Abstract
Alport syndrome is a hereditary nephropathy that results in irreversible, progressive renal failure. Recent reports suggested that bone marrow transplantation (BMT) has a beneficial, short-term effect on renal injury in Alport (Col4a3−/−) mice, but its long-term effects, especially with regard to survival, are unknown. In this study, Alport mice received a transplant of either wild-type or Col4a3−/− bone marrow cells. Surprising, laboratory evaluations and renal histology demonstrated similar findings in both transplanted groups. Transplanted cells accounted for >10% of glomerular cells at 8 wk, but type IV collagen α3 chains were not detected in glomerular basement membranes of either group by immunofluorescence or Western blot analysis, although Col4a3 mRNA in the kidney could be amplified by reverse transcription–PCR in knockout mice that received a transplant of wild-type bone marrow. Both transplanted groups, however, survived approximately 1.5 times longer than untreated knockout mice (log rank P < 0.05). These data suggested that irradiation, which preceded BMT, may have conferred a survival benefit; therefore, the survival time of knockout mice was assessed after sublethal irradiation (3, 6, and 7 Gy) without subsequent BMT. A strong positive correlation between irradiation dosage and survival time was identified (P < 0.0001). In conclusion, the improved survival observed in Alport mice that received a transplant of wild-type bone marrow might be primarily attributed to as-yet-unidentified effects of irradiation.
Alport syndrome (AS) is a progressive type of hereditary nephritis associated with genes coding for type IV collagen.1–3 Two major types exist in which an X-linked AS caused by mutations in COL4A5 includes >80% and an autosomal recessive form caused by mutations in COL4A3 or COL4A4 includes almost 15% in human.2,3 Regarding the optimal treatment of this disease, there is no viable strategy except for renal transplantation at present. There are some reports in which conservative therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blocker effectively attenuated renal deterioration in the mouse.4,5 There are also reports in which immunosuppressive therapy, such as cyclosporine A, has been shown to be useful for this syndrome in dogs and humans, but it is controversial to use an immunosuppressive agent in view of adverse effects.6,7 Gene therapy for this hereditary disease might thus be a potentially effective treatment modality. Heikkila et al.8,9 developed a method that delivered the target gene by organ perfusion and achieved high fixed rate to the kidney in the pig; however, the long-term effects were not evaluated, and, therefore, its efficacy remains unclear.
Recently, the possibility that bone marrow–derived cells can attenuate this nephropathy in the mouse was reported by two groups.10,11 Both groups showed some improvement in renal injury on the basis of the laboratory data and histology; however, so far, no report has addressed the survival time of these mice, which is essential to judge the effectiveness of bone marrow transplantation (BMT).12 Therefore, the short-term and long-term effects on BMT were analyzed in our study using larger sample sizes.
RESULTS
Survival Time
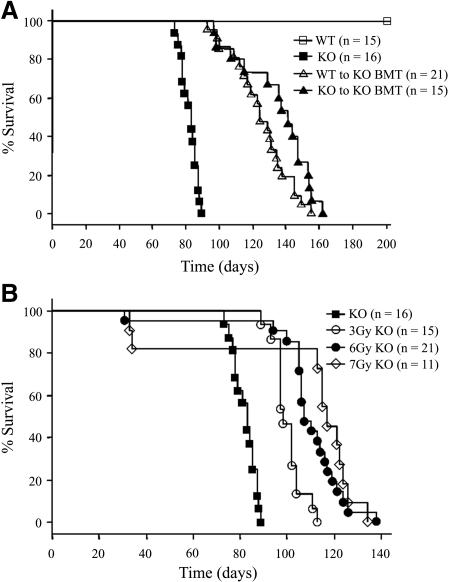
The survival times of Col4a3+/+ (wild-type [WT]) to Col4a3−/− BMT mice (knockout [KO]) and KO to KO BMT mice improved significantly in comparison with the KO mice (survival times 125 ± 4 and 135 ± 5 versus 82 ± 1 d; log rank test for trend, P < 0.05, respectively), whereas there was no significant difference in the mean survival times between the two BMT mice groups (z score = 1.48; P < 0.05; Figure 1A). The survival times of the irradiated KO mice without BMT were 100 ± 2, 108 ± 4, and 105 ± 11 d in 3, 6, and 7 Gy, respectively (Figure 1B). The positive correlation between the irradiation dosage and survival time was strongly significant (hazard ratio 0.553; 95% confidence interval 0.466 to 0.656; P < 0.0001).
Figure 1.
Survival time. (A) Survival times of WT to KO BMT and KO to KO BMT mice improved significantly in comparison with untreated KO mice (mean survival time 125 ± 4 and 135 ± 5 versus 82 ± 1 d; log rank test for trend, P < 0.05), whereas there was no significant difference of the mean survival times between the two BMT mouse groups (z score = 1.48; P < 0.05). (B) The survival times of the irradiated KO mice without BMT were 100 ± 2, 108 ± 4, and 105 ± 11 d in 3, 6, and 7 Gy, respectively. The positive correlation between the irradiation dosage and survival time was strongly significant (hazard ratio 0.553, 95% confidence interval [CI] 0.466 to 0.656; P < 0.0001). The data of the KO group were repeated in A and B.
