Abstract
An inverse relationship between arterial calcifications and bone activity has been documented in patients with ESRD. Calcium overload is associated with arterial calcification, which is associated with arterial stiffening. Whether bone activity interacts with calcium load, aortic stiffness, or arterial calcification is unknown. This study assessed the impact of bone activity on the relationships between the dosage of calcium-containing phosphate binders and aortic stiffness (measured by pulse wave velocity) or abdominal aorta calcification score. Aortic stiffness and calcification were both positively associated with calcium load and negatively associated with bone activity. A significant interaction was found between dosage of calcium-containing phosphate binders and bone activity such that calcium load had a significantly greater influence on aortic calcifications and stiffening in the presence of adynamic bone disease. Independent of any other factor, including dosage of calcium-containing phosphate binders, adynamic bone was associated with greater aortic stiffening, suggesting cross-talk between the bone and arterial walls.
Arterial calcifications (AC) are a common complication of chronic kidney (CKD) and ESRD. 1–4 In the general population and patients with ESRD, the extents of AC were associated with aortic stiffening and were predictive of subsequent cardiovascular disease and mortality beyond established conventional risk factors.5–8 The mechanisms responsible are complex, because AC is a regulated process with plasma constituents maintaining minerals in solution and inhibiting their deposition in tissues.9 An inverse relationship between AC and bone density was documented in general populations,10,11 and in patients with ESRD, the extent of AC was associated with low bone activity and adynamic bone disease (ABD).12 Disturbances in calcium (Ca) and phosphate (PO4) metabolism are associated with uremic bone disease, and the results of several studies indicated that Ca overload is associated with AC presence and progression.4,13 All of these observations strongly suggest complex interplay among bone activity, Ca load, and AC development, but whether the bone activity is involved in Ca load influence on AC is unknown. Aortic stiffness, a predictor of all-cause and cardiovascular mortality in ESRD, is associated with aortic calcifications (AoC), but whether it is influenced by Ca load and bone activity is not known. This study was designed to evaluate the relationships and interaction between bone activity, as assessed by bone histomorphometry, and the use of Ca-containing PO4 binders with the extent of abdominal aortic AoC and aortic stiffness assessed by pulse wave velocity (PWV) in patients who had ESRD and were undergoing chronic hemodialysis.
RESULTS
Characteristics of the Study Population
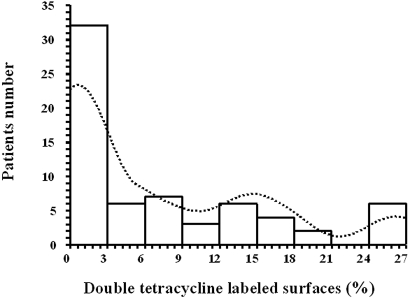
The distribution of the percentage of double tetracycline labeling is shown in Figure 1. Aortic, demographic, clinical, biochemical, and bone histomorphometry characteristics as a function of bone activity are summarized in Table 1. Patients with ABD were older and characterized by significantly higher abdominal aortic calcification scores (AoCS) and aortic PWV, lower serum albumin, higher C-reactive protein, and lower parathormone (PTH). Serum PO4 levels were comparable, but Ca-containing PO4 binder dosages were higher for patients with ABD, and more of them had surgical parathyroidectomy (PTX)-related hypoparathyroidism. Primary kidney diseases were comparably distributed in the two groups. All bone activity and remodeling indexes were significantly lower for patients with ABD, whereas their trabecular and osteoid volumes did not differ from those with active bone.
Figure 1.
Histogram showing the number of patients with double tetracycline–labeled surface values.
Table 1.
Aortic, clinical, biochemical, and bone characteristics of the 66 patients with ESRDa
| Variable | Active Bone(n = 33) | Adynamic Bone(n = 33) | P |
|---|---|---|---|
| AoCS (mean ± SEM) | 3.60 ± 0.80 | 12.00 ± 1.10 | <0.0001 |
| Aortic PWV (m/s; mean ± SEM) | 9.43 ± 0.35 | 12.52 ± 0.38 | <0.0001 |
| Age (yr; mean ± SEM) | 43.00 ± 2.60 | 53.60 ± 1.60 | <0.0010 |
| Gender (male/female) | 16/17 | 17/16 | NS |
| Smoking (pack-years; median [95% CI]) | 5.00 (0.00 to 16.00) | 3.50 (0.00 to 9.00) | NS |
| Vintage (mo; mean ± SEM) | 80.00 ± 8.50 | 90.00 ± 11.40 | NS |
| SBP (mmHg; mean ± SEM) | 154.00 ± 4.20 | 158.50 ± 3.60 | NS |
| DBP (mmHg; mean ± SEM) | 85.60 ± 2.40 | 84.20 ± 2.30 | NS |
| Antihypertensive drugs (no/yes) | 11/22 | 11/22 | NS |
| Total cholesterol (mmol/L; mean ± SEM) | 5.07 ± 0.25 | 5.21 ± 0.18 | NS |
| Serum albumin (g/L; mean ± SEM) | 39.7 ± 0.40 | 37.5 ± 0.40 | <0.0010 |
| CRP (mg/L; median [95% CI]) | 3.00 (2.00 to 5.00) | 9.00 (7.00 to 11.00) | <0.0010 |
| Serum Ca (mmol/L; mean ± SEM) | 2.43 ± 0.03 | 2.43 ± 0.03 | NS |
| Ionized Ca (mmol/L; mean ± SEM) | 1.23 ± 0.01 | 1.20 ± 0.01 | NS |
| Serum PO4 (mmol/L; mean ± SEM) | 1.92 ± 0.06 | 2.03 ± 0.07 | NS |
| Ca*PO4 (mmol2/L2; mean ± SEM) | 4.67 ± 0.15 | 4.95 ± 0.19 | NS |
| PTH (pg/ml; median [95% CI]) | 420.00 (327.00 to 549.00) | 142.00 (57.00 to 570.00) | <0.0001 |
| Serum Al (μmol/L; mean ± SEM) | 1.28 ± 0.14 | 1.60 ± 0.17 | NS |
| Deferoxamine test (ΔAl μmol/L; mean ± SEM) | 2.03 ± 0.28 | 2.60 ± 0.28 | NS |
| CaCO3 (g elemental Ca/d; mean ± SEM) | 1.47 ± 0.20 | 1.97 ± 0.14 | 0.0200 |
| 1α-OH-D3 (μg/d; median [95% CI]) | 0.25 (0.00 to 0.25) | 0.00 (0.00 to 0.25) | NS |
| Resorption surfaces (%; median [95% CI]) | 2.83 (2.18 to 3.47) | 0.19 (0.00 to 0.50) | <0.0001 |
| Osteoclasts/mm2; (median [95% CI]) | 2.14 (1.31 to 2.69) | 0.18 (0.09 to 0.40) | <0.0001 |
| Osteoblastic surfaces (%; median [95% CI]) | 13.20 (10.50 to 15.00) | 1.17 (0.42 to 2.12) | <0.0001 |
| Double tetracycline–labeled surfaces (%; median [95% CI]) | 13.50 (8.00 to 16.0) | 0.00 (0.00 to 0.00) | <0.0001 |
| Trabecular volume (%; median [95% CI]) | 21.2 (15.3 to 23.8) | 19.5 (15.4 to 29.1) | NS |
| Osteoid surface (%; median [95% CI]) | 53.5 (44.0 to 64.0) | 43.5 (33.0 to 58.0) | NS |
| Al-stained surfaces (%; median [95% CI]) | 6.0 (0.0 to 23.0) | 46.0 (17.0 to 75.0) | <0.0100 |
| Post-PTX hypoparathyroidism (yes/no) | 0/33 | 8/25 | 0.0020 |
| Glomerulonephritis (n) | 15 | 11 | NS |
| CPNPh, interstitial nephritis (n) | 11 | 10 | NS |
| Polycystic kidney disease (n) | 3 | 8 | NS |
| Nephroangiosclerosis (n) | 3 | 2 | NS |
| Diabetes (n) | 1 | 2 | NS |
Reference values: Resorption surfaces (%): 0.4 ± 0.3; osteoclasts/mm2: 0.15 ± 0.05; osteoblastic surfaces (%): 4 ± 2; trabecular volume (%): 15 to 22; osteoid surface (%): 15 ± 5. CI, confidence interval; CPNPh, chronic pyelonephritis; CRP, C-reactive protein; DBP, diastolic BP; SBP, systolic BP.
Table 2 gives the characteristics of AoC-negative and AoC-positive patients with ESRD. AoC-negative patients were younger, had shorter hemodialysis vintage, and smoked less. They had significantly higher bone activity, less pronounced microinflammation, and lower Ca-containing PO4 binder doses. Aortic PWV was significantly lower in AoC-negative than in AoC-positive patients.
Table 2.
