Abstract
Background and purpose:
Patients commonly take complementary medicines in conjunction with warfarin yet evidence supporting the safety or the risk of a herb–drug interaction is lacking. The aim of this study was to investigate the possible impact of two commonly used herbal medicines, garlic and cranberry, on the pharmacokinetics and pharmacodynamics of warfarin in healthy male subjects.
Experimental approach:
An open-label, three-treatment, randomized crossover clinical trial was undertaken and involved 12 healthy male subjects of known CYP2C9 and VKORC1 genotype. A single dose of 25 mg warfarin was administered alone or after 2 weeks of pretreatment with either garlic or cranberry. Warfarin enantiomer concentrations, INR, platelet aggregation and clotting factor activity were measured to assess pharmacokinetic and pharmacodynamic interactions between warfarin and herbal medicines.
Key results:
Cranberry significantly increased the area under the INR–time curve by 30% when administered with warfarin compared with treatment with warfarin alone. Cranberry did not alter S- or R-warfarin pharmacokinetics or plasma protein binding. Co-administration of garlic did not significantly alter warfarin pharmacokinetics or pharmacodynamics. Both herbal medicines showed some evidence of VKORC1 (not CYP2C9) genotype-dependent interactions with warfarin, which is worthy of further investigation.
Conclusions and implications:
Cranberry alters the pharmacodynamics of warfarin with the potential to increase its effects significantly. Co-administration of warfarin and cranberry requires careful monitoring.
Keywords: warfarin, garlic, cranberry, herb–drug interactions, pharmacokinetic, pharmacodynamic, platelet aggregation, genotype
Introduction
There is increasing evidence and clinical concern regarding herb–drug interactions (Mills et al., 2004). Warfarin has been implicated in almost 50 herb–drug interactions (Ernst, 2002), and Ramsay et al. (2005) found that 58% patients who take warfarin also consumed herbal medicines, which have the potential to interact with the anticoagulant. Despite increased awareness of the potential of herb–drug interactions, the lack of rigorous clinical evidence regarding the significance and possible mechanisms involved provides a challenge for clinicians and consumers to make rational decisions about the safe combination of complementary and conventional medicines.
Garlic is one of the most widely used herbal medicines and is commonly ingested by people receiving warfarin (Ramsay et al., 2005). In vitro studies with the garlic constituent, allicin, and its degradation products indicate that these compounds possess antiplatelet effects (Lawson et al., 1992; Briggs et al., 2000). The combination of garlic and warfarin has been implicated in episodes of bleeding in patient case reports (Burnham, 1995; German et al., 1995). There is conflicting evidence from in vitro (Ariga et al., 1981; Apitz-Castro et al., 1983) and in vivo (Piscitelli et al., 2002; Markowitz et al., 2003) studies concerning the effects of garlic formulations that release allicin on the inhibition of drug-metabolizing enzyme activity. Taken together, the evidence provides a basis for concern that allicin-containing garlic formulations have the potential to interact with warfarin through pharmacokinetic and/or pharmacodynamic mechanisms.
Cranberry is among the top 10 herbs sold in the United States (Blumenthal, 2003), where it is most commonly used to help prevent urinary tract infections (Bailey et al., 2007; Jepson and Craig, 2008). However, there has been an increasing number of case reports of life-threatening interactions between cranberry and warfarin (Suvarna et al., 2003; ADRAC, 2004; Grant, 2004; MHRA and CSM, 2004; Rindone and Murphy, 2006). One fatal case report described a patient with a significantly elevated international normalized ratio (INR) taking both warfarin and cranberry (Suvarna et al., 2003). Despite these data, cranberry and its constituents lack clear evidence to support a putative mechanism for an interaction with warfarin. Recent clinical studies, which have attempted to address the clinical significance of both pharmacokinetic and pharmacodynamic interactions between cranberry and warfarin, have been inconclusive (Greenblatt et al., 2006; Grenier et al., 2006; Li et al., 2006b; Lilja et al., 2007).
