Abstract
Attention is typically impaired in depression and may play a role in risk for suicidal behavior. In this study, 66 non-patients, 83 depressed subjects with no past history of suicide attempt, 53 depressed subjects with one or more low lethality suicide attempts, and 42 depressed subjects with at least one high lethality attempt were compared on two computerized measures of attention, a Continuous Performance Test (CPT) and a Stroop task. All subjects were medication free at the time of assessment. Attention was impaired in all depressed subjects but worse in those with a past history of suicidal behavior. CPT performance did not differ among the groups, but Stroop interference was significantly poorer in all depressed subjects relative to non-patients, and poorer still in high lethality suicide attempters relative to all other groups. Interference score correlated modestly with subjective depression, functional level, suicide ideation, number of past suicide attempts, and lethality of past attempts. Depression-related impairments of attention, especially susceptibility to interference, are accentuated in those with a past history of suicidal behavior. Fundamental deficits in attention control may play a role in risk for suicidal behavior, and may contribute to a variety of cognitive deficits in suicidal patients. Brain mechanisms subserving attention control, which overlap considerably with regions implicated in affective disorders, may be a useful target for studies seeking to characterize neuropsychological factors associated with suicidal behavior.
Keywords: Attention, Major Depression, Suicidal Behavior, Neuropsychology
1. INTRODUCTION
Attention deficits are common in major depression (Cornblatt et al., 1989; Lemelin et al., 1996, 1997; Thomas et al., 1997; Lemelin and Baruch, 1998; Cohen et al., 2001; Koetsier et al., 2002; Liu et al., 2002; Ottowitz et al., 2002; Den Hartog et al., 2003; Egeland et al., 2003; Siegle et al., 2004), and may also be associated with risk for suicidal behavior. Using a continuous performance task (CPT), Horesh (2001) found higher rates of both omission and commission errors in adolescent suicide attempters compared to hospitalized non-attempters. Tarter and colleagues (2004) included performance on a vigilance task and Stroop task in a composite measure of behavioral disinhibition that predicted risk for later substance use and suicide attempt in adolescents. Becker and colleagues (1999) found greater attentional interference in past suicide attempters compared to healthy controls on a Stroop task using suicide-related words as distractors. Our own previous work (Keilp et al., 2001), found that Stroop interference - using standard color/word stimuli in a single item, computerized format - differed significantly between depressed, high lethality past attempters and non-patients, with depressed non-attempters and less lethal attempters intermediate between these two groups. Deficits on the conflict condition of the Attention Network Test were found in patients with Borderline Personality Disorder, a disorder where risk for suicidal behavior is high (Posner et al., 2002). Impairments of attention, then, appear to be common in populations at risk for suicidal behavior, although their nature is not completely clear. Previous results suggest attention may be affected globally; own data suggests that performance on interference-type tasks may be more closely related to suicide attempt risk. Problems with the executive control of attention may be associated with risk for more severe attempts as well (Nasser & Overholzer, 1999; Keilp et al., 2001).
Other cognitive functions have been linked to suicidal behavior, including poor language fluency (Bartfai, et al, 1990), memory disturbance (Williams et al., 1996), problem solving (Pollock and Williams, 1998; Marzuk et al., 2005), decision-making (Jollant et al, 2006), and impulsiveness (Swann et al, 2005). There is little consistency across studies, however, and marked differences in sample sizes, nature of comparison groups and composition of test batteries. Attention disturbance is a common finding across studies that measure it adequately, and may play a role in other higher-order cognitive dysfunctions. As a first step in understanding the role of cognitive factors in suicidal behavior, it is reasonable to examine fundamental cognitive capacities such as attention, in as much detail and in samples as large as possible, to determine if or how they might be related to suicidal behavior – before invoking higher order cognitive constructs in these hypothetical models.
In the study reported here, performance data on the same attention tasks used in our initial study were examined in a significantly enlarged version (178 depressed patients; 66 non-patients) of our original sample (50 depressed patient; 22 non-patients). Tasks included a computerized CPT using 4 digit number strings as stimuli, and a computerized Stroop task using single-item presentation of color names and colors. These tasks are common elements in various batteries that have been employed in our center over a decade. The CPT and Stroop tasks are well-established paradigms that assess overlapping but complementary aspects of attention (Posner and Peterson, 1990; Mirsky et al., 1991; Parasuraman et al., 1998) typically impaired in psychiatric disease. Deficits on both types of task have been reported in major depression as well as in suicide attempters, as noted above. It is unclear, though, how well these tasks distinguish past attempters from comparably depressed subjects with no past history of attempt. Based on our own previous work and on the published literature, we hypothesized that past suicide attempters would perform more poorly on both. We also sought to determine if either one of these tasks was more sensitive to such deficits. Both CPT and Stroop tasks have been used extensively in functional imaging paradigms (i.e. Perlstein et al., 2003; Wagner et al., 2006), much is known about their neural circuitry (Posner & DiGirolomo, 1998; Cohen et al., 2004), and either could easily be adapted to examine attention systems in suicidal populations.
2. METHODS
2.1. Subjects
Subjects were 178 patients meeting DSM-IV criteria for a current Major Depressive Episode, and 66 non-patient comparison subjects. All patients were currently depressed, had a Hamilton Rating Scale for Depression (HRSD, 24-item) score > 16 at time of recruitment, did not meet criteria for psychotic depression (4.5% had a past history of psychotic symptoms), and had no current substance abuse or dependence (past history of abuse/dependence was present in 36.8% of patient subjects). Non-patients were free of current or past Axis I or Axis II disorders. All subjects were free of neurological disease and gross organic brain damage by clinical history and examination. The patient sample included 83 subjects with no past history of suicide attempt, 53 who had made one or more low lethality suicide attempts in the past, and 42 who had made at least one high lethality attempt in the past.
2.2. Instruments
Clinical Assessment
Diagnosis was established in patients using the Structured Clinical Interview for DSM-IV, Axes I (SCID-I; Spitzer et al., 1990) and II (SCID-II; First et al., 1996). Psychiatric illnesses were ruled out in non-patients using the non-patient version of the SCID (SCID-NP; First et al., 1997). Other clinical ratings have been described previously (Mann et al., 1999) and are listed in Table 1. The Vocabulary subtest from the Wechsler Adult Intelligence Scale, 3rd revision (WAIS-III; Wechsler, 1997) was used to estimate premorbid intellectual ability. History of past suicidal behavior was assessed via structured interview (Oquendo, Halberstam et al., 2003). Severity of past suicide attempts was quantified using Beck’s medical damage rating of physical injury resulting from an attempt (Beck et al., 1975). Scale ranges from 0 (no physical damage) to 8 (death). Low lethality attempts were defined as ratings between 0–3 (maximally, mild physical injury requiring medical intervention); high lethality attempts were rated 4–7 (minimally, serious injury requiring medical intervention).
Table 1.
Demographic and Clinical Rating Data
| Variable | Non-Patient Comparison | Depressed Non-Attempters | Low Lethality Attempters | High Lethality Attempters | p-value1 | Contrast2 |
|---|---|---|---|---|---|---|
| N | 66 | 83 | 53 | 42 | -- | |
| Age (yrs.) | 36.2 ± 14.2 | 41.2 ± 12.5 | 34.3 ± 10.9 | 39.2 ± 11.2 | .008 | LL < NA |
| Education (yrs.) | 16.7 ± 2.3 | 16.2 ± 2.7 | 15.5 ± 2.1 | 16.0 ± 2.5 | .08 | |
| WAIS-III Vocabulary | 13.8 ± 3.2 | 13.2 ± 3.4 | 13.3 ± 2.8 | 13.2 ± 2.8 | .68 | |
| (scaled score) | ||||||
| Duration of Current | -- | 49.8 ± 72.4 | 82.8 ± 150.1 | 92.8 ± 245.3 | .23 | |
| Episode (wks.) | [median=24] | [median=26] | [median=22] | |||
| Number Prior Episodes | -- | 7.2 ± 11.5 | 7.8 ± 13.0 | 10.6 ± 14.5 | .69 | |
| of Depression | [median=3] | [median=4] | [median=5] | |||
| Hamilton RSD (24-item) | 1.1 ± 1.5 | 24.8 ± 7.5 | 26.8 ± 7.5 | 26.0 ± 7.7 | <.001 | HL, LL, NA > C |
| Beck Depression | 2.0 ± 3.3 | 26.9 ± 9.5 | 31.0 ± 10.7 | 30.1 ± 12.1 | <.001 | HL, LL, NA > C |
| Inventory | LL > NA | |||||
| BPRS Total Score | 20.1 ± 2.6 | 33.5 ± 7.0 | 33.5 ± 7.1 | 33.8 ± 7.2 | <.001 | HL, LL, NA > C |
| Global Assessment of | 89.0 ± 6.3 | 49.2 ± 10.8 | 46.6 ± 8.7 | 39.7 ± 11.1 | <.001 | HL < LL, NA < C |
| Functioning (GAF) | ||||||
| Global Assessment of Functioning (GAF) without suicide item | 89.0 ± 6.3 | 49.2 ± 10.9 | 47.1 ± 8.4 | 41.5 ± 10.7 | <.001 | HL < LL, NA < C |
| Functioning (GAF) | ||||||
| without suicide item | ||||||
| Beck Hopelessness Scale | 1.6 ± 1.8 | 12.4 ± 5.7 | 14.3 ± 5.2 | 11.7 ± 5.4 | <.001 | LL > HL, NA > C |
| Scale for Suicide Ideation | 0.0 ± 0.0 | 7.7 ± 7.8 | 14.2 ± 9.3 | 16.3 ± 10.7 | <.001 | HL, LL > NA > C |
| (prior to hospitalization) | ||||||
| Scale for Suicide Ideation | 0.0 ± 0.0 | 5.3 ± 6.1 | 8.8 ± 7.7 | 7.5 ± 8.6 | <.001 | HL, LL, NA > C |
| (current) | LL > NA | |||||
| Barratt Impulsiveness | 36.2 ± 12.7 | 52.7 ± 17.9 | 58.8 ± 14.7 | 54.7 ± 19.7 | <.001 | HL, LL, NA > C |
| Scale | ||||||
| Buss-Durkee Hostility | 19.7 ± 9.2 | 33.6 ± 12.0 | 39.6 ± 11.6 | 37.6 ± 11.2 | <.001 | HL, LL, NA > C |
| Inventory | LL > NA | |||||
| Brown-Goodwin | 14.5 ± 3.6 | 16.8 ± 4.9 | 18.3 ± 4.8 | 18.9 ± 5.1 | <.001 | HL, LL, NA > C |
| Aggression History | HL > NA | |||||
| Number Previous Suicide | -- | -- | 1.9 ± 1.5 | 3.8 ± 2.9 | <.001 | HL > LL |
| Attempts | ||||||
| Time Since Most Recent | -- | -- | 69.9 ± 113.7 [median=12.9] | 43.3 ± 74.9 [median=6.9] | .40 | |
| Attempt (months) | [median=12.9] | [median=6.9] | ||||
| Lethality of Most Recent | -- | -- | 1.7 ± 1.3 | 4.6 ± 2.0 | <.001 | HL > LL |
| Attempt | ||||||
| Maximum Lethality of | -- | -- | 1.9 ± 1.1 | 5.6 ± 1.2 | <.001 | HL > LL |
| Attempt | ||||||
| Suicide Intent Scale, | -- | -- | 13.6 ± 5.4 | 19.0 ± 5.5 | <.001 | HL > LL |
| Most Recent Attempt | ||||||
| Suicide Intent Scale, | -- | -- | 13.6 ± 5.3 | 19.5 ± 4.9 | <.001 | HL > LL |
| Most Lethal Attempt | ||||||
| Sex (% Female) | 47.0% | 51.8% | 71.7% | 57.1% | .04 | LL > NA, C |
| Native English Speaking | 74.2% | 84.3% | 86.8% | 88.1% | .18 | |
| Axis I Diagnosis | -- | 73.5% | 67.9% | 57.1% | .18 | HL, LL > NA |
| (% Unipolar) | ||||||
| Axis II Diagnosis | -- | 17.1% | 48.1% | 38.1% | <.001 | HL, LL > NA |
| (% Borderline PD) | ||||||
| Past Substance | -- | 32.1% | 37.3% | 46.3% | .31 | |
| Abuse/Dependence | ||||||
| Inpatient Status | -- | 53.7% | 71.7% | 85.7% | .001 | HL, LL > NA |
| Violent Attempt Method | -- | -- | 36.0% | 17.5% | .05 | HL < LL |
Omnibus ANOVA with df=3,242 for comparisons with continuous variables, chi-squared for categorical variables.
Student/Newman-Keuls post-hoc test for continuous variables.
Attention Assessment
Attention tasks were selected to provide a rapid, clinical assessment of sustained (maintain focus over time) and selective (resolve perceptual conflict quickly) capacities. Tasks were programmed in the PsyScope programming language (Cohen et al., 1993) on a Macintosh computer. Sustained attention was assessed with the 4-digits fast condition of the Continuous Performance Test – Identical Pairs (Cornblatt et al., 1988), adapted for the Macintosh. Four digit number strings were presented for 50 msec, followed by 950 msec dark time. Subjects responded if the number string matched the string that had preceded it (same digits, same order). A total of 150 stimuli were presented: 28 “target” trials, 25 “false alarm” trials (three digits in the correct order and one incorrect digit, or all digits from the previous trial in an incorrect order), and 97 ”random” trials. Task ran 2.5 minutes. Performance was summarized by a sensitivity index, d-prime, a standardized difference between hit and false alarm rates. Response bias, beta, was computed as well.
The Stroop task was adapted from standard color/word versions of the task (MacLeod, 1991), using a single item presentation and a button press response. Subjects responded “1” for red, “2” for blue, “3” for green on a numeric keypad, using index, middle and ring fingers. Three conditions were administered in a blocked fashion, in a fixed order: the Word condition (identify color names in black letters), the Color condition (identify the color of a string of four X’s displayed in one of the three colors), and the Color/Word condition (identify display color of a stimulus containing an incongruous color name, ignoring the text). Stimuli were presented individually and cleared after subject response, with a 50 msec delay between stimuli. Auditory feedback was provided for all responses: correct (beep) and incorrect (buzz). Word and Color blocks included 45 stimulus trials (0.5–1.0 minute run time each); Color/Word block included 90 trials (1.0–2.0 minutes run time). Percent interference (percent change in median RT to color/word vs. color responses) was used to summarize performance; number of correct responses and median response times to correct items in each condition are reported as secondary measures.
Color/Word block included three embedded sub-blocks that manipulated negative priming, a common feature of selective attention tasks (May et al., 1995) that may contribute to overall Stroop effects. Negative priming occurs when a distractor stimulus on one trial becomes the target stimulus on the next trial, and results in subtle (~50–100 msec), but predictable slowing of reaction time. We manipulated the Color/Word stimuli so that these negative priming trials occurred in none of the trials (sub-block A), in all of the trials (sub-block B), and in 50% (sub-block C) of the trials. These sub-blocks were split and counterbalanced in the order A-B-C-C-B-A. In this way, we were able to compare the degree of negative priming that occurs between blocks (A vs. B) and within a block (C), and to determine if the negative priming effects contributed to the overall Stroop effect.
2.3. Procedures
Patient subjects were recruited by advertisement and referral from local clinicians; non-patients by advertisement. All subjects signed informed consent, and the study was approved by the local Institutional Review Board. Patient subjects underwent a medication washout as part of their participation in biological studies conducted in our Conte Center. Washout was two weeks, but extended to four weeks for oral neuroleptics, and five weeks for fluoxetine (three subjects were medication free due to prior non-compliance). Clinical ratings and neuropsychological testing were conducted at the end of washout. Attention measures were administered as part of a larger neuropsychological battery, and conducted blind to clinical assessments and past attempter status.
2.4. Statistical Analyses
Demographic and clinical variables were compared among groups using one-way analyses of variance and post-hoc Newman-Keuls tests for continuous variables, and chi-square analyses for categorical variables. Attention measures were first converted to normatively corrected z-scores based on an external normative sample (n=58; Keilp et al., 2005). These norms include adjustments for age, sex, and/or education effects and have been used previously (Keilp et al., 2001). All z-scores were positively scaled for analyses (lower scores = worse performance; raw scores for all tests are presented in Appendix Table 1.). Primary outcome measures were d-prime for the CPT-IP and percent interference for the Stroop. These scores were treated as repeated measures in a general linear model, to test the hypothesis of a differential effect based on the nature of the task (test by group interaction). An additional covariance analysis was conducted to assess the effects of five factors that might contribute to poor test performance: prior history of psychosis, prior history of substance abuse/dependence, presence of bipolar I subtype, presence of bipolar II subtype, and borderline personality disorder. Given a significant result in omnibus analyses, both primary and secondary measures (e.g. correct response rates and various reaction times for each task) were compared among groups using one-way analyses of variance with Student/Newman-Keuls post-hoc procedures. Alpha level was .05. Correlations between primary attention measures and clinical measures were computed on scores differentiating groups. Supplemental correlational analyses of scores assessing psychosis (Brief Psychiatric Rating Scale, BPRS) and motor retardation (items from BPRS and Hamilton Depression Scale) were conducted to determine if these symptoms were related to attention findings, as in previous studies (Benoit et al., 1992; Lemelin and Baruch, 1998; Nelson et al., 1998; Narita et al., 2004; Politis et al., 2004).