Laboratory Evaluation
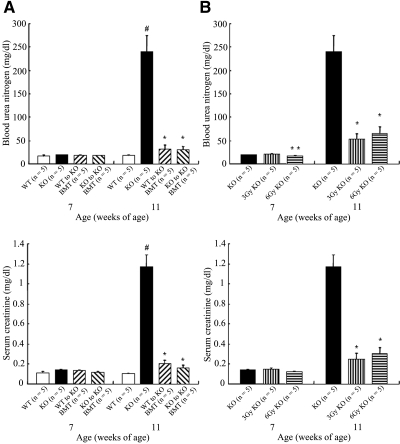
At 7 wk of age, no significant difference was observed in the blood urea nitrogen (BUN) and serum creatinine (Cr) levels among the four groups. The BUN levels of the WT, KO, WT to KO BMT, and KO to KO BMT were 16.78 ± 2.01, 18.92 ± 1.29, 17.84 ± 1.51, and 17.66 ± 0.82 mg/dl, whereas their Cr levels were 0.112 ± 0.010, 0.142 ± 0.006, 0.130 ± 0.010, and 0.120 ± 0.010 mg/dl, respectively (Figure 2A). At 11 wk of age, the BUN levels of these four different mouse groups were 18.40 ± 0.48, 239.32 ± 34.88, 32.22 ± 7.77, and 29.46 ± 7.86 mg/dl, whereas the Cr levels were 0.102 ± 0.002, 1.166 ± 0.120, 0.202 ± 0.040, and 0.162 ± 0.020 mg/dl, respectively. The BUN and Cr levels of the KO mice were significantly elevated in comparison to those of the WT mice (P < 0.05). The BUN and Cr levels of the two BMT groups significantly improved in comparison with those of the KO mice (P < 0.05; Figure 2A). The BUN levels of the KO, 3 Gy KO, and 6 Gy KO mice were 18.92 ± 1.29, 20.80 ± 1.02, and 16.66 ± 0.74 mg/dl, whereas their Cr levels were 0.142 ± 0.006, 0.144 ± 0.015, and 0.124 ± 0.004 mg/dl, respectively, at 7 wk of age. The BUN level of 3 Gy KO was elevated in comparison with that of 6 Gy KO mice (P < 0.05; Figure 2B). At 11 wk of age, the BUN levels of the KO, 3 Gy KO, and 6 Gy KO mice were 239.32 ± 34.88, 57.06 ± 13.14, and 63.82 ± 15.70 mg/dl, whereas the Cr levels were 1.166 ± 0.124, 0.248 ± 0.058, and 0.304 ± 0.057 mg/dl, respectively. These findings indicated the BUN and Cr levels of 3 and 6 Gy KO mice significantly improved in comparison with those of the untreated KO (P < 0.05; Figure 2B).
Figure 2.
Renal function at 7 and 11 wk of age. (A) The BUN and Cr levels of KO mice increased significantly in comparison with those of the WT mice at 11 wk of age (#P < 0.05). The BUN and Cr levels of two BMT mouse groups (each n = 5) significantly improved in comparison with those of untreated KO mice (n = 5) at 11 wk of age (*P < 0.05); no difference in the BUN and Cr levels was found between the two BMT mouse groups. (B) At 7 wk of age, the BUN level of 3 Gy KO mice (n = 5) was elevated in comparison with that of 6 Gy KO mice (n = 5; **P < 0.05). The BUN and Cr levels of 3 and 6 Gy KO mice significantly improved in comparison with those of the untreated KO mice (n = 5) at 11 wk of age (*P < 0.05). The data of the KO group were repeated in A and B.
Renal Histology
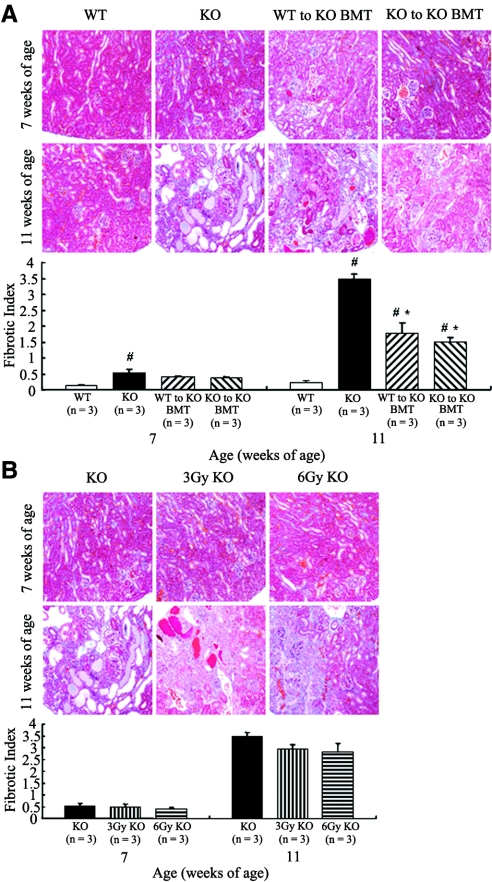
At 7 wk of age, the sclerotic indices of WT, KO, WT to KO BMT, and KO to KO BMT mice were 0.30 ± 0.04, 0.78 ± 0.10, 0.65 ± 0.10, and 0.60 ± 0.05. The sclerotic indices of KO and WT to KO BMT mice significantly increased in comparison with WT mice (P < 0.05; Figure 3A). At 11 wk of age, the sclerotic indices of the four different mouse groups were 0.62 ± 0.04, 2.59 ± 0.05, 1.41 ± 0.14, and 1.20 ± 0.07. A statistical analysis indicated that the sclerotic indices of KO, WT to KO BMT, and KO to KO BMT mice significantly increased in comparison with WT mice (P < 0.05). The sclerotic indices of the two BMT mouse groups significantly improved in comparison with the untreated KO mice (P < 0.05; Figure 3A). The sclerotic indices of KO, 3 Gy KO, and 6 Gy KO mice were 0.78 ± 0.10, 0.53 ± 0.08, and 0.61 ± 0.08 at 7 wk of age and 2.59 ± 0.05, 1.90 ± 0.14, and 1.64 ± 0.20 at 11 wk of age (Figure 3B). The sclerotic indices for 3 and 6 Gy KO mice significantly improved in comparison with those of the KO mice at 11 wk of age (P < 0.05). Conversely, the fibrotic indices of WT, KO, WT to KO BMT, and KO to KO BMT mice were 0.12 ± 0.04, 0.55 ± 0.10, 0.40 ± 0.03, and 0.38 ± 0.02 at 7 wk of age. The fibrotic index of KO mice significantly increased in comparison with WT mice (P < 0.05; Figure 4A). At 11 wk of age, the fibrotic indices of these four mouse groups were 0.22 ± 0.07, 3.45 ± 0.18, 1.78 ± 0.33, and 1.50 ± 0.15, respectively. These findings indicated that the fibrotic indices of KO, WT to KO BMT, and KO to KO BMT mice significantly increased in comparison with WT (P < 0.05). The fibrotic indices of the two BMT mouse groups significantly improved in comparison with the untreated KO mice (P < 0.05; Figure 4A). The fibrotic indices of KO, 3 Gy KO, and 6 Gy KO mice were 0.55 ± 0.10, 0.47 ± 0.15, and 0.38 ± 0.09 at 7 wk of age. The corresponding numbers at 11 wk of age were 3.45 ± 0.18, 2.95 ± 0.18, and 2.80 ± 0.39. There was no significant difference among the three groups at 7 and 11 wk of age (Figure 4B).