Genetic factors contribute to variability in warfarin response. Differences in disposition of S-warfarin have been attributed to genetic variability of CYP2C9 (cytochrome P450 2C9; Kirchheiner and Brockmoller, 2005). Studies on the genetic variability in vitamin K epoxide reductase subunit 1 gene (VKORC1) reported it to be involved in warfarin resistance. Patients with the variant type VKORC1 (CT or TT alleles) have been found to require a significantly lower warfarin dose compared with people with wild-type VKORC1 (CC allele) (Li et al., 2006a). However, there is no evidence regarding the possible impact of genotype on the interaction between warfarin and other drugs or herbal medicines.
The aim of this open-label randomized crossover study was to investigate the impact of two commonly used herbal medicines, garlic and cranberry, on the pharmacokinetics and pharmacodynamics (PKPD) of warfarin in healthy male subjects of known CYP2C9 and VKORC1 genotype.
This trial is registered with Australian New Zealand Clinical Trial Registry (ACTRN12607000054415), available at the URL ‘http://www.anzctr.org.au/trial_view.aspx?ID=81647'.
Methods
Herbal medicines
Garlic (Allium sativum) product selection involved analyses of a number of commercially available enteric-coated products. Tablets were assessed for their allicin-releasing characteristics using the USP dissolution Apparatus II according to the USP method described in delayed-release garlic tablets with a modified tablet/solution ratio. Garliplex 2000 enteric-coated garlic tablets, labelled as containing 2000 mg of fresh garlic bulb equivalent to 3.71 mg of allicin per tablet, were selected for use in this study. Subjects received one tablet twice daily in accordance with dosage recommended by the German Commission E (Blumenthal and Busse, 1998).
Cranberry (Vaccinium macrocarpon) juice concentrate formulations from a number of commercial products were qualitatively analysed using thin layer chromatography to identify the presence and to compare relative intensities of mercetin, quercetin, kaempherol, anthocyanins and resverterol as a marker for their relative composition in different commercial products (Upton et al., 2002). Quercetin and anthocyanins were found in all brands. The GNC cranberry juice concentrate (labelled to contain 500 mg of cranberry juice concentrate) was selected for use in this study as this product demonstrated physical stability and a thin layer chromatography fingerprint of comparatively high intensity for anthocyanins and quercetin. Subjects received two capsules three times daily, which is equivalent to 57 g of fruit per day.
Study design
The study was approved by the Human Research Ethics Committees at St Vincent's Hospital (Darlinghurst, NSW, Australia) and at the University of Sydney (Sydney, NSW, Australia). Twelve healthy male subjects, who were nonsmoking, not taking any medicines including herbal/vitamin supplements for at least 2 weeks and aged 18–34 years, were recruited. Subjects who gave written consent to participate in the study were selected after a full medical history, physical examination and clinical laboratory evaluation. Subjects with any medical condition that could alter warfarin effects, including any clotting disorders, hepatic dysfunction or platelet dysfunction were excluded from the study. Selected subjects were randomly allocated to three treatment groups to receive a single oral dose of 25 mg rac-warfarin (Coumadin 5 × 5 mg tablets) after an overnight fast (of at least 10 h) either alone or after 2 weeks of pretreatment with either cranberry juice concentrate capsules or enteric-coated garlic tablets. Dosing with herbal medicines continued for 7 days after warfarin administration until the last blood sample was drawn. Subjects were subsequently crossed over to other treatments following a washout period of 2 weeks. During the study, the subject's adherence to medicines, well-being and adverse events were assessed using regular mobile phone calls/text messages and e-mail contact. The study protocol indicated that any signs of bruising, bleeding or elevated INR (greater than 4.0) resulted in immediate referral for medical attention. Blood samples were collected before (−48, −24 and 0 h) and at 1, 2, 4, 8, 12, 24, 48, 72, 96, 120, 144 and 168 h after warfarin administration into sodium citrate tubes for INR and clotting factor activity measurement, and EDTA tubes for HPLC analysis of warfarin enantiomers. Plasma was harvested by centrifugation at 1500 g for 15 min and stored at −70 °C until further analysed.
