Abstract
Inflammation is a pathologic feature of a variety of chronic kidney diseases. Several lines of evidence suggest a potential anti-inflammatory role for vitamin D in chronic kidney disease, but the underlying mechanism remains unknown. Here, the effect of the synthetic vitamin D analogue paricalcitol on renal inflammation was investigated in a mouse model of obstructive nephropathy. Paricalcitol reduced infiltration of T cells and macrophages in the obstructed kidney. This inhibition of inflammatory cell infiltration was accompanied by a decreased expression of RANTES and TNF-α. Induction of RANTES was localized primarily to the tubular epithelium, underscoring a role for tubular cells in renal inflammation. In a human proximal tubular cell line (HKC-8), paricalcitol inhibited RANTES mRNA and protein expression and abolished the ability of tubular cells to recruit lymphocytes and monocytes after TNF-α stimulation. Although RANTES induction depended on NF-κB signaling, paricalcitol affected neither TNF-α–mediated IκBα phosphorylation and degradation nor p65 NF-κB activation and nuclear translocation. Instead, chromatin immunoprecipitation assay showed that paricalcitol abolished the binding of p65 to its cognate cis-acting element in the RANTES promoter. The vitamin D receptor (VDR) and p65 formed a complex in tubular cells after paricalcitol treatment, which inhibited the ability of p65 to trans-activate gene transcription. In vivo, paricalcitol did not block NF-κB nuclear translocation after obstructive injury but did increase the expression and nuclear distribution of VDR. These results suggest that paricalcitol inhibits renal inflammatory infiltration and RANTES expression by promoting VDR-mediated sequestration of NF-κB signaling.
Deficiency in vitamin D and its active metabolites is a common pathologic feature that occurs early in the pathogenesis of chronic kidney disease (CKD). Numerous clinical studies have shown a high prevalence of calcitriol deficiency in CKD, even in patients with reasonable GFR.1,2 In humans, active vitamin D reduces proteinuria and all-cause mortality in CKD, which is independent of serum parathyroid hormone, phosphorus, and calcium levels.3–5 Evidence is also mounting that vitamin D analogue is renoprotective in different experimental nephropathies.6 Although early studies largely focused on primary glomerular diseases,7–9 active vitamin D was shown to display beneficial effects in obstructive nephropathy,10 a model characterized by inflammatory infiltration, tubular atrophy, and interstitial fibrosis. The therapeutic effects of active vitamin D in obstructive nephropathy are thought to be mediated by its ability to preserve tubular epithelial integrity via inhibiting epithelial-to-mesenchymal transition (EMT)10; however, in view of its pleiotropic property, it is conceivable that vitamin D could elicit its renoprotection by a multitude of actions.11,12 One of the potential mechanisms could be related to its ability to modulate renal inflammation after injury.
Renal inflammation, characterized by the infiltration of inflammatory cells including T cells and macrophages to kidney parenchyma, is an imperative pathologic process in the evolution of CKD.13,14 Inflammatory infiltration contributes to the initiation and progression of CKD in several ways. On the one hand, inflammatory cells release proinflammatory, chemoattractant cytokines (chemokines), thereby leading to the formation of a vicious self-accumulation circle. On the other hand, production and secretion of profibrotic cytokines by inflammatory cells such as monocytes/macrophages and T cells create a fibrogenic microenvironment, leading to generation of the matrix-producing effector cells through fibroblast activation and tubular EMT. Not surprising, decline of renal function in patients with CKD often correlates closely to the extent of inflammation.15 Inhibition of renal inflammation by different maneuvers is therapeutically effective, resulting in an amelioration of renal fibrotic lesions in various experimental animal models.16,17
Several lines of evidence have suggested a potential anti-inflammatory activity of vitamin D in CKD.6,18 In animal models of primary glomerular diseases, administration of vitamin D reduces glomerular infiltration of inflammatory cells.6,9 Consistently, a decreased inflammation is associated with higher serum vitamin D level in patients with CKD.19 Vitamin D may exert its immunomodulatory action through regulating the activity of many types of immune cells such as macrophages, dendritic cells, and T cells.20–22 Furthermore, evidence also points to an inhibitory effect of vitamin D on the signaling of NF-κB, a key transcription factor that is thought to mediate acute and chronic inflammation by regulating the gene expression of cytokines, chemokines, and adhesion molecules14; however, it remains ambiguous as to the molecular mechanism by which vitamin D inhibits inflammation in the setting of CKD.
In this study, we demonstrated that paricalcitol, an active vitamin D analogue, reduces inflammatory cell infiltration and RANTES, also known as CC-chemokine ligand 5 (CCL5), expression in vivo. We further showed that paricalcitol induced a physical interaction of vitamin D receptor (VDR) and p65 NF-κB, resulting in sequestration of the ability of p65 to bind to cis-acting element, thereby causing the repression of NF-κB–mediated gene transcription. Our findings provide significant mechanistic insights into the understanding of the molecular details underlying vitamin D inhibition of renal inflammation.
RESULTS
Paricalcitol Inhibits Renal Inflammatory Infiltration
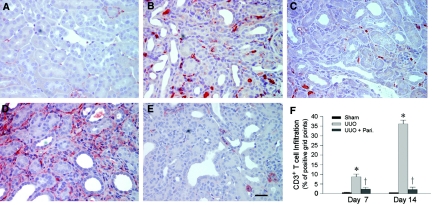
To assess the potential effect of paricalcitol on renal inflammation, we first examined renal infiltration of the CD3+ T cells in the obstructed kidney after unilateral ureteral obstruction (UUO). As shown in Figure 1, compared with sham controls, obstructive injury caused significant T cell infiltration in the kidney at 7 d after UUO, as illustrated by immunohistochemical staining for CD3 antigen. Prolonged, continuous obstruction for 14 d markedly increased renal infiltration of the CD3+ T cells in the obstructed kidney, when compared with 7 d after UUO (Figure 1, D versus B); however, paricalcitol treatment effectively inhibited the infiltration of T cells in the obstructed kidney at different time points (Figure 1, C and E). A computer-aided quantitative analysis also demonstrated a dramatic suppression of inflammatory T cell infiltration by paricalcitol at 7 and 14 d after UUO, respectively, in obstructive nephropathy (Figure 1F).
Figure 1.
Paricalcitol reduces renal inflammatory T cell infiltration. (A through E) Immunohistochemical staining revealed an increased infiltration of CD3+ T cells in the obstructed kidney at 7 d (B) and 14 d (D), respectively, after UUO, compared with sham control (A). Paricalcitol treatment at 0.3 μg/kg body wt effectively inhibited the infiltration of T cells in the obstructed kidneys at 7 d (C) and 14 d (E) after UUO, respectively, as indicated. Bar = 20 μm. (F) Graphic presentation of quantitative data. Data are means ± SEM of five animals per group. *P < 0.01 versus sham control; †P < 0.01 versus vehicle.
