creatinine values were varied until cost-effectiveness thresholds were reached. When mean serum creatinine concentrations for patients on SRL+ST and MMF+CsA+ST were actually greater than assumed in baseline (also assuming serum creatinine for patients on MMF+Tac+ST did not change), we observed the ranges over which SRL+ST and MMF+CsA+ST became less costly and less efficacious, were cost effective, and were dominated by other regimens (more costly and less efficacious). As shown in Figure 3A, we observed that SRL+ST and MMF+CsA+ST remained cost saving compared with MMF+Tac+ST even when mean serum creatinine increased by 13 and 10%, respectively, from baseline and when the mean serum creatinine of MMF+Tac+ST remained constant.
Figure 3.
One-way sensitivity analysis of changes in the incremental cost per QALY versus MMF+Tac+ST for increases and decreases in the mean serum creatinine concentrations for model immunosuppressive regimens. (A) Increase in mean serum creatinine concentration for SRL+ST and MMF+CsA+ST with a stable value for MMF+Tac+ST. (B) Decrease in mean serum creatinine concentration for MMF+Tac+ST with stable values for SRL+ST and MMF+CsA+ST. Figures show a threshold analysis of changes in cost-effectiveness as increases or decreases in mean serum creatinine levels occur. In A, changes in cost-effectiveness are shown as mean serum creatinine increases for patients treated with SRL+ST and MMF+CsA+ST, while mean serum creatinine is maintained at its baseline value for patients treated with MMF+Tac+ST. In B, changes in cost-effectiveness are shown as mean serum creatinine decreases for patients treated with MMF+Tac+ST, while mean serum creatinine is maintained at its baseline value for patients treated with SRL+ST and MMF+CsA+ST.
In a different sensitivity analysis, as mean serum creatinine level decreased for patients who were on MMF+Tac+ST (assuming serum creatinine for patients on SRL+ST and MMF+CsA+ST remained unchanged), SRL+ST and MMF+CsA+ST remained cost saving at decreases of 48 and 27% in baseline serum creatinine, respectively (Figure 3B).
DISCUSSION
A wide variety of specific immunosuppressive regimens are used in actual clinical practice. Our model examines the cost-effectiveness of treating an average de novo renal transplant patient with specific immunosuppressive regimens, based on published clinical evidence using a lifetime horizon. Specifically, we compare the cost-effectiveness of an approved CNI withdrawal regimen, SRL+ST, with that of other frequently prescribed CNI-containing immunosuppressive therapies in renal transplantation. Despite the potential reduced benefit of preventing early acute rejection associated with the CNI withdrawal regimen, we observed overall improved long-term patient and graft survival. In addition, the model demonstrated that the CNI withdrawal regimen was cost saving (more efficacious and less costly) because of the long-term benefit through improved renal function. The model also showed an improvement in the expected cost-effectiveness of the use of the CNI withdrawal regimen in higher risk donor types over CNI-containing regimens.
This model builds on the current research in the published literature. A key feature is that the model uses renal function to predict future clinical benefit. This is important because this replicates real-world patient management and follows treatment guidelines.1 In addition, the model builds on real-world data extracted from OPTN/SRTR and the US Renal Data System (USRDS).11,13 This adds a greater relevance of the results of the model to actual clinical practice.
As with any cost-effectiveness analysis, a number of limitations exist. Decision makers may consider the model's being built around renal function—in particular, serum creatinine level—one such limitation. Clinically, GFR is a better determinant of renal function than serum creatinine level; however, we chose to build the model around serum creatinine because serum creatinine is more frequently used in clinical practice and more frequently reported in the clinical trials. In addition, although renal function may be more accurately measured using iothalamate clearance testing, this test is costly, uncommon, and of limited practical value.
In this model, a central assumption is that the relationship between serum creatinine and graft survival as derived by Hariharan et al.12 for patients on CNI-containing regimens applies to patients on CNI withdrawal regimens. We fully acknowledge the limitation of the extrapolation of this outcome, because it may not be acceptable to assume that the serum creatinine relationship found when on a CNI-containing regimen is the same as when a CNI is withdrawn. We acknowledge, for example, that other clinical issues (e.g., acute rejection) in addition to serum creatinine may be affected as a result of withdrawing a CNI and therefore are important to consider when predicting long-term outcomes. Moreover, it may be thought that serum creatinine levels at 12 mo may be temporary and may deteriorate over time such that basing renal function on 12-mo serum creatinine measurements may be inappropriate. In a recent study by Legendre et al.,9 we observed that renal function as measured by GFR for a CNI-containing regimen did deteriorate from 12 to 60 mo; however, patients on the CNI withdrawal regimen, SRL+ST, saw a slight improvement. Regardless, if the extrapolation is not accurate, then the improved long-term outcome that was estimated on the basis of the assumed relationship between serum creatinine and graft survival derived by Hariharan et al.12 could be misrepresented; however, there is no evidence thus far to suggest whether and/how this relationship differs for CNI withdrawal regimens. As data become available, it will be important to validate this assumption.