Characteristics of the 66 patients with ESRD as a function of AoC status
| Variable | AoC Negative(n = 18) | AoC Positive(n = 48) | P |
|---|---|---|---|
| Age (yr; mean ± SEM) | 32.80 ± 2.50 | 53.60 ± 1.40 | <0.00010 |
| Aortic PWV (m/s; mean ± SEM) | 8.48 ± 0.38 | 11.63 ± 0.35 | <0.00010 |
| Smoking (pack-years; median [95% CI]) | 0.00 (0.00 to 5.00) | 5.00 (1.00 to 15.00) | <0.01000 |
| Vintage (mo; mean ± SEM) | 59.00 ± 7.90 | 94.00 ± 9.10 | 0.01000 |
| SBP (mmHg; mean ± SEM) | 151.00 ± 6.20 | 158.00 ± 3.10 | NS |
| DBP (mmHg; mean ± SEM) | 86.00 ± 3.80 | 86.00 ± 1.80 | NS |
| Total cholesterol (mmol/L; mean ± SEM) | 4.87 ± 0.30 | 5.18 ± 0.16 | NS |
| Serum albumin (g/L; mean ± SEM) | 40.80 ± 0.40 | 37.90 ± 0.30 | <0.00010 |
| CRP (mg/L) | 2.00 (1.00 to 3.00) | 8.00 (7.00 to 10.00) | <0.00010 |
| Serum Ca (mmol/L; mean ± SEM) | 2.40 ± 0.04 | 2.43 ± 0.02 | NS |
| Ionized Ca (mmol/L; mean ± SEM) | 1.23 ± 0.01 | 1.21 ± 0.01 | NS |
| Serum PO4 (mmol/L; mean ± SEM) | 1.81 ± 0.06 | 2.06 ± 0.06 | <0.01000 |
| Ca*PO4 (mmol2/L2; mean ± SEM) | 4.35 ± 0.17 | 5.00 ± 0.15 | <0.01000 |
| PTH (pg/ml; median [95% CI]) | 411.00 (229.00 to 670.00) | 206.00 (160.00 to 291.00) | <0.00001 |
| Serum Al (μ mol/L; mean ± SEM) | 0.96 ± 0.13 | 1.62 ± 0.14 | <0.01000 |
| Deferoxamine test (ΔAl μ mol/L; mean ± SEM) | 1.48 ± 0.26 | 2.70 ± 0.24 | <0.01000 |
| CaCO3 (g of elemental Ca/d; mean ± SEM) | 1.270 ± 0.300 | 1.900 ± 0.132 | 0.02000 |
| 1α-OH-D3 (μ g/wk; median [95% CI]) | 0.25 (0.00 to 0.50) | 0.00 (0.00 to 0.25) | NS |
| Resorption surfaces (%; median [95% CI]) | 2.56 (1.71 to 3.85) | 0.68 (0.27 to 1.40) | <0.00100 |
| Osteoclasts/mm2 (median [95% CI]) | 1.70 (1.05 to 2.70) | 0.47 (0.28 to 1.00) | <0.00100 |
| Osteoblastic surfaces (%; median [95% CI]) | 13.00 (10.20 to 15.20) | 3.40 (1.20 to 7.30) | <0.00100 |
| Double tetracycline–labeled surfaces (%; median [95% CI]) | 15.00 (9.00 to 18.50) | 1.30 (0.00 to 3.20) | <0.00100 |
| Al-stained surfaces (%; median [95% CI]) | 0.00 (0.00 to 7.50) | 28.50 (17.00 to 50.30) | <0.01000 |
| Post-PTX hypoparathyroidism (yes/no) (n) | 0/18 | 8/40 | <0.01000 |
Bone Activity, “Ca Load,” and Aortic Changes
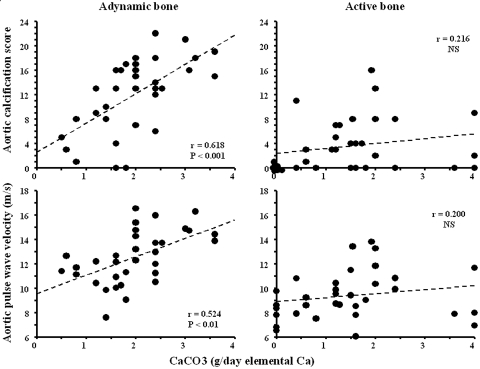
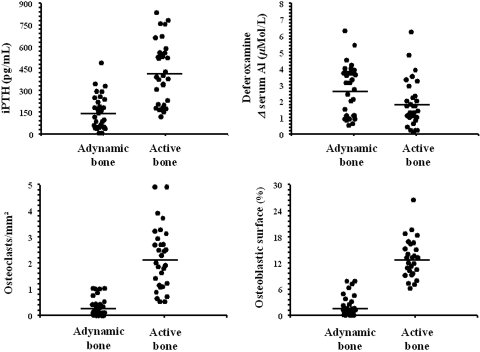
The univariate correlation between the daily elemental Ca dosage provided by CaCO3 and AoCS or aortic PWV according to bone activity is shown in Figure 2. Significant positive correlations between Ca dosage and AoCS or aortic PWV were observed for patients with ABD. Although biochemistry parameters, such as PTH, differed significantly between the two groups, Figure 3 shows that values overlapped between the two groups and a large proportion of patients with Kidney Disease Outcomes Quality Initiative (KDOQI)-recommended PTH levels (150 to 300 pg/ml) had ABD. The extent of overlapping was minimized when analyzed as a function of bone dynamics (Figure 3).
Figure 2.
Correlations between daily CaCO3 dosage expressed in grams of elemental Ca and AoCS or aortic PWV for patients with active bone and those with ABD.
Figure 3.
Biochemistry and bone histomorphometry differences between patients with active bone and those with ABD.
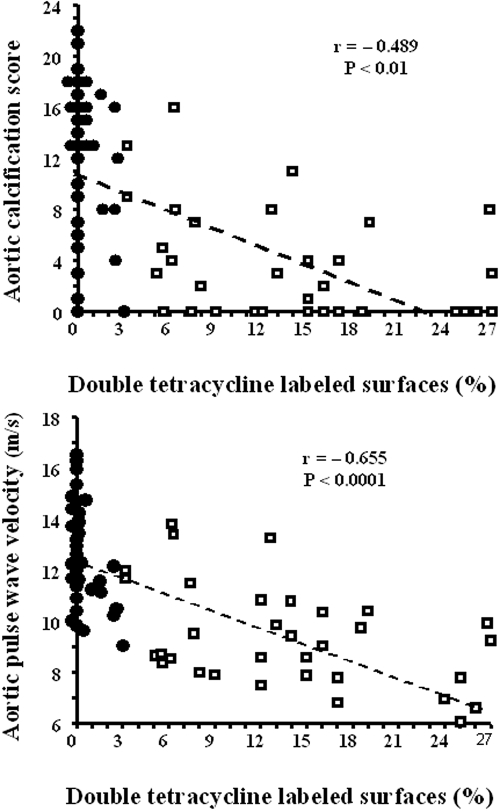
Analyses of variance data (Table 3) showed significant interaction between Ca load (elemental Ca in g/d), bone activity (ABD, 0; active bone, 1), and AoCS (P = 0.002) or aortic PWV (P = 0.003) in the entire population. The adjusted multivariate analyses of variables associated with AoCS or aortic PWV for the entire population of 66 patients with ESRD are reported in Table 4. AoCS was negatively correlated with serum albumin, bone activity (ABD, 0; active bone, 1), gender (male, 0; female, 1), and Ca dosage*bone activity interaction. Positive correlations were observed with CaCO3 dosage, age, and vintage. Double tetracycline–labeled surfaces were negatively associated with age (P = 0.04) and positively associated with resorption surface (P = 0.03) and osteoblastic surface (P < 0.0001) but not with aluminum (Al)-stained surfaces (P = 0.3). Aortic PWV was positively associated with age and Ca dosage and negatively with bone activity (ABD, 0; active bone, 1) and Ca-dosage*bone activity interaction (Table 4). In multivariate analysis, the association with BP was NS. A significant inverse correlation existed between aortic PWV and double tetracycline–labeled surface (Figure 4).
Table 3.
Analysis of covariance with abdominal AoCS or aortic PWVa
| Term | F ratio | P |
|---|---|---|
| Abdominal AoCS | ||
| X1 vintage (mo) | 15.79 | 0.0003 |
| X2 age (yr) | 52.90 | <0.0001 |
| A CaCO3 (g elemental Ca/d) | 10.57 | <0.0001 |
| B bone activity (0, ABD; 1, active bone) | 6.50 | 0.0200 |
| AB interaction (bone status*CaCO3) | 3.28 | 0.0020 |
| Aortic PWV (m/s) | ||
| X1 age (yr) | 27.65 | <0.0001 |
| X2 mean BP (mmHg) | 6.34 | 0.0100 |
| A CaCO3 (g elemental Ca/d) | 7.37 | <0.0001 |
| B bone activity (0, ABD; 1, active bone) | 9.44 | 0.0400 |
| AB interaction (bone status*CaCO3) | 4.64 | 0.0030 |
A, abdominal AoCS; B, aortic PWV; X, covariate.
Table 4.
Multivariate analysis of variables associated with abdominal AoCS or aortic PWV (n = 66)
| Variable | β Coefficient | T | P |
|---|---|---|---|
| Abdominal AoCSa | |||
| intercept | 16.100 | 1.856 | 0.0700 |
| serum albumin (g/L) | −0.530 | −2.661 | 0.0100 |
| gender (0, male; 1, female) | −2.330 | −3.188 | 0.0020 |
| bone activity (0, ABD; 1, active bone) | −2.820 | −2.801 | 0.0070 |
| bone/Ca interaction | −0.130 | −2.075 | 0.0400 |
| CaCO3 (g elemental Ca/d) | 2.710 | 5.573 | <0.0001 |
| age (yr) | 0.130 | 3.639 | <0.0010 |
| vintage (mo) | 0.020 | 3.486 | <0.0010 |
| Aortic PWV (m/s)b | |||
| intercept | 4.540 | 2.911 | 0.0050 |
| age (yr) | 0.070 | 4.410 | <0.0001 |
| mean BP (mmHg) | 0.020 | 1.721 | 0.0900 |
| CaCO3 (g elemental Ca/d) | 1.010 | 4.389 | <0.0001 |
| bone activity (0, ABD; 1, active bone) | −1.130 | −2.244 | 0.0300 |
| bone/Ca interaction | −0.100 | −2.346 | 0.0200 |
Adjusted r2 for the model 0.8258; F ratio 45.01; P < 0.00001.
Adjusted r2 for the model 0.7020; F ratio 31.61; P < 0.00001.
Figure 4.
Correlations for the entire population (n = 66) between double tetracycline–labeled surfaces and abdominal aortic calcification score or aortic PWV. •, adynamic bone; □, active bone.
DISCUSSION
The results of several previous cross-sectional and longitudinal studies indicated that vascular calcifications were associated with the use of Ca-containing PO4 binders, but the interaction between bone activity and Ca load on PWV and vascular calcifications were not analyzed.1,2,4 The results of this study highlighted the complexity of the relationship between Ca load and aortic stiffness or AoCS, with bone turnover playing an important role. In patients with ESRD, aortic PWV and AoCS depended significantly on CaCO3 dosage and bone activity, and the dosage of Ca-containing PO4 binders and bone activity interacted significantly. The positive associations between Ca load and PWV or AoCS were stronger in patients with ABD, indicating that the presence of ABD conferred significantly greater influence of Ca load on aortic calcifications and stiffening. The presence of an active bone was associated with lower aortic stiffness and better aortic capacitive function.
ABD was associated with aging, microinflammation and hypoalbuminemia, and more frequent surgical PTX-related hypoparathyroidism. Although the role of PTX is more specific to patients with ESRD, the associations of age or microinflammation with bone density and activity have been observed in nonuremic populations. The results of several studies showed that chronic inflammation is associated with bone loss and heightened fracture risk14,15 and cardiovascular calcifications.16,17
Although the serum PO4 level did not differ as a function of bone activity, for maintaining similar PO4 levels, patients with ABD received higher dosages of Ca-containing PO4 binders. As shown in Table 3, AoCS was significantly associated with Ca load and bone activity, with a significant Ca dosage*bone activity interaction. In multivariate analyses, the β coefficient of the interaction term was negative, indicating that the correlation between Ca load and AoCS differed according to bone activity and was stronger in patients with ABD, indicating that the presence of ABD conferred significantly greater influence of Ca load. Vintage was longer for patients with ABD, and a higher percentage of those patients had been treated with Al-containing PO4 binders in the past. Although the prescription of Al-containing PO4 binders was stopped several years before the bone biopsy, patients with ABD still had higher bone Al-stained surfaces but comparable serum Al concentrations and deferoxamine test. Several clinical and experimental studies demonstrated that Al reduced circulating PTH by decreasing its synthesis and release18–20 and could induce ABD. Bone Al deposition rises after subtotal PTX, and it is likely that low PTH and low remodeling could facilitate bone Al deposition.21
In this study, the extent of double tetracycline labeling was negatively correlated with age and positively with osteoclastic resorption and osteoblastic surfaces but not directly with serum Al, deferoxamine-induced serum Al changes, and Al-stained surfaces. A significant negative, albeit weak, correlation was observed between Al-stained surfaces and osteoblastic surfaces, suggesting that even an ancient Al overload could still influence bone activity. The results of multivariate analyses indicated that, after adjustment for significant covariates including hemodialysis vintage, Al-overload indices were not correlated with AoCS or aortic PWV (Table 4). The data published to date do not favor a possible association between Al and aortic stiffness or AoCS, because elastin calcifications and elastolysis by matrix metalloproteinases are prevented by Al chloride.22,23 Furthermore, several patients with active bone, including those without AoC, had received Al-containing PO4 binders (Figure 3).
The principal novelty of this study concerns the relationships between Ca load, bone activity, and aortic stiffness. The most important determinants of aortic stiffness are age, BP, and composition of arterial walls. The influence of these factors on aortic PWV are shown in Table 4, and the relationship between abdominal AoCS and PWV confirmed previous observations.24 The results of several studies documented an inverse relationship between arterial stiffness and vertebral bone density25 or osteoporosis.26,27 These associations could, for the most part, be related to common effects of factors influencing both PWV and bone density, such as the aging process, microinflammation, or smoking.28 Aortic PWV was significantly associated with Ca load and bone activity, with a significant Ca dosage*bone activity interaction. In multivariate analyses, the β coefficient of the interaction term was negative, indicating that the correlation between Ca load and PWV differed according to bone activity and that the presence of ABD conferred significantly greater influence of Ca load. Independent of any other factor, including dosage of Ca-containing PO4 binders, adynamic bone status was associated with greater aortic stiffening (Figure 4).
We can only speculate about the mechanisms linking bone activity with aortic stiffening and arteriosclerosis. Bone remodeling is regulated by multiple hormones, including those involved in endocrine regulation of energy metabolism, such as leptin.29 In chronic renal failure, serum leptin is increased and inversely correlated to histomorphometric parameters of bone turnover and PTH,30,31 suggesting that leptin might be implicated in low bone turnover and AC.32 In a recent study, Lee et al.33 showed that osteoblasts exert an endocrine regulation on energy metabolism. Those authors showed that mice lacking the protein tyrosine phosphatase OST-PTP are hypoglycemic and protected from obesity and glucose intolerance associated with β cell proliferation and insulin secretion. All of these phenotypic characteristics could be corrected by removing one osteocalcin allele. Osteocalcin can improve glucose tolerance and stimulate insulin expression in β cells and adiponectin in adipocytes.33 Adiponectin protects arteries against hypertension, suppresses atherosclerosis, and increases bone mass by activating osteoblastogenesis.34
The clinical relevance of bone–Ca interaction needs further research. Recent randomized study of hemodialysis patients did not demonstrate a higher mortality in patients who were treated with Ca-containing PO4 binders35 but indicated a significant age effect in patients who were older than 65 yr. Aging adversely affects bone formation. An association of Ca supplementation with upward trend in cardiovascular event rates was recently observed in elderly healthy postmenopausal women.36
Patients with ESRD and active bone were less sensitive to the use of Ca-containing PO4 binders, and many patients had no AoC even after 15 yr on hemodialysis (Table 2). These latter were younger, did not have malnutrition inflammation, and took lower dosages of Ca-containing PO4 binders. The practical clinical problem remains how to identify precisely patients who are more prone to aortic calcifications. Patients with active bone differ from those with ABD, because they have significantly higher PTH (and other biochemical markers), but as shown in Figure 3, serum PTH levels overlapped between the two groups, and its specificity and sensitivity to predict the degree of bone turnover has been questioned. It is excluded on ethical grounds to biopsy bone merely to evaluate the need for PO4 control, and it is essential to design studies to identify and define the most specific and sensitive noninvasive markers of bone turnover in patients with ESRD.37
This study has several limitations. The first is the observational cross-sectional nature of the study and that prevalent patients were evaluated, thereby making it difficult to reconstruct the natural history of bone disease and its association with AoCS and stiffness. The second concerns some clinical characteristics of this population that were typical for patients treated in the Ile-de-France/Paris region in the late 1980s and in 1990s, before the introduction of non–Ca-containing PO4 binders and calcimimetics.38 Many included patients had been on hemodialysis for many years and had begun replacement therapy when some therapeutic approaches, no longer recommended today, were used, principally exposure to Al-containing PO4 binders and total surgical PTX. Although Al-containing PO4 binders are no longer recommended, they are still frequently used, and Al still seems to be implicated in a high percentage of low-turnover bone disease.39,40 With the introduction of calcimimetics into the therapeutic armamentarium, the incidence of surgical PTX has decreased but not disappeared, and postsurgical hypoparathyroidism is still seen and represents a major cause of hypoparathyroidism.41
In conclusion, in hemodialysis patients with ESRD, aortic stiffness and calcifications were significantly associated with both Ca load and bone activity, with a significant Ca load*bone activity interaction. The positive associations between Ca load and PWV or AoCS were stronger in patients with ABD, indicating that the presence of ABD conferred significantly greater influence of Ca load on aortic calcifications and stiffening. Independent of any other factor, including dosage of Ca-containing PO4 binders, adynamic bone status was associated with greater aortic stiffening, suggesting a direct bone–arterial cross-talk.
CONCISE METHODS
Patients
Inclusion criteria were (1) hemodialysis vintage at least 12 mo (median 72; range 12 to 214); (2) age ≥18 and ≤70; (3) absence of clinical history of cardiovascular disease; and (4) complete set of results including blood chemistries, AoCS, bone biopsy, and aortic PWV. Between 1986 and 1996, 66 patients who had ESRD and fulfilled these criteria (48 patients were also part of a previous cohort12) were included. Dialysis duration was individually tailored (4 to 6 h thrice weekly) to control body fluids and blood chemistries and to achieve a Kt/V >1.2 (1.42 ± 0.11). Bicarbonate dialysate was prepared using double reverse osmosis-treated water with 1.25, 1.5, or 1.75 mmol/L of Ca, according to the serum Ca-PO4 equilibrium and the need to use vitamin D3 (1α-OH-D3) and CaCO3. Although CaCO3 was used exclusively as a PO4 binder at the time of the study, 28 patients had taken Al hydroxide [Al(OH)3] in the past. Twelve patients underwent subtotal PTX and 10 patients total PTX with heterotopic autotransplantation into the forearm. PTX had been performed 20 to 70 mo before the study. Eight patients had post-PTX hypoparathyroid activity (PTH <100 pg/ml). Patients regularly took iron and vitamin supplements.
Abdominal Aortic Calcification Score
Lateral lumbar spine radiographs were acquired in the standing position, as described previously.40 An AoCS was developed to grade AoC severity at the level of the first four (L1 through L4) lumbar vertebrae. Radiographs were read by two independent observers with no knowledge of the patients’ clinical histories. The radiodensity of the aortic wall was systematically assessed at each vertebral segment, and calcific deposits were considered present when densities were visible in an area parallel to the lumbar spine and anterior to the lower part of the spine. Calcific densities were graded 0 to 3 at each lumbar vertebral segment: 0, no calcific deposits; 1, small scattered calcific deposits filling less than one third of the longitudinal aorta wall; 2, one third or more but less than two thirds of the longitudinal aorta wall calcification; and 3, two thirds or more of the longitudinal aorta wall calcification. A separate score was determined for the anterior and posterior aorta, and the values were summed across the four vertebral levels, yielding in an abdominal AoCS that could range from 0 to 24 points, as described previously.5,42 It has been shown that the AoCS is an important predictor of vascular morbidity and mortality, and the results of one study demonstrated very good correlation between AoCS and coronary calcification scores using electron-beam computed tomography.43
Aortic PWV, as a surrogate of aortic stiffness, was determined using the foot-to-foot method, as described previously.44 Simultaneously recorded pulse waveforms were obtained transcutaneously over the common carotid and femoral groin arteries. PWV was calculated as the distance between suprasternal notch and femoral artery recording site measured over the surface of the body, divided by the time interval between the feet of the flow waves. This interval was averaged over 10 cardiac cycles. Aortic PWV (and corresponding BP) was measured at monthly intervals twice before bone biopsy and twice after bone biopsy. Reported PWV and BP values are the average of these multiple measurements.
Bone Histomorphometry
Diagnostic anterior iliac crest bone biopsies were taken after double tetracycline labeling according to the schedule of 2 d on tetracycline, 10 d off, and 2 d on.12,45 On three 5-μm-thick sections stained with Toluidine blue, trabecular bone volume (%), osteoid surface and volume (%), osteoblast surface (%), osteoclast resorption surface (%), and osteoclast number (n/mm2) were assessed. On two unstained 10-μm-thick sections, the bone mineralization rate and the extent of double and total tetracycline-labeled surfaces (%) were evaluated. Bone Al-staining was evaluated according to the method described by Maloney et al.46 and expressed as the percentage of the trabecular surface stained. All measurements were made using an eye-piece reticle (Zeiss integral plate II, Oberkochen, Germany). The double tetracycline–labeled surfaces were used to distinguish between patients with ABD and those with active bone. The median value of double tetracycline–labeled surfaces (3.1%; range 0 to 27) was used to classify patients with ABD <3.1% (n = 33) and those with active bone ≥3.1% (n = 33).
Blood Chemistries
Blood chemistries including serum Ca and PO4, blood lipids, intact PTH (Nichols Institute; N-IRMA), serum albumin, and serum Al were determined the week preceding the bone biopsy. Serum Al was measured with atomic absorption spectrophotometry and graphite furnace. Plasma samples for quantification of the Al concentration were obtained before routine hemodialysis and 40 h after deferoxamine infusion (40 mg/kg) after hemodialysis. Smoking habits, prescriptions for 1α-OH-D3 (μg/wk), and the CaCO3 dosage expressed in grams of elemental Ca/d prescribed to each patient were obtained from the patients’ files. The average blood chemistry values and mean daily CaCO3 dosage over the 12 mo preceding bone biopsy are reported. All patients gave informed written consent to participate in the study, which was approved by our institutional review board.
Statistical Analysis
Data are expressed as means ± SEM or medians (95% confidence intervals) when appropriate. The primary analysis concerned patient subgroup comparison (i.e., active bone versus ABD). The secondary analyses concerned comparisons of patients with positive versus negative AoCS. Between-group comparisons for quantitative variables were performed using the Mann-Whitney U test, and χ2 test was used for categorical variables. Pearson or Spearman correlation coefficient was used to assess the relationships between AoCS or aortic PWV and clinical or biochemistry parameters. The impact of interactions between Ca load (elemental Ca in g/d) and bone activity (expressed as dummy variables [i.e., ABD, 0; active bone, 1]) on AoCS or aortic PWV was tested using ANOVA adjusted for covariates. The CaCO3 load*bone activity interaction term was included in the multivariate regression analysis with the subset of independent clinical and biochemistry variables (P < 0.05 after correction for the number of correlations studied) associated with AoCS or PWV. All tests were performed using NCSS 7.0 software (J. Hintze, Kaysville, UT).
DISCLOSURES
None.
Acknowledgments
This work was supported by Groupe d’Etude de Physiopathologie de l’Insuffisance Rénale, INSERM U632, and U606. Sponsors have not been involved in any way in the study design, data interpretation, and manuscript editing.
We thank Mrs. Janet Jacobson for editorial assistance.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RB, Salusky I: Coronary artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Guérin AP, London GM, Marchais SJ, Métivier F: Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Oldendorf M, Moshage W, Heidler F, Zeitler E, Luft FC: Electron-beam computed tomography in the evaluation of cardiac calcifications in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Block GA, Spiegal DM, Ehrlich J, Ravindra M, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel PD, Hannan M, Polak JM, Cupples A: Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 103: 1529–1534, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, Rumberger JA, Sheedy PF, Peyser PA, Schwartz RS: Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 104: 412–417, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Guérin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 8.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Davies MR, Hruska KA: Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int 60: 472–479, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC: Progression of aortic calcification is associated with metacarpal bone loss during menopause: A population-based longitudinal study. Arterioscler Thromb Vasc Biol 20: 1926–1931, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW: Bone loss and the progression of abdominal aortic calcification over a 25-year period: The Framingham Heart Study. Calcif Tissue Int 68: 271–276, 2001 [DOI] [PubMed] [Google Scholar]
- 12.London GM, Marty C, Marchais SJ, Guérin AP, Métivier F, de Vernejoul M-C: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Chertow GM, Burke SK, Raggi P, for the Treat-to-Goal Working Group: Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Schett G, Kiechl S, Weger S, Pederiva A, Mayr A, Petrangeli M, Oberhollenzer F, Lorenzini R, Redlich K, Axmann R, Zwerina J, Willeit J: High-sensitivity C-reactive protein and risk of nontraumatic fractures in the Bruneck study. Arch Intern Med 166: 2495–2501, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Koh JM, Khang YH, Jung CH, Bas S, Kim DJ, Chung YE, Kim GS: Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: Evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int 16: 1263–1271, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Wang AY, Woo J, Wang M, Sea MM, Ip R, Li PK, Lui SF, Sanderson JE: Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol 12: 1927–1936, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D'Agostino RB, Wilson PW, O'Donnell CJ: C-reactive protein is associated with subclinical epicardial coronary calcification in men and women the Framingham Heart Study. Circulation 106: 1189–1191, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Morrissey J, Slatopolsky E: Effect of aluminum on parathyroid hormone secretion. Kidney Int 29: S41–S44, 1986 [PubMed] [Google Scholar]
- 19.Diaz-Corte C, Fernandéz-Martin JL, Barreto S, Gomez C, Fernandéz-Coto T, Braga S, Cannata JB: Effect of aluminum load on parathyroid hormone synthesis. Nephrol Dial Transplant 16: 742–745, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Piraino B, Chen T, Puschett JB: Elevated bone aluminum and suppressed parathyroid hormone levels in hypercalcemic dialysis patients. Am J Nephrol 9: 190–197, 1989 [DOI] [PubMed] [Google Scholar]
- 21.de Vernejoul M-C, Marchais S, London G, Morieux C, Bielakoff G, Miravet L: Increased bone aluminum deposition after subtotal parathyroidectomy in dialyzed patients. Kidney Int 27: 785–791, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Vyavahare N, Ogle M, Schoen FJ, Levy RJ: Elastin calcification and its prevention with aluminum chloride pretreatment. Am J Pathol 155: 973–982, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey M, Xiao H, Ogle M, Vyavahare N: Aluminum chloride pretreatment of elastin inhibits elastolysis by matrix metalloproteinases and leads to inhibition of elastin-oriented calcification. Am J Pathol 159: 1981–1986, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raggi P, Bellasi A, Ferramosca E, Islam T, Muntner P, Block GA: Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Raggi P, Bellasi A, Ferramosca E, Islam T, Block GA, Muntner P: Pulse wave velocity is inversely related to vertebral bone density in hemodialysis patients. Hypertension 49: 1278–1284, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Joki N, Hase H, Shirataka M, Kishi N, Tochigi S, Imamura Y: Calcaneal osteopenia is a new marker for arterial stiffness in chronic hemodialysis patients. Am J Nephrol 25: 196–202, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Sumimo H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Sakamaki T, Kurabayashi M: Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas 55: 212–218, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Gerdhem P, Obrant KJ: Effects of cigarette smoking on bone mass as assessed by dual-energy X-ray absorptiometry and ultrasound. Osteoporos Int 13: 932–936, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Ae Kim CA, Ogawa Y, Liu X, Ware SM, Craigen WJ, Robert JJ, Vinson S, Capeau J, Karsenty G: Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A 101: 3258–3263, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coen G, Ballanti P, Fischer MS, Balducci A, Calabria S, Colamarco L, Di Zazzo G, Lifrieri F, Manni M, Sardella D, Nofroni I, Bonucci E: Serum leptin in dialysis renal osteodystrophy. Am J Kidney Dis 42: 1036–1042, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Mallamaci F, Tripepi G, Zoccali C: Leptin in end-stage renal disease (ESRD): A link between fat mass, bone and cardiovascular system. J Nephrol 18: 464–468, 2005 [PubMed] [Google Scholar]
- 32.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL: Leptin enhances the calcification of vascular cells: Artery wall as a target of leptin. Circ Res 88: 954–960, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Young DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G: Endocrine regulation of energy metabolism by the skeleton. Cell 130: 456–469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I: Adiponectin increases bone mass by suppressing osteoclasts and activating osteoblasts. Biochem Biophys Res Commun 331: 520–526, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK: Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int 72: 1130–1137, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, Gamble GD, Grey A, Reid IR: Vascular events in healthy older women receiving calcium supplementation: Randomised controlled trial. BMJ 336: 262–266, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G: Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Renal Epidemiology and Information Network & Agence de la Biomédecine: REIN annual report 2005. Nephrol Ther 3[Suppl 1]: S1–S82, 2007 [PubMed] [Google Scholar]
- 39.Araujo SM, Ambrosoni P, Lobao RR, Caorsi H, Moysés RM, Barreto FC, Olaizola I, Cruz EA, Petraglia A, Dos Reis RM, Duarte ME, Jorgetti V, Carvalho AB: The renal osteodystrophy pattern in Brazil and Uruguay: An overview. Kidney Int 63[Suppl 85]: S54–S56, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Avila-Diaz M, Matos M, Garcia-Lopez E, Prado MD, Castro-Vasquez F, Ventura MD, Gonzales E, Amato D, Paniagua R: Serum markers of low-turnover bone disease in Mexican children with chronic kidney disease undergoing dialysis. Perit Dial Int 26: 78–84, 2006 [PubMed] [Google Scholar]
- 41.Dussol B, Morand P, Martinat C, Lombard E, Portugal H, Brunet P, Berland Y: Influence of parathyroidectomy on mortality in hemodialysis patients: A prospective observational study. Ren Fail 29: 579–586, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kauppila LJ, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW: New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis 25: 245–250, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Bellasi A, Ferramosca E, Muntner P, Ratti C, Wildman RP, Block GA, Raggi P: Correlation of simple imaging tests and coronary artery calcium measurement by computed tomography in hemodialysis patients. Kidney Int 70: 1623–1628, 2006 [DOI] [PubMed] [Google Scholar]
- 44.London GM, Marchais SJ, Safar ME, Genest AF, Guérin AP, Métivier F, Chedid K, London AM: Aortic and large artery compliance in end-stage renal failure. Kidney Int 37: 137–142, 1990 [DOI] [PubMed] [Google Scholar]
- 45.London GM, de Vernejoul M-C, Fabiani F, Marchais SJ, Guérin AP, Métivier F, London AM, Llach F: Secondary hyperparathyroidism and cardiac hypertrophy in hemodialysis patients. Kidney Int 32: 900–907, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Maloney NA, Ott SM, Alfrey AC, Miller NJ, Coburn JW, Sherrard DJ: Histological quantitation of aluminum in iliac bone from patients with renal failure. J Lab Clin Med 99: 206–216, 1982 [PubMed] [Google Scholar]