CYP2C9 genotyping
EDTA blood samples were stored at −20 °C until genomic DNA extraction (Qiagen, Doncaster, VIC, Australia). The polymorphisms of CYP2C9 were detected by PCR-based on restriction fragment length polymorphism (PCR-RFLP) analysis. The primers and restriction sites for CYP2C9*2 and CYP2C9*3 allele used in this study were as described by Sullivan-Klose et al. (1996).
VKORC1 genotyping
The VKORC1 1173T>C polymorphism was detected using allelic discrimination real-time PCR as described by Li et al. (2006a) with minor modifications. TaqMan Universal PCR master mix was used with cycle conditions at 50 °C for 2 min, 95 °C for 10 min, followed by 50 cycles at 92 °C for 15 s, 60 °C for 90 s, and an end point plate read performed on an 7000 real-time PCR system (Applied Biosystems, Melbourne, VIC, Australia).
Warfarin enantiomer concentration analysis
Plasma samples obtained from each of the blood samples taken at different times after warfarin dosing in each treatment were analysed using a validated chiral HPLC assay (Jiang et al., 2004). In brief, plasma (0.5 mL) was acidified with 0.5 M sulphuric acid (350 μL) and extracted with an organic solvent mixture (hexane: dichloromethane, 5:1 v/v). After roller bed mixing and centrifugation, the organic layer was removed, evaporated to dryness under nitrogen and reconstituted with acetonitrile (200 μL) before HPLC analysis. The HPLC system comprised a silica-bonded β-cyclodextrin column with a mobile phase of acetonitrile/acetic acid/triethylamine (500:1.35:1.00, v/v/v) pumped at a flow rate of 1 mL min−1 with fluorescence detection (excitation and emission wavelengths 310 and 400 nm, respectively). Inter- and intra-assay precision and accuracy for warfarin enantiomers were within 15% when expressed as coefficient of variation. Extraction recovery of both S-warfarin and R-warfarin was within the range of 86–93%. The lower limit of quantification was 36.9 ng mL−1 for both enantiomers of warfarin.
Plasma protein binding
The unbound fraction of S-warfarin and R-warfarin in plasma samples was investigated using a validated ultrafiltration method (Jiang et al., 2004) based on the assumption that warfarin fraction unbound concentration is independent of concentration up to 25 μg mL−1 of rac-warfarin as demonstrated by (Banfield et al., 1983). In brief, rac-warfarin (15 μg) was added to plasma samples (1 mL) from each subject obtained by pooling samples collected between 1 and 8, 12 and 72, and 96 and 168 h after the warfarin dose. Unbound warfarin enantiomers were separated by ultrafiltration (Centrifree YM-30; Millipore; Australia Pty Ltd, North Ryde, NSW, Australia) with centrifugation at 1500 g for 20 min. Ultrafiltrate was assayed using the validated chiral HPLC assay described above. The unbound fraction was calculated as the ratio of ultrafiltrate to plasma warfarin enantiomer concentrations.
Platelet aggregation
Platelet aggregation was assessed on blood samples (within 3 h of blood collection) collected 24 h prior to warfarin administration (−24 h) in each treatment period using a whole blood aggregometer (Chrono-par; Chrono-log Corp., Havertown, Pennsylvania, USA; Edward Keller Australia Pty Ltd, Hallam, VIC, Australia). Briefly, whole blood (0.5 mL) was diluted twofold with freshly prepared normal saline and incubated at 37 °C for at least 6 min. Platelet aggregation was induced by four different agents; ADP (10 μM), arachidonic acid (0.5 mM), collagen (2 μg mL−1) and ristocetin (1 mg mL−1). A change in impedance (Ω) was recorded for at least 6 min.
International normalized ratio
International normalized ratio was measured at each blood sampling time before and after warfarin dosing using a BFT II analyser. Briefly, plasma (50 μL) was incubated (1 min, 37 °C) in a cuvette, to which human thromboplastin reagent (100 μL, thromborel S) was added. INR was calculated and displayed as a function of time taken for a change in impendence.