Another limitation is the interpretation of the renal function clinical outcome for patients on SRL+ST. The clinical study for SRL+ST was based on a randomized, controlled study by Johnson et al.6 in which the trial design randomly assigned patients at 3 mo after transplantation. Given this randomization approach, we adjusted serum creatinine projections for SRL+ST patients to include serum creatinine of non–randomly assigned patients within the trial to minimize bias toward better renal function in these patients. This adjustment placed serum creatinine for these patients in conformance with other clinical studies in which randomization was at the time of transplantation. A separate run of the cost-effectiveness analysis without the non–randomly assigned patient data showed further improvement in graft loss, patient survival, and cost-effectiveness for SRL+ST versus MMF+Tac+ST; however, we prefer the intention-to-treat analysis that included the outcomes of the non–randomly assigned patients; thus, we include these data in our primary analysis.
Additional attention may need to be paid to the concept of better renal function in common SRL-containing regimens. Multiple clinical studies that show better renal function with SRL and CNI withdrawal exist.3–5,14–17 Some recent studies examining CNI-free regimens have shown renal function similar to other regimens.18–20 For example, Larson et al.18 reported that there was no difference in corrected and uncorrected iothalamate clearance measurements at year 1 or 2 for patients on SRL+MMF+ST compared with patients on Tac+MMF+ST. The SYMPHONY trial reported that the low-dosage SRL arm (complete avoidance of a CNI) had the best GFR numberwise, but, statistically, GFR was the same as that seen in the usual-dosage CsA arm.19 Despite that these studies contain CNI-free regimens, the issue of improved serum creatinine for SRL-containing regimens without CNI may be in question.
Overall, because the level of uncertainty around 12-mo serum creatinine as a predictor of graft survival, sensitivity analyses around serum creatinine concentration were performed to examine the potential impact of alternative relationships on the results. Specifically, a threshold sensitivity analysis showed changes in the cost-effectiveness as the 12-mo mean serum creatinine increased or decreased from its assumed baseline values. Decision makers are cautioned to consider the assumptions around renal function and sensitivity analysis results when basing decisions on cost-effectiveness.
In addition to the key limitations discussed, this study has several other limitations. For example, we approached the analysis from a payer perspective rather than a societal perspective. Indirect costs, such as employment impact and caregiver burden, could have a substantial effect on the results of a parallel societal analysis; however, we chose not to consider indirect costs because of the limited availability of data and the number of assumptions that would be needed. Overall, in cases in which assumptions were necessary, we performed the base analysis with conservative estimates to avoid exaggeration of the potential benefits of treatment with a CNI withdrawal regimen, and we then performed extensive sensitivity analyses. As more data become available, it will be possible to improve further the model assumptions.
In conclusion, this study found that a CNI withdrawal regimen is expected to dominate the most commonly prescribed CNI-containing regimens (i.e., to produce superior outcomes at lower cost) over the life of a patient. The mechanism of this long-term advantage is due, in part, to the clinical benefit of improved renal function. In making decisions based on cost-effectiveness, transplant centers should consider the impact that improved renal function may have on lifetime costs and outcomes. Potential benefits of immunosuppressive regimens that may affect renal function could be substantial. The emphasis in kidney transplantation has shifted from short- to long-term outcomes, and clinicians consider the lifetime of the patient to be more important than the year that follows transplantation. As such, CNI withdrawal regimens should be considered as options that may maximize the lifetime benefits of renal transplantation.
CONCISE METHODS
We developed a decision-analytic model in Microsoft Excel to compare the cost-effectiveness of commonly prescribed immunosuppressive therapies with that of a CNI withdrawal therapy. The model was used to simulate a cohort of adult de novo renal transplant patients from the period after their first transplant until death (i.e., a lifetime horizon). Costs were examined from a payer perspective.