3. RESULTS
3.1. Demographic and Clinical Measures
Subject groups were comparable in education and estimated intellectual level (Table 1.). Low lethality past attempters were younger than nonattempters, more likely to be female than non-attempters and non-patients, and more likely to have Borderline Personality Disorder than other depressed patient groups. Percentage of unipolar and bipolar depressed subjects was comparable among patient groups. Overall severity and chronicity of depression were also comparable among patient groups, although past attempters tended to report higher levels of subjective depression (Beck Depression Inventory). Past attempters also reported the highest levels of suicidal ideation, more so for the period immediately prior to study entry. High lethality past attempters had the poorest level of functioning, and had made more attempts than low lethality attempters. In this attempter sample overall, past attempts tended to be by non-violent methods (i.e. drug overdose); violent attempts in the low lethality group primarily involved superficial cutting, which accounted for 80% of violent attempts in this group. Depressed patients reported greater hopelessness, lifetime impulsiveness, hostility, and past aggressive behavior than non-patients. Low lethality attempters reported the highest levels of current hopelessness and trait hostility, and high lethality attempters the highest levels of lifetime aggressive behavior.
3.2. Attention Measures
Significant effects for subject group (F[3, 239]= 5.39, P=0.001), test (CPT vs. Stroop; F[1, 239]= 3.96, P <0.05), and the interaction of subject group and test (F[3, 239]= 3.67, P =0.01) were found in the analysis of primary attention measures. Given differences in gender distribution of the samples, sex tested as a covariate and was not significant (F[1, 238]= 0.41, P =0.521), and had no effect on group (F[1, 238]= 5.11, P =0.002) or group by test interaction (F[3, 238]= 4.18, P =0.007) effects. If covariates for past history of psychosis, past history of substance abuse/dependence, bipolar I disorder, bipolar II disorder, and borderline personality disorder were all entered into the analysis, the main effect for subject group (F[3, 225]= 5.84, P =0.001) and the subject group by test interaction (F[3, 225]= 2.70, P =0.046) remained significant. Only the past history of substance abuse/dependence was a significant covariate (F[1, 225]= 4.73, P =0.031): those with a past history outperformed those without on the CPT (t[168]=2.02, P = 0.045) but not on Stroop (t[169]=1.34, P = 0.182).
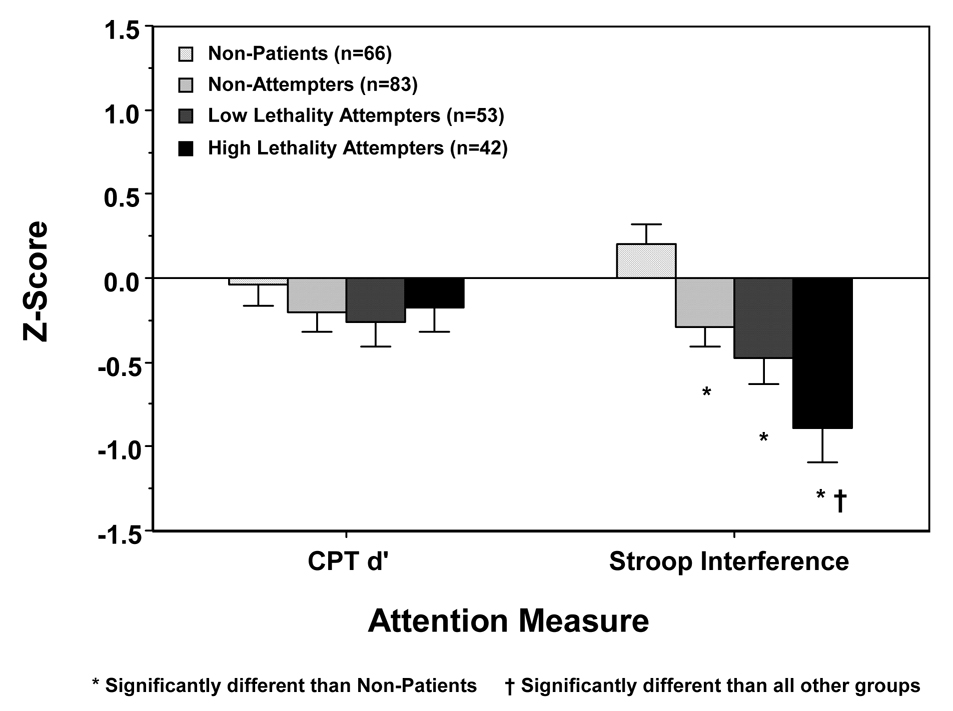
Univariate tests revealed that differences between the tests and between subjects groups were primarily attributable to the poorer performance by depressed and suicide attempter groups on the Stroop (Figure 1.). In one-way analyses, neither CPT d-prime nor any secondary measure from the CPT differentiated groups (Table 2.). In analyses of the Stroop, the percent interference score, its primary outcome measure, differentiated non-patients from all depressed groups, and high lethality attempters from all other groups (Table 2.). Group differences were generally restricted to this interference condition. Base reaction times to the Word and Color conditions did not differ across groups, and group differences in RT only occurred in the Color/Word condition. Slowing of reaction time in the critical interference condition, then, was not a function of generalized slowing across all conditions. Although error rates were lower in the Word condition in patient subjects, error rates were comparable across groups in the Color and Color/Word conditions, the conditions that were used in the calculation of the interference effect.
Figure 1.
Primary attention test scores in non-patient and depressed patient subject groups, divided by past suicide attempt status (Z-score ± Standard Error of Mean).
Table 2.
Attention Test Scores
| Variable | Non-Patient Comparison | Depressed Non-Attempters | Low Lethality Attempters | High Lethality Attempters | p-value1 | Contrast2 |
|---|---|---|---|---|---|---|
| Continuous Performance Test | ||||||
| d’ | −.04 ± 1.0 | −.20 ± 1.1 | −.26 ± 1.1 | −.17 ± 1.0 | .72 | |
| Secondary Measures | ||||||
| Log Beta | .05 ± 1.1 | −.05 ± 1.0 | .15 ± 1.0 | −.09 ± 1.1 | .65 | |
| Hits | −.02 ± 1.0 | −.21 ± 1.2 | −.43 ± 1.3 | −.03 ± 1.1 | .20 | |
| False Alarms | −.09 ± 1.0 | −.15 ± 1.0 | .01 ± 1.1 | −.21 ± 1.0 | .75 | |
| Random Responses | −.54 ± 1.5 | −.66 ± 1.9 | −.23 ± 1.4 | −.92 ± 2.0 | .26 | |
| RT Hits | .22 ± 0.9 | −02 ± 1.0 | .05 ± 1.1 | .07 ± 1.1 | .55 | |
| Stroop Task | ||||||
| Percent Interference | .20 ± 1.0 | −.29 ± 1.1 | −.47 ± 1.2 | −.89 ± 1.3 | <.001 | HL < LL, NA < C |
| Secondary Measures | ||||||
| RT Word Items | .47 ± 1.0 | .09 ± 1.5 | .05 ± 1.1 | .11 ± 1.4 | .21 | |
| RT Color Items (X’s) | .33 ± 1.0 | −.03 ± 1.2 | −.11 ± 1.3 | .05 ± 1.2 | .16 | |
| RT Color/Word Items | .36 ± 1.1 | −.35 ± 1.5 | −.58 ± 1.8 | −.71 ± 1.5 | <.001 | HL, LL, NA < C |
| # Correct Word Items | −.07 ± 1.1 | .38 ± 0.8 | .44 ± 0.7 | .39 ± 0.9 | .004 | HL, LL, NA < C |
| # Correct Color Items | −.18 ± 1.2 | .12 ± 1.1 | .25 ± 1.2 | .17 ± 1.0 | .17 | |
| # Correct Color/Word | −.41 ± 1.7 | .04 ± 1.4 | −.40 ± 2.0 | −.35 ± 2.0 | .35 | |
| Blocked Negative | .08 ± 0.7 | −.01 ± 0.8 | −.00 ± 1.2 | −.11 ± 0.7 | .41 | |
| Priming | ||||||
| Mixed Negative Priming | −.37 ± 1.3 | −.12 ± 1.3 | −.04 ± 1.3 | .09 ± 2.3 | .92 | |
Omnibus ANOVA with df=3,242 for CPT and df=3,243 for Stroop.
Student/Newman-Keuls post-hoc test.
Negative priming effects were found in both the blocked (90.4 ± 104.6 msec) and mixed (98.9 ± 140.6 msec) sub-blocks across all groups. There were, however, no group differences found for the negative priming measures (Table 2). Stroop effects were greater in blocks containing negative priming items (43.7 ± 27.2% in sub-block B; 40.9 ± 27.7% in sub-block C vs. 28.0 ± 23.8% in sub-block A; F[2, 480]= 114.6, P <0.001), but group differences in Stroop interference were comparable across all sub-blocks (F[3, 240]= 8.90, P <0.001) with no interaction by block (F[6, 480]= 1.14, P =0.338).
3.3. Correlations with Clinical Measures
Stroop performance (the positively scaled z-score for interference: higher score, less interference) was not correlated with the Hamilton (rho=−0.11, P =0.13) but was weakly correlated with Beck Depression Inventory (rho=−0.16, P =0.04), GAF score (with or without suicide item, rho=0.18, P =0.02), length of current episode of depression (rho=−0.18, P =0.02), and suicide ideation prior to admission (rho=−0.19, P =0.01). In all cases, poorer performance was associated with greater severity, although associations were modest. Stronger associations were found among suicide attempters with maximum lethality (rho=−0.27, P=0.008) and recent lethality (rho=−0.24, P=0.02) of past suicide attempts, and with number of past attempts (rho=−0.25, P=0.02). Poorer performance was associated with more frequent and more lethal attempts. Test performance was uncorrelated with time since most recent attempt (rho=−0.04, P=0.71).
Stroop performance did not correlate with impulsiveness (BIS; rho=0.04, P=0.64), hostility (BDHI; rho=0.01, P=0.92), or history of aggressive behavior (rho=−0.01, P=0.92). Stroop performance was not significantly correlated in patients with BPRS total score (rho=0.08, P=0.30), a rating sensitive to the presence of psychotic symptoms. Ratings of motor retardation differed between patients and non-patients on both the BPRS (F[3,228]=6.46, P <0.001) and Hamilton Depression Scale (F[3,229]=7.63, P <0.001), but did not differ among depressed groups (patient means for BPRS motor retardation, 1.6 ± 0.9; for Hamilton retardation item, 0.5 ± 0.7), nor did it correlate with Stroop interference in patients (for BPRS motor retardation, rho=0.06, P=0.45; for Hamilton retardation, rho=0.00, P=0.96).
4. DISCUSSION
Deficits in attention were apparent in all depressed subjects, but significantly greater in past suicide attempters and primarily attributable to their greater susceptibility to interference on the Stroop. In our previous study with a much smaller sample (Keilp et al., 2001), Stroop performance was poor in past suicide attempters, but only distinguished them from non-patients. Increased statistical power in this study enabled us to demonstrate that this impairment in attention is worse in those with a past history of more severe suicidal behavior, relative to other depressed patients. Moreover, deficits were evident without any type of explicit emotional provocation or emotionally biased stimuli, suggesting that cognitive control mechanisms themselves are dysfunctional, and not simply susceptible to specific types of emotional arousal. Stroop performance did not clearly distinguish all attempters from non-attempters, but the general trend of this difference was consistent with an association with suicide attempt.
Stroop performance is typically impaired in depression, most likely because it relies on many of the same brain areas affected by depression (Mayberg, 2003). The circuitry of the emotion regulation system, involving the amygdala, anterior cingulate, and prefrontal cortex (Ochsner and Gross, 2005), overlaps considerably with the selective attention system and is affected by genetic risk for depressive disorders (Pezawas et al., 2005). Wagner and colleagues (2006) found that overactivation of rostral cingulate and dorsolateral prefrontal cortex during Stroop performance distinguished depressed subjects from healthy volunteers. However, metabolic deficits in prefrontal cortex and cingulate are accentuated in high lethality suicide attempters (Oquendo et al., 2003). Posner and colleagues (2002) suggest that deficits on attentional conflict tasks may be characteristic of conditions where emotional dysregulation and suicide risk is prominent, such as Borderline Personality Disorder. These deficits are apparent on Stroop tasks (LeGris and van Reekum, 2006). Our data are consistent with this hypothesis, but suggest these deficits are most pronounced among those with a past history of suicidal behavior - even among those without BPD. Poor performance on conflict tasks, then, may be more closely associated with suicide risk than with personality disorder per se.
Deficits related to past history of suicidal behavior were also comparable in unipolar and bipolar depression, and were not affected by bipolar disorder subtype. In a previous report of a preliminary analysis of past suicide attempters with Bipolar I and Bipolar II disorder from a separate cohort (Harkavy-Friedman et al., 2006), we had found differences in Stroop performance between these subtypes, with Bipolar II subjects worse. However, the preliminary nature of that analysis, differences in enrollment criteria (intercurrent substance abuse and medication both allowed in that study) and the greater representation of higher lethality subjects in this study eliminated these differences here.
Neither past suicide attempters nor depressed subjects in general were significantly impaired on the CPT used in this study. This is consistent with one other study that used the 4-digits fast condition of the CPT-IP in a depressed sample, although, in that same study, deficits were found on a similar task using spatial stimuli (Cornblatt et al., 1989). The sensitivity of the CPT task used in this study is well established in psychotic and pure attention disorders, both in the published literature (Cornblatt and Keilp, 1994) as well as in our own laboratory. Clinically stable schizophrenic subjects (n=106) administered the same task in our lab performed significantly more poorly than any group in this study (z=−1.1 ± 1.0; raw d’=1.28 ± 0.83; Keilp, unpublished data). However, our review of the literature suggests that deficits on CPT tasks are most likely to be found in depression when tasks use spatial stimuli (Cornblatt et al., 1989; Liu et al., 2002) or run longer than 7 minutes (Cohen et al., 2001; Koetsier et al., 2002; Egeland et al., 2003), over twice the length of the task used here. Deficits in depressed patients, then, only tend to emerge when stimuli are more difficult to verbalize or in later blocks of trials; whereas deficits in schizophrenic subjects are often evident in the earliest blocks of trials and consistent over all blocks. This coincides with theories linking cognitive impairments in depression to failures of right-sided cortical and subcortical arousal mechanisms (Liotti and Mayberg, 2001) rather than in the more basic information processing capabilities that are impaired in psychosis. Depressed suicide attempters clearly performed no differently than other depressed subjects, and very much unlike the subjects with psychosis in our other studies.
It is possible that subtle brain injuries sustained by higher lethality attempters may have contributed to poorer Stroop performance in this study. Impairments of selective attention in depression have been linked to white matter abnormalities (Videbech et al, 2004), but appear related to global slowing. The lack of impairment in CPT performance, and in reaction times to Word and Color conditions of the Stroop indicate that is not the case here. Similarly, impairments of Stroop performance in depression have been linked to intercurrent psychosis (Nelson et al., 1998; Politis et al., 2004) and psychomotor retardation (Benoit et al., 1992; Lemelin and Baruch, 1998; Narita et al., 2004), but neither type of symptom was more pronounced in our past attempter samples nor correlated with performance.
Overall, data suggest that subjects with a past history of suicide attempt are more severely impaired in aspects of attention affected by depression, despite no differences in observer-rated symptom severity (i.e. Hamilton). Risk for suicidal behavior, therefore, may be associated with more extensive dysfunction in cortical control mechanisms despite no differences in overt depressive symptomatology. Less obvious, subjective aspects of depression, however, may be related to these impairments. Psychological aspects of depression are related to elevated glucose metabolism in dorsal cingulate (Milak et al., 2005), a region commonly activated by Stroop tasks (Posner & DiGirolomo, 1998; Cohen et al., 2004; Wagner et al., 2006).
At a neuropsychological level, data support Posner and colleagues’ hypothesis (2002) that defects in emotion regulation networks, which are most severe when risk for suicidal behavior is high, will be manifest in persistent impairments of attention control. At a phenomenological level, impairments on conflict tasks such as the Stroop suggests a difficulty shifting attention from compelling but inappropriate stimuli. These impairments may predispose suicidal individuals to attend to prepotent emotional states, such as intense feelings of pessimism (Oquendo et al., 2004) or self-blame (Grunebaum et al., 2005), and to formulate actions based on a narrow view of their condition. Impaired selective attention performance, then, may underlie the “cognitive rigidity” that is a common clinical feature of suicide attempters (Pollock & Williams, 1998). Attention control is a prominent feature of current psychotherapeutic treatments for suicidality, such as Dialectical Behavior Therapy (Linehan et al., 2006) and Mindfulness-based Cognitive Therapy (Williams et al., 2006), which train patients to redirect attention at moments of crisis.
Previous research suggests that decision-making is impaired in suicidal individuals (Jollant et al., 2005), but decision processes may be corrupted by a failure to adequately focus attention and control the information on which they are based. At least one study of subjects with Human Immunodeficiency Virus found that poor decision-making task performance (Iowa Gambling Task) was associated with impairments of selective attention and memory (Hardy et al., 2006). Further work is needed to understand the relationship between these measures - particularly how the narrowing of the focus of attention affects decision processes leading to suicidal acts - and to examine the neural circuitry common to selective attention processes and suicidal behavior.
ACKNOWLEDGEMENTS
Supported by National Institute of Mental Health grants MH-062185 and MH-062155, the National alliance for Research on Schizophrenia and Depression (NARSAD), and the American Foundation for Suicide Prevention (AFSP).
Appendix
Appendix 1.
Raw Attention Test Scores
| Variable | Non-Patient Comparison | Depressed Non-Attempters | Low Lethality Attempters | High Lethality Attempters |
|---|---|---|---|---|
| Continuous Performance Test | ||||
| d’ | 2.26 ± .89 | 2.06 ± .95 | 2.03 ± .83 | 2.08 ± .81 |
| Secondary Measures | ||||
| Log Beta | .16 ± .77 | .09 ± .74 | .24 ± .75 | −.09 ± 1.1 |
| Hits | 22.79 ± 4.26 | 21.61 ± 5.15 | 21.33 ± 5.28 | 22.29 ± 4.93 |
| False Alarms1 | 3.45 ± 2.87 | 3.82 ± 2.91 | 3.63 ± 3.14 | 4.07 ± 2.88 |
| Random Responses1 | .48 ± .92 | .67 ± 1.48 | .42 ± .91 | 1.10 ± 2.35 |
| RT Hits1 | 530.7 ± 77.3 | 551.9 ± 92.4 | 546.1 ± 95.3 | 544.3 ± 95.6 |
| Stroop Task | ||||
| Percent Interference1 | 26.67 ± 20.93% | 40.89 ± 22.78% | 36.38 ± 22.79% | 51.49 ± 28.86% |
| Secondary Measures | ||||
| RT Word Items1 | 529.5 ± 83.9 | 575.5 ± 128.3 | 554.6 ± 86.5 | 566.6 ± 116.4 |
| RT Color Items (X’s)1 | 532.8 ± 91.7 | 578.8 ± 118.3 | 559.8 ± 99.0 | 566.3 ± 113.8 |
| RT Color/Word Items1 | 677.1 ± 171.9 | 820.6 ± 240.7 | 767.7 ± 207.5 | 861.2 ± 261.2 |
| Raw Stroop (C/W – C)1 | 144.3 ± 120.9 | 241.9 ± 158.5 | 208.0 ± 141.6 | 294.9 ± 190.4 |
| # Correct Word Items | 41.26 ± 2.35 | 42.35 ± 1.66 | 42.47 ± 1.38 | 42.37 ± 1.85 |
| # Correct Color Items | 41.14 ± 2.33 | 41.80 ± 2.06 | 41.91 ± 2.27 | 41.86 ± 1.97 |
| # Correct Color/Word | 83.48 ± 4.59 | 84.58 ± 4.70 | 83.32 ± 6.90 | 83.38 ± 6.50 |
| Blocked Negative Priming | 91.4 ± 78.9 | 98.2 ± 122.4 | 83.5 ± 117.7 | 81.9 ± 84.5 |
| Mixed Negative Priming | 71.0 ± 94.8 | 104.0 ± 127.4 | 107.6 ± 107.3 | 121.9 ± 232.4 |
Scores are negatively scaled. Higher scores indicate poorer performance.
All RT, raw Stroop, and negative priming scores in millisecond.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- Bartfai A, Winborg IM, Nordstrom P, Asberg M. Suicidal behavior and cognitive flexibility: design and verbal fluency after attempted suicide. Suicide and Life Threatening Behavior. 1990;20(3):254–266. [PubMed] [Google Scholar]
- Beck AT, Beck R, Kovacs M. Classification of suicidal behaviors: I. Quantifying intent and medical lethality. American Journal of Psychiatry. 1975;132:285–287. doi: 10.1176/ajp.132.3.285. [DOI] [PubMed] [Google Scholar]
- Becker ES, Strohbach D, Rinck M. A specific attentional bias in suicide attempters. Journal of Nervous and Mental Disease. 1999;187(12):730–735. doi: 10.1097/00005053-199912000-00004. [DOI] [PubMed] [Google Scholar]
- Benoit G, Fortin L, Lemelin S, Laplante L, Thomas J, Everett J. Selective attention in major depression: clinical retardation and cognitive inhibition. Canadian Journal of Psychology. 1992;46(1):41–52. [PubMed] [Google Scholar]
- Cohen JD, Aston-Jones G, Gilzenrat MS. A systems-level perspective on attention and cognitive control. In: Posner MI, editor. Cognitive Neuroscience of Attention. New York, NY: Guilford Press; 2004. pp. 71–90. [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods, Instruments, & Computers. 1993;25:257–271. [Google Scholar]
- Cohen R, Lohr I, Paul R, Boland R. Impairments of attention and effort among patients with major affective disorders. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(3):385–395. doi: 10.1176/jnp.13.3.385. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Research. 1989;29(1):65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Den Hartog HM, Derix MM, Van Bemmel AL, Kremer B, Jolles J. Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: testing the effort and cognitive speed hypotheses. Psychological Medicine. 2003;33(8):1443–1451. doi: 10.1017/s003329170300833x. [DOI] [PubMed] [Google Scholar]
- Egeland J, Rund BR, Sundet K, Landro NI, Asbjornsen A, Lund A, Roness A, Stordal KI, Hugdahl K. Attention profile in schizophrenia compared with depression: differential effects of processing speed, selective attention and vigilance. Acta Psychiatrica Scandanavica. 2003;108(4):276–284. doi: 10.1034/j.1600-0447.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Loma B. Structured Clinical Interview for DSM-IV Axis II Personality Disorders(SCID-II), (Version 2.0) New York, NY: Biometric Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, Oquendo MA, Mann JJ. Symptoms components of standard depression scales and past suicidal behavior. Journal of Affective Disorders. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20(3):355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkavy-Friedman JM, Keilp JG, Grunebaum MF, Sher L, Printz D, Burke AK, Mann JJ, Oquendo M. Are BPI and BPII suicide attempters distinct neuropsychologically? Journal of Affective Disorders. 2006;94(1–3):255–259. doi: 10.1016/j.jad.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Horesh N. Self-report vs. computerized measures of impulsivity as a correlate of suicidal behavior. Crisis. 2001;22(1):27–31. doi: 10.1027//0227-5910.22.1.27. [DOI] [PubMed] [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162(2):304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. American Journal of Psychiatry. 2001;158(5):735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135(3):191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Koetsier GC, Volkers AC, Tulen JHM, Passchier J, van den Broek WW, Bruijn JA. CPT performance in major depressive disorder before and after treatment with fluvoxamine. Journal of Psychiatric Research. 2002;36:391–397. doi: 10.1016/s0022-3956(02)00026-2. [DOI] [PubMed] [Google Scholar]
- LeGris J, van Reekum R. The neuropsychological correlates of borderline personality disorder and suicidal behavior. Canadian Journal of Psychiatry. 2006;51(3):131–142. doi: 10.1177/070674370605100303. [DOI] [PubMed] [Google Scholar]
- Lemelin S, Baruch P. Clinical psychomotor retardation and attention in depression. J Psychiatry Research. 1998;32:81–88. doi: 10.1016/S0022-3956(98)00002-8. [DOI] [PubMed] [Google Scholar]
- Lemelin S, Baruch P, Vincent A, Everett J, Vincent P. Distractibility and processing resource deficit in major depression. Evidence for two deficient attentional processing models. Journal of Nervous and Mental Disease. 1997;185(9):542–548. doi: 10.1097/00005053-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Lemelin S, Baruch P, Vincent A, Laplante L, Everett J, Vincent P. Attention disturbance in clinical depression. Deficient distractor inhibition or processing resource deficit? Journal of Nervous and Mental Disease. 1996;184(2):114–121. doi: 10.1097/00005053-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, Korslund KE, Tutek DA, Reynolds SK, Lindenboim N. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Archives of General Psychiatry. 2006 Jul;63(7):757–766. doi: 10.1001/archpsyc.63.7.757. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. Journal of Clinical and Experimental Neuropsychology. 2001;23(1):121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- Liu SK, Chiu CH, Chang CJ, Hwang TJ, Hwu HG, Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. American Journal of Psychiatry. 2002;159(6):975–982. doi: 10.1176/appi.ajp.159.6.975. [DOI] [PubMed] [Google Scholar]
- MacLeod C. A half-century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156:181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatrica Scandinavica. 2005;112(4):294–301. doi: 10.1111/j.1600-0447.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- May CP, Kane MJ, Hasher L. Determinants of negative priming. Psychological Bulletin. 1995;118(1):35–54. doi: 10.1037/0033-2909.118.1.35. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Archives of General Psychiatry. 2005;62(4):397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychology Review. 1991;2(2):109–145. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Narita H, Odawara T, Iseki E, Kosaka K, Hirayasu Y. Psychomotor retardation correlates with frontal hypoperfusion and the Modified Stroop Test in patients under 60-years-old with major depression. Psychiatry and Clinical Neurosciences. 2004;58(4):389–395. doi: 10.1111/j.1440-1819.2004.01273.x. [DOI] [PubMed] [Google Scholar]
- Nasser EH, Overholzer JC. Assessing varying degrees of lethality in depressed adolescent suicide attempters. Acta Psychiatrica Scandinavica. 1999;99(6):423–431. doi: 10.1111/j.1600-0447.1999.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Nelson EB, Sax KW, Strakowski SM. Attentional performance in patients with psychotic and nonpsychotic major depression and schizophrenia. American Journal of Psychiatry. 1998;155(1):137–139. doi: 10.1176/ajp.155.1.137. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: The utility and limitations of research instruments. In: First M, editor. Standardized Evaluation in Clinical Practice, Review of Psychiatry. Volume 22. Washington, DC: American Psychiatric Publishing; 2003. pp. 103–130. [Google Scholar]
- Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Archives of General Psychiatry. 2003;60(1):14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. American Journal of Psychiatry. 2004;161(8):1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorder: implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry. 2002;10(2):86–99. doi: 10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Warm J, See J. Brain systems of vigilance. In: Parasuraman R, editor. Varieties of Attention. Cambridge, MA: MIT Press; 1998. pp. 221–256. [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003;53(1):25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinsky BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impact human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Politis A, Lykouras L, Mourtzouchou P, Christodoulou GN. Attentional disturbances in patients with unipolar psychotic depression: a selective and sustained attention study. Comprehensive Psychiatr. 2004;45(6):452–459. doi: 10.1016/j.comppsych.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Pollock LR, Williams JM. Problem solving and suicidal behavior. Suicide and Life Threatening Behavior. 1998;28(4):375–387. [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman R, editor. The Attentive Brain. Cambridge, MA: MIT Press; 1998. pp. 401–423. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neurosciiences. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Vizueta N, Levy K, Thomas KM, Clarkin J. Attentional mechanisms of borderline personality disorder. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16366–16370. doi: 10.1073/pnas.252644699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Milstein JA, Dalley JW. Neuropharmacology of attention. In: Posner MI, editor. Cognitive Neuroscience of Attention. New York, NY: Guilford Press; 2004. pp. 283–293. [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52(1):63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction Manual for the Structured Clinical Interview for the DSM-IV (SCID-P) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. American Journal of Psychiatry. 2005;162(9):1680–1687. doi: 10.1176/appi.ajp.162.9.1680. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Reynolds M, Mezzich A. Neurobehavior disinhibition in childhood predicts suicide potential and substance use disorder by young adulthood. Drug and Alcohol Dependence. 2004;76 Suppl:S45–S52. doi: 10.1016/j.drugalcdep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Thomas J, Raoux N, Everett J, Dantchev N, Widlocher D. Deficit in selective attention and its evolution in depression. Encephale. 1997;23(2):108–112. [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Gammelgaard L, Egander A, Clemmensen K, Rasmussen NA, Gjedde A, Rosenberg R. The Danish PET/depression project: performance on Stroop's test linked to white matter lesions in the brain. Psychiatry Research. 2004;130(2):117–130. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG. Cortical inefficiency in patients with unipolar depression: an event-related FMRI study with the Stroop task. Biological Psychiatry. 2006 May 15;59(10):958–965. doi: 10.1016/j.biopsych.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale – Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Williams JM, Duggan DS, Crane C, Fennell MJ. Mindfulness-based cognitive therapy for prevention of recurrence of suicidal behavior. Journal of Clinical Psychology. 2006;62(2):201–210. doi: 10.1002/jclp.20223. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imageability of the future. Memory and Cognition. 1996;24(1):116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]