Abstract
Induced in high glucose-1 (IHG-1) is an evolutionarily conserved gene transcript upregulated by high extracellular glucose concentrations, but its function is unknown. Here, it is reported that the abundance of IHG-1 mRNA is nearly 10-fold higher in microdissected, tubule-rich renal biopsies from patients with diabetic nephropathy compared with control subjects. In the diabetic nephropathy specimens, in situ hybridization localized IHG-1 to tubular epithelial cells along with TGF-β1 and activated Smad3, suggesting a possible role in the development of tubulointerstitial fibrosis. Supporting this possibility, IHG-1 mRNA and protein expression also increased with unilateral ureteral obstruction. In the HK-2 proximal tubule cell line, overexpression of IHG-1 increased TGF-β1–stimulated expression of connective tissue growth factor and fibronectin. IHG-1 was found to amplify TGF-β1–mediated transcriptional activity by increasing and prolonging phosphorylation of Smad3. Conversely, inhibition of endogenous IHG-1 with small interference RNA suppressed transcriptional responses to TGF-β1. In summary, IHG-1, which increases in diabetic nephropathy, may enhance the actions of TGF-β1 and contribute to the development of tubulointerstitial fibrosis.
Diabetic nephropathy (DN) is a leading cause of kidney disease, accounting for more than one third of all new cases of end-stage renal failure in Western society.1, 2 In DN, glomerulosclerosis precedes and primes for progressive accumulation of extracellular matrix in the interstitial space, resulting in the development of tubulointerstitial fibrosis (TIF).3 TIF is a final common pathway of injury in DN and other renal diseases of diverse etiology; the extent of tubular fibrosis mirrors closely loss of renal function.3, 4
TGF-β1 plays a key role in regulating the pathologic changes of kidney disease, resulting in the development of TIF.3–5 TGF-β1 mediates interstitial myofibroblast activation, a critical event in the pathogenesis of interstitial fibrosis, and also induces epithelial-to-mesenchymal transformation (EMT) of tubular epithelial cells into myofibroblast cells, further contributing to renal interstitial fibrogenesis.6, 7 TGF-β1 mediates its effects principally via activation of Smad proteins.8–10 TGF-β1 receptor activation triggers phosphorylation of the receptor-regulated Smad (R-Smad) 2 and 3.8–10 Phosphorylated R-Smad proteins bind to Smad4 and accumulate in the nucleus, where they activate transcription. The inhibitory Smad (I-Smad) 6 and 7 act in a negative feedback loop to inhibit TGF-β1 activity by preventing phosphorylation and/or nuclear accumulation of R-Smad proteins.11
The critical role of Smad signaling in renal fibrogenesis is demonstrated by a number of in vivo studies. Renal fibrosis did not develop in Smad3 knockout mice with streptozotocin-induced diabetes or after unilateral ureteric obstruction (UUO), an acute model of TIF.12, 13 In addition, overexpression of Smad7 has been shown to protect against kidney fibrosis in a number of animal models, including DN14 and UUO.15
In this article, we report that induced in high glucose-1 (IHG-1), a novel, highly conserved transcript, is associated with DN and UUO and is localized to renal tubules. Overexpression of IHG-1 amplifies TGF-β1–induced transcriptional activation in kidney tubule cells and enhances Smad3 phosphorylation. Inhibition of endogenous IHG-1 expression suppresses transcriptional responses to TGF-β1 and Smad3 phosphorylation. These data suggest that increased IHG-1 levels are likely to contribute to the TGF-β1–induced profibrotic changes in tubular cells that prime for TIF.
RESULTS
IHG-1 Is a Conserved Gene Transcript Induced by High Extracellular Glucose
IHG-1 (NCBI accession no. AF110136), identified by suppression subtractive hybridization16–18 is homologous to THG1L (Genbank no. NM_017872, Unigene HS 353090).19, 20
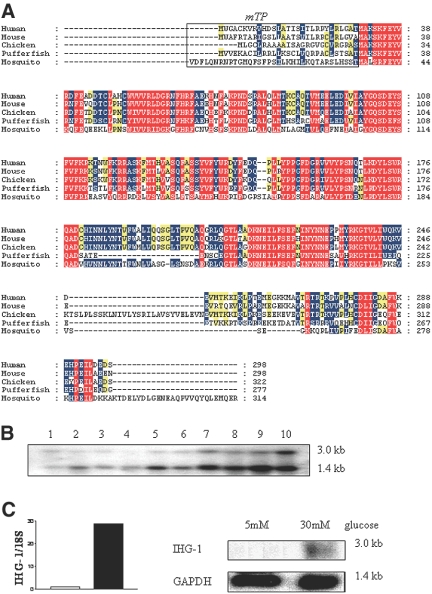
The IHG-1 amino acid sequence contains a number of regions with >90% amino acid conservation between eukaryotic species (Figure 1A). This suggests that these regions are necessary for function and therefore may represent or contain functional motifs; however, the only predicted functional domain to date is that of a mitochondrial localization signal (Figure 1A). On discovery, therefore, IHG-1 had no known function or functional classification.
Figure 1.