Factor II, Factor VII and Factor X activity
The activity of the clotting Factor II, Factor VII and Factor X was measured in pooled plasma samples obtained over the collection periods from −48 to 0, 1 to 12, 24 to 72 and 96 to 168 h after warfarin dosing either alone or after pretreatment with cranberry juice extract. Clotting factor activity was assessed using the BFT II analyser. In brief, clotting time of the diluted pooled plasma sample (1:20) was measured following the addition of factor-deficient plasma. Percentage factor activity for each sample was obtained from a standard curve constructed using different dilutions of standard human plasma of known factor activity and clotting time. Control plasma of known activity for all factors was used to establish the accuracy of the method, which was found to be within 2% of the nominal value.
Data analysis
The pharmacodynamic response to warfarin was assessed using the area under the international normalized ratio–time curve (AUCINR) calculated using the trapezoidal rule, INR at baseline (INR0) before warfarin administration and maximum observed INR (INRmax) after warfarin administration. Clotting factor activities are presented as the percentage decrease in activity from baseline for each pooled sample in different treatment periods.
The pharmacokinetic parameters for the warfarin enantiomers were estimated using both non-compartmental and population PKPD modelling methods. The elimination rate constant (kel) was obtained from the slope of the terminal portion of the logarithmic concentration–time curve for S-warfarin and R-warfarin. The area under the warfarin enantiomer concentration–time curve (AUC) to the last quantifiable concentration (AUC0−t) was calculated using the trapezoidal rule and extrapolated to infinity (AUC0−∞) by adding Ct/kel, where Ct is the concentration of the last quantifiable sample. The half-life was calculated as ln 2/kel, the apparent clearance (CL/F) as dose/AUC0−∞ and the apparent volume of distribution (V/F) as CL/kel. The maximum concentration (Cmax) and the time it occurred (tmax) were determined by observation.
To elucidate the mechanism of the herb–drug interaction further, pharmacokinetic and pharmacodynamic data from each arm of the study were analysed using the nonlinear mixed-effects modelling programme (NONMEM VI), using the first-order conditional estimation method with interaction. The modelling approach used in this study has been described by Jiang et al. (2006). In brief, a two-compartment pharmacokinetic model was used to describe S-warfarin concentration–time data and an indirect pharmacodynamic model described the relationship between percentage of prothrombin complex activity (PCA; which was derived from the INR data in this study) and S-warfarin concentration. The development and evaluation of this model has been described previously (Jiang et al., 2006). Herbal medicine treatment was evaluated as a possible influential covariate on both pharmacokinetic and pharmacodynamic parameters.
Materials
Garliplex 2000 enteric-coated garlic tablets (Golden Glow Pty Ltd, VA, QLD, Australia), GNC cranberry juice concentrate (General Nutrition Company, Melbourne, VIC, Australia) and rac-warfarin (Coumadin 5 × 5 mg tablets; Boots HealthCare Australia Pty Ltd, North Ryde, NSW, Australia). TaqMan Universal PCR master mix (Applied Biosystems); silica-bonded β-cyclodextrin column (Cyclobond I 2000; Astec, Alltech Associates Australia Pty Ltd, Baulkham Hills, NSW, Australia). BFT II analyzer, thromborel S and factor-deficient and control plasma (Dade Behring Diagnostics Pty Ltd, Lane Cove, NSW, Australia).
Statistical analysis
Preliminary analyses indicated that 12 subjects in a crossover study provides an 80% chance of detecting a 20% difference in the AUC0−∞ of S-warfarin at P<0.05 level of significance, which is in agreement with our previous study (Jiang et al., 2004). A difference of greater than 20% was considered to be clinically significant. Pharmacokinetic and pharmacodynamic parameters calculated after warfarin alone (control) and after warfarin with herbal medicines (intervention) were logarithmically transformed and reported as the geometric mean ratio (intervention to control) and 90% confidence interval (CI) of the ratio. If 90% CI of ratios fell within the range of 0.8–1.25, then the variation in the parameter was considered to be clinically insignificant. The residual mean square error used to calculate the 90% CI for each logarithmically transformed parameter for each treatment was obtained using ANOVA (Stata 5.0; Stata Corp., College Station, Texas, USA) with nested parameters in treatment and sequence order.