Figure 3.
Periodic acid-Schiff stain and sclerotic index. (A) The sclerotic indices of KO and WT to KO BMT mice significantly increased in comparison with the WT group at 7 wk of age (#P < 0.05). The sclerotic indices of KO, WT to KO BMT, and KO to KO BMT significantly increased in comparison to the WT group at 11 wk of age (#P < 0.05). Although the sclerotic indices of the two BMT mice groups significantly improved in comparison with KO mice at 11 wk of age (*P < 0.05), there was no significant difference between the two BMT mouse groups. (B) There was no significant difference among the three groups receiving radiation at 7 wk of age. The sclerotic indices of 3 and 6 Gy KO significantly improved in comparison with those of the KO mice at 11 wk of age (*P < 0.05). The data of the KO group were repeated in A and B.
Figure 4.
Masson-Trichrome stain and fibrotic index. (A) The fibrotic index of KO mice significantly increased in comparison with the WT group at 7 wk of age (#P < 0.05). The fibrotic indices of KO, WT to KO BMT, and KO to KO BMT mice significantly increased in comparison with the WT group at 11 wk of age (#P < 0.05). Although the fibrotic indices of the two BMT mouse groups significantly improved in comparison with the KO mice (*P < 0.05), no significant difference was observed between the two BMT mouse groups at 11 wk of age. (B) There was no significant difference among the three groups receiving radiation at 7 and 11 wk of age. The data of the KO group were repeated in A and B.
Immunofluorescence for Type IV Collagen α1 through 6 Chains
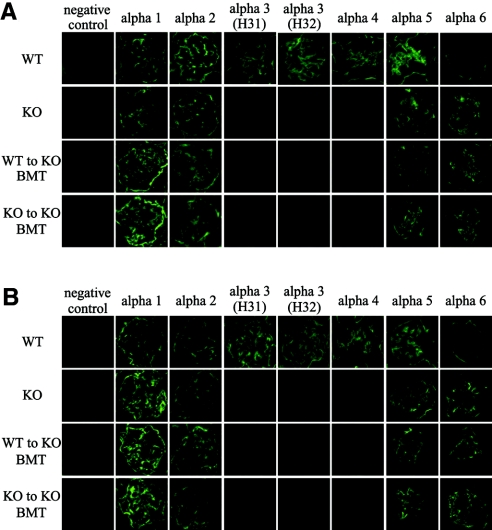
At 7 and 11 wk of age, α1 and 2 staining in the glomerular basement membrane (GBM) was positive in all groups (Figure 5, A and B). In contrast, α3 and 4 staining in the GBM was positive only in the WT mice at these time points. α3 staining was performed with two different mAb (H31 and H32). α5 staining was positive in all groups at 7 and 11 wk of age, and the strength indices in WT, KO, WT to KO BMT, and KO to KO BMT mice were 4.00 ± 0.00, 2.58 ± 0.04, 2.70 ± 0.03, and 2.77 ± 0.06 at 7 wk of age. Although the strength indices of the three KO groups decreased significantly in comparison with the WT mice (P < 0.05), no significant difference was observed within the three KO groups. At 11 wk of age, the strength indices of type IV collagen α5 chains in these four mouse groups were 4.00 ± 0.00, 3.10 ± 0.08, 3.43 ± 0.03, and 3.40 ± 0.05. The strength indices of KO, WT to KO BMT, and KO to KO BMT mice decreased significantly in comparison with WT (P < 0.05), and the strength indices of WT to KO BMT and KO to KO BMT mice increased significantly in comparison with KO mice (P < 0.05). Although α6 staining was negative in the GBM of WT mice, it was positive in the other three groups at 7 and 11 wk of age. The strength indices of type IV collagen α6 chains in WT, KO, WT to KO BMT, and KO to KO BMT mice were 0.00 ± 0.00, 3.12 ± 0.04, 3.27 ± 0.03, and 3.15 ± 0.06 at 7 wk of age. There was no significant difference within the three KO groups. The corresponding numbers at 11 wk of age were 0.00 ± 0.00, 3.63 ± 0.03, 3.67 ± 0.03, and 3.73 ± 0.03. The strength index of KO to KO BMT mice increased significantly in comparison with that of KO mice (P < 0.05). The strength indices of α5 staining increased significantly from 7 to 11 wk of age in these three KO groups (P < 0.05), whereas the strength indices of α6 staining did not change substantially between 7 and 11 wk of age.
Figure 5.
(A and B) Immunofluorescence for type IV collagen α1 through 6 chains at 7 wk of age (A) and at 11 wk of age (B). At 7 and 11 wk of age, α1 and 2 staining in the GBM was positive in all groups. At 7 and 11 wk of age, α3 and 4 staining in the GBM was positive only in the WT mice. α3 staining was done with two different mAb (H31 and H32). α5 staining in the GBM was positive in all groups at 7 and 11 wk of age. Although α6 staining was negative in the GBM of WT mice, it was positive in the other three groups at 7 and 11 wk of age.
Reverse Transcription–PCR Analysis
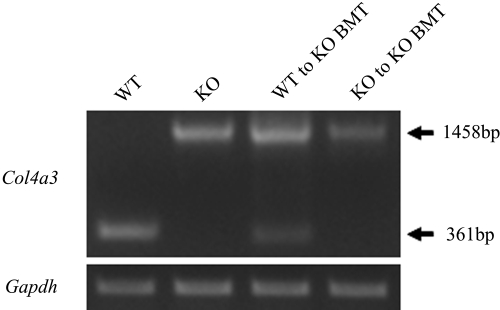
A 361-bp product of Col4a3 representing exon 46 through 48 was detected in whole kidney of WT mice at 11 wk of age. As expected, this band was not detected in KO mice. Instead, we detected a 1458-bp product of Col4a3 in KO mice, which agreed well with the fact that gene ablation was achieved by insertion of the neo cassette into exon 48. Although the 361-bp band of Col4a3 was weaker than that of the WT mice, there were 361- and 1458-bp bands of Col4a3 in WT to KO BMT mice (Figure 6). The Gapdh levels were similar among the four groups.
Figure 6.
Reverse transcription–PCR analysis. At 11 wk of age, a 361-bp product of Col4a3 representing exons 46 through 48 was detected in the WT group. A 1458-bp product representing the same region of Col4a3 with an inserted neo cassette was detected in the KO, WT to KO BMT, and KO to KO BMT groups. Although the 361-bp band of Col4a3 was weaker than that of the WT group, a 361-bp band of Col4a3 was detected in the WT to KO BMT group. The Gapdh levels were similar among the four groups.
Western Blot Analysis
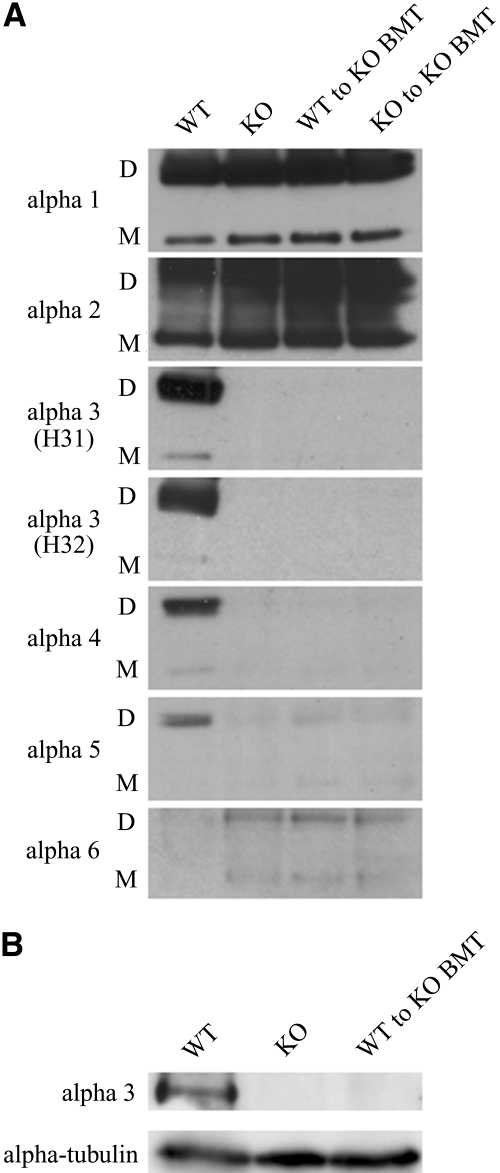
After collagenase treatment of the whole kidney, the type IV collagen α1 and 2 chains were detected as a monomer and a dimer in all four groups. Type IV collagen α3 and 4 chains were detected only in the WT mice. The type IV collagen α5 chains were detected in all groups with the highest levels in WT. The level was very low in the untreated KO group and somewhat higher in the two BMT groups. Although the type IV collagen α6 chains were hardly detected in WT mice, there were a monomer and a dimer in the three KO groups (Figure 7A). We also analyzed the presence of the full-length type IV collagen α3 chain (a molecular mass of approximately 180 kD), but the polypeptide was detected only in the total kidney lysate from the WT mice, not in the kidney lysates from the other three KO groups (data of KO to KO BMT mice not shown; Figure 7B).
Figure 7.
Western blot analysis. (A) Type IV collagen α1 and 2 chains were detected as a monomer and a dimer in all four groups. Type IV collagen α3 and 4 chains were detected only in the WT group. The appearance of type IV collagen α5 chains was most strongly detected in the WT group and most weakly in the KO group. The signal in the two BMT groups was somewhat stronger than in the KO group. Although the type IV collagen α6 chain was hardly detected in the WT group, the signal was stronger in the kidney samples from the three KO groups. D, noncollagenous domain 1 (NC1) dimer (molecular mass of approximately 44 to 50 kD); M, NC1 monomer (molecular mass of approximately 24 to 28 kD). (B) The full-length type IV collagen α3 chain with a molecular mass of approximately 180 kD was detected only in the total kidney lysate from the WT mice. α-Tubulin was used as a loading control.
Fluorescence In Situ Hybridization for the Male Y Chromosome
Transplanted cells in the WT to KO BMT mice accounted for >99% of the cells in the bone marrow from 4 wk after transplantation (4 wk 99.2%, 8 wk 99%, 12 wk 99.5%, 15 wk 100%). In glomeruli, the transplanted cells in WT to KO BMT mice gradually increased up to nearly 13% (4 wk 5.7 ± 0.1%, 8 wk 10.4 ± 0.3%, 12 wk 11.9 ± 2.7%, 15 wk 12.7%) in the paraffin sections. Moreover, the transplanted cells in WT to KO BMT and KO to KO BMT mice in frozen sections at 8 wk after transplantation were 11.5 ± 0.4 and 11.0 ± 0.4% in the glomeruli and 6.4 ± 0.3 and 6.3 ± 0.5% in the tubuli, respectively.
Electron Microscopy
Although the GBM remained intact in the WT mice at 11 wk of age, the GBM of the KO mice was severely damaged with thickening and splitting. In the WT to KO BMT, KO to KO BMT, and 6 Gy KO mice, the damage of GBM was weaker than in the KO (Supplemental Figure 1). The thicknesses of GBM of WT, KO, WT to KO BMT, KO to KO BMT, and 6 Gy KO mice were 94.6 ± 0.4, 347.0 ± 65.5, 210.3 ± 10.0, 226.2 ± 15.4, and 304.6 ± 79.9 nm, respectively.
DISCUSSION
We found in this study a significant improvement in the survival time of the AS mice that receive a transplant of bone marrow cells from the WT mice, and a similar improvement in the survival time was achieved when the mice received KO bone marrow cells. Because the recipient mice were irradiated before BMT, these findings led to the examination of the effects of the irradiation itself. The correlation between the irradiation dosage and survival time was found to be strongly significant. Although the transplanted cells in the glomeruli accounted for >10% at 8 wk after transplantation, no expression of type IV collagen α3 chains was detected using immunofluorescence and a Western blot analysis. The results therefore suggest that the major reason for an improved survival time by BMT might be attributed to the dosage-dependent irradiation effect.
Two recent article reported evidence for the GBM expression of type IV collagen α3 chains and improved renal function after wild-type BMT to the Col4a3-deficient AS mice10,11; however, Sugimoto et al.10 did not present any data of KO to KO BMT mice, and those of Prodromidi et al.11 were also limited (the study group was composed of three mice). Those studies differ from this one in regard to the time of transplantation and the strain. We chose 3 wk of age for the timing of BMT in 129 × 1/SvJ, whereas they chose a BMT time point of approximately 8 wk of age in C57BL6. Although the typical thickening of GBM with splitting had already started from 3 wk of age in the 129 × 1/SvJ strain, the change was still mild (Supplemental Figure 2); therefore, the damage to the GBM might be milder than that to the C57BL6 strain at 8 wk of age, which may affect the capacity of the bone marrow cells to migrate through the GBM, thus explaining, at least in part, the different outcome of the studies. Attempts were made to identify what the transplanted cells in the glomeruli were, but it was technically impossible. In addition, we could detect only very low levels of Col4a3 mRNA in the kidney of WT to KO BMT mice in all (n = 3), which may very likely be the reason that the type IV collagen α3 chains were undetectable according to the findings of both immunofluorescence and a Western blot analysis in our study. Prodromidi et al.11 also showed the partial restoration of Col4a3 mRNA after WT BMT into KO mice in only two of five samples.
Recently, the GBM deposition of type IV collagen α5 chain was recognized in Col4a3-deficient AS mice, which occurred as a type IV collagen α5:α5:α6 dimer.13 The immunofluorescence findings of this study were compatible with their result, because we detected type IV collagen α5 and 6 chains in the GBM of all KO groups with or without BMT. The GBM deposition of type IV collagen α5 and 6 chains increased slightly more in the two BMT groups than in the KO mice, on the basis of the results of immunofluorescence and a Western blot analysis. This increase might play a partial role in the improved survival time.
Several studies have shown that radiation can induce radiation nephropathy,14,15 which can be typically observed in a mouse model receiving >14 Gy, and the onset appears from approximately 24 wk after irradiation.16 The long-term harmful effects of irradiation itself could not be elucidated in this study because AS mice treated by BMT could not live >20 wk after irradiation. Because the KO mice at 3 wk of age, which received >8.5 Gy irradiation, died within 2 wk after irradiation (Table 1), the dosage-dependent irradiation effects might only be observed at levels <8 Gy without BMT. The lack of any improvement in the tubulointerstitial compartment in the irradiation-only groups compared with the two BMT mouse groups might thus be partially attributed to the rapid progression to end-stage renal failure; however, there is a recent report that irradiation induces an improvement in another mouse model of kidney disease.17,18 Because irradiation itself has multifactorial effects, the precise mechanism behind the improved survival time in the AS mice remains to be elucidated. One possible approach might be to perform a microarray analysis.19
Table 1.
All KO mice received irradiation at 3 wk of age (21 d)
| Irradiation Dosage (Gy) | Survival Time (d) |
|---|---|
| 6.0 | >49 |
| 6.0 | >49 |
| 8.0 | >49 |
| 8.0 | >49 |
| 8.5 | 34 |
| 8.5 | 34 |
| 9.0 | 30 |
| 9.0 | 34 |
| 9.5 | 28 |
| 9.5 | 28 |
| 10.0 | 26 |
| 10.0 | 26 |
In conclusion, the significant improvement in the survival time observed in AS mice by BMT may primarily be attributed to the dosage-dependent irradiation effects.
CONCISE METHODS
Experimental Design
Col4a3+/− mice whose background was 129 × 1/SvJ were purchased from the Jackson Laboratory (Bar Harbor, ME).20 The mice were bred in specific pathogen-free areas, and all of these experiments were approved by Institutional Animal Care and Use Committee regulations. Typing was performed using the PCR with tail DNA. A mouse BMT model was modified and used for the study.21 Three-week-old KO female mice were the recipients, and the donors were 8-wk-old WT or KO male mice. Bone marrow cells were harvested from the ileum, femur, and tibia and suspended in DMEM with 10% FBS. The most suitable irradiation dosage was determined to be 8 Gy after checking the range of 6 to 10 G irradiation (Table 1). After total irradiation of 8 Gy, 1 × 107 cells were transplanted via the supraorbital vein indirectly within 12 h.
Survival Time
The survival time of WT (n = 15), KO (n = 16), WT to KO BMT (n = 21), and KO to KO BMT mice (n = 15) was checked until 200 d and evaluated using the Kaplan-Meier method. Next, the survival time of KO mice irradiated with 3, 6, and 7 Gy (n = 15, 21, and 11, respectively) without BMT was evaluated using the Kaplan-Meier method.
Laboratory Evaluation
BUN and Cr levels were examined at 7 and 11 wk of age in each group (each n = 5). BUN and Cr were measured by using a urease and glutamate dehydrogenase assay (UN-S kit; Denka Seiken, Tokyo, Japan) and an enzymatic assay (VL II CRE kit; Alfresa Pharma Corp., Osaka, Japan), respectively.
Renal Histology
The renal histology was assessed at 7 and 11 wk of age in each group. The kidney specimens were formalin-fixed, paraffin-embedded and stained with periodic acid-Schiff base to analyze the sclerotic changes (each n = 5). For analysis of the tubulointerstitial fibrotic changes, Masson-Trichrome stain was used (each n = 3). The sclerotic index was examined in 20 glomeruli randomly selected from each mouse in a blinded manner. The fibrotic index to assess the tubulointerstitial damage rather than glomerular damage was examined in 20 areas randomly selected from each mouse in a blinded manner. The sclerotic and fibrotic indices were divided into five categories: 0 (no apparent damaged area), +1 (1 to 25% damaged area), +2 (26 to 50% damaged area), +3 (51 to 75% damaged area), and + 4 (76 to 100% damaged area) as described previously.22
Immunofluorescence for Type IV Collagen α1 through 6 Chains
Immunofluorescence was assessed at 7 and 11 wk of age in each group (n = 3). Cryosections (4 μm) were collected onto silanized slides. The slides were treated with acetone for 10 min at −20°C. After washing with PBS (pH 7.4), the sections were blocked with 10% normal goat serum for 30 min. Next, the slides were incubated with primary antibodies for 60 min at room temperature after washing with PBS. Monoclonal primary antibodies of type IV collagen α1 (H11), 2 (H22), 3 (H31 and H32), 4 (RH42), 5 (b14), and 6 (B66) from Shigei Medical Research Institute were diluted 1:100 with PBS containing 1% BSA except for H22, which was diluted 1:50. All of these primary antibodies were made against noncollagenous domain 1 (NC1) of each type IV collagen αchain. Sections stained with H11, H22, H31, H32, and b14 were treated with 6 M urea in 0.05 M Glycine/HCl (pH 3.5) for 10 min and B66 for 1 min before blocking. After washing with PBS, the specimens were incubated with FITC-conjugated affinity-purified antibody to rat IgG (Cappel 55760; Cappel, Solon, OH) for 30 min at room temperature. The specimens were mounted after washing with PBS. Nonspecific staining was ruled out by the use of negative controls. To evaluate the results of type IV collagen α5 and 6 chains, 20 glomeruli were randomly selected from each mouse in a blinded manner, and the strength of signals of GBM with same exposure times were divided into five categories (score 0 to 4) and expressed as strength index as described previously.23
Reverse Transcription–PCR Analysis
Total RNA was isolated from frozen kidneys at 11 wk of age (each n = 3) using TRIzol Reagent (Invitrogen, Tokyo, Japan) and reverse transcription was carried out at 42°C for 50 min in 20 μl of reverse transcription mixture containing 5 μg of total RNA, SuperScript II (Invitrogen), and oligo dT primers. The primer sequences were as follows: Col4a3, 5′-GACAGCCAGGAATGAAAGGA-3′ (forward in exon 46) and 5′-TCTTGTCCATGTGCACGTTT-3′ (reverse in exon 48); Gapdh, 5′-TTCACCACCATGGAGAAGGC-3′ (forward) and 5′-GGCATGGACTGTGGTCATGA-3′ (reverse).24 PCR was performed under the following conditions: Initial denaturation temperature at 96°C for 15 min, 40 cycles for Col4a3 and 25 cycles for Gapdh of denaturation at 96°C for 45 s, annealing at 59°C for Col4a3 and 51°C for Gapdh for 45 s, extension at 72°C for 1 min, and final extension at 72°C for 15 min. The predicted band sizes of Col4a3 were 361 bp in WT mice and 1458 bp in KO mice because a 1097-bp neo cassette was inserted into the exon 48 to generate the KO mice.20
Western Blot Analysis
The presence of type IV collagen α3 chains in total kidney lysates was investigated at 11 wk of age under nonreducing and reducing conditions (each n = 3). Under nonreducing conditions, the mouse kidneys were minced with blades, incubated at 37°C for 24 h with 0.5 mg of collagenase I (Worthington Biochemical Corp., Lakewood, NJ) and 2 Vol of digestion buffer (0.05 M HEPES [pH 7.5], 0.01 M CaCl2, 4 mM N-ethylmaleimide, 1 mM PMSF, 5 mM benzamidine HCl, and 25 mM 6-aminohexanoic acid) to solubilize the NC1 of type IV collagen.25 Five micrograms of supernatants was loaded onto the 4 to 12% NuPAGE Bis-Tris Gel (Invitrogen). Under reducing conditions, the kidneys were homogenized into a reductive SDS sample buffer and the polypeptides were separated by electrophoresis in 7.5% SDS–polyacrylamide gels. Thereafter, the gels were transferred to polyvinylidene difluoride membrane. The membranes were blocked overnight at 4°C and incubated with primary antibodies at room temperature for 1 h. Under nonreducing conditions, H11, H22, H31, H32, and RH42 were diluted 1:100 and M54 and M69 were diluted 1:50 as the primary antibodies. Under reducing conditions, H31 and α-tubulin antibody (Calbiochem #CP06; Calbiochem, Darmstadt, Germany) were diluted 1:10 and 1:1000 as the primary antibodies. Horseradish peroxidase–linked secondary antibodies were anti-rat (H11, H22, H31, H32, RH42, M54, and M69) and anti-mouse (α-tubulin) and diluted 1:3000. Immunodetection was performed using an enhanced chemiluminescence kit (Amersham Biosciences, Tokyo, Japan). The blots were then exposed to film for various times.
Fluorescence In Situ Hybridization for the Male Y Chromosome
The paraffin sections were rehydrated after deparaffinization in xylene for a total of 10 min. Next, the sections were boiled in 0.01 M citrate buffer solution for 20 min and washed in 0.1% Triton X-100/2× SSC (300 mM NaCl and 30 mM sodium citrate) at 37°C for 30 min. After washing in 2× SSC three times for 5 min, the sections were then incubated in 3 mg/ml pepsin/10 mM HCl at 37°C for 10 min. After washing in PBS, the sections were incubated in 50 mM MgCl2/PBS for 5 min. Then, the sections were incubated in 4% paraformaldehyde in 50 mM MgCl2/PBS for 10 min and dehydrated in 70% ethanol for 5 min and in 100% ethanol for 5 min. The sections were denatured by incubating in 70% formamide/2× SSC at 85°C for 10 min. After washing in cold 70 and 100% ethanol, respectively, the sections were then dried and put on a 45°C slide warmer for 20 s. Thereafter, the probes for the X and Y chromosomes were applied and incubated at 37°C overnight. The mouse XA (473L8) and YA (Z22F22) probes were labeled and prepared using Nick Translation Kit (Abbott Molecular, Chicago, IL). After washing in 50% formamide/2× SSC at 37°C for 15 min, 2× SSC at room temperature for 15 min, 0.1% Triton X-100/2× SSC at room temperature for 15 min, and 2× SSC at room temperature for 5 min, 10 μl of 125 ng/ml 4′,6-diamino-2-phenylindole (DAPI) in 1,4-diazabicyclo[2.2.2]octane (DABCO)/90% glycerol was applied. Thereafter, the slides were examined to count Y-positive cells using triple band pass filter for FITC, Texas Red, and DAPI. The time course of the transplanted cells in the WT to KO BMT group was monitored in both bone marrow (each n = 1) and glomeruli (each n = 2 except for 15 wk after BMT [n = 1]) at 4, 8, 12, and 15 wk after BMT. One thousand cells in bone marrow and the glomeruli were counted in each mouse, and the Y probe–positive cells were expressed as a percentage. In frozen sections, the Cy5-labeled X probe and Cy3-labeled Y probe (Chromosome Science Lab, Sapporo, Japan) were used. In frozen sections, 1000 cells were counted in the glomeruli and tubulointerstitial areas in WT to KO BMT and KO to KO BMT mice (each n = 2) at 8 wk after transplantation, which means 11 wk of age, and the Y probe–positive cells were expressed as a percentage.
Electron Microscopy
For electron microscopy, kidney specimens at 11 wk of age were fixed in 4% paraformaldehyde/2.5% glutaraldehyde in 0.1 M phosphate buffer and processed with standard methods in WT, KO, WT to KO BMT, KO to KO BMT, and 6 Gy KO mice (each n = 2). The thickness of GBM was calculated in 20 different points randomly selected from each mouse in a blinded manner.
Statistical Analysis
The data are expressed as means ± SEM. A comparison of the survival time was performed using the Kaplan-Meier method, and log-rank test for trend was used to compare the survival curves among more than two groups. We used the Cox proportional hazards regression model to calculate the hazard ratio and its 95% confidence interval and to assess whether the survival time correlates with irradiation in dosage-dependent manner. Two-way ANOVA was performed to examine the separate effects of the experimental groups and postpartum day on each parameter. This was followed by one-way ANOVA in the case of the presence of a significant interaction between the experimental groups and the postpartum day. Furthermore, one-way ANOVA was followed by the post hoc test in cases in which a significant difference was recognized. Differences were considered to be statistically significant at a value of P < 0.05, and the Statview 5.0 software package (SAS Institute, Cary, NC) was used for all calculations.
DISCLOSURES
None.
Acknowledgments
This work was supported in part by grants-in-aid for Scientific Research in Japan.
Parts of this article were previously published in the abstract presented at the annual meeting of the American Society of Nephrology; November 8 through 13, 2005; Philadelphia, PA.
We thank Karl Tryggvason and Timo Pikkarainen for valuable comments.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Stem Cell–Based Therapy for Glomerular Diseases: An Evolving Concept,” on pages 1621–1623.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Alport AC: Hereditary familial congenital haemorrhagic nephritis. BMJ 1: 504–506, 1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K: Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248: 1224–1227, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan D, Schroder CH, Smeets HJ, Reeders ST: Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet 8: 77–81, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addicks K, Smyth N, Schulze-Lohoff E, Weber M: Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int 63: 438–446, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Gross O, Schulze-Lohoff E, Koepke ML, Beirowski B, Addicks K, Bloch W, Smyth N, Weber M: Antifibrotic, nephroprotective potential of ACE inhibitor vs AT1 antagonist in a murine model of renal fibrosis. Nephrol Dial Transplant 19: 1716–1723, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Jefferson B, Harvey SJ, Zheng K, Gartley CJ, Jacobs RM, Thorner PS: Cyclosporin a slows the progressive renal disease of Alport syndrome (X-linked hereditary nephritis): Results from a canine model. J Am Soc Nephrol 14: 690–698, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Charbit M, Gubler MC, Dechaux M, Gagnadoux MF, Grunfeld JP, Niaudet P: Cyclosporin therapy in patients with Alport syndrome. Pediatr Nephrol 22: 57–63, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Heikkila P, Parpala T, Lukkarinen O, Weber M, Tryggvason K: Adenovirus-mediated gene transfer into kidney glomeruli using an ex vivo and in vivo kidney perfusion system: First steps towards gene therapy of Alport syndrome. Gene Ther 3: 21–27, 1996 [PubMed] [Google Scholar]
- 9.Heikkila P, Tibell A, Morita T, Chen Y, Wu G, Sado Y, Ninomiya Y, Pettersson E, Tryggvason K: Adenovirus-mediated transfer of type IV collagen alpha 5 chain cDNA into swine kidney in vivo: Deposition of the protein into the glomerular basement membrane. Gene Ther 8: 882–890, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Floege J, Kunter U, Weber M, Gross O: Bone marrow transplantation rescues Alport mice. Nephrol Dial Transplant 21: 2721–2723, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Borza DB: Loss of alpha3/alpha4 (IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6 (IV) collagen associated with longer renal survival in Col4a3−/− Alport mice. J Am Soc Nephrol 17: 1962–1969, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Molteni A, Moulder JE, Cohen EP, Fish BL, Taylor JM, Veno PA, Wolfe LF, Ward WF: Prevention of radiation-induced nephropathy and fibrosis in a model of bone marrow transplant by an angiotensin II receptor blocker. Exp Biol Med 226: 1016–1023, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Moulder JE, Fish BL, Regner KR, Cohen EP, Raife TJ: Retinoic acid exacerbates experimental radiation nephropathy. Radiat Res 157: 199–203, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Safwat A, Nielsen OS, El-Badawy S, Overgaard J: Late renal damage after total body irradiation and bone marrow transplantation in a mouse model: Effect of radiation fractionation. Eur J Cancer 31A: 987–992, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Guo JK, Schedl A, Krause DS: Bone marrow transplantation can attenuate the progression of mesangial sclerosis. Stem Cells 24: 406–415, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Guo JK, Ardito TA, Kashgarian M, Krause DS: Prevention of mesangial sclerosis by bone marrow transplantation. Kidney Int 70: 910–913, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kruse JJ, te Poele JA, Velds A, Kerkhoven RM, Boersma LJ, Russell NS, Stewart FA: Identification of differentially expressed genes in mouse kidney after irradiation using microarray analysis. Radiat Res 161: 28–38, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC: Collagen COL4A3 knockout: A mouse model for autosomal Alport syndrome. Genes Dev 10: 2981–2992, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Yokoo T, Ohashi T, Utsunomiya Y, Shen JS, Hisada Y, Eto Y, Kawamura T, Hosoya T: Genetically modified bone marrow continuously supplies anti-inflammatory cells and suppresses renal injury in mouse Goodpasture syndrome. Blood 98: 57–64, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C: Regression of renal vascular and glomerular fibrosis: Role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol 14: 1132–1144, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto T, Karasawa T, Saito A, Miyauchi N, Han GD, Hayasaka K, Shimizu F, Kawachi H: Ephrin-B1 localizes at the slit diaphragm of the glomerular podocyte. Kidney Int 72: 954–964, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Overbergh L, Valckx D, Waer M, Mathieu C: Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11: 305–312, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Langeveld JP, Wieslander J, Timoneda J, McKinney P, Butkowski RJ, Wisdom BJ Jr, Hudson BG: Structural heterogeneity of the noncollagenous domain of basement membrane collagen. J Biol Chem 263: 10481–10488, 1988 [PubMed] [Google Scholar]