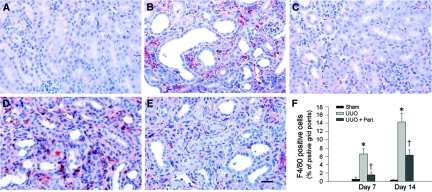
We also examined the effects of paricalcitol on monocyte/macrophage infiltration in the obstructed kidney. As shown in Figure 2, UUO instigated significant infiltration of the F4/80 antigen–positive myeloid cells, including macrophages and dendritic cells in renal interstitium. Similarly, administration of paricalcitol substantially reduced F4/80-positive cell infiltration at different time points after UUO (Figure 2, C, E, and F).
Figure 2.
Paricalcitol inhibits renal inflammatory macrophage infiltration. (A through E) Immunohistochemical staining showed an increased infiltration of F4/80+ myeloid cells including macrophages and renal dendritic cells in the obstructed kidney at 7 d (B) and 14 d (D), respectively, after UUO, compared with sham control (A). Paricalcitol at 0.3 μg/kg body wt inhibited the infiltration of the F4/80+ cells in the obstructed kidneys at 7 d (C) and 14 d (E), respectively, after UUO as indicated. Bar = 20 μm. (F) Graphic presentation of quantitative data. Data are means ± SEM of five animals per group. *P < 0.01 versus sham control; †P < 0.01 versus vehicle.
Paricalcitol Inhibits Renal mRNA Expression of RANTES and TNF-α
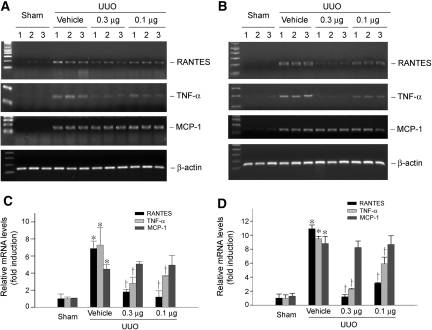
We next examined the expression of RANTES, an important proinflammatory chemokine that plays a crucial role in inciting infiltration of T cells and other inflammatory cells such as monocytes, in the obstructed kidney. As shown in Figure 3, reverse transcriptase–PCR (RT-PCR) results revealed that approximately seven- and 11-fold induction of RANTES/CCL5 mRNA was observed in the obstructed kidney at 7 and 14 d, respectively, when compared with sham controls. Interestingly, we found that paricalcitol markedly inhibited RANTES mRNA expression in different time points (Figure 3, A and B); however, paricalcitol seemed to have little effect on monocyte chemoattractant protein-1 (MCP-1), also known as CCL2, mRNA expression in the obstructed kidney (Figure 3).
Figure 3.
Paricalcitol inhibits RANTES/CCL5 and TNF-α but not MCP-1/CCL2 mRNA expression in obstructive nephropathy. (A and B) RT-PCR results showed that paricalcitol suppressed renal RANTES and TNF-α mRNA expression at 7 d (A) and 14 d (B), respectively, after UUO in a dosage-dependent manner; however, no significant difference in MCP-1 mRNA abundance was observed after paricalcitol treatment, compared with vehicle controls. Numbers (1, 2, and 3) denote each individual animal in a given group. Dosages of paricalcitol were 0.3 and 0.1 μg/kg body wt, respectively. (C and D) Renal mRNA levels of RANTES, TNF-α, and MCP-1 in various groups. Relative mRNA levels at 7 d (C) and 14 d (D) after UUO were calculated and expressed as fold induction over sham controls (value = 1.0) after normalization with β-actin. Data are means ± SEM of five animals per group. *P < 0.01 versus sham control; †P < 0.01 versus vehicle.
We further investigated the expression of TNF-α, a key inflammatory cytokine that is produced primarily by the infiltrated cells, in the obstructed kidney after UUO. As presented in Figure 3, UUO dramatically induced renal TNF-α mRNA expression at 7 and 14 d after UUO, respectively. Similarly, paricalcitol inhibited TNF-α expression in the obstructed kidney in a dosage-dependent manner.
RANTES Protein Is Induced Specifically in Renal Tubules after Obstructive Injury
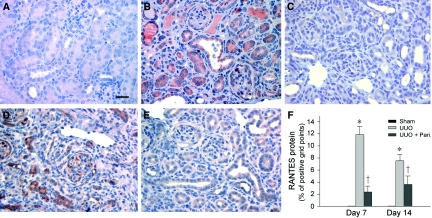
We also examined RANTES protein expression and its localization in the obstructed kidney by immunohistochemical staining. As shown in Figure 4A, no or little RANTES protein was observed in normal, sham-operated kidney; however, RANTES protein expression was markedly induced in the obstructed kidney at 7 and 14 d, respectively. RANTES protein was localized predominantly in renal tubular epithelia (Figure 4, B and D). Consistent with the mRNA data (Figure 3), paricalcitol treatment significantly reduced RANTES protein expression in the obstructed kidney at different time points (Figure 4, C and E).
Figure 4.
Paricalcitol inhibits renal RANTES protein expression in the obstructed kidney after UUO. (A through E) Immunohistochemical staining demonstrated that RANTES protein was induced predominantly in renal tubular epithelium in the obstructed kidney at 7 d (B) and 14 d (D) after UUO, compared with sham control (A). Tubular expression of RANTES was largely suppressed by paricalcitol treatment (0.3 μg/kg body wt) at 7 d (C) and 14 d (E), respectively, after UUO. Bar = 20 μm. (F) Quantitative determination of RANTES protein expression in various groups as indicated. Data are means ± SEM of five animals per group. *P < 0.05 versus sham control; †P < 0.05 versus vehicle.
Paricalcitol Inhibits Tubular RANTES Induction In Vitro
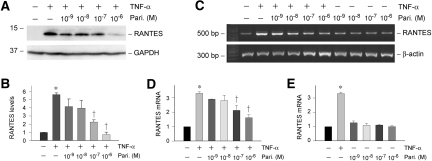
Given that tubular epithelial cells are the primary sites of RANTES production in vivo in obstructive nephropathy, we next examined the regulation of renal RANTES expression by paricalcitol using an in vitro cell culture system. To this end, human proximal tubular epithelial cells (HKC-8) were incubated with TNF-α in the presence or absence of paricalcitol, and RANTES expression was examined. As shown in Figure 5, A and B, Western blot analysis showed that proinflammatory cytokine TNF-α dramatically induced RANTES expression in HKC-8 cells. Simultaneous incubation with paricalcitol, however, inhibited the TNF-α–mediated RANTES expression (Figure 5, A and B). Similarly, RT-PCR results demonstrated that paricalcitol reduced RANTES mRNA expression induced by TNF-α in a dosage-dependent manner (Figure 5, C and D). Of note, paricalcitol did not affect basal RANTES expression under unstimulated conditions in HKC-8 cells (Figure 5E).