Results
Twenty-three subjects were screened for entry into the study and of these seven subjects did not meet the entry criteria. Sixteen subjects were randomized to treatment, two were lost to follow-up and two chose to discontinue. Twelve subjects completed the study (four subjects per treatment arm). The mean (and range) age, weight and height of the subjects were 23 years (20–35 years), 68 kg (54–81 kg) and 1.75 m (1.65–1.85 m), respectively. Seven subjects were Caucasians and five subjects were Asian (including three people of Indian origin). Nine subjects were of the CYP2C9*1/*1 genotype and three were of the CYP2C9*1/*2 genotype. Four subjects were VKORC1 wild-type (CC) and eight subjects carried variant VKORC1 alleles including six with CT and two with TT alleles.
No subjects experienced major bleeding or INR readings above 4. Two subjects in the cranberry–warfarin treatment arm experienced rashes and one subject had evidence of nasal bleeding (presence of dried blood in his nose) at approximately 72 h after warfarin treatment. Subjects on garlic reported the characteristic garlic odour and one subject complained of lip dryness. No adverse events were reported with warfarin-alone treatment.
Pharmacodynamic end points
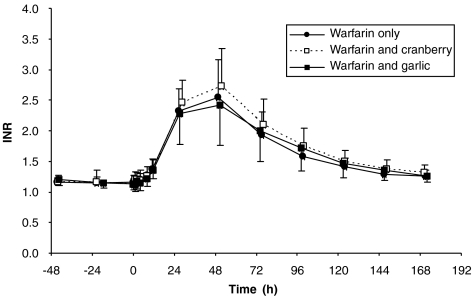
Figure 1 shows the INR–time profile after warfarin dosing either alone or following pretreatment with cranberry or garlic. Neither garlic nor cranberry pretreatment affected the baseline INR, suggesting that these herbal medicines do not have a significant independent effect on the clotting system (Table 1).
Figure 1.
International normalized ratio of prothrombin time (INR) vs time profile (n=12) after a single oral dose of warfarin (25 mg) alone, and after multiple dose of cranberry juice concentrate capsules and after multiple dose of enteric-coated garlic tablets.
Table 1.
Warfarin pharmacodynamic end points following the different treatments
|
Mean (95% CI) |
Geometric mean ratio (90% CI) |
||||
|---|---|---|---|---|---|
| Warfarin alone | Warfarin and cranberry | Warfarin and garlic | Warfarin and cranberry/warfarin only | Warfarin and garlic/warfarin only | |
| INR0 | 1.2 (1.1–1.2) | 1.2 (1.1–1.2) | 1.2 (1.1–1.2) | 0.98 (0.94–1.03) | 1.00 (0.97–1.02) |
| INRmax | 2.6 (2.3–3.0) | 2.8 (2.5–3.1) | 2.5 (2.1–2.8) | 1.08 (0.98–1.19) | 0.95 (0.86–1.04) |
| AUCINR | 96.0 (72.1–119.8) | 119.2 (97.0–141.4) | 100.3 (70.9–129.8) | 1.28* (1.06–1.53) | 0.98 (0.82–1.19) |
Abbreviations: CI, confidence interval; INR, international normalized ratio of prothrombin time; INR0, INR of subjects before administration of warfarin; INRmax, maximum INR achieved; AUCINR, area under the INR–time curve.
*Statistically and clinically significant difference.