IHG-1 is an evolutionarily conserved ubiquitously expressed transcript. (A) IHG-1 encodes an evolutionarily conserved protein. The predicted coding sequence of IHG-1 was aligned with homologous proteins from mouse, chicken, pufferfish, and mosquito. Conservation is indicated by text and background color: 90%, red; 70%, blue; 50%, yellow. Mitochondrial targeting and cleavage sequences that were predicted by the programs Target P server and MitoProt II 1.0a4 are boxed and labeled mTP. (B) IHG-1 expression in normal human tissues. Autoradiograph of IHG-1 mRNA levels analyzed by Northern blot (FirstChoice Human Blot 1; Ambion, Austin, TX). The blot contained 2 μg of poly A RNA from the following human tissues: brain (1), placenta (2), skeletal muscle (3), heart (4), kidney (5), pancreas (6), liver (7), lung (8), spleen (9), and colon (10). Bands at approximately 3 and 1.4 kb were detected after hybridization with the IHG-1 probe. (C) The influence of high ambient glucose on IHG-1 mRNA levels in human MC. MC were exposed to 5 mM glucose (lane 1) and 30 mM glucose (lane 2) for 7 d. (Left) Real-time analysis of IHG-1 expression. Data are presented as fold change after normalization to ribosomal RNA control. IHG-1 levels are increased approximately 29-fold after exposure to 30 mM glucose for 7 d. (Right) Autoradiograph of IHG-1 mRNA levels analyzed by Northern blot. A band of approximately 3 kb was detected after hybridization with the IHG-1 probe.
Analysis by Northern blot identified two IHG-1–related transcripts of approximately 3 and 1.4 kb (Figure 1B) in 10 human tissues, suggesting that IHG-1 was ubiquitously expressed. Although only the larger transcript (encoding IHG-1) was detected as being induced in cultured mesangial cells (MC) treated with high extracellular glucose (Figure 1C), both transcripts are detected in normal human kidney tissue, the smaller transcript being more abundant (Figure 1B). Induction of IHG-1 mRNA expression in primary human MC cultured in high glucose was confirmed by Northern blotting and quantitative real-time reverse transcription–PCR with primers directed against the open-reading frame (Figure 1C).
Increased Expression of IHG-1 Is Associated with Human DN
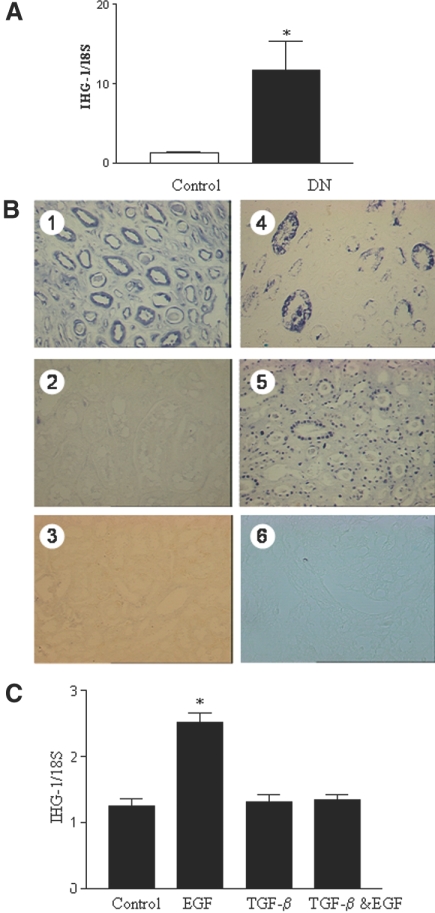
IHG-1 mRNA levels were significantly higher in tubule-rich microdissected renal biopsies from patients with DN (n = 13) as compared with those taken from control kidney (mean 9.7-fold increase over control; Figure 2A). Control tissue was from both individuals without diabetes (n = 4) and from normal regions of tumor nephrectomies (n = 4).
Figure 2.
Increased expression of IHG-1 mRNA is associated with DN. (A) IHG-1 mRNA levels are increased in DN. RNA was isolated from in tubule-rich microdissected renal biopsies taken from four living donors and four tumor nephrectomies (controls) and 11 patients with DN. Total RNA was reverse-transcribed into cDNA and analyzed for the expression of IHG-1 by quantitative real-time PCR (Taqman). Data are presented as fold change after normalization to ribosomal RNA control. Differences in means are significant (*P < 0.05). (B) IHG-1 expression in DN. In situ hybridization: (1) Sections obtained from patients with advanced DN showed marked IHG-1 staining in the tubules; (2) IHG-1 expression in normal kidney; no staining is seen; (3) IHG-1 sense probe; (4) TGF-β1 staining in DN showing strong tubular staining; (5) Southwestern (SW) showing activated Smad3 in tubules in DN; and (6) control for Smad3 (SW). (C) EGF but not TGF-β1 increases IHG-1 mRNA levels. RNA was isolated from HK-2 cells treated with EGF (10 ng/ml) or TGF-β1 (3 ng/ml) for 48 h. Total RNA was reverse-transcribed into cDNA and analyzed for the expression of IHG-1 by quantitative real-time PCR (Taqman). Data are presented as fold change after normalization to ribosomal RNA control ± SEM. Differences in means are significant (*P < 0.05).
IHG-1 expression in normal kidneys and in human DN was also assessed by means of in situ hybridization (ISH). Sections hybridized with sense probes showed no staining (e.g., Figure 2B, 3). Marked expression of IHG-1 was observed in tubular epithelial cells in biopsy specimens from patients with advanced DN (Figure 2B, 1). TGF-β1 was also expressed abundantly in tubular epithelial cells in DN sections and seemed to have a similar pattern of expression as IHG-1 (Figure 2B, 4). In both cases, some tubules stained more abundantly for these transcripts than others, most likely reflecting variations in cellular/regional responses to disease stress. Activated Smad3 assessed by Southwestern analysis was also observed to have a similar pattern of expression in renal tubular cells in DN (Figure 2B, 5). Activated Smad3 was localized to the nucleus as expected, whereas IHG-1 was not. These data suggest an association between expression of this gene and tubulointerstitial change in DN. This is an area critical to disease progression and fibrosis and is associated with significant TGF-β1 presence and Smad3 function.21, 22 IHG-1 expression was also increased and localized to renal tubules in progressive cases of the following fibrotic kidney diseases: Systemic lupus erythematosus, IgA nephropathy, and membranous nephropathy (S.M. et al., unpublished data). In contrast to Northern analysis and reverse transcription–PCR, IHG-1 mRNA was not detected by ISH in normal kidney (Figure 2B, 2). This is most likely due to reduced sensitivity of the ISH technique.
