Abstract
Epidemiologic studies have linked fructose intake with the metabolic syndrome, and it was recently reported that fructose induces an inflammatory response in the rat kidney. Here, we examined whether fructose directly stimulates endothelial inflammatory processes by upregulating the inflammatory molecule intercellular adhesion molecule-1 (ICAM-1). When human aortic endothelial cells were stimulated with physiologic concentrations of fructose, ICAM-1 mRNA and protein expression increased in a time- and dosage-dependent manner, which was independent of NF-κB activation. Fructose reduced endothelial nitric oxide (NO) levels and caused a transient reduction in endothelial NO synthase expression. The administration of an NO donor inhibited fructose-induced ICAM-1 expression, whereas blocking NO synthase enhanced it, suggesting that NO inhibits endothelial ICAM-1 expression. Furthermore, fructose resulted in decreased intracellular ATP; administration of exogenous ATP blocked fructose-induced ICAM-1 expression and increased NO levels. Consistent with the in vitro studies, dietary intake of fructose at physiologic dosages increased both serum ICAM-1 concentration and endothelial ICAM-1 expression in the rat kidney. These data suggest that fructose induces inflammatory changes in vascular cells at physiologic concentrations.
We are witnessing an epidemic of the metabolic syndrome, which in turn is a risk factor for hypertension, kidney disease, diabetes, and cardiovascular disease.1 Although the obesity epidemic likely has many causes, including excessive caloric intake and physical inactivity, an increase in fructose intake has also been implicated.2 In particular, there is a marked increase in daily intake of high-fructose corn syrup in the past four decades.3 A causal role of fructose in the metabolic syndrome is supported by studies demonstrating that fructose causes some features of metabolic syndrome in humans as well as in rodents.4–6
Chronic inflammation and endothelial dysfunction are commonly observed in the metabolic syndrome.7 Fructose-fed rats develop endothelial dysfunction after 2 wk.8 We have also reported that a high-fructose diet may induce inflammation, because fructose accelerates renal disease in rats in association with an increase in monocyte-macrophage infiltration.9 In this study, we tested the hypothesis that fructose can induce proinflammatory changes in human endothelial cells, specifically using concentrations of fructose that can be achieved in the current American diet.
RESULTS
Fructose Induces Intercellular Adhesion Molecule-1 Expression in Human Aortic Endothelial Cells
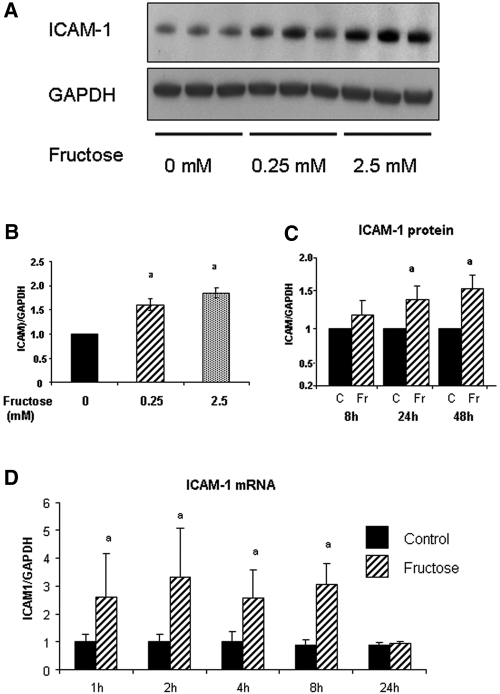
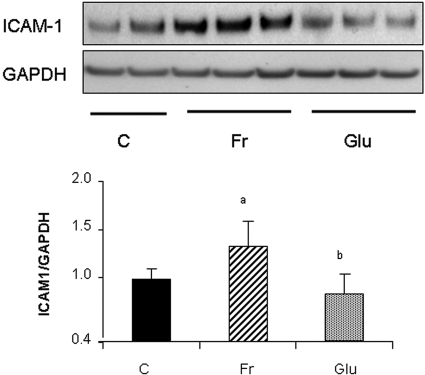
All cells were cultured in media containing physiologic (5 mM) concentrations of glucose. As shown in Figure 1A, the addition of fructose stimulated endothelial intercellular adhesion molecule-1 (ICAM-1) expression in a dosage-dependent manner at 48 h. Although the effect of fructose on ICAM-1 expression was modest, even dosages as low as 0.25 mM fructose significantly stimulated its expression (Figure 1B). Fructose also stimulated ICAM-1 protein level in a time-dependent manner with a significant increase noted at 24 h (Figure 1C). Real-time PCR documented an increase in ICAM-1 mRNA with 1 mM fructose as early as 1 h, which was sustained by 8 h (Figure 1D). In contrast, the addition of equivalent concentrations of glucose to the medium (which already contained 5 mM glucose) did not induce ICAM-1 expression (Figure 2).
Figure 1.
Fructose induces ICAM-1 expression in HAEC. (A) Fructose dosage-dependently induces ICAM-1 protein expression at 48 h by Western blot analysis. (B) Quantification by image analysis demonstrates the significant increase of ICAM-1 expression by 0.25 and 2.5 mM fructose at 48 h. aP < 0.05 versus fructose 0 mM. (C) Fructose-induced ICAM-1 protein level is significantly increased at 24 and 48 h compared with control (C; 0 mM fructose). aP < 0.05 versus control at each time point. (D) ICAM-1 mRNA expression. Real-time PCR demonstrated that ICAM-1 mRNA expression is significantly induced as early as 1 h after exposure to fructose, and it is sustained by 8 h. aP < 0.05 versus control at each time point.