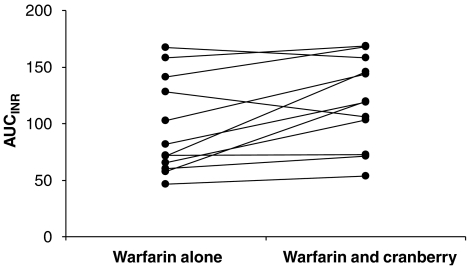
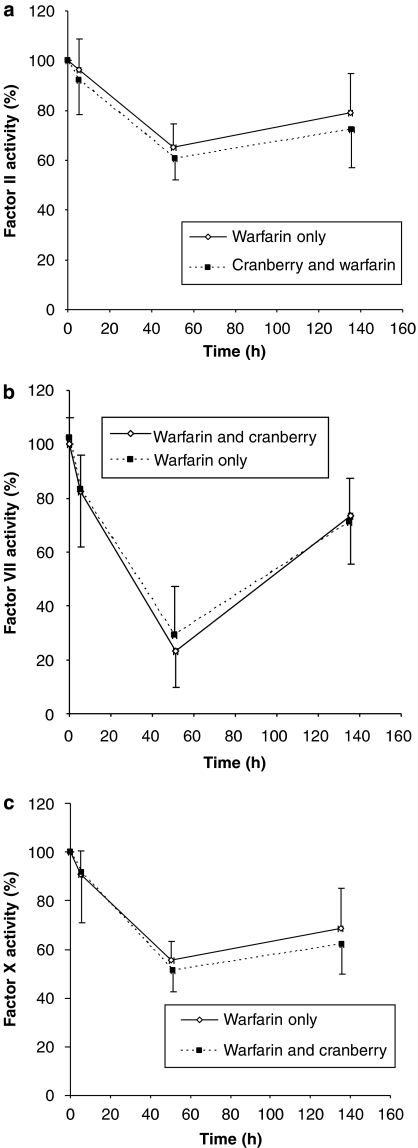
Cranberry juice concentrate pretreatment significantly increased the mean AUCINR by approximately 30% (Table 1). Moreover, the majority of the subjects displayed an increase in AUCINR in response to warfarin after cranberry pretreatment (Figure 2). There was a trend towards a greater decrease in the activity of clotting Factor II, Factor VII and Factor X when warfarin was co-administered with cranberry (Figures 3a–c). However, this did not reach statistical significance (data not shown). Co-administration of garlic and warfarin did not significantly affect AUCINR (Table 1). Furthermore, 2 weeks of pretreatment with garlic or cranberry had no effect on platelet aggregation.
Figure 2.
Individual area under the international normalized ratio (INR)–time curve (AUCINR) of subjects receiving warfarin alone or after pretreatment with cranberry. A majority of subjects showed a tendency towards an increase in AUCINR following cranberry and warfarin treatment in comparison with warfarin alone. However, two subjects exhibited a decrease in AUCINR.
Figure 3.
Percentage clotting activity vs midpoint time profiles for (a) Factor II, (b) Factor VII and (c) Factor X during warfarin treatment alone or after cranberry pretreatment. Mean and s.d. (error bars) are presented.
Pharmacokinetic end points
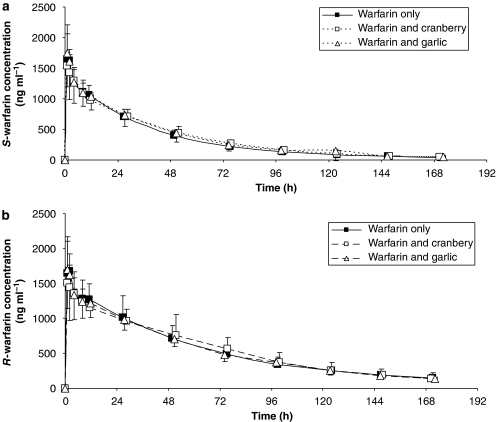
The plasma concentration–time profiles of S-warfarin or R-warfarin were unaltered when rac-warfarin was co-administered with either cranberry juice concentrate or garlic (Figures 4a and b; Table 2). Plasma unbound fraction of warfarin enantiomers was not affected by concomitant administration of garlic or cranberry juice concentrate (Table 2).
Figure 4.
Plasma concentration–time profile of (a) S-warfarin and (b) R-warfarin after a single oral dose of warfarin (25 mg) alone, or after multiple doses of cranberry juice concentrate capsules given along with single dose of warfarin and after multiple doses of enteric-coated garlic tablets.
Table 2.