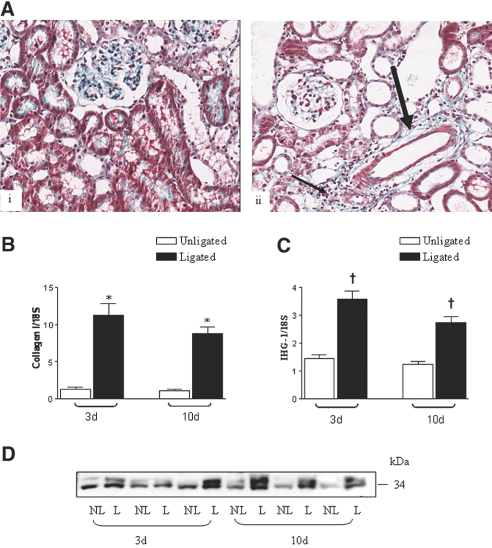
Figure 3.
IHG-1 expression is increased in rat UUO. (A) Histologic detection of TIF in UUO using Gomorri trichrome staining (magnification, ×20) of a 10-d nonligated (NL; i) and ligated (L; ii) rat kidney illustrating mature collagen fibrils present in the expanded tubulointerstitium (arrows in ii). (B) Collagen 1 mRNA levels are increased in UUO. RNA was isolated from rat L and NL kidneys 3 (n = 6) and 10 d (n = 5) after UUO. Total RNA was reverse-transcribed into cDNA and analyzed for the expression of collagen I by quantitative real-time PCR (Taqman). Data are presented as fold change after normalization to ribosomal RNA control ± SEM. Differences in means are significant (*P < 0.05). (C) IHG-1 mRNA levels are increased in UUO. Experimental analysis and presentation of results is as in B. Differences in means are significant (†P < 0.005). (D) IHG-1 protein levels are increased in UUO. Protein extracts were prepared from rat L and NL kidneys 3 (n = 3) and 10 d (n = 3) after UUO. Shown are Western analyses of these extracts probed for IHG-1.
EGF expression has been reported to be increased in experimental DN23; in addition, high extracellular glucose transactivates the EGF receptor in proximal tubule cells.24 EGF receptor activation is believed to contribute to TIF.5 EGF but not TGF-β1 induced the expression of IHG-1 in HK-2 cells, a human proximal tubule cell line (Figure 2C). We previously reported both treatments to increase fibronectin expression and decrease E-cadherin expression, consistent with changes associated with fibrosis.7 When EGF and TGF-β1 were added in combination, IHG-1 expression was not stimulated.
IHG-1 Expression Is Increased in Rat Kidneys after UUO
We decided to use the UUO model of renal fibrosis to study further the role of IHG-1 in TIF. TIF is the final pathway leading to end-stage renal disease in DN and in many chronic kidney diseases.21, 22 Because fibrosis in this model does not occur secondary to a preexisting systemic disorder, it allowed us to examine whether increased expression of IHG-1 was a feature of TIF per se.
Three days after UUO, tubular dilation and interstitial inflammation was evident in the affected kidney. At 10 d after UUO, the contralateral nonligated kidneys of rats showed a staining pattern with Gomorri trichrome consistent with a normal renal morphology. The mesangial matrix and brush border of proximal tubules was clearly evident (Figure 3A, i). In the ligated kidney, TIF was evident (thin arrow, Figure 3A, ii) in areas of expanded and inflamed tubulointerstitium. Discrete areas of heavy collagen deposition were particularly notable in perivascular regions (thick arrow, Figure 3A, ii). Collagen I mRNA levels were also significantly increased in UUO both at day 3 (8.3-fold; Figure 3B) and at day 10 after UUO (eight-fold; Figure 3B). IHG-1 mRNA levels were significantly increased in UUO both at day 3 (2.8-fold; Figure 3C), before development of tubulointerstitial fibrosis, and were still significantly increased at day 10 after UUO (2.2-fold; Figure 3C), when the tubulointerstitial lesion was well advanced (Figure 3A, ii). IHG-1 protein was increased in UUO at both day 3 and day 10 (Figure 3D).
IHG-1 Expression Amplifies TGF-β1 Signal Transduction
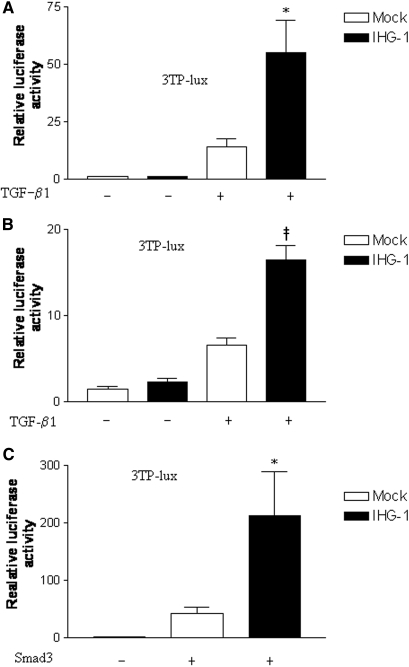
Similar to DN, activation of the TGF-β1 pathway in UUO is a pivotal event leading to development of TIF.5 Because IHG-1 expression was increased in kidney tubules in advanced DN and in the rat model of TIF, we examined the impact of IHG-1 overexpression on TGF-β1 signal transduction. Mv 1 Lu cells have been widely used to analyze transcriptional responses to TGF-β1.25–28 IHG-1 overexpression significantly enhanced TGF-β1 mediated transcription from a transfected TGF-β1–sensitive plasminogen activator inhibitor (PAI) promoter reporter construct. Levels of reporter gene expression were on average four-fold greater in cells overexpressing IHG-1 after TGF-β1 stimulation as compared with mock-transfected cells (Figure 4A). IHG-1 overexpression had no effect on PAI promoter activity in the absence of TGF-β1 stimulation.
Figure 4.
IHG-1 overexpression amplifies TGF-β1 signal transduction. (A) IHG-1 enhances 3TP-Lux activity in response to TGF-β1. Mv 1 Lu cells were co-transfected with the 3TP-Lux reporter (A through C) and phRL-CMV, an internal control reporter driving the expression of Renilla luciferase (Promega, Madison, WI), with or without pcDNA6-IHG-1-V5 expression plasmid, as indicated. Firefly luciferase activity normalized to Renilla luciferase activity was determined as directed by the manufacturer (Promega). Where indicated, cells were stimulated with 5 ng/ml TGF-β1 24 h after transfection. Luciferase activity was measured 24 h later. The results shown are means ± SEM of at least three independent experiments. IHG-1 significantly enhances TGF-β1 induced luciferase activity (*P < 0.05). (B) IHG-1 enhances 3TP-Lux activity in response to TGF-β1 in HK-2 cells stably transfected with pIRESpuro3-IHG-1-V5. Stably transfected HK-2 cells were co-transfected with the 3TP-Lux reporter and phRL-CMV. Experimental analysis and presentation of results is as in A. IHG-1 significantly enhances TGF-β1–induced luciferase activity (‡P < 0.0001). (C) IHG-1 enhances 3TP-lux activity in response to Smad3 stimulation. Mv 1 lu cells were co-transfected with p3TP-lux reporter, phRL-CMV, and combinations of pcDNA6-IHG-1-V5 and pRK5-Smad3 as indicated. Experimental analysis and presentation of results is as in A. IHG-1 significantly enhances Smad3-induced luciferase activity. IHG-1 significantly enhances Smad3-induced luciferase activity (*P < 0.05)
To investigate whether IHG-1 modulated TGF-β1 activity in a similar manner in kidney proximal tubule cells, we generated a stable cell line overexpressing IHG-1 in human renal tubular HK-2 cells. IHG-1 overexpression also increased levels of reporter gene expression after TGF-β1 stimulation in HK-2 cells and again had no effect on PAI-1 promoter activity in the absence of TGF-β1 (Figure 4B). These data suggest that IHG-1 protein amplifies the activity of TGF-β1. Because TGF-β1 is believed to be the key mediator of TIF, these data suggest that IHG-1 may contribute to kidney disease by amplifying TGF-β1 action.
Overexpressed Smad3 can activate a TGF-β1–sensitive promoter in the absence of TGF-β1–induced Smad3 phosphorylation. It is suggested that under such conditions, multimerization of Smad3 monomers induces activation.29 IHG-1 further enhanced activation of the PAI-1 promoter by overexpressed Smad3, further suggesting that IHG-1 may modulate Smad signal transduction in more than one way (Figure 4C).
IHG-1 Expression Prolongs Smad3 Phosphorylation and Increases Fibronectin and Connective Tissue Growth Factor Expression in Response to TGF-β1
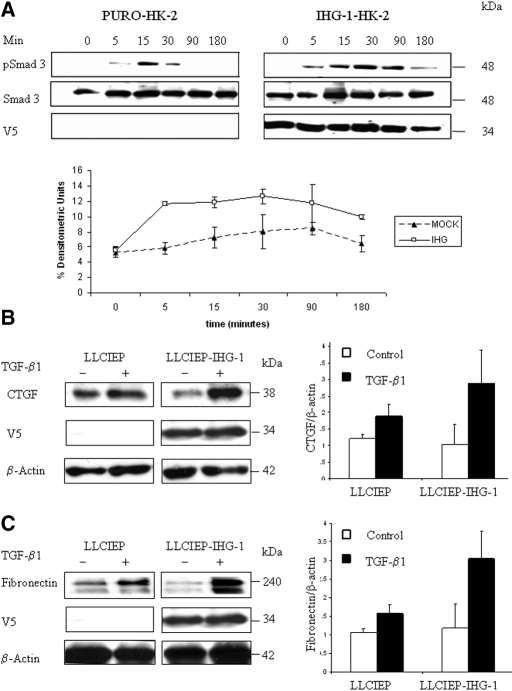
Smad-dependent responses to TGF-β1 are mediated by TGF-β1 receptor–dependent Smad2 and/or Smad3 phosphorylation.10 Overexpression of IHG-1 in stably transfected HK-2 cells resulted in increased levels of phosphorylated Smad3 after stimulation with TGF-β1 (Figure 5A, right) as compared with mock-transfected cells (Figure 5A, left): Higher levels of phosphorylated Smad3 were seen in the IHG-1–overexpressing cells at each time point during the 180-min response observed, whereas total Smad3 levels were unchanged by IHG-1 expression in this system (similar results were obtained in HeLa cells; data not shown). Overexpression of IHG-1 in HK-2 cells did not alter levels of pSmad2, p38, p42/44, or pAKT after stimulation with TGF-β1 (data not shown). These data suggest that IHG-1 facilitates increased levels and temporal prolongation of Smad3 phosphorylation. It is proposed that induction of the profibrotic mediator connective tissue growth factor (CTGF) by TGF-β1 is Smad3 dependent,30 whereas TGF-β1–induced fibronectin expression may be both Smad dependent and Smad independent.31–34 Overexpression of IHG-1 after transduction of HK-2 cells resulted in increased levels of both CTGF (2.8-fold; Figure 5B) and fibronectin (2.6-fold; Figure 5C) protein after stimulation with TGF-β1 as compared with mock-transduced cells (CTGF 1.56-fold, fibronectin 1.48-fold). These data suggest that IHG-1 facilitated increases and prolonged phosphorylation of Smad3, enhancing transcriptional responses to TGF-β1 along a fibrotic pathway.
Figure 5.
IHG-1 overexpression increases levels of phosphorylated Smad3, CTGF, and fibronectin. (A, top) IHG-1 expression increases cellular phospho-Smad3 levels after TGF-β1 stimulation of HK-2 cells stably transfected with pIRESpuro3-IHG-1-V5. Protein extracts were prepared from stably transfected HK-2 cells in the presence or absence of TGF-β1 (5.0 ng/ml) stimulation for the times indicated. Shown are Western analyses of these extracts probed for V5, phospho-Smad3, and Smad3. The IHG-1-V5 fusion protein was detected using a V5 antibody. Results shown are representative blots. (Bottom) Alterations in phosphorylation for Smad3 levels were measured using densitometric analysis (n = 3). Shown are percentage of densitometric units ± SEM after normalization to total Smad3 protein. (B) IHG-1 expression increases CTGF levels after TGF-β1 stimulation. HK-2 cells were transduced by a lentivirus overexpressing IHG-1 (LLCIEP-IHG-1) and by a lentivirus with no insert (LLCIEP) for 24 h before serum starvation. Transduced cells were serum starved for 24 h before stimulation with TGF-β1. Protein extracts were prepared from transduced HK-2 cells in the presence or absence of TGF-β1 stimulation as indicated. Shown are Western analyses (left) of these extracts probed for CTGF, V5, and β-actin. The IHG-1-V5 fusion protein was detected using a V5 antibody. β-Actin was measured to control for equal loading of protein. Results shown are representative blots. Alterations in protein levels for CTGF were measured using densitometric analysis (right). IHG-1 enhanced TGF-β1–induced CTGF expression. Shown is fold change ± SEM after normalization to β-actin (n = 3). (C) IHG-1 expression increases fibronectin levels after TGF-β1 stimulation. Experimental analysis and presentation of results are as in A. Results shown are representative blots (left). Alterations in protein levels for fibronectin were measured using densitometric analysis (right). IHG-1 enhanced TGF-β1–induced fibronectin expression. Shown is fold change ± SEM after normalization to β-actin (n = 3).
Loss of IHG-1 Expression Inhibits TGF-β1 Signal Transduction
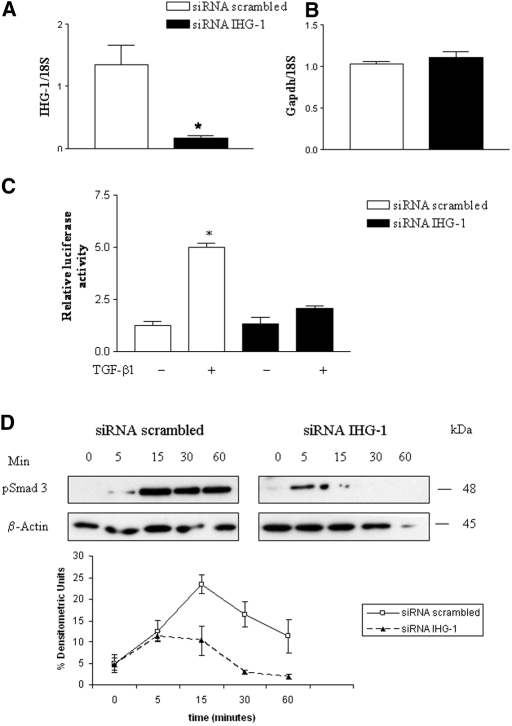
To determine whether endogenously expressed IHG-1 modulates TGF-β1 signal transduction, we used small interfering RNA (siRNA) to achieve selective knockdown of IHG-1 in HK-2 cells. IHG-1–directed siRNA (10 nM) led to an eight-fold decrease in IHG-1 expression in transfected HK-2 cells as compared with cells transfected with scrambled siRNA (Figure 6A), whereas it had no effect on the expression of nontarget transcript glyceraldehyde-3-phosphate dehydrogenase (Figure 6B). Transfection of IHG-1 siRNA also led to a consistent 50% decrease in PAI-luciferase reporter gene expression after TGF-β1 stimulation (Figure 6C) and decreased levels and time of presence of phosphorylated Smad3 (Figure 6D) after TGF-β1 stimulation, when compared with scrambled siRNA-transfected cells. These data demonstrate that endogenous IHG-1 potentially plays an active role in regulating the level of TGF-β1 signaling responses in renal tubular epithelial cells.
Figure 6.
siRNA directed against IHG-1 inhibits TGF-β1 activity. (A) HK-2 cells were transfected with either siRNA targeted against IHG-1 or scrambled siRNA. Total RNA was reverse-transcribed into cDNA and analyzed for the expression of IHG-1 by quantitative real-time PCR (Taqman). Data are presented as fold change after normalization to ribosomal RNA control. The results shown are means ± SEM of at least three independent experiments. IHG-1 mRNA is significantly decreased; differences in means are significant (*P < 0.05). (B) Total RNA was reverse-transcribed into cDNA and analyzed for the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by quantitative real-time PCR (Taqman). Experimental analysis and presentation of results are as in A. No change in GAPDH mRNA levels was observed on transfection with IHG-1–specific siRNA. (C) HK-2 cells were co-transfected with the 3TP-Lux reporter, phRL-CMV, and either siRNA targeted against IHG-1 or scrambled siRNA. Firefly luciferase activity normalized to Renilla luciferase activity was determined as directed by the manufacturer (Promega). Where indicated, cells were stimulated with 5 ng/ml TGF-β1 24 h after transfection. Luciferase activity was measured 24 h later. The results shown are means ± SEM of a representative experiment of three independent analyses. In all cases, siRNA directed against IHG-1 decreased TGF-β1 induction of the 3TP-lux reporter by at least 50%. (D) Loss of IHG-1 expression inhibits Smad3 phosphorylation. HK-2 cells were transfected with siRNA for IHG-1 or scrambled siRNA in serum-free medium for 24 h, before TGF-β1 stimulation. Protein extracts were prepared from these cells after TGF-β1 (5.0 ng/ml) stimulation for the times indicated. Shown are Western analyses (top) of these extracts probed for phospho-Smad3 and β-actin. β-Actin was analyzed to control for equal loading of protein. Results shown are representative blots (n = 3). (Bottom) Alterations in phosphorylation for Smad3 levels were measured using densitometric analysis (n = 3). Shown are percentage of densitometric units ± SEM.
DISCUSSION
We previously reported the identification of a novel gene transcript, IHG-1, in an in vitro screen for genes associated with development of DN.15, 16 IHG-1 transcript levels were significantly upregulated in MC cultured in high glucose conditions, leading us to investigate whether the expression of this gene also occurred in human DN. IHG-1 transcript levels were significantly increased in tubule-rich microdissected renal biopsies from patients with DN, with clear expression being localized to the tubule in the diabetic kidney. The expression pattern was similar to that of TGF-β1 and of activated Smad3. TGF-β1 is believed to be the key mediator of fibrosis in the kidney.5, 21, 22, 35 Increased activity of TGF-β1 in the tubulointerstitium resulted in increased expression and accumulation of extracellular matrix proteins, resulting in compartment-specific pathologic matrix remodeling and scarring.5
In advanced DN, we hypothesized that increased IHG-1 levels are likely to contribute to the TGF-β1–induced profibrotic changes in tubular cells that prime for TIF. The significant increase in expression of IHG-1 in the UUO model adds further support to our hypothesis that IHG-1 is a mediator of TIF. UUO leads directly to TIF, in contrast to DN, in which changes in the glomeruli come first and lead to the development of the tubulointerstitial lesion. Increased expression of IHG-1 in this model of renal fibrosis suggests that this novel gene may contribute to TIF per se and may not be restricted to DN. Observations of increased IHG-1 expression in the tubules in other fibrotic diseases add further weight to our hypothesis.
Although the initiating stresses in DN and UUO are different, the development of TIF is associated with common cytokine/growth factor stimuli.5 For instance, both conditions have been successfully treated experimentally with bone morphogenic protein-7.36, 37 EGF receptor activation has been implicated in tubulointerstitial fibrogenesis.5 It is transactivated by high extracellular glucose24 and has been proposed to assist in the selective survival of a transdifferentiated, profibrotic cell type.7 One of the ways in which it may facilitate the profibrotic effects on tubule cells may be via induction of IHG-1 expression.
Our investigations of IHG-1's effect on responses to TGF-β1 stimulation in renal tubule cells clearly points to IHG-1's being an amplifier of TGF-β1 action in the tubule. Its overexpression-induced increases in luciferase reporter activity from a TGF-β1 responsive region of the PAI-1 promoter. PAI-1 is strongly induced in various kidney pathologies, including DN and UUO, and is considered an important factor in the development of renal fibrosis.21, 22, 38 IHG-1 had no effect on basal levels of reporter expression, suggesting that signal transduction must be first initiated for IHG-1 to mediate its effects. Activated Smad3 co-localized with IHG-1 in DN, suggesting that IHG-1 might influence TGF-β1 signaling by targeting Smad3.
TGF-β1 stimulation of epithelial cells causes a transient phosphorylation of R-Smad evident within 10 min, peaking between 30 and 60 min and persisting for up to 5 h.10, 39 Both R-Smad and Smad nuclear accumulation are maintained only when receptors are active.8–11 As soon as receptor activity decreases, R-Smad phosphorylation decreases and nuclear accumulation is lost. Dephosphorylation is proposed as the main mechanism of deactivation.8–11 The majority of phospho-Smad are believed to be recycled after dephosphorylation, phospho-Smad3 by the phosphatase PPM1A.10, 40 The early and sustained increase in Smad3 phosphorylation in epithelial cells overexpressing IHG-l coupled with the rapid loss of phosphorylation with IHG-1 knockdown suggests that IHG-1 may function by inhibiting the activity of a phosphatase, which may target either the activated receptor (e.g., GADD34)41 or the R-Smad itself. How this may function will be the subject of our future investigations. It is also possible that IHG-1 may modulate the activity of the I-Smad, Smad7, which not only targets TGF-β1 receptors for dephosphorylation but also both receptors and R-Smad for proteosomal degradation.11 The dephosphorylation machinery is believed to be active and in place from onset to termination of signaling; thereby, signal transduction and consequent gene transcription relies on a dynamic balance between phosphorylation and dephosphorylation.8–11 Increased expression of IHG-1, leading to increased phosphorylation of Smad3, may tip this balance in favor of fibrosis.
Smad3 is required for TGF-β1–induced fibrosis.12, 13, 42 An increasing body of evidence demonstrates that decreasing TGF-β1 signaling through blocking Smad3 can protect against fibrosis both in vivo and in vitro.12, 13, 38 It has been reported that renal fibrosis did not develop in Smad3 knockout mice with streptozotocin-induced diabetes or after UUO. In addition, cells from these mice failed to undergo an EMT when stimulated with TGF-β1.12, 13 Interestingly, we detected no amplification of Smad2 activation by IHG-1. Why the effect of IHG-1 is Smad3 specific remains unknown and will be the subject of our future investigations.
Expression of CTGF, a profibrotic mediator, is increased in the tubular epithelium by TGF-β1 and is proposed to play a key role in renal fibrogenesis.43 Overexpression of IHG-1 increased TGF-β1–induced CTGF expression, further strengthening our hypothesis that IHG-1 contributes to the development of TIF. IHG-1 expression also increased TGF-β1–induced fibronectin expression, which has been reported to be induced by both Smad-dependent and Smad-independent pathways.31–34 Our data suggest that IHG-1 amplifies TGF-β1–induced fibronectin expression by a Smad-dependent mechanism; however, there is also the possibility that this induction is Smad dependent and indirect, mediated, for instance, by CTGF.
Suppression of IHG-1 expression using siRNA led to reduced TGF-β1 induction of the PAI promoter activation and reduced levels of Smad3 phosphorylation, suggesting that IHG-1 has an important role in promoting TGF-β1 responses in renal tubular cells, and given the ubiquitous nature of tissue IHG-1 expression, this may be a general phenomenon. Thus, we describe IHG-1, a novel protein-encoding transcript, whose increased expression is associated with renal tubular elements in human DN and in kidney tissue in the rat UUO model of interstitial fibrosis. We show, on overexpression, that IHG-1 is a novel amplifier of a TGF-β1 transcriptional response possibly through increasing and/or maintaining phosphorylated Smad3 protein levels after receptor activation by TGF-β1. In addition, knockdown of endogenous IHG-1 blunts Smad3 phosphorylation and a TGF-β1 transcriptional response. Considering TGF-β1's central role in the development of fibrotic renal disease, IHG-1 may well constitute a novel profibrotic mediator.
CONCISE METHODS
IHG-1 cDNA Assembly
Database searching was performed using BLAST.44 Suppression subtractive hybridization analysis16–18 yielded a 198-bp cDNA fragment that we have called IHG-1 (Genbank accession no. AF110136). A sequence identical to UniGene cluster HS353090 that encoded a complete open-reading frame of 894 bp was generated by expressed sequence tag walking.45
Northern Blot Analysis and Real-Time (Taqman) PCR
Northern blots were performed using formaldehyde denaturation according to standard protocols. Transcript levels were determined by quantitative real-time Taqman PCR, as described previously.46 Probe and primer sequences were Pre-Developed Assay Reagent (PDAR kit; Applied Biosystems, Foster City, CA).
Human DN Kidney Biopsies
Human biopsy segments were obtained from patients after informed consent and with the approval of their local ethical committees.47 ISH and Southwestern analyses were as described previously.48, 49 Ureteric obstruction was performed in rats anaesthetized using isofluorane inhalation. Following laparotomy, the proximal portion of the left ureter was ligated with a 6/0 silk, the laparotomy closed and animals allowed to recover for 3 days (n = 6) or 10 days (n = 5). Animals were then anaesthetized and underwent a second laparotomy.
Transfection
Stably transfected cell lines were generated with plasmid pIRESpuro3 and pIRESpuro3-IHG-1-V5 (Clontech, Paola Alto, CA). Transfection of HK-2 cells with siRNA (Dharmacon, Chicago, IL; SMARTpool reagent) was as described.50
Recombinant Lentivirus Production
HEK 293T cells were transfected with (pCMVΔR8.9), (pMD.2G), and LLCIEP or IHG-1-V5-LLCIEP using a calcium phosphate transfection kit (Invitrogen, Paisley, UK).
DISCLOSURES
None.
Acknowledgments
S.M. is a recipient of Fondecyt 1080083. This research was funded by the Wellcome Trust (M.M., F.M., and C.G.) and Science Foundation Ireland (M.M., F.M., and C.G.). N.G.D., B.G., H.R.B., F.M., and C.G. are recipients of grants from the Health Research Board of Ireland. C.G. is a member of EU EICOSANOX consortium (ISHM-CT-2004-005033).
We express our thanks to Catherine Moss, Laura Connole, and Janet McCormack (Transcriptomic Unit, Conway Institute, UCD, Dublin, Ireland) for performing real-time PCR analyses. We acknowledge receipt of reagents from Dr. Rik Derynck (University of California, San Francisco, CA), Dr. Joan Massague (Sloan Kettering Institute, New York, NY), and Dr. Ed Leof (Mayo Clinic College of Medicine, Rochester, MN). Renal biopsy profiling was performed in the ERCB.47
Published online ahead of print. Publication date available at www.jasn.org.
J.H.'s current affiliation is Division of Experimental Pathology, Lund University, University Hospital, Malmö, Sweden. M.K.'s current affiliation is Division of Nephrology, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan.
REFERENCES
- 1.Excerpts from United States Renal Data System Annual Data Report. Am J Kidney Dis 34[2 Suppl 1]: s1–S176, 1999 [PubMed] [Google Scholar]
- 2.Parring HH, Osterby R, Anderson PW, Hsueh WA: Diabetic nephropathy. In Brenner and Rector's The Kidney, 5th Ed., edited by Brenner BM, Philadelphia, W.B. Saunders, 1996, pp 1864–1883
- 3.Mason RM, Abdel Wahab N: Extracellular matrix metabolism in diabetic nephropathy J Am Soc Nephrol 14: 1358–1373, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bottinger EP, Bitzer M: TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Docherty NG, O’Sullivan OE, Healy DA, Fitzpatrick JM, Watson RW: Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol 290: F4–F13, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Liu Y: Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 159: 1465–1475, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty NG, O’Sullivan OE, Healy DA, Murphy M, O’Neill AJ, Fitzpatrick JM, Watson RW: TGF-beta1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. Am J Physiol Renal Physiol 290: F1202–F1212, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Moustakas A, Souchelnytskyi S, Heldin CH: Smad regulation in TGF-beta signal transduction. J Cell Sci 114: 4359–4369, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Attisano L, Wrana JL: Signal transduction by the TGF-beta superfamily. Science 296: 1646–1647, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Itoh S, ten Dijke P: Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol 19: 176–184, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 2: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto M, Maezawa Y, Yokote K, Joh K, Kobayashi K, Kawamura H, Nishimura M, Roberts AB, Saito Y, Mori S: Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun 305: 1002–1007, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lund RJ, Davies MR, Hruska KA: Bone morphogenetic protein-7: An anti-fibrotic morphogenetic protein with therapeutic importance in renal disease. Curr Opin Nephrol Hypertens 11: 31–36, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ: Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD: Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci U S A 93: 6025–6030, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy M, Godson C, Cannon S, Kato S, Mackenzie HS, Martin F, Brady HR: Suppression subtractive hybridization identifies high glucose levels as a stimulus for expression of connective tissue growth and other genes in human mesangial cells. J Biol Chem 274: 5830–5834, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Clarkson M, Murphy M, Gupta S, Lambe T,Godson C, Mackenzie HS, Martin F, Brady HR: High glucose-altered gene expression in mesangial cells: Actin-regulatory protein gene expression is triggered by oxidative stress and cytoskeletal disassembly J Biol Chem 277: 9707–9712, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Gu W, Jackman JE, Lohan AJ, Gray MW, Phizicky EM: tRNAHis maturation: An essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis Genes Dev 17: 2889–2901, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo D, Hu K, Lei Y, Wang Y, Ma T, He D: Identification and characterization of a novel cytoplasm protein ICF45 which is involved in cell cycle regulation. J Biol Chem 279: 53498–53505, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Koka V, Lan HY: Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology 10: 48–56, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Gilbert RE, Cox A, McNally PG, Wu LL, Dziadek M, Cooper ME, Jerums G: Increased epidermal growth factor in experimental diabetes related kidney growth in rats. Diabetologia 40: 778–785, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Saad S, Stevens VA, Wassef L, Poronnik P, Kelly DJ, Gilbert RE, Pollock CA: High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int 68: 985–997, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Weis-Garcia F, Massagué J: Complementation between kinase-defective and activation-defective TGF-beta receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J 15: 276–289, 1996 [PMC free article] [PubMed] [Google Scholar]
- 26.Feng XH, Derynck R: Ligand-independent activation of transforming growth factor (TGF) beta-signaling pathways by heteromeric cytoplasmic domains of TGF-beta receptors. J Biol Chem 271: 13123–13129, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL: SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95: 779–791, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Angelis K, Danielpour D, Haddad MM, Bischof O, Campisi J, Stavnezer E, Medrano EE: Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type beta transforming growth factor. Proc Natl Acad Sci U S A 97: 5924–5929, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moustakas A, Heldin CH: From mono- to oligo-Smads: The heart of the matter in TGF-beta signal transduction. Genes Dev 16: 1867–1871, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME: The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J 393: 601–607, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niculescu-Duvaz I, Phanish MK, Colville-Nash P, Dockrell ME: The TGFbeta1-induced fibronectin in human renal proximal tubular epithelial cells is p38 MAP kinase dependent and Smad independent. Nephron Exp Nephrol 105: e108–e116, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD: Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in Adriamycin-induced nephropathy. Am J Pathol 169: 1527–1540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isono M, Chen S, Hong SW, Iglesias-de la Cruz MC, Ziyadeh FN: Smad pathway is activated in the diabetic mouse kidney and Smad3 mediates TGF-beta-induced fibronectin in mesangial cells. Biochem Biophys Res Commun 296: 1356–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Yang J, Dai C, Wu C, Liu Y: Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 112: 503–516, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN: Transcriptional activation of transforming growth factor-beta1 in mesangial cell culture by high glucose concentration. Kidney Int 54: 1107–1116, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J: Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol 279: F130–F143, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto H, Grahovac G, Zeisberg M, Kalluri R: Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56: 1825–1833, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Matsuo S, Lopez-Guisa JM, Cai X, Okamura DM, Alpers CE, Bumgarner RE, Peters MA, Zhang G, Eddy AA: Multifunctionality of PAI-1 in fibrogenesis: Evidence from obstructive nephropathy in PAI-1-overexpressing mice. Kidney Int 67: 2221–2238, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Inman GJ, Nicolas FJ, Hill CS: Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol Cell 10: 283–294, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Duan X, Liang YY, Su Y, Wrighton KH, Long J, Hu M, Davis CM, Wang J, Brunicardi FC, Shi Y, Chen YG, Meng A, Feng XH: PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 125: 915–928, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi W, Sun C, He B, Xiong W, Shi X, Yao D, Cao X: GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J Cell Biol 164: 291–300, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flanders KC: Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85: 47–64, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H: Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ: Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon R, Murphy M, Clarkson M, Taal M, Mackenzie HS, Godson C, Martin F, Brady HR: IHG-2, a mesangial cell gene induced by high glucose, is human gremlin. Regulation by extracellular glucose concentration, cyclic mechanical strain, and transforming growth factor-beta1. J Biol Chem 275: 9901–9904, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR: The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem 277: 44187–44194, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Cohen CD, Frach K, Schlondorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: A novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Mezzano SA, Droguett MA, Burgos ME, Ardiles LG, Aros CA, Caorsi I, Egido J: Overexpression of chemokines, fibrogenic cytokines, and myofibroblasts in human membranous nephropathy. Kidney Int 57: 147–158, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Mezzano SA, Barria M, Droguett MA, Burgos ME, Ardiles LG, Flores C, Egido J: Tubular NF-kappaB and AP-1 activation in human proteinuric renal disease. Kidney Int 60: 1366–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Healy DA, Daly PJ, Docherty NG, Murphy M, Fitzpatrick JM, Watson RW: Heat shock-induced protection of renal proximal tubular epithelial cells from cold storage and rewarming injury. J Am Soc Nephrol 17: 805–812, 2006 [DOI] [PubMed] [Google Scholar]