Figure 5.
Paricalcitol inhibits the TNF-α–mediated RANTES expression in tubular epithelial cells in vitro. Human proximal tubular epithelial (HKC-8) cells were treated with 2 ng/ml TNF-α in the presence or absence of various concentrations of paricalcitol as indicated. (A and B) Representative Western blot (A) and graphic presentation (B) showed that paricalcitol inhibited RANTES expression induced by TNF-α in HKC-8 cells. (C, D, and E) RT-PCR showed that paricalcitol inhibited TNF-α–induced RANTES mRNA expression in HKC-8 cells. Data are means ± SEM of three experiments. *P < 0.05 versus control; †P < 0.05 versus TNF-α treated alone.
Paricalcitol Reduces the Recruitment of Inflammatory Cells In Vitro
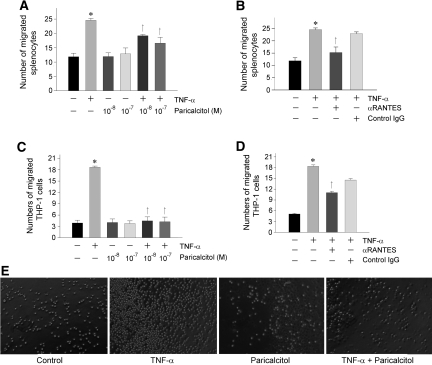
To evaluate the significance of tubular expression of RANTES in recruiting inflammatory cells, we examined the chemoattractant ability of the supernatants of tubular cells by using chemotaxis assay. To this end, HKC-8 cells were treated with TNF-α in the absence or presence of paricalcitol for 24 h. The supernatants of these cultures were used to assess their ability to attract lymphocyte or monocyte migration across the Transwell filter. A shown in Figure 6, conditioned medium derived from TNF-α–stimulated HKC-8 cells were more potent in chemoattracting mouse splenocytes (Figure 6A) or THP-1 cells (Figure 6C), compared with controls. These chemoattractant activities of the conditioned medium were largely abolished when incubated with anti-RANTES antibody (Figure 6, B and D), suggesting that RANTES is a key chemotactic component secreted by tubular cells. Not surprising, treatment of HKC-8 cells with paricalcitol significantly blocked the chemotaxis of splenocytes and THP-1 cells in this assay (Figure 6, A and C).
Figure 6.
Paricalcitol reduces the chemoattraction of tubular epithelial cells to splenocytes and THP-1 cells in vitro. HKC-8 cells were treated without or with TNF-α (2 ng/ml) in the absence or presence of various concentrations of paricalcitol for 24 h. The supernatants of these cultures were used in a chemotaxis assay to assess their ability to attract splenocyte (A and B) or THP-1 cell (C and D) migration across the filter of Transwell chambers. Freshly isolated mouse splenocytes (A and B) and cultured THP-1 cells (C and D) were used. (A and C) Paricalcitol treatment reduced the ability of HKC-8 cell supernatants to attract both splenocyte (A) and THP-1 cell (C) migration. (B and D) Neutralization of RANTES in HKC-8 cell supernatants with specific anti-RANTES antibody (2 μg/ml) reduced splenocyte (B) and THP-1 cell (D) migration. The same amount of normal IgG was used as controls. Data are expressed as the percentages of migrated cells in total cells added and presented as means ± SEM of three experiments. *P < 0.05 versus control; †P < 0.05 versus TNF-α treated alone. (E) Representative pictures show the migrated THP-1 cells in the bottom chambers of the Transwell plates in various groups as indicated.
NF-κB Signaling Is Critical for Mediating RANTES Induction In Vitro
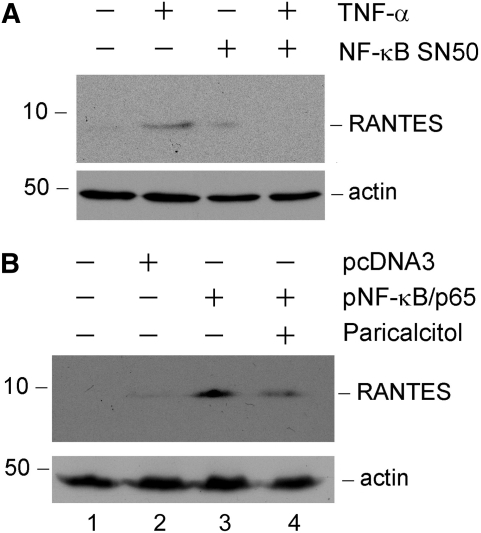
We next sought to investigate the mechanism underlying RANTES induction in tubular epithelial cells. Because NF-κB signaling is known to be important in the process of inflammation, we speculated that RANTES expression may be regulated by this signaling pathway. To test this, we treated HKC-8 cells with TNF-α in the absence or presence of specific NF-κB inhibitor (NF-κB SN50), a cell-permeable inhibitor peptide. As shown in Figure 7A, inhibition of NF-κB signaling abolished RANTES expression induced by TNF-α in tubular cells, indicating that an intact NF-κB signaling is required for RANTES induction. In reciprocal experiments, ectopic expression of p65 NF-κB sensitized tubular epithelial cells to express RANTES in response to a low level of TNF-α (0.2 ng/ml) stimulation (Figure 7B, lane 2 versus lane 3). Interestingly, paricalcitol (10−7 M) also inhibited RANTES expression in the tubular cells overexpressing p65 NF-κB (Figure 7B, lane 3 versus lane 4).
Figure 7.
NF-κB signaling is critical for mediating RANTES induction in tubular epithelial cells. (A) Inhibition of NF-κB signaling abrogated RANTES induction by TNF-α in tubular epithelial cells. HKC-8 cells were treated with 2 ng/ml TNF-α in the absence or presence of NF-κB inhibitor (NF-κB SN50). Cell lysates were immunoblotted with antibodies against RANTES and actin, respectively. (B) Ectopic expression of p65 NF-κB sensitized tubular epithelial cells to express RANTES in response to a low level of TNF-α stimulation. HKC-8 cells were transfected with either p65 NF-κB expression vector or pcDNA3 empty vector as indicated, followed by incubation with a low level of TNF-α (0.2 ng/ml) in the presence or absence of paricalcitol (10−7 M). Western blot showed that ectopic expression of p65 NF-κB rendered HKC-8 cells more sensitive to the stimulation with low concentration of TNF-α to express RANTES, whereas this effect was largely blocked by paricalcitol.
Paricalcitol Does not Affect NF-κB Early Activation and Its Nuclear Translocation
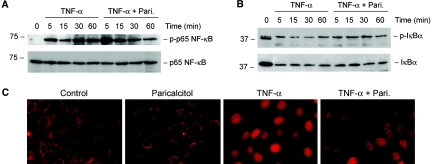
Having established that NF-κB signaling is important in mediating RANTES induction (Figure 7), we reasoned whether paricalcitol inhibits RANTES expression and renal inflammation by modulating NF-κB pathway. To test this hypothesis, we first examined the effect of paricalcitol on IκBα phosphorylation and its subsequent degradation, as well as p65 NF-κB phosphorylation and activation. When HKC-8 cells were incubated with proinflammatory TNF-α, IκBα and p65 NF-κB were rapidly phosphorylated starting at the time point as early as 5 min (Figure 8, A and B); however, treatment of HKC-8 cells with paricalcitol did not significantly affect the kinetics and magnitude of IκBα and p65 NF-κB phosphorylation. Likewise, paricalcitol also did not influence IκBα degradation triggered by TNF-α in HKC-8 cells (Figure 8B). We next assessed the p65 NF-κB nuclear translocation after various treatments. Immunofluorescence staining demonstrated that upon stimulation with TNF-α, p65 NF-κB rapidly translocated into the nuclei in HKC-8 cells, and paricalcitol did not affect the nuclear translocation of p65 NF-κB (Figure 8C). These results suggest that paricalcitol inhibits tubular RANTES expression by a mechanism independent of NF-κB early activation and its nuclear translocation.
Figure 8.
Paricalcitol does not affect NF-κB activation and its nuclear translocation induced by TNF-α. HKC-8 cells were pretreated with or without 10−7 M paricalcitol for 0.5 h, followed by incubation with 2 ng/ml TNF-α for various periods as indicated. (A) Western blot showed that paricalcitol did not significantly affect p65 NF-κB phosphorylation and activation induced by TNF-α. (B) Paricalcitol also did not significantly alter IκBα phosphorylation and subsequent degradation induced by TNF-α. Cell lysates were immunoblotted with specific antibodies against phospho- and total p65 NF-κB and against phospho- and total IκBα, respectively. The low band in the doublet (B, lane 1) likely represents the unphosphorylated IκBα. (C) Immunofluorescence staining demonstrated that paricalcitol pretreatment did not affect p65 NF-κB to undergo nuclear translocation upon TNF-α stimulation in HKC-8 cells.
Paricalcitol Abolishes NF-κB Binding to RANTES Promoter by Facilitating VDR/p65 Interaction
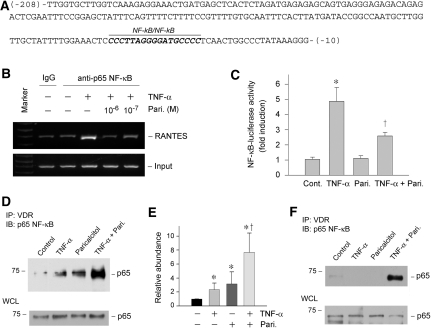
The inability of paricalcitol to affect NF-κB activation prompted us to examine whether it modulates the signaling events after p65 nuclear translocation. To test this, we first investigated the possibility that paricalcitol might negatively influence the in vivo binding of p65 NF-κB to RANTES promoter region. As shown in Figure 9, A and B, TNF-α induced p65 binding to its cognate cis-acting NF-κB element in human RANTES promoter, as revealed by chromatin immunoprecipitation (ChIP) assay; however, treatment of HKC-8 cells with paricalcitol abolished the binding of p65 to RANTES promoter induced by TNF-α. To assess the consequence of this paricalcitol-mediated inhibition of p65/DNA binding, we examined the effect of paricalcitol on NF-κB–mediated gene expression by using a luciferase reporter assay. As demonstrated in Figure 9C, paricalcitol effectively repressed the NF-κB–mediated reporter gene expression after TNF-α stimulation. These results indicate that paricalcitol can specifically target a key event in NF-κB signaling.
Figure 9.
Paricalcitol induces a physical association between VDR and p65 NF-κB, inhibits its binding to RANTES promoter, and represses NF-κB–mediated gene transcription. (A) Partial sequence of human RANTES gene promoter region. Highlighted is the NF-κB element in this region. (B) ChIP assay demonstrated that paricalcitol abrogated the binding of p65 to its cognate cis-acting element in the RANTES promoter. HKC-8 cells were treated with TNF-α and paricalcitol as indicated. (C) Luciferase reporter assay demonstrated that paricalcitol repressed the NF-κB–mediated gene transcription in response to TNF-α treatment. Relative luciferase activity (with control group = 1.0) is presented. Data are means ± SEM from three experiments. *P < 0.01 versus control; †P < 0.01 versus TNF-α treatment group. (D and E) Co-immunoprecipitation revealed that paricalcitol induced an increased binding of VDR to p65 NF-κB. HKC-8 cells were transfected with p65 NF-κB expression vector, followed by treatment without or with TNF-α, paricalcitol, or both as indicated. Cell lysates were then immunoprecipitated with anti-VDR antibody, followed by immunoblotting with anti-p65. Whole-cell lysates were also immunoblotted with anti-p65 for normalizing p65 abundance. Data from three independent experiments are shown (E). *P < 0.05 versus control; †P < 0.05 versus TNF-α or paricalcitol treated alone. (F) Paricalcitol also induced a physical interaction between VDR and endogenous p65 NF-κB. HKC-8 cells were treated without or with TNF-α, paricalcitol, or both and then assessed for endogenous p65/VDR complex formation by co-immunoprecipitation.
We next investigated the potential mechanism underlying the paricalcitol inhibition of p65 binding to RANTES promoter. By co-immunoprecipitation, we found that paricalcitol facilitated the interaction between VDR and p65 NF-κB in HKC-8 cells that overexpressed p65. As shown in Figure 9, D and E, after stimulation by TNF-α and paricalcitol, increased p65 was detectable in the immunocomplexes precipitated by anti-VDR antibody. Similarly, in HKC-8 cells without ectopic expression of exogenous p65, paricalcitol induced a physical interaction between VDR and endogenous p65 NF-κB (Figure 9F). This suggests that an increased VDR/p65 complex formation after paricalcitol treatment would lead to a reduced availability of free p65, thereby sequestering its ability to bind to cis-acting element, causing a repression of p65-mediated gene transcription.
Paricalcitol Does not Affect NF-κB Nuclear Translocation but Restores VDR Expression In Vivo
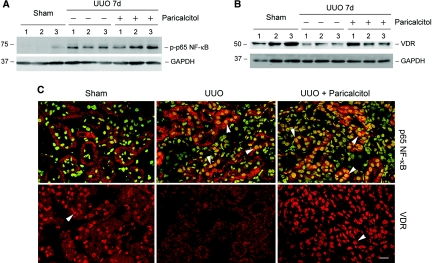
We further investigated how paricalcitol inhibits NF-κB signaling and renal inflammation in obstructive nephropathy in vivo. As showed in Figure 10A, UUO markedly induced p65 phosphorylation and activation, as revealed by Western blot analysis of whole-kidney lysates using phospho-specific anti-p65 antibody; however, paricalcitol did not inhibit p65 phosphorylation in the obstructed kidney at 7 d after UUO (Figure 10A). Immunofluorescence staining demonstrated that p65 NF-κB was localized largely in the cytoplasm of the tubular cells in normal kidney (Figure 10C); however, obstructive injury apparently induced its nuclear translocation. Similar to an in vitro situation, administration of paricalcitol did not affect the activation and nuclear translocation of p65 in obstructed kidney (Figure 10C).
Figure 10.
Paricalcitol does not block NF-κB activation and its nuclear translocation but restores VDR expression and enhances its nuclear translocation in the obstructed kidney after UUO in vivo. (A) Western blot demonstrated that UUO induced p65 NF-κB phosphorylation and paricalcitol treatment did not block p65 NF-κB activation in vivo. Kidney lysates were immunoblotted with anti–phospho-specific p65 NF-κB antibody. (B) Western blot showed that VDR expression was reduced significantly in the obstructed kidney and paricalcitol treatment largely restored VDR expression in vivo. Numbers (1, 2, and 3) denote each individual animal in a given group. (C) Immunofluorescence staining also demonstrated that paricalcitol restored VDR expression but did not block p65 NF-κB nuclear translocation in the obstructed kidney in vivo. Kidney sections were immunostained for total p65 (red) and the nuclei (green; top). (Bottom) Staining for VDR (red). Arrowheads indicate the nuclear staining of VDR and p65 NF-κB. Bar = 20 μm.
We also examined the expression and localization of VDR after different treatments. Consistent with a previous report,10 obstructive injury caused a suppression of VDR, and paricalcitol largely restored VDR expression after UUO (Figure 10B). Interestingly, immunostaining exhibited that a large proportion of VDR was localized in the nuclei in normal kidney (Figure 10C), suggesting an active role of the vitamin D system in the maintenance of normal tubular integrity. After UUO, the overall level of VDR was reduced and little nuclear VDR was observed; however, paricalcitol not only restored VDR protein level but also induced VDR to redistribute completely into the nuclei. It is plausible, therefore, that the nuclear VDR after paricalcitol treatment would bind to the activated p65 in the nuclei and sequester its gene-transactivating ability.
DISCUSSION
The results presented in this study demonstrate that paricalcitol, an active vitamin D analogue, displays a potent anti-inflammatory activity by effectively inhibiting T lymphocyte and macrophage infiltration and proinflammatory cytokines RANTES and TNF-α expression in a mouse model of obstructive nephropathy. Mechanistically, paricalcitol specifically target NF-κB, a principal signaling transducer that is involved in mediating proinflammatory responses in virtually all circumstances. Paricalcitol induces VDR binding to the p65 subunit of NF-κB and prevents it from interacting with cis-acting DNA element, thereby sequestering its ability to transactivate the transcription of its targeted genes. Because chronic inflammation is a critical process that contributes to the pathogenesis of CKD in many ways,23,24 inhibition of inflammation could be an important mechanism by which vitamin D exerts its beneficial activity in ameliorating renal interstitial fibrosis after obstructive injury; therefore, our findings shed new light on the understanding of the therapeutic effects of active vitamin D in CKD.
Although an anti-inflammatory effect of vitamin D is well documented, previous studies largely emphasized its role in modulating immune cell activity22; however, paricalcitol inhibits RANTES expression that is localized almost exclusively in renal tubules after injury, suggesting that tubular epithelial cells are likely the primary target of vitamin D in eliciting its anti-inflammatory action. Tubular epithelial cells, the largest cell population in kidney parenchyma, are not a bystander in the development of renal inflammation. Instead, increasing evidence indicates that they contribute directly and actively to renal inflammatory response after injury. Tubular production of RANTES inevitably builds up the chemokine gradient, which serve as chemotactic signals to attract the migrating leukocytes to move along the gradient and eventually reach the tubulointerstitial site of inflammation.25 In this regard, tubular expression and secretion of RANTES is a critical step that sets in motion toward the peritubular infiltration of inflammatory cells. As shown by chemotaxis assay, RANTES is a major chemotactic component in the supernatants of tubular cells after TNF-α stimulation, which plays an important role in attracting both splenocyte (rich in lymphocytes) and THP-1 cell (monocytic cell line) migration (Figure 6); therefore, by inhibiting RANTES expression in tubular cells, paricalcitol directly targets a key event in the circuit of inflammatory responses in obstructive nephropathy.
The influx of inflammatory cells from the peripheral circulation to the injured sites is a complex process in which chemokines play a fundamental role. Chemokines not only act as the directional signals to sort and guide effector leukocyte migration but also are instrumental in modulating immune cell activation.13,26 Among many chemokines, RANTES/CCL5 is one of the best characterized.25 Increased RANTES expression has been documented in a variety of kidney disorders ranging from acute kidney injury and renal transplant rejection to chronic renal fibrosis.23,27,28 RANTES is a broad chemoattractant that is capable of recruiting many types of immune cells, including T lymphocytes, monocytes/macrophages, and natural killer cells, and promoting the chemotaxis of these cells along its gradient25,29; therefore, RANTES induction in tubular cells after obstructive injury not only is important for T cell infiltration but also is critical for the influx of monocytes/macrophages.29 Of note, although the importance of macrophages in renal inflammation is widely recognized, the role of T cells in the tubulointerstitial inflammation and injury that follows UUO is poorly understood. Our study clearly implicates T cells as a major constituent of the infiltrated inflammatory cells in this model (Figure 1), suggesting a potential role of T cells in obstructive nephropathy. It is worthwhile to point out that inhibition of RANTES expression by paricalcitol in tubular cells is compatible with several previous observations performed in cultured human dermal fibroblasts and vascular endothelial cells,30,31 in which active vitamin D also suppresses this chemokine expression; however, MCP-1/CCL2 mRNA is not significantly inhibited by paricalcitol (Figure 3), suggesting that its effect on chemokine expression seems to be gene specific. Interestingly, the expression of TNF-α, another proinflammatory cytokine that is produced predominantly by the infiltrated cells, is markedly induced in the obstructed kidney. TNF-α, in turn, stimulates tubular epithelial cells to produce RANTES in vitro. This would create a vicious circuit between tubular RANTES induction and inflammatory infiltration, leading to a sustained, chronic inflammation, a characteristic feature of CKD. That administration of paricalcitol inhibits both RANTES and TNF-α expression in vivo underscores its ability to break up such a vicious cycle, resulting in effective inhibition of renal inflammation in this model. Notably, because TNF-α is mainly produced by infiltrating leukocytes, a reduction of TNF-α expression in vivo probably reflects a decreased infiltration of leukocytes after paricalcitol treatment.