Figure 2.
Glucose does not stimulate ICAM-1 expression in HAEC. In contrast to fructose, glucose (0.25 mM) does not induce ICAM-1 expression at 24 h in HAEC. aP < 0.05 versus control; bP < 0.05 versus fructose at 24 h.
Endothelial ICAM-1 Expression in Response to Fructose Is NF-κB Independent
NF-κB is a major intracellular mediator of vascular inflammation; therefore, the involvement of NF-κB p65 phosphorylation on this process was examined. We found that 1 mM fructose did not induce p65 phosphorylation in endothelial cells, whereas TNF-α (a positive control) was very effective (Figure 3).
Figure 3.
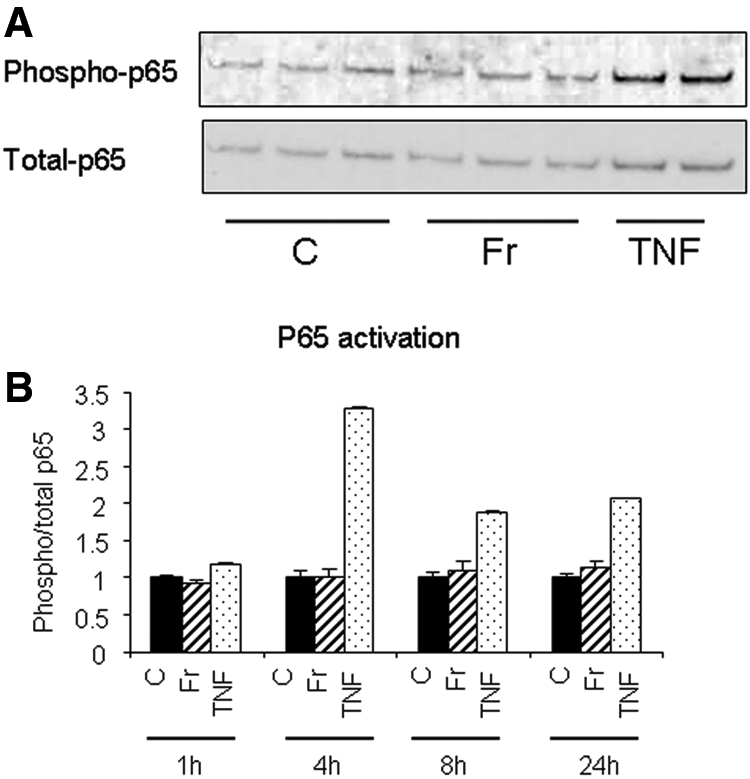
Fructose does not activate NF-κB on endothelial cell. (A) Western blotting demonstrated that 1 mM fructose did not phosphorylate NF-κB p65 on HAEC as opposed to rat TNF-α (10 ng/ml) at 4 h. TNF-α was used as a positive control for p65 activation. (B) Quantification of the ratio of phosphorylated p65/total p65 at various time points using NIH image. C, control; Fr, 1 mM fructose.
Reduced Nitric Oxide Levels by Fructose Could Cause ICAM-1 Expression
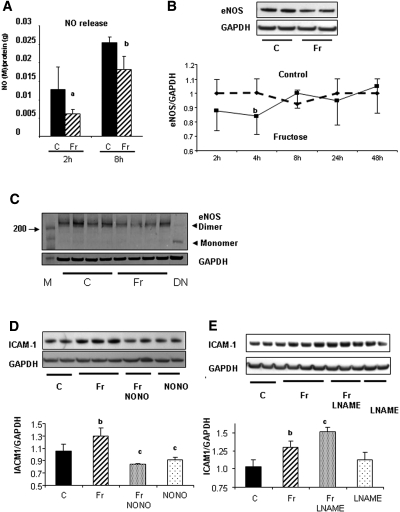
Endothelial nitric oxide (NO) is known to have both vasodilatory and anti-inflammatory effects10,11 and has been reported to be inhibited in fructose-fed rats.8 We therefore examined whether fructose might inhibit NO levels as a mechanism for inducing ICAM-1 expression. Indeed, 1 mM fructose reduced endothelial-derived NO level in human aortic endothelial cells (HAEC) at 2 h, and this was sustained for 8 h (Figure 4A). Compatibly, we also found that 1 mM fructose downregulated total endothelial NO synthase (eNOS) protein expression at 4 h, although eNOS protein level returned to normal after 8 h (Figure 4B). Because a reduced level of NO could be caused by uncoupling of eNOS, we examined whether 1 mM fructose induces the uncoupling of eNOS. In this experiment, eNOS protein was denatured by heating at 95°C and was used as a positive control for the monomeric form. We found that eNOS protein was not uncoupled by fructose at 4 h, whereas the dimer of eNOS protein was reduced by fructose (Figure 4C). Next, for examination of the role of NO on ICAM-1 expression, cells were stimulated by the NO donor (NONOate 10−5 M) in addition to 1 mM fructose. We found that NO donors inhibited ICAM-1 expression induced by 1 mM fructose at 24 h (Figure 4D). In contrast, NO inhibition by N-ω nitro-l-arginine methyl ester (L-NAME; 1 mM) enhanced ICAM-1 expression in response to 1 mM fructose (Figure 4E). These data suggest that eNO negatively regulates ICAM-1 expression in HAEC. In turn, a reduced NO level caused by fructose could be a mechanism by which fructose induces ICAM-1 expression in endothelial cells.
Figure 4.
Role of eNO on ICAM-1 expression. (A) NO levels in the culture medium is significantly lower in response to fructose (Fr) than control (C) at 2 and 8 h. aP < 0.01 versus control at 2 h; bP < 0.05 versus control at 8 h. (B) Total eNOS protein expression is significantly reduced by 1 mM fructose at 4 h, but it returns to normal level after 8 h. bP < 0.05 versus control. (C) Immunoblotting for dimer and monomer of eNOS protein. A total of 1 mM fructose administration does not result in the monomer formation of eNOS protein. M, molecular marker; DN, denatured protein as positive control for monomer. (D) Increased ICAM-1 expression in response to fructose is inhibited by 10−5M NONOate (NONO) at 24 h. Quantification by image analyzer shows that NONOate significantly reduces ICAM-1 expression induced by 1 mM fructose at 24 h. (E) L-NAME (10−3 M) significantly enhances ICAM-1 expression induced by 1 mM fructose at 24 h. bP < 0.05 versus control; cP < 0.05 versus fructose.
Fructose-Induced ATP Depletion Can Cause Reduced eNO Levels and Stimulate ICAM-1 Expression
On the one hand, ATP is known to stimulate NO production in endothelial cells.12,13 On the other hand, fructose causes ATP depletion in a variety of cell types.14 Thus, we next determined whether the mechanism by which 1 mM fructose reduces NO level in endothelial cells involves ATP depletion. As shown in Figure 5A, 1 mM fructose caused modest ATP depletion at 2 h (23% decrease versus control; P < 0.05) and 4 h compared with control. The effect of 1 mM fructose on ATP depletion was temporary and was not observed at 24 h. Interestingly, exogenous ATP administration restored NO levels (Figure 5B) and blocked the induction of ICAM-1 (Figure 5, C and D).
Figure 5.
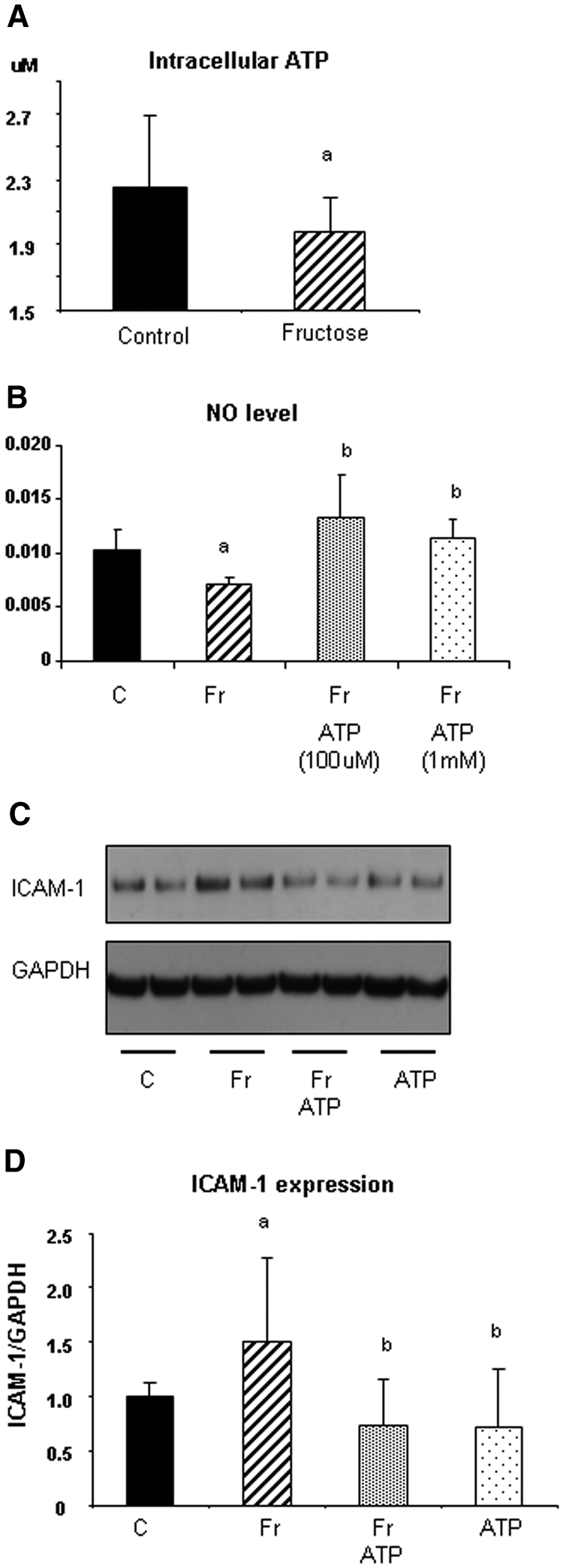
Role of ATP on NO availability and ICAM-1 expression in HAEC. (A) Fructose (1 mM) decreases intracellular ATP content in HAEC at 4 h. (B) Exogenous ATP (100 μM and 1 mM) significantly blocks the reduction of NO level in response to 1 mM fructose at 4 h. (C) ATP (10 μM) prevents an induction of ICAM-1 expression in response to fructose at 24 h. (D) Quantification demonstrated that ATP significantly inhibits fructose-induced ICAM-1 expression in HAEC.
Other Inflammatory Factors Induced by Fructose
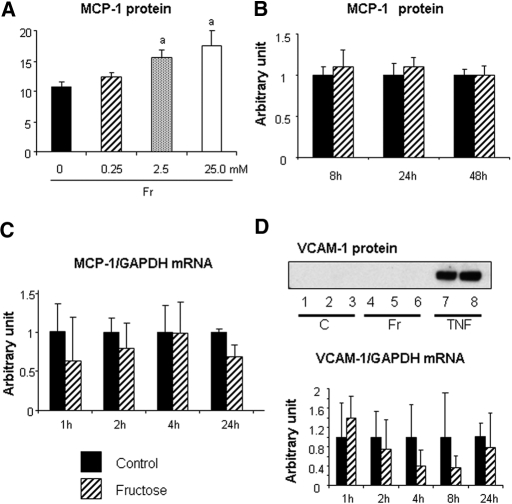
We also examined whether fructose induces other inflammatory factors in endothelial cells, including vascular cell adhesion molecule-1 (VCAM-1) and monocyte chemoattractant protein-1 (MCP-1). Endothelial MCP-1 protein level was increased by fructose at 48 h in a dosage-dependent manner; however, 1 mM fructose did not increase MCP-1 protein level at 48 h or alter mRNA levels at 24 h (Figure 6, A through C). Fructose at a concentration of 1 mM also did not alter VCAM-1 mRNA or protein levels in HAEC (Figure 6D).
Figure 6.
Endothelial MCP-1 and VCAM expressions in response to fructose. (A) Total MCP-1 protein level in culture medium is divided by total cell protein. Fructose at 2.5 and 25.0 mM significantly increased MCP-1 protein level in culture medium at 48 h. aP < 0.05 versus fructose at 0 mM. (B) Time course of MCP-1 protein expression in response to 1 mM fructose. Data are shown as arbitrary units. (C) Time course of MCP-1 mRNA level in response to 1 mM fructose. Data are shown as arbitrary units. (D) VCAM-1 protein and mRNA expression. The immunoblotting shows no VCAM expression in control and fructose stimulation as opposed to a stimulation with 10 ng/ml rat TNF-α (positive control). Samples 1 through 3, control; samples 4 through 6, 1 mM fructose; samples 7 and 8, TNF-α. (Bottom) Time course of VCAM-1 mRNA expression under fructose stimulation or control. Data are shown as arbitrary units.
Fructose Induces Endothelial ICAM-1 Expression in an Animal Model
Finally, we examined whether fructose intake can increase ICAM-1 expression in the rat. Two sets of studies were performed. In the first set, we revisited our previous study in which male Sprague-Dawley rats were fed control (n = 7; 46% non–fructose-containing carbohydrate) or 60% fructose diet (n = 7; Harlan, Madison, WI) for 10 wk.6 Because a 60% fructose diet is not physiologic, we also performed a second study in which we gave either a 20% fructose diet or starch diet for 33 wk.
The general characteristics of the study are shown in Tables 1 and 2. Consistent with in vitro data, both 20 and 60% fructose diet increased serum ICAM-1 levels compared with the control diet (Tables 3 and 4). In the kidney, both fructose diets but not control diets significantly increased the expression of ICAM-1 in vascular endothelial cells of peritubular capillaries, glomerular capillaries, and arteries (Figure 7). Conversely, serum MCP-1 concentration was not increased by fructose intake (Tables 3 and 4).
Table 1.
General characteristics in rats fed 60% fructose diet for 10 wka
| Characteristic | Control (n = 7) | Fructose (n = 7) | P |
|---|---|---|---|
| Body weight (g) | 474 ± 28 | 522 ± 57 | NS |
| Systolic BP (mmHg) | 125 ± 7 | 142 ± 4 | <0.05 |
| Triglyceride (mg/dl) | 95 ± 18 | 233 ± 81 | <0.01 |
| Insulin (pg/ml) | 450 ± 330 | 1575 ± 696 | <0.01 |
| Uric acid (mg/dl) | 1.3 ± 0.4 | 1.7 ± 0.2 | <0.05 |
Data obtained from our previous study.6
Table 2.
General characteristics in rats fed 20% fructose diet for 33 wk
| Characteristic | Starch (n = 5) | Fructose (n = 5) | P |
|---|---|---|---|
| Body weight (g) | 437 ± 15 | 450 ± 33 | NS |
| Systolic BP (mmHg) | 127 ± 4 | 142 ± 13 | <0.05 |
| Triglyceride (mg/dl) | 158 ± 34 | 165 ± 34 | NS |
| Insulin (pg/ml) | 353 ± 216 | 456 ± 184 | NS |
| Uric acid (mg/dl) | 2.0 ± 0.3 | 2.0 ± 0.1 | NS |
Table 3.
Serum concentrations of ICAM-1 and MCP-1 in rats fed 60% fructose diet for 10 wk
| Parameter | Control (n = 7) | Fructose (n = 7) | P |
|---|---|---|---|
| ICAM-1 (ng/ml) | 26.0 ± 8.3 | 34.8 ± 3.1 | <0.05 |
| MCP-1 (ng/ml) | 19.8 ± 4.9 | 21.1 ± 7.8 | NS |
Table 4.
Serum concentrations of ICAM-1 and MCP-1 in rats fed with 20% fructose diet for 33 wk
| Parameter | Starch (n = 5) | Fructose (n = 5) | P |
|---|---|---|---|
| ICAM-1 (ng/ml) | 20.3 ± 0.8 | 33.9 ± 12.0 | <0.05 |
| MCP-1 (ng/ml) | 26.2 ± 11.8 | 35.3 ± 12.0 | NS |
Figure 7.
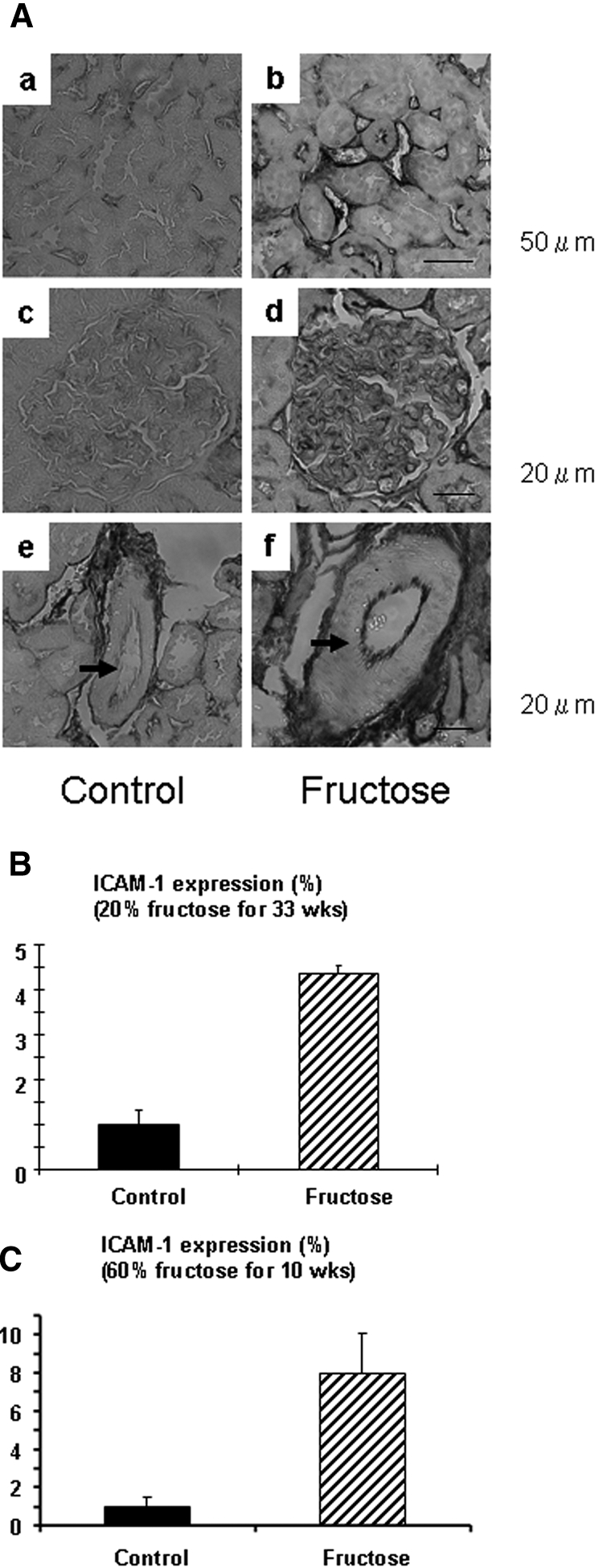
(A) Immunohistochemistry for ICAM-1 in 20% fructose-fed rat kidney. (a and b) Tubulointerstitium. (c and d) Glomerulus. (e and f) Arcuate artery. Bar = 20 μm. Compared with rats on control diet (a, c, and e), ICAM-1 expression (brown color) was induced in endothelial cell in the peritubular capillary (b), glomerulus (d), and arcuate artery (f; arrow) in 20% fructose-fed rat kidney. (B) Quantification of renal ICAM-1 expression in rat with 20% fructose diet for 33 weeks. (C) Quantification of renal ICAM-1 expression in rat with 60% fructose diet for 10 weeks.
DISCUSSION
In this study, we examined whether fructose at blood concentrations that are achieved in the current American diet can induce inflammatory changes in human vascular cells. Our primary finding is that fructose can induce ICAM-1 expression in a time- and dosage-dependent manner in HAEC. The mechanism was shown to be due to the ability of fructose to reduce eNO levels, resulting from a reduced eNOS expression and ATP depletion. Compatibly, serum ICAM-1 concentration was elevated in fructose-fed rats compared with control rats. Furthermore, we found that fructose intake induced endothelial ICAM-1 expression in the rat kidney. These studies provide the first evidence that fructose can induce inflammatory changes in human vascular cells at concentrations achieved in the normal American diet.
Previous studies showed that fructose can induce endothelial dysfunction and metabolic syndrome in rats8; however, most animal studies used very high concentrations of fructose (60%) compared with the current American diet (which is closer to 12 to 15%), thereby raising issues of clinical significance. Nevertheless, Some studies showed that even concentrations of 15 to 20% fructose can induce features of metabolic syndrome if the diet is prolonged.15
The concentration of fructose (0.25 and 1 mM) we used in this study can be regarded to be relevant to the human condition. For example, plasma fructose concentrations are usually in the range of 0.1 to 0.25 mM but can rise to levels of 0.25 to 0.8 mM at 30 to 60 min after the oral administration of dosages ranging from 18 to 100 g of fructose.16,17 In other words, 1 mM serum fructose concentration can be achieved when a large amount of fructose is ingested.16 As such, a 20-oz soft drink, which contains 32.6 g of fructose, would be expected to increase serum fructose concentrations in this range. We found that concentrations as low as 0.25 mM fructose could induce ICAM-1 expression in HAEC, suggesting potential physiologic effects from daily consumption of high fructose–containing beverages. Nevertheless, we should be cautious to translate the in vitro studies to the human condition, because the peak fructose concentration after ingestion usually lasts for only 1 to 2 h,18 whereas we evaluated the effect of fructose for 24 to 48 h in this study.
Nevertheless, we also demonstrated that fructose induces endothelial ICAM-1 expression in vivo. Both 60% fructose diet and 20% fructose diet were found to increase both serum ICAM-1 in the plasma and ICAM-1 expression in the endothelium of blood vessels in the kidney. Similarly, Havel's19 group recently investigated the effects of 10 wk of fructose intake compared with glucose consumption in overweight/obese (body mass index 25 to 35 kg/m2) adults and found that the plasma level of serum ICAM-1 increased by 8 ± 3% (P < 0.05) in individuals consuming fructose but not in those consuming glucose. Interestingly, serum ICAM-1 and kidney ICAM-1 expression were induced by 20% fructose diet while it did not cause the development of metabolic syndrome in rat. These data suggest that endothelial ICAM-1 induction could occur before development of or independent of the metabolic syndrome. Interestingly, fructose at physiologic concentration did not induce other proinflammatory mediators, such as MCP-1 and VCAM-1, or stimulate the NF-κB pathway in endothelial cell. Given that NF-κB can be a major mediator for vascular inflammation, fructose may engage a unique pathway on inflammation.
Fructose is distinct from other sugars because it can rapidly cause ATP depletion in cells. Unlike the glucose-glucokinase pathway, which is tightly regulated, the phosphorylation of fructose by fructokinase is not effectively regulated, and, thus, rapid ATP depletion can occur.20 It has been shown that as little as 50 g of fructose (which is slightly more than that present in two 12-oz soft drinks) can induce hepatic ATP depletion in humans when given intravenously.21–23 In our study, we showed that concentrations as low as 0.25 mM fructose could also reduce ATP level transiently in vascular endothelial cells. In addition, we found that fructose-induced endothelial dysfunction was strongly linked with ATP depletion and ICAM-1 induction.
The manner in which fructose is given could be important in these effects. Fructose is usually ingested in the form of sucrose (a disaccharide of fructose and glucose) or high-fructose corn syrup (which contains glucose and fructose in monosaccharide form). Although both preparations have similar amounts of fructose, sucrose must first be hydrolyzed before being absorbed in the intestine, and, as such, there may be a delay or reduction in the peak plasma fructose concentration achieved. Gaby18 mentioned in his review article that the mean peak in serum fructose concentrations after sucrose ingestion was 36 to 41% lower than after equivalent amounts of fructose. Because we demonstrated that ICAM-1 expression is dosage-dependently induced by fructose, this difference in the manner of fructose intake could result in pathophysiologic differences.
The current American is ingesting 70 to 80 g/d fructose, which composes nearly 12 to 15% of their diet.24,25 In as many as 20% of the population, fructose intake may exceed 100 g/d. An increase of fructose intake has also paralleled the obesity epidemic since it first began in the late 1800s.26 In turn, the administration of fructose (or sucrose that contains 50% fructose) to humans can cause all features of the metabolic syndrome (weight gain, rise in BP, rise in blood glucose and insulin resistance, elevated triglycerides, and low HDL cholesterol).18,25,27 Although some studies in humans have reported negative results, in most cases this can be attributed to the dosage and duration of therapy.21 In fact, we found that a 60% fructose diet causes metabolic syndrome in 10 wk in rat, whereas 33 wk still was not long enough for the metabolic syndrome to develop with a 20% fructose diet; however, 15 mo is an adequate period of time for a 15% fructose diet to induce insulin resistance in rats.15
Interestingly, the inability of 20% fructose to induce metabolic syndrome in rats could be attributed to a low level of uric acid in this species as a result of the presence of uricase. High concentrations of fructose (60%) can induce hyperuricemia in rats, which has been shown to have a causal role in the development of metabolic syndrome in rat6; however, the effect of fructose to cause hyperuricemia in rats is markedly increased when uricase is inhibited.28 Furthermore, rats given 20% fructose and a uricase inhibitor develop hypertension and hyperinsulinemia after several weeks.29
In conclusion, we present the first evidence that fructose, at concentrations achieved in the current American diet, can induce inflammatory changes in human vascular cells. The potential importance of these findings to human disease remains to be established.
CONCISE METHODS
Cell Culture
HAEC (Cambrex, Walkersville, MD) were cultured in endothelial basal medium (EBM) using the Bullet kit (Cambrex), containing FBS, bovine brain extract, hydrocortisone, EGF, gentamicin/amphotericin B, and 5 mM glucose as conditioned medium. All experiments were performed without starvation. Fructose, glucose, and other factors were added in baseline condition medium to stimulate cells. Cells between passages 4 and 6 were used for experiments. Three to six wells were used for each experimental condition, and all experiments were repeated at least three times.
Measurement of NO in Culture Medium
HAEC were seeded at 100,000 cells/well in six-well plates and grown to confluence, which rendered them quiescent in endothelial basal medium with Bullet kit. Twenty-four hours later, cells were stimulated with physiologic concentrations of fructose or glucose (0.25 to 2.5 mM) in conditioned medium. Reactions were terminated by removal of the supernatant that was subsequently centrifuged and stored at −80°C for NO analysis. By using the model 280i nitric oxide (NOA) chemiluminescence analyzer (Analytix, Durham, UK), the levels of total NO were measured after medium nitrite was converted with acetic acid/sodium iodine/nitrogen to NO. Results were corrected for background levels of NO present in culture medium alone and were expressed as nM/μg (NO/total protein).
Western Blot Analysis
As described previously, 20 μg of cell protein samples were resolved on NuPAGE Bis-Tris Gel (4 to 12%) and transferred to polyvinylidene difluoride membranes by electroblotting.30 Each primary antibody was incubated at 4°C overnight. Anti-human ICAM-1 or anti-human VCAM-1 mouse mAb (Santa Cruz Biotechnology, Santa Cruz, CA), anti-human mouse monoclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Chemicon, Temecula, CA), anti-human phospho–NF-κB p65 (Ser536) rabbit mAb, and anti-human NF-κB p65 antibody (Cell Signaling, Beverly, MA) were used. As a positive control for NF-κB activation, rat TNF-α (10 ng/ml; PeproTech, Rocky Hill, NJ) was used. Polyclonal antibodies to total eNOS were obtained from Cell Signaling. After washing with TBST, membrane was rocked with secondary antibody (anti-mouse IgG or anti-rabbit IgG, horseradish peroxidase–linked antibody (Cell Signaling). The blot was then developed using the ECL detection kit (Amersham International, Bucks, UK). Ratios of ICAM-1 to GAPDH were calculated for each sample and expressed as mean ± SD.
Determination of eNOS Dimers and Monomers
For immunoblot analysis of the monomer of eNOS, samples were heated at 95°C for 5 min before electrophoresis. For the dimer of eNOS, samples were not heated and the temperature of the gel was maintained below 15°C during electrophoresis (low-temperature SDS-PAGE).31
Real-Time PCR
To quantify mRNA expression for ICAM-1, we performed real-time PCR as described previously.30 Briefly, 1 μg of total RNA was converted to cDNA with Platinum PCR supermix (Bio-Rad, Hercules, CA), and PCR was performed as follows: 94°C for 5 min, then 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 60 s, and extension at 72°C for 90 s. The sizes of amplicons were 200 bp (human ICAM-1: GGCCTCAGTCAGTGTGA as forward primer, AACCCCATTCAGCGTCA as reverse primer), 141 bp (human VCAM-1: CCGGATTGCTGCTCAGATTGGA as forward primer, AGCGTGGAATTGGTCCCCTCA as reverse primer), 137 bp (human MCP-1: TGCAGAGGCTCGCGAGCTA as forward primer, CAGGTGGTCCATGGAATCCTGA as reverse primer), and 225 bp (human GAPDH: GCCAAAAGGGTCATCATCTC as forward primer, GGCCATCCACAGTCTTCT as reverse primer). Reaction specificity was confirmed by electrophoretic analysis of products in 2% agarose gel before real-time RT-PCR, and bands of expected size were detected. Ratios of ICAM-1 to GAPDH mRNA were calculated for each sample and expressed as means ± SD.
Intracellular ATP Concentration in Endothelial Cells
Intracellular ATP was measured in HAEC according to the manufacturer's protocol (PerkinElmer, Boston, MA). Briefly, cells (1 × 104) were seeded on 96-well plates with conditioned medium. After overnight incubation, cells were stimulated with 1 mM fructose for 2, 4, and 24 h. Afterward, 50 μl of cell lysis buffer containing an inhibitor of ATPase was added in each well, and the luminescence signals were measured. Basal values in the medium were subtracted from each value. Cells were also stimulated with ATP (1 μM to 1 mM), which has been shown to increase endothelial cGMP level or other vasodilation factors that could be theoretically associated with NO production.32–34 Intracellular ATP concentration was thought to be approximately 1.0 to 30 μM.35,36
ELISA for MCP-1
The expression of MCP-1 in the culture medium was examined at 48 h after fructose stimulation using a human MCP-1 ELISA kit (BD Bioscience, San Jose, CA). The total amount of MCP-1 level (pg) in culture medium was divided by total cell protein (μg) and expressed as mean ± SD.
Other Materials
NONOate, L-NAME, and adenosine 5 triphosphate (magnesium salt and disodium salt; all from Sigma-Aldrich, St. Louis, MO) were used.
ICAM-1 Expression in Fructose-Fed Rats
We examined two sets of animal experiments. First, we examined Sprague-Dawley rats (300 g) that were fed 20% fructose diet or 20% starch diet (Harlan) for 33 wk. We also revisited our previous study6 in which male Sprague-Dawley rats (150 to 200 g) were housed in standard conditions and fed control (n = 7) or 60% fructose diet (Harlan; n = 7) for 10 wk. “Control diet” contained 46% carbohydrate, which is mainly composed of starch, whereas the fructose diet contained 60% fructose as the carbohydrate. General characterization is shown in Tables 1 and 2.6 For determination of serum ICAM-1 and MCP-1 concentrations at 10 wk, ELISA kits for rat ICAM-1 (BD Bioscience, San Jose, CA) and rat MCP-1 (R&D Systems, Minneapolis, MN) were used. Kidneys were fixed in 10% formalin and embedded in paraffin. Immunohistochemistry was performed with anti-rat ICAM-1 antibody (BD Bioscience). Percentage positive area in renal cortex was quantified in light microscopy using Axioplan2 imaging system (Carl Zeiss, Thornwood, NY) with digital imaging software. Quantification was performed by two independent people in a blinded manner.
Statistical Analysis
All values presented are expressed as means ± SD. ANOVA followed by Bonferroni correction (ANOVA) or t test was used. Significance was defined as P < 0.05.
DISCLOSURES
T.N. and R.J.J. are listed as inventors on several patent applications from the University of Florida or University of Washington related to uric acid and cardiovascular disease.
Acknowledgments
This study was supported by National Institutes of Health grant DK-52121.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S: Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa T, Tuttle KR, Short RA, Johnson RJ: Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 1: 80–86, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Nielsen SJ, Popkin BM: Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79: 537–543, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Beck-Nielsen H, Pedersen O, Lindskov HO: Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr 33: 273–278, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L: Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 54: 1907–1913, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ: A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290: F625–F631, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV: Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: Results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation 111: 343–348, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R: Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes 48: 2437–2445, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T: Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 293: F1256–F1261, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Fleming I, Busse R: Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Heeringa P, van Goor H, Itoh-Lindstrom Y, Maeda N, Falk RJ, Assmann KJ, Kallenberg CG, Jennette JC: Lack of endothelial nitric oxide synthase aggravates murine accelerated anti-glomerular basement membrane glomerulonephritis. Am J Pathol 156: 879–888, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll J, Raththagala M, Subasinghe W, Baguzis S, D’Amico Oblak T, Root P, Spence D: An altered oxidant defense system in red blood cells affects their ability to release nitric oxide-stimulating ATP. Mol Biosyst 2: 305–311, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Konduri GG, Mital S: Adenosine and ATP cause nitric oxide-dependent pulmonary vasodilation in fetal lambs. Biol Neonate 78: 220–229, 2000 [DOI] [PubMed] [Google Scholar]
- 14.van den Berghe G, Bronfman M, Vanneste R, Hers HG: The mechanism of adenosine triphosphate depletion in the liver after a load of fructose: A kinetic study of liver adenylate deaminase. Biochem J 162: 601–609, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakely SR, Hallfrisch J, Reiser S, Prather ES: Long-term effects of moderate fructose feeding on glucose tolerance parameters in rats. J Nutr 111: 307–314, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Bohannon NV, Karam JH, Forsham PH: Endocrine responses to sugar ingestion in man: Advantages of fructose over sucrose and glucose. J Am Diet Assoc 76: 555–560, 1980 [PubMed] [Google Scholar]
- 17.Macdonald I, Keyser A, Pacy D: Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood. Am J Clin Nutr 31: 1305–1311, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Gaby AR: Adverse effects of dietary fructose. Altern Med Rev 10: 294–306, 2005 [PubMed] [Google Scholar]
- 19.Stanhope K, Griffen S, Keim N, Ai M, Otokozawa S, Nakajima K, Schaefer E, Havel P: Consumption of fructose-, but not glucose-sweetened beverages produces an atherogenic lipid profile in overweight/obese men and women [Abstract]. Diabetes 56: A16, 2007 [Google Scholar]
- 20.Maenpaa PH, Raivio KO, Kekomaki MP: Liver adenine nucleotides: Fructose-induced depletion and its effect on protein synthesis. Science 161: 1253–1254, 1968 [DOI] [PubMed] [Google Scholar]
- 21.Bode JC, Zelder O, Rumpelt HJ, Wittkamp U: Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest 3: 436–441, 1973 [DOI] [PubMed] [Google Scholar]
- 22.Nair S, V PC, Arnold C, Diehl AM: Hepatic ATP reserve and efficiency of replenishing: Comparison between obese and nonobese normal individuals. Am J Gastroenterol 98: 466–470, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Li Calzi S, Harrison JK, Tran-Son-Tay R, Grant MB: Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 55: 102–109, 2006 [PubMed] [Google Scholar]
- 24.Dwyer JT, Evans M, Stone EJ, Feldman HA, Lytle L, Hoelscher D, Johnson C, Zive M, Yang M: Adolescents’ eating patterns influence their nutrient intakes. J Am Diet Assoc 101: 798–802, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Havel PJ: Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63: 133–157, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG: Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ: Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr 76: 911–922, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Stavric B, Johnson WJ, Clayman S, Gadd RE, Chartrand A: Effect of fructose administration on serum urate levels in the uricase inhibited rat. Experientia 32: 373–374, 1976 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Lozada L, Soto V, Tapia E, Avila-Casado C, Bautista R, Nakawaga T, Franco M, Johnson R: Low fructose caloric intake concomitant to hyperuricemia induces hyperinsulinemia and renal structural damage in rats [Abstract]. J Am Soc Nephrol 18: 184A, 2007 [Google Scholar]
- 30.Nakagawa T, Lan HY, Zhu HJ, Kang DH, Schreiner GF, Johnson RJ: Differential regulation of VEGF by TGF-beta and hypoxia in rat proximal tubular cells. Am J Physiol Renal Physiol 287: F658–F664, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Venema RC, Ju H, Zou R, Ryan JW, Venema VJ: Subunit interactions of endothelial nitric-oxide synthase: Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem 272: 1276–1282, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Boeynaems JM, Boutherin-Falson O, Lagneau C, Galand N: Enhancement of the endothelial production of prostacyclin by inhibitors of protein synthesis. Br J Pharmacol 101: 799–802, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsberg EJ, Feuerstein G, Shohami E, Pollard HB: Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A 84: 5630–5634, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JA, Lang D: Release of endothelium-derived relaxing factor from pig cultured aortic endothelial cells, as assessed by changes in endothelial cell cyclic GMP content, is inhibited by a phorbol ester. Br J Pharmacol 99: 565–571, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beis I, Newsholme EA: The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J 152: 23–32, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, Venkatachalam MA: Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol 140: 457–471, 1992 [PMC free article] [PubMed] [Google Scholar]