Data for the model were obtained from the published literature. Specifically, the literature was searched for clinical trials of commonly prescribed immunosuppressive therapies. In addition, general clinical issues associated with the use of immunosuppressive therapies in renal transplantation, not typically studied in most clinical trials (e.g., posttransplantation diabetes), and previous cost-effectiveness analyses were reviewed. Medline (1990 to 2006) was searched, using search terms such as “renal transplant,” “kidney transplant,” “mycophenolate mofetil,” “tacrolimus,” “sirolimus,” “diabetes,” “economic model,” and “costs.” Searches of bibliographies and expert opinions were used to identify additional published studies of relevance.
Model Structure
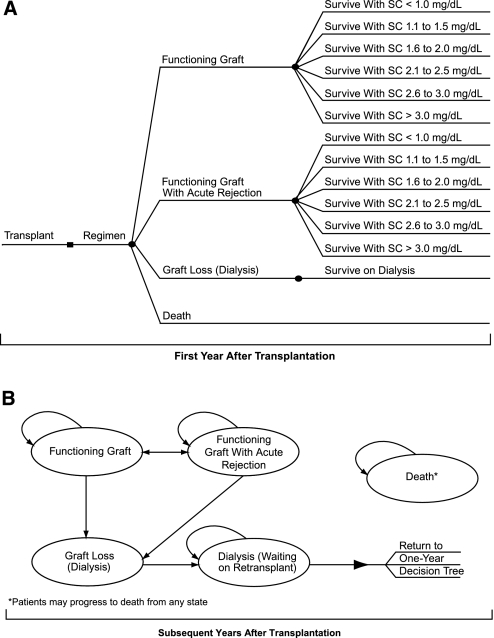
On the basis of the recommended clinical management of renal transplant patients,1 the model was composed of two parts. The first part focused on the treatment of patients in the first year after transplantation (Figure 4A) with specific reference to the management of acute rejection. One-year clinical outcomes in de novo patients were modeled within a decision-tree framework. On the basis of their renal function, patients surviving the first year after transplantation were transitioned into the second part of the model that focused on long-term patient and graft survival. The second part of the model used a Markov approach to transition patients annually into health states that were clinically and/or economically important to the patient and payer (Figure 4B). The full model structure is presented in Figure 4.
Figure 4.
Two-part model structure of maintenance of renal transplant: First-year and subsequent-year management. (A) Decision tree for the first year after transplantation. (B) Markov model for years subsequent to the first year after transplantation. Adult de novo transplant patients progress through one focused phase of treatment. In the first year after transplantation (phase 1: decision tree [A]), patient outcomes result in one of four outcomes. Surviving patients enter phase 2 (Markov [B]), on the basis of renal function measures, and progress through four health states for the remainder of their lifetimes. SC, serum creatinine level.
Model health states, similar to previously published economic analyses of renal transplantation,21–24 included functioning graft, functioning graft with acute rejection, graft loss (dialysis), dialysis (waiting on transplant), and death. Patients could transition back and forth from functioning graft and functioning graft with acute rejection in the Markov portion of the model. Patients in either of these states could transition to graft loss (dialysis) upon beginning dialysis treatment. Patients returning to dialysis subsequently transitioned to a waiting dialysis state (waiting on transplant) and could remain on dialysis treatment until death or retransplantation. Patients were allowed one additional transplant graft in the model. If a patient had a second transplant, then the individual entered the 1-yr decision tree again and the process repeated. Patients could enter the death state from any model state, including the decision tree permitting death with graft function, at any time.
Input Parameters
Input parameters such as mortality, clinical efficacy (acute rejection, graft loss, 12-mo serum creatinine level), other clinical events and treatments (e.g., diabetes or lipid-lowering medication), costs, and utilities were extracted from a variety of sources, including clinical trial data, the 2004 annual report of the OPTN/SRTR,11 the 2004 annual report of the USRDS,13 and other published literature. Commonly prescribed immunosuppressive regimens were chosen for this analysis. These included MMF+Tac+ST and MMF+CsA+ST. These regimens were compared with a CNI withdrawal regimen currently indicated for treatment in renal transplant patients, SRL+ST.