This study has unraveled the molecular details in which paricalcitol inhibits RANTES expression, thereby providing significant mechanistic insights into how vitamin D inhibits renal inflammation. RANTES expression in tubular epithelial cells is controlled primarily by NF-κB, a “master” transcription factor that is silenced by its specific inhibitor, IκB, in the cytoplasm in normal resting state. Upon activation, IκB undergoes phosphorylation, ubiquitination, and subsequent degradation.14,32 Thus, NF-κB can be released and translocated into the nucleus, where it dictates the transcription of its targeted genes. Presumably, the activity of NF-κB can be regulated either positively or negatively at multiple sites along this signaling pathway; however, paricalcitol treatment influences neither NF-κB phosphorylation nor its nuclear translocation (Figures 8 and 10), suggesting that any opposing effect of paricalcitol on NF-κB signaling is operated at the postnuclear translocation stage. Of note, several reports suggested that vitamin D inhibits NF-κB signaling by increasing IκBα and reducing p65 NF-κB nuclear translocation in mesangial cells, mouse embryonic fibroblasts, and pancreatic islet cells.33–35 The reason behind this discrepancy remains unknown, but it could be related to the cell-type specificity. In this study, we demonstrated that paricalcitol induced a complex formation between VDR and p65 NF-κB, consistent with previous reports in fibroblasts and osteoblastic cells.34,36 It should be noted that in the p65-overexpressing tubular cells, addition of TNF-α (or paricalcitol) alone slightly induced the p65/VDR interactions (Figure 9). This could be attributable to the presence of a trivial amount of vitamin D in the cultured conditions, which promoted the p65/VDR interaction when the cells overproduce p65. Neither TNF-α nor paricalcitol alone induced endogenous p65/VDR interaction (Figure 9F), when these cells did not overexpress p65. The physical interaction between VDR and p65 evidently prevents activated NF-κB from binding to the DNA element in the promoter of RANTES gene (Figure 9B). These findings underscore that paricalcitol targets specifically and directly a key nuclear event in NF-κB signal circuit, resulting in an effective sequestration of this proinflammatory signaling.
The data presented here strongly suggest that paricalcitol inhibits renal inflammation primarily by triggering a direct, VDR-mediated sequestration of NF-κB signaling; however, although the in vitro data are clear, it remains to be determined whether this mechanism is operative in vivo and whether the VDR ligation is necessary for the paricalcitol effect. In addition, we cannot exclude the possibility that paricalcitol may regulate RANTES and TNF-α expression by a NF-κB–independent mechanism. Furthermore, paricalcitol may exert its anti-inflammatory actions by other indirect routes as well, given that vitamin D has pleiotropic effects.37,38 Along this line, Li et al.39 reported that vitamin D is a negative regulator of renin gene expression, and, therefore, vitamin D analogue could inhibit renin expression, resulting in a reduced activation of the renin-angiotensin system in diseased kidney. Because angiotensin II is a known proinflammatory stimulus, it is conceivable that paricalcitol could inhibit renal inflammation by targeting the renin-angiotensin system in CKD. In addition, vitamin D is shown to induce the expression of hepatocyte growth factor,40 a cytokine that has potent anti-inflammatory and antifibrotic properties.41–43
In summary, we have shown in this study that paricalcitol attenuates renal infiltration of inflammatory cells and inhibits tubular RANTES expression in obstructive nephropathy. This anti-inflammatory effect of paricalcitol seems to be mediated by its ability to induce the VDR-mediated sequestration of NF-κB signaling; therefore, in addition to preserving tubular epithelial integrity by blocking EMT, as previously reported,10 active vitamin D also exerts its beneficial activities in CKD by inhibiting renal inflammation through directly disrupting NF-κB signaling.
CONCISE METHODS
Animals
Male CD-1 mice that weighed approximately 18 to 22 g were purchased from Harlan Sprague-Dawley (Indianapolis, IN). UUO was performed using an approved protocol by the Institutional Animal Care and Use Committee at the University of Pittsburgh, as described previously.10,44 Sham-operated mice were used as normal controls. Paricalcitol (provided by Abbott laboratories, Abbott Park, IL) was administered by daily subcutaneous injection at the dosages of 0.1 and 0.3 μg/kg body wt, respectively. Mice that underwent UUO and received an injection of the same volume of vehicle (ethanol) were used as vehicle controls. Groups of mice (n = 5) were killed at 7 and 14 d after UUO, respectively, and the kidneys were removed for various analyses.
Cell Culture and Cytokine Treatment
Human proximal tubular epithelial cells (HKC, clone-8) were provided by Dr. L. Racusen (Johns Hopkins University, Baltimore, MD). Cell culture and cytokine treatments were carried out according to the procedures described previously.44 Briefly, HKC-8 cells were seeded in complete medium that contained 10% FBS at approximately 70% confluence. After an overnight incubation, cells were serum-starved in serum-free medium for 24 h before addition of cytokines. Recombinant human TNF-α was purchased from R&D Systems (Minneapolis, MN). HKC-8 cells were incubated with various concentrations of paricalcitol in the absence or presence of TNF-α (2 ng/ml) for 24 h, unless otherwise indicated. For some experiments, such as assessing NF-κB activation, HKC-8 cells were pretreated with paricalcitol for 0.5 h, followed by incubation with TNF-α for various periods as indicated. For blocking NF-κB signaling, HKC-8 cells were pretreated with a cell-permeable inhibitor peptide NF-κB SN50 (Calbiochem, La Jolla, CA; 20 μM) for 1 h and then incubated with TNF-α. In some experiments, HKC-8 cells were transfected with either p65 NF-κB expression vector (pNF-κB/p65; provided by Dr. J.A. Schmid, Medical University Vienna, Vienna, Austria) or empty pcDNA3 control vector for 48 h and then treated with low concentration of TNF-α (0.2 ng/ml). Whole-cell lysates or conditioned media were prepared and then subjected to various analyses.
Western Blot Analysis
The preparation of whole-cell lysates and kidney tissue homogenates and Western blot analysis of protein expression were carried out by using routine procedures as described previously.10 The primary antibodies were obtained from following sources: Anti-RANTES (sc-1410) and anti-VDR (sc-1008; Santa Cruz Biotechnology, Santa Cruz, CA); anti-p65 NF-κB, anti–phospho-p65 NF-κB (Ser536), anti-IκBα, and anti–phospho-IκBα (Ser32; Cell Signaling Technology, Danvers, MA); anti–glyceraldehyde-3-phosphate dehydrogenase (Ambion, Austin, TX).
Immunohistochemical and Immunofluorescence Staining
Immunohistochemical staining of kidney sections was performed by an established protocol.10 In brief, paraffin-embedded sections were stained with anti-CD3 (sc-20047; Santa Cruz Biotechnology), anti-F4/80 (14-4801-82; eBioscience, San Diego, CA), and anti-RANTES (500-P118; PeproTech, Rocky Hill, NJ) antibodies using the Vector M.O.M. immunodetection kit, according to the protocol specified by the manufacturer (Vector Laboratories, Burlingame, CA). Indirect immunofluorescence staining was carried out according to the procedures described previously.45 Briefly, cells or kidney cryosections were incubated with the specific primary anti-p65 and anti-VDR antibodies, followed by staining with cyanine Cy3–conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Some slides were double stained with sytox green (Invitrogen, Carlsbad, CA) to visualize the nuclei. Slides were viewed with a Nikon Eclipse E600 microscope equipped with a digital camera (Melville, NY) or Leica TCS-SL confocal microscope (Microsystems, Heidelberg, Germany). Nonimmune normal control IgG was used to replace the primary antibody as negative control, and no staining occurred. CD3, F4/80, and RANTES staining were semiquantified by a computer-aided morphometric analysis (MetaMorph; Universal Imaging Co., Downingtown, PA). Briefly, a grid containing 117 (13 × 9) sampling points was superimposed on images of cortical high-power field (×400). The number of grid points overlying positive area (except tubular lumen and glomeruli) was counted and expressed as a percentage of all sampling points, as described previously.10 For each kidney, 10 randomly selected, nonoverlapping fields were analyzed in a blinded manner.
RT-PCR
For determination of RANTES and TNF-α mRNA expression, a semiquantitative RT-PCR was used. Total RNA was prepared from kidney tissue and cultured HKC-8 cells. After reverse transcription of the RNA, cDNA was used as a template in PCR reactions using gene-specific primer pairs. Approximately 20 to 25 cycles for amplification in the linear range were used. After quantification of band intensities by use of densitometry, the relative steady-state level of mRNA was calculated after normalization to β-actin. The sequences of the primer sets were as follows: RANTES (human), 5′-ACC CTG CTG CTT TGC CTA C (sense), and 5′-GGT TCA CGC CAT TCT CCT G (antisense); RANTES (mouse), 5′-GTG CCC ACG TCA AGG AGT AT (sense) and 5′-GGG AAG CGT ATA CAG GGT CA (antisense); TNF-α, 5′-CTG GGA CAG TGA CCT GGA CT-3′ (sense) and 5′-GCA CCT CAG GGA AGA GTC TG-3′ (antisense); MCP-1, 5′-CCC ACT CAC CTG CTG CTA C-3′(sense) and 5′-TTC TTG GGG TCA GCA CAG A-3′ (antisense). The sequences of β-actin primer set were described previously.46
Chemotaxis Assay
Cell chemotaxis assay was performed using a 24-well Transwell plate as described previously.29 Human monocytic cell line (THP-1; American Type Culture Collection, Manassas, VA) was maintained in RPMI-1640 medium supplemented with 10% FCS. Splenocytes, which contain lymphocytes, as well as macrophages, natural killer cells, and dendritic cells, were freshly prepared from mouse spleen using an established procedure as described previously.47 Briefly, spleens were removed from CD1 mice and a single-cell suspension in Hank's buffer prepared using 40-μm cell strainers. Red blood cells were lysed using standard lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA [pH 7.4]). Splenocytes were then washed and used in chemotaxis assays. Cultured THP-1 cells and mouse splenocytes (5 × 105 in 100 μl) were added onto the upper chamber of the Transwell insert (5-μm polycarbonate filter; Corning, Corning, NY). HKC-8 cell–conditioned media (0.5 ml) were added in the lower chamber of the Transwell. For the neutralizing experiments, anti-RANTES antibody (5 μg/ml; Santa Cruz Biotechnology) or control goat IgG was added to the conditioned media in the lower chamber. After incubation at 37°C for 4 h, the number of cells migrated to the lower chamber of the Transwell was counted. Data are expressed as percentage of the migrated cells in total number of input cells.
ChIP Assay
ChIP assay was performed to analyze in vivo interactions of NF-κB and its cognate cis-acting element in RANTES promoter. This assay was carried out essentially according to the protocols specified by the manufacturer (ChIP assay kit; Upstate, Charlottesville, VA). Briefly, HKC-8 cells after various treatments as indicated were cross-linked with 1% formaldehyde and then resuspended in SDS lysis buffer containing protease inhibitors. The chromatin solution was sonicated, and the supernatant was diluted 10-fold. An aliquot of total diluted lysate was used for total genomic DNA as input DNA control. The anti-p65 NF-κB antibody was added and incubated at 4°C overnight, followed by incubation with protein A–agarose for 1 h. The precipitates were washed, and chromatin complexes were eluted. After reversal of the cross-linking at 65°C for 4 h, the DNA was purified, and ChIP samples were used as a template for PCR using the primer sets for human RANTES promoter regions (from −208 to −10) containing NF-κB response element.48 The sequences of primers used for ChIP assay were as follows: Forward, 5′-TTGGTGCTTGGTCAAAGAGG-3′; and reverse, 5′-CCCTTTATAGGGCCAGTTGA-3′.
Transient Transfection and Luciferase Reporter Assays
The effect of paricalcitol on NF-κB–mediated gene transcription was assessed by using the PathDetect NF-κB-Luc cis-reporter system (Stratagene, La Jolla, CA), in which NF-κB response element was linked to the firefly luciferase gene. HKC-8 cells were co-transfected by using Lipofectamine 2000 reagent (Invitrogen) with pNF-κB-Luc plasmid (1 μg). An internal control reporter plasmid (0.05 μg) Renilla reniformis luciferase driven under thymidine kinase (TK) promoter (pRL-TK; Promega, Madison, WI) was also co-transfected for normalizing the transfection efficiency. The transfected cells were incubated in serum-free medium without or with TNF-α (2 ng/ml) in the absence or presence of paricalcitol (10−7 M) as indicated. Luciferase assay was performed using the Dual Luciferase Assay System kit according to the manufacturer's protocols (Promega). Relative luciferase activity (arbitrary unit) was reported as fold induction over the controls after normalization for transfection efficiency.
Immunoprecipitation
Immunoprecipitation was carried out by using an established method.49 Briefly, HKC-8 cells were transfected with p65 NF-κB expression vector (pNF-κB/p65) by using lipofectamine 2000 reagent (Invitrogen) and then incubated with or without 10−7 M paricalcitol in the absence or presence of 2 ng/ml TNF-α for 1 h. In the experiments for assessing endogenous p65/VDR interaction, HKC-8 cells were directly treated with paricalcitol, TNF-α, or both and then subjected to co-immunoprecipitation. Cells were lysed on ice in 1 ml of nondenaturing lysis buffer that contained 1% Triton X-100, 0.01 M Tris-HCl (pH 8.0), 0.14 M NaCl, 0.025% NaN3, 1% protease inhibitors cocktail, and 1% phosphatase inhibitors cocktails I and II (Sigma). After preclearing with normal IgG, cell lysates (0.5 mg of protein) were incubated overnight at 4°C with 4 μg of anti-VDR (Santa Cruz Biotechnology), followed by precipitation with 30 μl of protein A/G Plus-Agarose for 1 h at 4°C. The precipitated complexes were separated on SDS–polyacrylamide gels and immunoblotted with anti-p65 NF-κB antibody.
Statistical Analysis
Statistical analysis of the data was carried out using SigmaStat software (Jandel Scientific, San Rafael, CA). Comparison between groups was made using one-way ANOVA followed by Student-Newman-Keuls test. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by National Institutes of Health grants DK061408, DK064005, and DK071040 and a Grant-in-Aid from Abbott Laboratories. X.T. was supported by a postdoctoral fellowship from the American Heart Association Great Rivers Affiliate.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 2.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R: Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 349: 446–456, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E: Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 18: 1796–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hirata M, Makibayashi K, Katsumata K, Kusano K, Watanabe T, Fukushima N, Doi T: 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant 17: 2132–2137, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G: Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int 60: 87–95, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Tan X, Li Y, Liu Y: Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Tian J, Liu Y, Williams LA, de Zeeuw D: Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant 22: 321–328, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Andress DL: Vitamin D in chronic kidney disease: A systemic role for selective vitamin D receptor activation. Kidney Int 69: 33–43, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Segerer S, Nelson PJ, Schlondorff D: Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol 11: 152–176, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Guijarro C, Egido J: Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int 59: 415–424, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Lo WK: Serum parameters, inflammation, renal function and patient outcome. Contrib Nephrol 150: 152–155, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D: A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest 109: 251–259, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM, Zatz R: Chronic inhibition of nuclear factor-kB attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol 292: F92–F99, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T: A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158: 1733–1741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zehnder D, Eardley KS, Quinkler M, Lepenies J, Howie AJ, Adu D, Cockwell PM, Stewart PM, Hewison M: Modulation of inflammation in situ by the vitamin D hormonal system in human renal disease [Abstract]. J Am Soc Nephrol 15: 503A, 2004 [Google Scholar]
- 20.Veldman CM, Cantorna MT, DeLuca HF: Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374: 334–338, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Mathieu C, Adorini L: The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8: 174–179, 2002 [DOI] [PubMed] [Google Scholar]
- 22.van Etten E, Mathieu C: Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol 97: 93–101, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlondorff D: Obstructive nephropathy in the mouse: Progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol 12: 1173–1187, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Eddy AA: Progression in chronic kidney disease. Adv Chronic Kidney Dis 12: 353–365, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Krensky AM, Ahn YT: Mechanisms of disease: Regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol 3: 164–170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson Z, Schwarz M, Power CA, Wells TN, Proudfoot AE: Multi-faceted strategies to combat disease by interference with the chemokine system. Trends Immunol 26: 268–274, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Roson MI, Cavallero S, Della Penna S, Cao G, Gorzalczany S, Pandolfo M, Kuprewicz A, Canessa O, Toblli JE, Fernandez BE: Acute sodium overload produces renal tubulointerstitial inflammation in normal rats. Kidney Int 70: 1439–1446, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Nelson PJ, Krensky AM: Chemokines, chemokine receptors, and allograft rejection. Immunity 14: 377–386, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Lai KN, Leung JC, Chan LY, Guo H, Tang SC: Interaction between proximal tubular epithelial cells and infiltrating monocytes/T cells in the proteinuric state. Kidney Int 71: 526–538, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Equils O, Naiki Y, Shapiro AM, Michelsen K, Lu D, Adams J, Jordan S: 1,25-Dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol 143: 58–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuoka M, Ogino Y, Sato H, Ohta T, Komoriya K: Regulation of RANTES and IL-8 production in normal human dermal fibroblasts by active vitamin D3 (tacalcitol). Br J Pharmacol 124: 1433–1438, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins ND: Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8: 49–62, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC: 1,25-Dihydroxyvitamin D(3) targeting of NF-κB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int 72: 193–201, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC: Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab 291: E315–E322, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Giarratana N, Penna G, Amuchastegui S, Mariani R, Daniel KC, Adorini L: A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J Immunol 173: 2280–2287, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lu X, Farmer P, Rubin J, Nanes MS: Integration of the NF-κB p65 subunit into the vitamin D receptor transcriptional complex: Identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J Cell Biochem 92: 833–848, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Dusso AS, Thadhani R, Slatopolsky E: Vitamin D receptor and analogs. Semin Nephrol 24: 10–16, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Tan X, Li Y, Liu Y: Therapeutic role and potential mechanisms of active vitamin D in renal interstitial fibrosis. J Steroid Biochem Mol Biol 103: 491–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Spataro BC, Yang J, Dai C, Liu Y: 1,25-Dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int 68: 1500–1510, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y: Hepatocyte growth factor exerts its anti-inflammatory action by disrupting NF-κB signaling. Am J Pathol 173: 2008, doi: 10.2353/ajpath.2008.070583 [DOI] [PMC free article] [PubMed]
- 42.Liu Y: Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol Renal Physiol 287: F7–F16, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD: Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol 15: 2868–2881, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Liu Y: Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13: 96–107, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Yang J, Luo JH, Dedhar S, Liu Y: Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol 18: 449–460, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y: Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol 17: 2781–2791, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Dong H, Osmanova V, Epstein PM, Brocke S: Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated lymphocytes. Biochem Biophys Res Commun 345: 713–719, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Nelson PJ, Kim HT, Manning WC, Goralski TJ, Krensky AM: Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol 151: 2601–2612, 1993 [PubMed] [Google Scholar]
- 49.Zhang X, Yang J, Li Y, Liu Y: Both Sp1 and Smad participate in mediating TGF-β1-induced HGF receptor expression in renal epithelial cells. Am J Physiol Renal Physiol 288: F16–F26, 2005 [DOI] [PubMed] [Google Scholar]