Pharmacokinetic parameters of S-warfarin and R-warfarin
|
Mean (95% CI) |
Geometric mean ratio (90% CI) |
||||
|---|---|---|---|---|---|
| Warfarin only | Warfarin and cranberry | Warfarin and garlic | Warfarin and cranberry/warfarin only | Warfarin and garlic/warfarin only | |
| S-warfarin | |||||
| tmax (h) | 1.4 (1.1–1.6) | 1.9 (1.1–2.7) | 1.3 (1.1–1.6) | NA | NA |
| Cmax (μg mL−1) | 1.8 (1.7–1.9) | 1.7 (1.5–2.0) | 1.9 (1.5–2.2) | 0.94 (0.67–1.29) | 1.06 (0.77–1.47) |
| t1/2 (h) | 38.6 (35.7–41.5) | 34.9 (31.0–38.8) | 41.2 (35.1–47.3) | 0.94 (0.79–1.12) | 0.99 (0.83–1.18) |
| AUC (μg mL−1 h) | 60.3 (54.5–66.1) | 63.0 (57.3–68.8) | 65.5 (55.3–75.6) | 1.04 (0.94–1.67) | 1.06 (0.95–1.19) |
| CL/F (mL h−1) | 213 (193–232) | 204 (183–224) | 202 (175–229) | 0.89 (0.80–0.99) | 1.05 (0.94–1.17) |
| Fraction unbound (fu) | 0.010 (0.007–0.013) | 0.010 (0.007–0.014) | 0.011 (0.008–0.014) | 1.00 (0.75–1.25) | 1.12 (0.39–1.84) |
| R-warfarin | |||||
| tmax (h) | 1.4 (1.1–1.6) | 1.9 (1.1–2.7) | 1.8 (0.7–2.9) | NA | NA |
| Cmax (μg mL−1) | 1.8 (1.7–2.0) | 1.7 (1.5–1.9) | 1.9 (1.5–2.2) | 0.90 (0.67–1.21) | 1.02 (0.8–1.4) |
| t1/2 (h) | 55.6 (46.2–65.0) | 47.7 (41.5–53.9) | 52.6 (47.4–57.9) | 0.93 (0.80–1.08) | 0.98 (0.84–1.14) |
| AUC (μg mL−1 h) | 108.7 (90.0–127.3) | 105.8 (91.7–119.9) | 105.0 (96.3–113.7) | 1.0 (0.9–1.1) | 0.99 (0.87–1.13) |
| CL/F (mL h−1) | 123 (106–140) | 124 (109–139) | 121 (112–131) | 0.87 (0.75–0.99) | 0.96 (0.84–1.11) |
| Fraction unbound (fu) | 0.006 (0.004–0.007) | 0.005 (0.004–0.007) | 0.006 (0.004–0.007) | 0.95 (0.72–1.18) | 1.04 (0.80–1.29) |
Abbreviations: AUC, area under the plasma concentration–time curve; CI, confidence interval; CL, clearance; CL/F, apparent clearance; Cmax, maximum concentration; F, bioavailability; NA, not applicable; tmax, time to Cmax.
Pharmacokinetic–pharmacodynamic modelling
The population PKPD parameter estimates for warfarin are presented in the Table 3. The NONMEM analysis confirmed the lack of an effect of herbal medicine treatment on pharmacokinetic parameters and highlighted a significant effect of cranberry treatment on warfarin pharmacodynamic parameters. The PKPD model displayed a significantly lower NONMEN-derived objective function (ΔOFV=−6.765) when cranberry treatment was included as covariate on S-warfarin EC50. The mean ratio of S-warfarin EC50 (90% CI; n=12) for cranberry vs control was 0.85 (0.77–0.94) indicating that treatment with cranberry juice concentrate significantly increased the sensitivity of S-warfarin, reflecting a lower EC50 value for S-warfarin. Using the final PKPD model, it was possible to generate posterior Bayesian estimates of S-warfarin EC50 (presented as mean±s.d., n=12), which were 443±212 ng mL−1 in subjects receiving warfarin only, 376±184 ng mL−1 when warfarin was co-administered with cranberry and 486±275 ng mL−1 during garlic co-administration.
Table 3.
Population pharmacokinetic and pharmacodynamic parameter estimates derived from the combined data from all treatment arms
|
Parameter |
Estimates |
Between-subject variability (CV %) |
Between-occasion variability (CV %) |
|---|---|---|---|
| Structural model | |||
| ka (h−1) | 3.15 (fixed) | 59% (fixed) | — |
| CL/F (mL h−1) | 208 | 13% | 10% |
| VC/F (mL) | 6440 | 15% | 14% |
| