Abstract
Inflammation following ischemic stroke is known to contribute to injury. NADPH oxidase (NOX) is a major enzyme system originally studied in immune cells that leads to superoxide (O•-) generation. Apocynin is a NOX inhibitor that has been studied as a potential treatment in experimental stroke. Here we explored the effect of different doses of apocynin in a mouse model of 2 h transient middle cerebral artery occlusion (tMCAO) followed by 22 h reperfusion. Apocynin, given intravenously at a dose of 2.5 mg/kg 30 minutes before reperfusion, improved neurological function (P<0.01), reduced infarct volume (P<0.05), and the incidence of cerebral hemorrhage (P<0.05), but not at higher doses of 3.75 and 5 mg/kg, where it actually increased brain hemorrhage. Apocynin also tended to reduce mortality at the lower dose, but not at higher doses. Using hydroethine fluorescence to delineate O•- in the brain, neurons and some microglia/macrophages, but not vascular endothelial cells were found to contain O•-. Apocynin at protective doses markedly prevented ischemia induced increases in O•-. Our data suggested that apocynin can protect against experimental stroke, but with a narrow therapeutic window.
Keywords: apocynin, superoxide, NADPH oxidase, stroke, brain hemorrhage
INTRODUCTION
There is increasing evidence that inflammation accompanying ischemic stroke accounts for some of its progression, at least acutely (Zheng and Yenari, 2004, Chamorro and Hallenbeck, 2006, Wang et al., 2007). A robust inflammatory reaction characterized by peripheral leukocyte influx into the cerebral parenchyma and activation of endogenous microglia follows focal cerebral ischemia. This leads to the generation of reactive oxygen species (ROS) which can then stimulate ischemic cells, even ischemic neurons, to secrete inflammatory factors. Once activated, inflammatory cells can release a variety of cytotoxic agents including more cytokines, matrix metalloproteinases (MMPs), nitric oxide (NO) and more ROS. These substances may induce more cell damage as well as disruption of the blood-brain barrier (BBB) and extracellular matrix (Emsley and Tyrrell, 2002, Danton and Dietrich, 2003). BBB disruption can further potentiate brain injury and contribute to secondary ischemic brain damage by permitting serum elements and blood to enter the brain (Siesjo and Siesjo, 1996, Rosenberg, 1999).
Generation of ROS by inflammatory cells occurs via several enzyme systems. NADPH oxidase (NOX) is a major enzyme that generates superoxide. While numerous forms of the enzyme have now been described (Lambeth, 2004), phagocytic NOX, also referred to as NOX2, is associated with immune cells. It consists of two membrane bound subunits, gp91 and p22, and three cytosolic subunits, p67, p47 and p40 plus Rac, a small GTPase (Groemping and Rittinger, 2005). With appropriate stimuli, phosphorylation of the p47 subunit by protein kinase C (PKC) (Reeves et al., 1999, Noh and Koh, 2000) is followed by the cytosolic subunits translocating to the membrane where they interact with the membrane bound subunits to transfer electrons from NADPH to oxygen to form superoxide. Prior work has shown that mice deficient in the gp91 subunit of NOX2 have smaller infarcts than wild type mice (Walder et al., 1997, Kahles et al., 2007), and microglia potentiate injury to the blood brain barrier due to superoxide produced by NOX2 in brain ischemia models (Yenari et al., 2006).
Apocynin is an inhibitor of NOX2, and has been shown to reduce histologic injury following global (Wang et al., 2006) and focal (Tang et al., 2007) cerebral ischemia. Here we explore the dose response of apocynin on neurological outcome and BBB disruption and brain hemorrhage in experimental stroke.
EXPERIMENTAL PROCEDURES
All reagents were obtained from Sigma-Aldrich, except where noted. Animals were obtained from Jackson Labs.
Mouse model of experimental stroke
C57BL/6 male mice, 25–30 gram, were housed and treated in a humane manner in strict accordance with Department of Agriculture and institutional animal subjects’ guidelines (NIH publication No. 86-23). Mice were anesthetized with 5% isofluorane delivered into an anesthesia induction chamber with oxygen and air supplied at a ratio of 0.2 L/min oxygen: 0.8 L/min air. Once surgical planes of anesthesia were attained (assessed by absence of hind leg withdrawal to pinch), isoflurane was decreased to 2–2.5% throughout the remainder of the surgery. Strokes were created in mice by transient middle cerebral artery occlusion (tMCAO) as previously described (Yenari et al., 2006). Briefly, a poly-L-lysine coated monofilament suture (Ethicon) was introduced to the middle cerebral artery and left for 2 h. A total of 56 mice were used in this study.
Treatment
Mice were randomly assigned to various treatment groups: apocynin 2.5 mg/kg, n =9; 3.75 mg/kg, n=9; 5 mg/kg, n=7; and vehicle, n=16. All drugs were administered intravenously (I.V.) through a tail vein, and given 30 min before the onset of reperfusion. Animals that died were excluded from histological, behavioral and biochemical analyses. Those that died for reasons not related to anesthetic or surgical complications were counted to estimate the mortality rate.
To determine whether apocynin can enter the brain, apocynin was labeled by dissolving in dichloromethane containing triethylamine and Texas Red for 1 h, then partitioned, dried and filtered. The solid fraction was then subjected to conventional flash chromatography to yield the desired Texas Red labeled apocynin. 3 uninjured mice received 5 mg/kg Texas red-tagged apocynin I.V., and brains were harvested 3 h later. As a control, 3 mice received only Texas red suspended in PBS.
Behavior Studies
Neurological deficits were assessed 24 h after ischemia onset using a modified scale where 0 indicates no detectable deficit; 1, unable to extend the contralateral forelimb; 2, flexion of the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side (Yenari et al., 2006, Zheng et al., 2008).
Mortality was also reported in this study. Mice that died before the end of the 24 h observation period were counted only if they expired after recovery from anesthesia, thus eliminating the possibility that they died due to anesthetic or surgical complications.
Infarct volume and gross hemorrhage assessment
After sacrifice, brains were removed, and cut into 2 mm coronal slices in a mouse brain matrix (World Precision Instruments, Inc., Sarasota, FL). Sections were inspected for gross hemorrhage, photographed, then immersed in 2,3,5-triphenyltetrazolium (TTC) chloride at 37°C for 20 min. Brain sections from the photographs were visually inspected for gross hemorrhage and scored by a rater blinded to treatment using methods previously published by our group (Yenari et al., 1997a, Yenari et al., 1997b, Yenari et al., 2006). Only cerebral hemorrhages visible to the naked eye were scored: 0, if there was no gross hemorrhage, 1=small amount of hemorrhage (present on no more than 1 brain section), 2=medium amount of hemorrhage (present on 2–3 sections), 3=large amount of hemorrhage (present on >3 sections, but no subarachnoid blood, and no hemorrhage in the contralateral hemisphere), 4=severe hemorrhage (same as 3 but brain also has subarachnoid blood and/or hemorrhage in the contralateral hemisphere).
Infarct volume was determined from the TTC stained sections using previously published methods (Maier et al., 2001). Afterwards, brains were fixed and prepared for histochemistry (Wang et al., 2002).
BBB permeability
BBB permeability was evaluated by the detection of Evans blue dye extravasation as previously described (Yenari et al., 2006). 100 µL of 2% Evans blue in PBS was injected via the tail vein 3 h before euthanasia. At the time of sacrifice, mice were anesthetized and transcardially perfused with ice-cold PBS until the outflow of perfusate was clear. The volume of extravasated dye was determined by tracing the region of blue-stained tissue from coronal sections and normalized to the infarct volume to correct for the smaller lesions seen among treated animals. This method is highly correlated to a spectrophotometric method (data not shown) (Belayev et al., 1996).
In situ superoxide detection
The production of superoxide (O•-) after ischemia was detected in situ by a method previously described with minor modification (Maier et al., 2002). Hydroethidine (HEt, Invitrogen, Cat. D11347) was dissolved in 0.1% dimethyl sulfoxide (DMSO) then diluted with phosphate-buffered saline (PBS) to a concentration of 1 mg/mL. The HEt solution was prepared in a hypoxia chamber (Coy Laboratory, Grass Lake, MI), and 150 µl freshly prepared HEt was given I.V. with the animal in a mouse restrainer (Kent Scientific Corporation No.:RSTR552. USA). Animals were euthanized one hour later with an overdose of isoflurane, followed by transcardial perfusion of 200 mL of PBS. Brains were harvested and sunk in 20% sucrose in PBS overnight, then cut into 25 µm thick sections on a cryostat (Leica CM 1850) and placed on a glass slide (Fisherbrand, superfrost/plus, Cat. 12-550-15). The sections were mounted with Mounting Medium containing DAPI (Vectashield, Cat. H-1200, Burlingame, CA) and visualized under a fluorescent microscope (Axiovert 40 CFL, Carl Zeiss)).
Fluorescence was assessed microscopically at an excitation of 540–552 nm and emission >590 nm for ethidium detection. The intensity of oxidized HEt (red fluorescence) was compared between apocynin and vehicle treated animals. In order to quantify the production of O•-, high magnification (400 X) pictures were taken randomly at 3 sites within the peri-infarct region and 3 anatomically comparable sites within the contralateral hemisphere. The amount of relative fluorescence was measured from the entire field of view, and the percent increase in fluorescent intensity relative to background values was measured using Adobe Photoshop 6.0 (Adobe Systems, Inc., San Jose, CA) (Lu et al., 1998).
For in situ superoxide detection, 9 more mice were studied. 3 groups were compared: sham operated, tMCAO plus vehicle treatment, tMCAO plus apocynin 2.5 mg/kg I.V. (n=3 in each group). Brains were harvested 24 h after the onset of ischemia.
Double immunofluorescent labeling of HEt and brain cells
After collecting brain sections in animals given HEt, sections were fixed with acetone (75%)/ethanol (25%) for 15 minutes, then blocked in 5% normal goat serum, 5% normal horse serum and 1% BSA in PBS. Sections were reacted with primary antibodies conjugated to a fluorescent probe overnight at 4°C in a foil wrapped box. Neurons were detected by mouse anti-neuronal nuclei (NeuN) conjugated to Alexa Fluor 488 (1:100, Cat.# MAB377X, mouse monoclonal, Chemicon, Temecula, CA); endothelial cells were detected by rat anti-mouse CD31 monoclonal antibody conjugated to Alexa Fluor® 488 (1:200, Cat.# 102514, Biolegend, San Diego, CA); microglia/macrophages were detected by FITC-conjugated Rat Anti-Mouse CD11b (1:200, Cat.# 553310, BD Pharmingen,San Jose, CA). After washing, brain sections were mounted with VECTASHIELD Mounting Medium with DAPI (Cat. # H-1200, Burlingame, CA) and visualized under a fluorescent microscope as described above.
Statistical Analysis
All statistical analyses were performed using Sigma Stat 2.03 (Systat Software, Inc., San Jose, CA). Quantitative data were presented as mean ± S.D. Two-group comparisons were performed by two-tailed student’s t-test. Multiple-group comparisons were performed using one-way ANOVA followed by Tukey HSD post hoc test. Chi square tests or Kruskal-Wallis ANOVA on Ranks were used for noncontinuous data. P<0.05 was considered statistically significant.
RESULTS
Apocynin penetrates the intact BBB
Texas Red-labeled apocynin administered to uninjured mice was readily detected 3 h later in the brain (Fig 1A). In comparison, injection of Texas Red led to little detection of the fluorescent label (Fig 1B). Thus, after systemic administration, apocynin is capable of crossing the BBB and entering the brain.
Fig. 1. Apocynin can penetrate the intact BBB.
Texas Red labeled apocynin (A) or Texas Red alone (B) was injected intravenously into uninjured mice. Three hours later, brains were harvested. Apocynin was detected in the brain, often within cells. Sections were counter stained with DAPI to delineate nuclei. (scale bar=50µm)
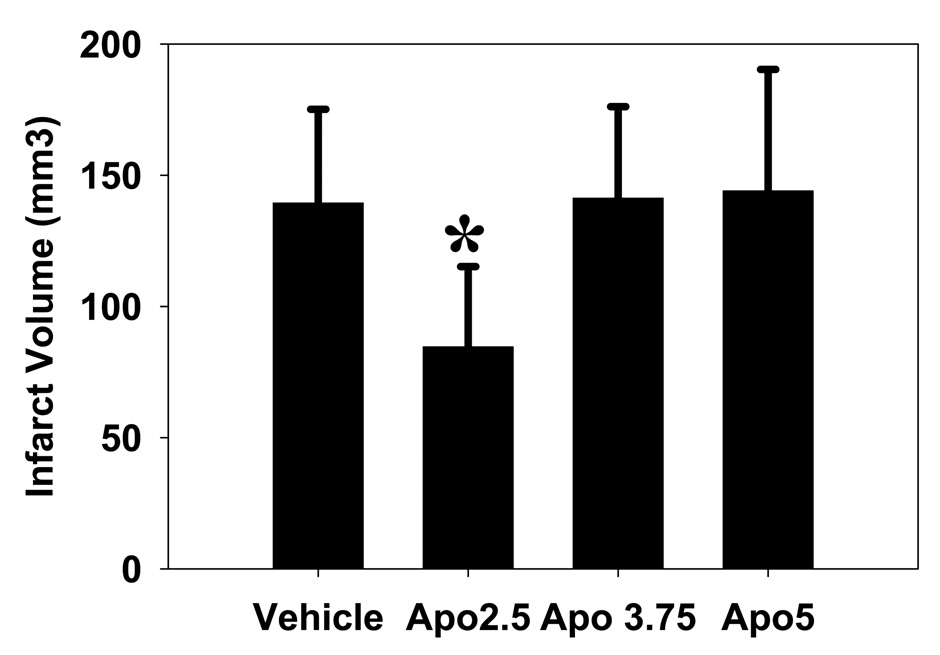
Apocynin reduces infarct size, but not at higher doses
Compared to vehicle treated animals, apocynin 2.5 mg/kg significantly reduced infarct volume by about 40% (84.6 ± 30.6 mm3 vs 139.4 ± 35.7 mm3, P<0.05). However, at higher doses, apocynin treatment had no effect on infarct size (3.75 mg/kg: 141.3 ± 34.9 mm3, 5 mg/kg: 144.0 ± 46.3 mm3, NS) (Fig 2).
Fig 2. Apocynin reduces infarct volume.
Compared to vehicle treated controls, apocynin treatment reduces infarct volume at a dose of 2.5 mg/kg, but not 3.75 or 5 mg/kg (*P<0.05).
Effect of apocynin treatment on cerebral hemorrhage and mortality
Apocynin 2.5 mg/kg also markedly reduced the incidence and severity of brain hemorrhage after stroke compared to the vehicle treated animals. However, apocynin treatment at doses of 3.75 and 5 mg/kg did not reduce the incidence of brain hemorrhage. In fact, apocynin at higher doses resulted in larger cerebral hemorrhages in the ischemic hemisphere with hemorrhage in the contralateral non-ischemic hemisphere compared to vehicle treated controls (Fig 3). Using a semi-quantitative score and non-parametric statistical tests, higher doses significantly increased the severity of hemorrhage (Table).
Fig 3. Apocynin’s effect on brain hemorrhage depends on the dose used.
Apocynin at a dose of 2.5 mg/kg (Apo2.5) reduced the extent of brain hemorrhage compared to vehicle treated controls (Vehicle). In contrast, a mouse given apocynin at a dose of 5.0 mg/kg (Apo5.0) had a much larger hemorrhage, even extending into the contralateral hemisphere.
Table.
Effect of Apocynin on Cerebral Hemorrhage and Mortality
| Veh | Apo2.5 | Apo3.75 | Apo5.0 | ||
|---|---|---|---|---|---|
| Incidence of Hemorrhage | # bleed | 7 | 1 | 4 | 3 |
| total # | 12 | 8 | 7 | 5 | |
| % | 58 | 12.5* | 57 | 60 | |
| Hemorrhage Score | mean ± SE | 2.0±0.21 | 0.50±0.19* | 3.14±0.26* | 3.40±0.40* |
| Mortality | # died | 4 | 1 | 2 | 3 |
| # with bleed | 2 | 0 | 1 | 2 | |
| total # | 16 | 9 | 9 | 8 | |
| % | 25 | 13 | 22 | 38 | |
Veh = vehicle treated animals; Apo2.5 = apocynin 2.5 mg/kg i.v.; Apo3.75 = apocynin 3.75 mg/kg; Apo5.0 = apocynin 5.0 mg/kg.
P < 0.05 vs Veh.
Mice treated with apocynin 2.5 mg/kg showed a trend towards decreased mortality (13%) compared to the vehicle treated animals (25%). Apocynin 3.75 mg/kg (22%) showed a similar mortality rate as vehicle treated mice, but apocynin 5.0 mg/kg treated mice tended to have a higher mortality rate (38%) (Table). All animals counted in the mortality tabulations had frank brain edema, and 50% of them had gross hemorrhage. The distribution of brain hemorrhages among animals that died was not affected by dose. None of these data were statistically different, but a lack of significance could be due to the relatively small numbers, and that the current study was not powered to specifically test this hypothesis.
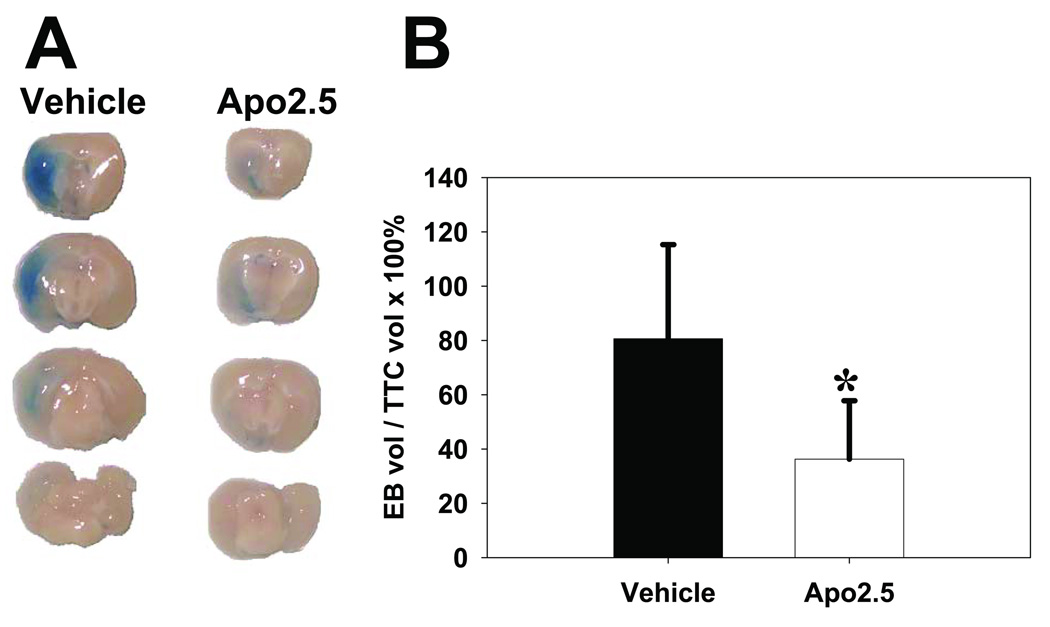
Apocynin 2.5 mg/kg treatment reduces BBB disruption and improves neurological outcome after stroke
Apocynin 2.5 mg/kg reduced the amount of Evans blue dye extravasation in the brain compared with vehicle treatment. Since apocynin may lessen the extent of BBB disruption simply by its effect on reducing infarct size, the volume of Evans blue (EB) dye extravasations was expressed relative to the infarct size as delineated by TTC staining. Compared with the control animals, the EB/TTC ratio was still reduced in apocynin 2.5 mg/kg treated animals (36.2 ± 21.5 mm3 Evans blue/100 mm3 infarct vs 80.6 ± 34.7 Evans blue/100 mm3 infarct, P<0.05) (Fig 4).
Fig 4. Apocynin reduces BBB disruption.
A: Apocynin 2.5 mg/kg reduced Evans blue dye extravasation into the brain 24 h after tMCAO, compared to vehicle treated controls. Representative brain sections following perfusion with Evan’s blue shows less extravasation of dye in an animal treated with apocynin (Apo2.5) compared to vehicle. B: The accompanying graph shows that even when the area of Evan’s blue dye (EBvol) extravasation in normalized to the size of the infarct (TTCvol), the extent of BBB disruption is less among the apocynin treated group (*P<0.05).
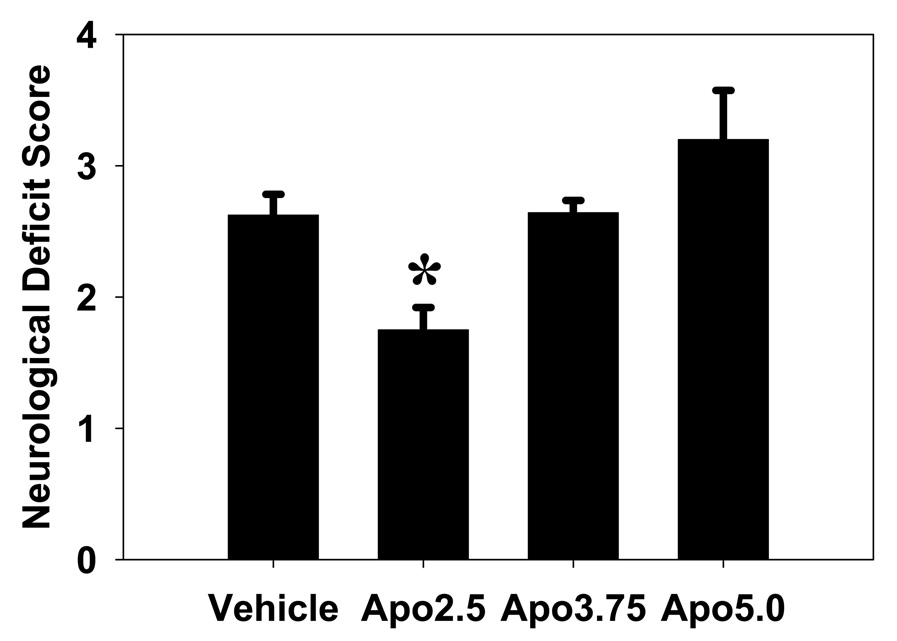
Compared to the vehicle treated animals, apocynin 2.5 mg/kg treated animals showed improved neurological outcome as assessed by modified Bederson’s score (1.75 ± 0.42 vs 2.63 ± 0.44, P<0.01). However, apocynin 3.75 mg/kg and apocynin 5.0 mg/kg had no effect on neurological outcome (Fig 5).
Fig 5. Apocynin improves neurological function following experimental stroke.
Apocynin 2.5 mg/kg (Apo2.5) significantly improved the neurological function 24 h after stroke onset compared to vehicle treated mice. Higher doses of apocynin (Apo3.75 and Apo5.0) had no effect on neurological outcome (*P<0.05).
Superoxide is generated by neurons and some microglial/macrophages: reduction by apocynin
Superoxide was detected mostly in the peri-infarct area, and was found mostly in neurons and some microglia/macrophages, but no endothelial cells (Fig 6). Compared to sham operated animals, tMCAO markedly increased O•- as estimated by the intensity of red fluorescence by hydroethidine (100.0±1.7 vs. 22.3±1.8 in shams, P<0.01), and this was reduced by apocynin 2.5 mg/kg treatment to levels even below that of sham (1.1 ± 0.2, P<0.05) (Fig 7).
Fig 6. Superoxide is found mostly in neurons and some microglia/macrophages.
Following 2 h tMCAO and 22 hours reperfusion, superoxide was visualized by hydroethidine (HEt). Images are taken from peri-infarct areas. Most O•- is found in neurons (NeuN), with some detected in microglia/macrophages (CD11b), including microglia/macrophages surrounding vessel-like structures (arrows). No HEt was found in vascular endothelial cells (CD31) (scale bar=125µm).
Fig 7. Apocynin treatment reduces superoxide anion (O•-) generation.
A: Superoxide detection using hydroethidine (HEt) was increased following 2 h tMCAO and 22 h reperfusion (Vehicle) compared to sham operated controls (Sham). Apocynin treatment at a dose of 2.5 mg/kg (Apo2.5) dramatically reduced the generation of superoxide anions (O•-). Brain regions within the cortical borderzone of infarction are shown (scale bar=75µm). B: Relative fluorescence intensity of entire high power fields sampled within the peri-infarct regions were significantly increased by ischemia (Vehicle), and markedly reduced by apocynin treatment (Apo2.5). (*P<0.01 vs. vehicle).
DISCUSSION
In this study, we show that apocynin at a dose of 2.5 mg/kg given just prior to reperfusion, or 1.5 h after ischemia onset, resulted in reduced infarct volume, BBB disruption and cerebral hemorrhage, and improved neurological outcome. We also found that in this model of stroke, O•- is largely generated by neurons and some microglia/monocytes, with no generation in brain vascular endothelial cells. Apocynin at protective doses markedly reduced O•- in the brain. Interestingly, apocynin at higher doses failed to show any benefit, and increased the severity of brain hemorrhage.
Apocynin is generally thought to be a drug of very low toxicity with a LD50 of about 9 kg/kg when adminstered orally (E. van den Worm, 1996). Recent work has shown that apocynin was protective at a dose of 5 mg/kg given intraperitoneally (I.P.) 30 min prior to ischemia (Wang et al., 2006) in a rat model of global cerebral ischemia. In another study of tMCAO in the rat, apocynin 50 mg/kg I.P. given 30 minutes before reperfusion also reduced infarct size (Tang et al., 2007). This is somewhat in contrast to the findings reported here where doses higher than 3.75 mg/kg had no effect, and the higher doses studied tended to worsen brain hemorrhage and death. Apocynin at higher doses actually worsened the severity of hemorrhage, with blood appearing even within the contralateral, non ischemic hemisphere.
The reasons for this narrow dosage range are unclear, but a few factors should be pointed out. In our study, apocynin was administered intravenously, in comparison to the other studies where drug was given I.P. Thus, absorption in our model may have been more complete. We also studied brain ischemia in the mouse, whereas the other studies used rats. Anecdotal experience from our own lab has shown that brain hemorrhage occurs more frequently following tMCAO in mice (Yenari et al., 2006) compared to rats (Neumann-Haefelin et al., 2001, Lee et al., 2005). Thus, it is possible that mice are more sensitive to insults that lead to brain hemorrhage. Also, the duration of ischemia was longer in our study than in the study by Tang et al. (Tang et al., 2007) (2 hours MCAO vs 90 minutes MCAO). Furthermore, the occurrence of hemorrhage following global cerebral ischemia is unusual. Thus, apocynin may be better tolerated in models that are relatively resistant to bleeding. The injection of Texas red-tagged apocynin to healthy animals at the highest concentration studied here did not lead to any death or brain hemorrhage, suggesting that the narrow dose range applies only in the setting of brain ischemia. Further, pilot in vitro studies in our laboratory suggest that at higher concentrations, apocynin begins to have pro-oxidant properties. Regardless, our findings here would have significant implications should apocynin ever be considered for treating stroke patients in the future. While the effects of inhibiting NADPH oxidase appear effective, safer inhibitors should be developed.
Apocynin is a potent inhibitor of NADPH oxidase which is a major enzyme that generates O•- when stroke occurs (Kahles et al., 2007). Consistent with numerous prior reports, we found marked increases in the generation of O•- after stroke, and most O•- was found in neurons. Furthermore, apocynin markedly inhibited neuronal O•- generation, even though NADPH oxidase is thought to be found in immune cells such as microglia. However, we found relatively little O•- generation in microglia/macrophages, and might suggest that NOX is present in neurons as well. In fact, a few studies have documented NOX in neurons (Serrano et al., 2003, Tejada-Simon et al., 2005), and NOX4 appears to increase in neurons after brain ischemia (Vallet et al., 2005). Interestingly, we did not find any O•- generation in vascular structures, even though prior work has documented NOX or functional NOX in endothelial cells (Jones et al., 1996, Park et al., 2005, Park et al., 2008). There are also reports of salutary effects of blocking O•- in brain vessels or blocking NOX in microglia associated with the BBB (Yenari et al., 2006). However, we did not look at earlier time points to see if O•- was generated in other cell types, but it is unlikely that we would have seen O•- in microglia or vessels as neurons are the most vulnerable cells to succumb to ischemia, and tend to generate ROS earlier than other cells. We also previously examined O•- generation using this same method in a similar rat stroke model, and found (Maier et al., 2002) generation as early as 10 min after reperfusion that was also largely confined to neurons. Furthermore, since NOX2’s activity occurs at the cell membrane to generate extracellular O•-, it is not surprising that microglia or vessels themselves lack intracellular O•-. Microglial and endothelial NOX could still be capable of increasing oxidative stress in neurons through O•- production. Thus, it is possible that apocynin showed protective effects by reducing O•- generation in neurons, although not necessarily due to a direct effect on neuronal NOX. Neuron derived O•-, also acting as a signaling molecule, could also lead to the activation of microglia and other immune cells, as well as upregulation of proteases and other immune mediators that not only contribute to brain cell death, but BBB disruption as well. On the other hand, apocynin may have other anti-oxidant properties leading to reduction of O•-, independent of the O•- generating mechanism.
Regardless, we suggest that NOX as a therapeutic target should be explored for treatment of stroke in humans. Because of the potential beneficial effects of reducing cerebral hemorrhage, inhibiting NOX may be useful to use in combination with thrombolytic agents. However, agents safer than apocynin with a broader dosing range should be identified and explored prior to testing in humans.
CONCLUSIONS
In conclusion, our data showed that apocynin, a NADPH oxidase inhibitor, reduces infarct volume, cerebral hemorrhage, and BBB disruption and improves neurological function following experimental stroke but with a narrow therapeutic window. We suggest that this is a relevant therapeutic target, but safer inhibitors should be developed.
Acknowledgments
This work was supported by NIH NINDS grant R01 NS40516 (MAY), P50 NS014543 (MAY), P01 NS37520 (MAY), and an American Heart Association Established Investigator Award (MAY)‥
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- E. van den Worm CJB, van den Berg AJJ, Kroes BH, van der Wal D, van Dijk H, Labadie RP. Apocynin: a lead-compound for new respiratory burst inhibitors ? In: Cruijff J, editor. Vaak wordt een uitslag verward met de situatie. 1996. pp. 48–58. [Google Scholar]
- Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–1419. doi: 10.1097/01.WCB.0000037880.62590.28. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, O'Donnell VB, Wood JD, Broughton JP, Hughes EJ, Jones OT. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am J Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005;103:289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- Lu G, Greene EL, Nagai T, Egan BM. Reactive oxygen species are critical in the oleic acid-mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension. 1998;32:1003–1010. doi: 10.1161/01.hyp.32.6.1003. [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Cheng D, Yenari MA, Chan PH, Steinberg GK. Effects of mild hypothermia on superoxide anion production, superoxide dismutase expression, and activity following transient focal cerebral ischemia. Neurobiol Dis. 2002;11:28–42. doi: 10.1006/nbdi.2002.0513. [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurg. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Ringer TM, Sun GH, Yenari MA, Moseley ME. MRI of subacute hemorrhagic transformation in the rat suture occlusion model. Neuroreport. 2001;12:309–311. doi: 10.1097/00001756-200102120-00025. [DOI] [PubMed] [Google Scholar]
- Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci. 2000;20:RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Dekker LV, Forbes LV, Wientjes FB, Grogan A, Pappin DJ, Segal AW. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem J. 1999;344(Pt 3):859–866. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Siesjo P. Mechanisms of secondary brain injury. Eur J Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Deng HY, Maier CM, Sun GH, Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Beaulieu C, Steinberg GK, Moseley ME. Diffusion-weighted magnetic resonance imaging characteristics of hemorrhagic transformation in experimental embolic stroke. J Neuroimaging. 1997a;7:227–231. doi: 10.1111/jon199774227. [DOI] [PubMed] [Google Scholar]
- Yenari MA, de Crespigny A, Palmer JT, Roberts S, Schrier SL, Albers GW, Moseley ME, Steinberg GK. Improved perfusion with rt-PA and hirulog in a rabbit model of embolic stroke. J Cereb Blood Flow Metab. 1997b;17:401–411. doi: 10.1097/00004647-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–1093. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Yenari MA. Post-ischemic inflammation: molecular mechanisms and therapeutic implications. Neurol Res. 2004;26:884–892. doi: 10.1179/016164104X2357. [DOI] [PubMed] [Google Scholar]