Patient Population
The model allowed for various subgroup population analyses. Specifically, three donor types were considered in the model: Living, deceased ECD, and deceased non-ECD. The distribution of patients obtaining grafts from each donor source was extracted from the OPTN/SRTR 2004 annual report.11 No induction use was assumed in this analysis. The cohort age at the start of the model is set at the average age (45.89 yr) of first-transplant patients.25 The probability of receiving a second transplantation each year after the failure of the first graft (12.75%) was estimated from the median waiting time of 5.08 yr from listing to transplantation, assuming an exponential distribution.11
Clinical Efficacy
The primary clinical effect of treatment regimens was assumed to be on first-year acute rejection and on graft loss in subsequent years. First-year acute rejection was estimated from published literature for each treatment regimen and adjusted for differences in patient populations. For comparison of immunosuppressive regimens on the basis of their clinical efficacy alone (i.e., no boost in reduction in acute rejection), acute rejection between immunosuppressive regimens was normalized to include no induction use. Specifically, acute rejection was adjusted from the percentage of patients taking and not taking specific induction agents as reported within the trials and by the expected reduction in acute rejection for a specific induction agent. Patients taking IL-2 receptor antagonists could expect their no-induction probability of acute rejection to be reduced by 28%,26 and patients treated with muromonab CD3 and equine antithymocyte globulin could expect their no-induction probability to be reduced by 9%.27–29 Calculated first-year, no-induction acute rejection probabilities for each regimen are presented in Table 2. The probability of acute rejection in year 2 after transplantation was assumed to be 2.86%30 and was assumed to decline linearly to a probability of 0% over a 10-yr time horizon. Although acute rejection may be considered a predictor of long-term outcome, we made the simplifying assumption in this analysis that the use of induction agents did not affect long-term clinical efficacy. Instead, we assumed that induction affected acute rejection as examined in the induction agent clinical trials.
Table 2.
Clinical efficacy inputs
| Regimen-Specific Inputs | SRL+ST | MMF+CsA+ST | MMF+Tac+ST | Sources |
|---|---|---|---|---|
| First-year acute rejection (%) | 21.80 | 19.00 | 17.10 | 6,30,46,47 |
| Serum creatinine concentration (mg/dl) 1 yr after transplantation (SD) | 1.78 (0.66) | 1.78 (0.76) | 1.20 (1.40) | 6,32,33 |
| Subsequent-year graft failure (%) | 7.20 | 7.62 | 7.60 | Calculation from mean serum creatinine 12 |
| Statin use (%) | 72.00 | 53.30 | 16.00 | 6,32,33 |
| Diabetes (%) | ||||
| year 1 | 14.20 | 14.20 | 22.10 | 34 |
| year 2 | 19.10 | 19.10 | 28.20 | 34 |
| year 3 and subsequent | 21.00 | 21.00 | 31.80 | 34 |
Because the focus of the first part of the model was acute rejection, probabilities for graft loss in the first year after transplantation were assumed to be the same for all immunosuppressive regimens and were extracted from the OPTN/SRTR 2004 annual report for each donor type.11 Graft loss in subsequent years was predicted by renal function as measured by serum creatinine levels.12,31 Renal function was represented by serum creatinine at 12 mo, because it is a commonly used measure in clinical practice and more frequently reported in the randomized, controlled trials. As a result, randomized trials reporting serum creatinine were selected. Specifically, means and SD for patient serum creatinine concentration at 12 mo were extracted from published clinical trial data for each treatment regimen (see Table 2).6,32,33 For patients on SRL+ST, serum creatinine was obtained from the study by Johnson et al.6 In this study, patients were randomly assigned at 3 mo after transplantation when they met eligibility criteria. Thus, biases in renal function in these patients may exist. For adjustment for any bias, serum creatinine concentrations for these patients were adjusted to reflect de novo randomization as in the clinical trials for the comparator immunosuppressive regimens. Specifically, the mean serum creatinine concentration and SD of randomly assigned patients at 12 mo were weighted with the mean serum creatinine concentration and SD of non–randomly assigned patients who were alive at 12 mo.
Serum creatinine was used to predict graft survival as derived by the relationship estimated by Hariharan et al.12 Assuming a normal distribution around the mean, the probability of patients’ being in each serum creatinine range reported by Hariharan et al.12 was calculated. Median graft half-life for each serum creatinine group and for each donor type was converted into an annual probability of graft loss, assuming time to graft loss followed an exponential distribution. We recognized that the relationship of renal function and graft survival, as derived by Hariharan et al.,12 was based on CNI-containing immunosuppressive regimens; however, evidence to suggest the extent to which the relationship of serum creatinine on graft survival differs for patients on a CNI withdrawal regimen is limited. In the absence of a predictor of long-term graft survival without CNI, we made the simplifying assumption that this relationship held true for all immunosuppressive regimens. Extensive sensitivity analyses around serum creatinine were performed to examine the impact of this assumption. Calculated graft failure probabilities for subsequent model years are displayed in Table 2. Graft failure was increased for patients with a second transplant by a relative risk (RR) of 1.09.13
Other Clinical Events
The model incorporated other important clinical events, such as increased triglyceride and/or cholesterol levels. We included these events by modeling the cost of lipid-lowering agents for each regimen. The need for lipid-lowering agents (assumed to be statins) was used as a surrogate marker of increased triglyceride and/or cholesterol levels. Prevalence of statin use for each immunosuppressive regimen was obtained from the published literature (see Table 2).6,32,33 In the cost-effectiveness model, it was assumed that patients on statin therapy at 12 mo remained on statin therapy until either graft loss or patient death. Long-term effects of cardiovascular disease, such as myocardial infarction, stroke, and death, were not modeled because of limited availability of data. Rather, long-term effects of cardiovascular disease between the CNI withdrawal and CNI-containing regimens were assumed to be controlled through the use of statins.
The model considered the incidence of and outcomes attributed to diabetes among patients treated with immunosuppressive drugs. These data were derived from Kasiske et al.,34 who report the cumulative incidence of diabetes at months 3, 12, and 36 after transplantation for patients receiving either Tac or no Tac. For estimation of the cumulative incidence of diabetes at 24 mo after transplantation, a logarithmic equation based on the 3-, 12-, and 36-mo values was used. The probability of diabetes at years 1, 2, and 3 are reported in Table 2 for each immunosuppressive regimen. The percentage of patients with diabetes at 3 yr after transplantation was assumed to remain constant throughout the life of the graft. Thus, conservatively, patients were assumed not to have developed diabetes when diabetes had not occurred within the first 3 yr after transplantation. For patients who developed diabetes, an RR for graft failure of 1.46 was applied.34
Mortality
Age-specific mortality for living-donor, deceased-ECD, and deceased–non-ECD transplant patients as well as for wait-listed patients was estimated from the OPTN/SRTR 2004 annual report,11 the USRDS 2004 annual report,13 and Wolfe et al.2 These data are summarized in Table 3. Because mortality increases as a result of the occurrence of diabetes, a mortality RR of 1.87 was applied to all age-specific base mortality rates for patients developing diabetes.34
Table 3.
Mortality by patient age group
| Mortality (Age Group) | Living Donor (%) | Deceased Non-ECD (%) | Deceased ECD (%) | Wait-List Dialysis (%) | Sources |
|---|---|---|---|---|---|
| 0 to 19 | 0.45 | 0.78 | 1.42 | 2.17 | 2,11,13 |
| 20 to 29 | 0.58 | 1.00 | 1.82 | 2.13 | |
| 30 to 39 | 0.91 | 1.58 | 2.89 | 3.37 | |
| 40 to 49 | 1.55 | 2.69 | 4.91 | 4.86 | |
| 50 to 59 | 2.40 | 4.16 | 7.59 | 7.51 | |
| 60 to 64 | 3.58 | 6.20 | 11.32 | 9.55 | |
| ≥65 | 5.52 | 9.56 | 17.47 | 14.74 |
Costs
Health-state costs, not including costs of immunosuppressive regimens, were derived from previous published literature and inflated to 2005 US dollars, using the medical component of the Consumer Price Index (Table 4).16,35–37 Costs were obtained primarily from a previous cost-effectiveness analysis reporting health-state costs obtained from the USRDS.35 These costs are Medicare-based costs and include all costs of care for patients within these health states. Patients treated with lipid-lowering agents were assumed to have been prescribed a generic statin (pravastatin [Pravachol] 40 mg/d). Additional annual medical costs as a result of diabetes in renal transplant patients (inflated to 2005 US dollars using the medical component of the Consumer Price Index, or $14,966) were obtained from Woodward et al.38 For examination of drug costs in a comparable setting, immunosuppressive drug costs were estimated from the daily allowable consumption, in milligrams, for each regimen from the Surveillance Data, Inc., data set of March 2005.39 The annual treatment regimen cost then was obtained from wholesale acquisition costs.40
Table 4.
Health-state costs and utility valuesa
| Health-State Costs and Utility Values | Cost | Utility Value | Sources |
|---|---|---|---|
| Functioning graft | $7473 | 0.84 | 24,35,41 |
| Functioning graft with acute rejection | $36,936 | 0.84 | 35,41 |
| Graft loss (dialysis) | $168,959 | 0.44 | 36,41 |
| Dialysis (waiting on retransplantation) | $47,855 | 0.44 | 35,41,43 |
| Transplantation | $75,379 | N/A | 35,41 |
| Death (with functioning graft) | $87,563 | 0.00 | 35,41 |
| Death (on dialysis) | $64,634 | 0.00 | 35 |
N/A, not applicable.
Utility Weights
Utility weights allow an objective measurement of the desirability of a health state in cost-effectiveness analyses. A utility of 1.0 represents perfect health, whereas a value of 0.0 represents death. When combined with life-years, utilities produce QALY.
In renal transplantation, few studies have specifically assessed utilities for patients. For economic evaluations of renal transplantation, two studies are often used: Churchill et al.41 and Laupacis et al.42 This analysis uses values from the study by Churchill et al.41 because it is the only study that separated utilities on the basis of the location and type of dialysis performed. Specifically, the utility for dialysis was calculated by the weighted average of dialysis types reported by Churchill et al.41 and the proportions of dialysis usage obtained from the USRDS annual report.13 Similar to other recent cost-effectiveness analyses in renal transplantation, no utility decrement was assumed to be associated with acute rejection, because the event is thought to be acute and transient.23,34,36,43,44 Utility values for the model are presented in Table 4.
Model Outcomes
The model estimated the following outcomes: Average immunosuppressive drug and other medical costs, patient survival (life-years), QALY, and the incremental cost per QALY gained. The incremental cost-effectiveness ratio (ICER) was used to compare the cost-effectiveness of treatment regimens and was calculated as follows:
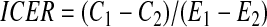 |
where C1 is the total costs incurred by patients on the regimen of interest, C2is the total costs incurred by patients on the base regimen, E1 is the total measure of effectiveness (e.g., QALY) accrued by patients on the regimen of interest, and E2 is the total measure of effectiveness accrued by patients on the base regimen. All costs are reported in 2005 US dollars. A discount rate of 3% per annum for both costs and out comes was assumed.45
Sensitivity Analyses
One-way sensitivity analyses were performed with the incremental cost per QALY as the outcome measure for the following parameters: Serum creatinine concentrations, diabetes probabilities and costs, health-state costs, discount rates, and utility values. Comparisons of SRL+ST versus MMF+Tac+ST and of MMF+CsA+ST versus MMF+Tac+ST were performed. The effect of varying individual parameters was examined using plausible ranges from values in the literature or an increase or a decrease of 20% of the baseline value for all model parameters except serum creatinine concentrations. One-way sensitivity analyses were presented in the form of tornado diagrams that show the range of cost-effectiveness values over the range of each input value. Because of the uncertainty around the effects of serum creatinine and long-term outcomes, we examined increases in SRL+ST and MMF+CsA+ST serum creatinine concentrations versus a static MMF+Tac+ST serum creatinine concentration as well as static SRL+ST and MMF+CsA+ST serum creatinine concentrations versus a decrease in MMF+Tac+ST serum creatinine concentration in a threshold analysis. Mean serum creatinine concentrations were increased or decreased to points at which the incremental cost per QALY changed quadrants in the cost-effectiveness plane (e.g., from cost saving to cost-effective, from cost-effective to less costly and less efficacious).
DISCLOSURES
Funding for this analysis was provided by Wyeth Pharmaceuticals (Collegeville, PA).
Acknowledgments
We acknowledge the following individuals who contributed to the Australia and UK economic models for sirolimus, which provided the basis for constructing the US model: Adam Gordois, Michael Nobes, Michaela Toohey, and Graeme Russ from Australia and Phil McEwan, Keshwar Baboolal, Pete Conway, and Craig J. Currie from the United Kingdom.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Practical Utility of an Economic Analysis of Calcineurin Withdrawal following Renal Transplantation,” on pages 1627–1628.
REFERENCES
- 1.Kasiske BL, Vazquez MA, Harmon WE, Brown RS, Danovitch GM, Gaston RS, Roth D, Scandling SD Jr, Singer GG: Recommendations for the outpatient surveillance of renal transplant recipients. J Am Soc Nephrol 11[Suppl 15]: S1–S86, 2000 [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Mulay AV, Hussain N, Fergusson D, Knoll BA: Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: A systematic review of randomized trials. Am J Transplant 5: 1748–1756, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Kreis H, Oberbauer R, Campistol JM, Mather T, Daloze P, Schena FP, Burke JT, Brault Y, Gioud-Paquet M, Scarola JA, Neylan JF: Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol 15: 809–817, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Oberbauer R, Kreis H, Johnson RW, Mota A, Claesson K, Ruiz JC, Wilczek H, Jamieson N, Henriques AC, Paczek L, Chapman J, Burke JT: Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-Year results of the Rapamune maintenance regimen study. Transplantation 76: 364–370, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Johnson RW, Kreis H, Oberbauer R, Brattstrom C, Claesson K, Eris J: Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation 72: 777–786, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Lengendre C, Claesson K, Castagneto M, Campistol JM, Hutchison B, Burke JT, Yilmaz S, Hayry P, Neylan JF: Sirolimus-based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. Am J Transplant 4: 953–961, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Oberbauer R, Segoloni G, Campistol JM, Kreis H, Mota A, Lawen J, Russ G, Grinyo JM, Stallone G, Hartmann A, Pinto JR, Chapman J, Burke JT, Brault Y, Neylan JF: Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transplant Int 18: 22–28, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Legendre C, Brault Y, Morales JM, Oberbauer R, Altieri P, Riad H, Mahony J, Messina M, Pussell B, Martinez JG, Lelong M, Burke JT, Neylan JF: Factors influencing glomerular filtration rate in renal transplantation after cyclosporine withdrawal using sirolimus-based therapy: A multivariate analysis of results at five years. Clin Transplant 21: 330–336, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Sirolimus [package insert], Philadelphia, Wyeth Pharmaceuticals Inc., 2007
- 11.US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: 2004 Annual Report, Transplant Data 1994–2003, Rockville, Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, and Richmond, United Network for Organ Sharing, and Ann Arbor, University Renal Research and Education Association, 2004
- 12.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System: 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2004
- 14.Weir MR, Ward MT, Blahut SA, Klassen DK, Cangro CB, Bartlett ST, Fink JC: Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int 59: 1567–1573, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Velosa JA, Larson TS, Gloor JM, Stegall MD: Cyclosporine elimination in the presence of TOR inhibitors: Effects on renal function, acute rejection, and safety. Am J Kidney Dis 38[Suppl 2]: S3–S10, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Suwelack B, Gerhardt U, Hohage H: Withdrawal of cyclosporine or tacrolimus after addition of mycophenolate mofetil in patients with chronic allograft nephropathy. Am J Transplant 4: 655–662, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Weir MR, Blahut S, Drachenburg C, Young C, Papademitriou J, Klassen DK, Cangro CB, Bartlett ST, Fink JC: Late calcineurin inhibitor withdrawal as a strategy to prevent graft loss in patients with suboptimal kidney transplant function. Am J Nephrol 24: 379–386, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, Gloor JM, Cosio FG, Lund WJ, Kremers WK, Nyberg SL, Ishitani MB, Prieto M, Velosa JA: Complete avoidance of calcineurin inhibitors in renal transplantation: A randomized trial comparing sirolimus and tacrolimus. Am J Transplant 6: 514–522, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kahn K: Low-dose tacrolimus superior to other immunosuppressives after renal transplant. Available at: http://www.medscape.com/viewarticle/542257. Accessed November 19, 2007
- 20.Flechner SM, Glyda M, Steinberg S, Copley JB, the ORION Trial Investigators: The efficacy of sirolimus (SRL) and tacrolimus (TAC) withdrawal versus SRL and mycophenolate mofetil (MMF) compared with TAC and MMF in de novo renal allograft recipients: Interim results from the Orion study [abstract]. J Am Soc Nephrol 18: 95A, 2007 [Google Scholar]
- 21.Gordois A, Nobes M, Tohhey M, Russ G: Cost-effectiveness of sirolimus therapy with early cyclosporine withdrawal vs. long-term cyclosporine therapy in Australia. Clin Transplant 20: 526–536, 2006 [DOI] [PubMed] [Google Scholar]
- 22.McEwan P, Baboolal K, Conway P, Currie CJ: Evaluation of the cost-effectiveness of sirolimus versus cyclosporine for immunosuppression after renal transplantation in the United Kingdom. Clin Ther 27: 1834–1846, 2005 [DOI] [PubMed] [Google Scholar]
- 23.McEwan P, Dixon S, Baboolal K, Conway P, Currie C: Evaluation of the cost-effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the United Kingdom. Pharmacoeconomics 24: 67–79, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Jassal SV, Krahn MD, Nagle G, Zaltzman JS, Roscoe JM, Cole EH, Redelmeier DA: Kidney transplantation in the elderly: A decision analysis. J Am Soc Nephrol 14: 187–196, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Irish W, Sherrill B, Brennan DC, Lowell J, Schnitzler M: Three-year post-transplant graft survival in renal transplant patients receiving tacrolimus or cyclosporine microemulsion within a triple drug regimen. Transplantation 76: 1686–1690, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Bunnapradist S, Takemoto SK: Multivariate analysis of antibody induction therapy and their associated outcomes in deceased donor transplants. Transplant Proc 37: 889–891, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Castro MC, Araujo LM, Nahas WC, Arap S, David-Neto E, Ianhez LE: Induction versus noninduction therapy in kidney transplantation: Considering different PRA levels and different induction therapies. Transplant Proc 36: 874–876, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Kode R, Fa K, Chowdhury S, Ranganna K, Fyfe B, Stabler S, Damask A, Laftavi MR, Kumar AM, Pankewycz O: Basiliximab plus low-dose cyclosporin vs. OKT3 for induction immunosuppression following renal transplantation. Clin Transplant 17: 369–376, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Heifets M, Saeed MI, Parikh MH, Sierka D, Kumar MS: Induction immunosuppression in kidney transplant recipients older than 60 years of age: Safety and efficacy of ATGAM, OKT3 and Simulect. Drugs Aging 21: 747–756, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Pascual J, Marcen R, Ortuno J. Renal function: Defining long-term success. Nephrol Dial Transplant 19[Suppl 6]: vi3–vi7, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Flechner SM, Goldfarb D, Modline C, Feng J, Krishnamurthi V, Mastroianni B, Savas K, Cook DJ, Novick AC: Kidney transplantation without calcineurin inhibitor drugs: A prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 74: 1213–1220, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, Nicolas M, Ruiz P, Rosen A, Miller J: A randomized long-term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation, II: Survival, function, and protocol compliance at 1 year. Transplantation 77: 252–258, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ: Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 3: 178–185, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Matas AJ, Schnitzler MA: Payment for living donor (vendor) kidneys: A cost-effectiveness analysis. Am J Transplant 4: 216–221, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Schnitzler MA, Whiting JF, Brennan DC, Lin G, Chapman W, Lowell J, Boxerman S, Hardinger KL, Kalo Z: The expanded criteria donor dilemma in cadaveric renal transplantation. Transplantation 75: 1940–1945, 2003 [DOI] [PubMed] [Google Scholar]
- 37.US Department of Labor, Bureau of Labor Statistics: Consumer Price Index—All Urban Consumers (Current Series). Available at: http://data.bls.gov/PDQ/outside.jsp?survey=cu. Accessed July 15, 2006
- 38.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC: Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant 3: 590–598, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Surveillance Data Inc.: Rapamune Dosing Study [data on file], Plymouth Meeting, PA, Surveillance Data Inc., 2005
- 40. Red Book for Windows, version 61127 (CD-ROM), Greenwood Village, CO, Thomson Healthcare
- 41.Churchill DN, Torrance GW, Taylor DW, Barnes CC, Ludwin D, Shimizu A, Smith EK: Measurement of quality of life of end-stage renal disease: The time trade-off approach. Clin Invest Med 10: 14–20, 1987 [PubMed] [Google Scholar]
- 42.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Mutinga N, Brennan DC, Schnitzler MA: Consequences of eliminating HLA-B in deceased donor kidney allocation to increase minority transplantation. Am J Transplant 5: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yen E, Hardinger K, Brennan DC, Woodward RS, Desai NM, Crippin JS, Gage BF, Schnitzler MA: Cost-effectiveness of extending Medicare coverage of immunosuppressive medications to the life of a kidney transplant. Am J Transplant 4: 1703–1708, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Gold MR, Siegel JE, Russell LB, Weinstein MC: Cost-Effectiveness in Health and Medicine, New York, Oxford University Press, 1996
- 46.Meier-Kriesche HU, Steffen BJ, Chu AH, Loveland JL, Gordon RD, Morris JA, Kaplan B: Sirolimus with NEORAL versus mycophenolate mofetil with NEORAL is associated with decreased renal allograft survival. Am J Transplant 4: 2058–2066, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S, the Prograf Study Group: A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: Results at 1 year. Transplantation 80: 303–309, 2005 [DOI] [PubMed] [Google Scholar]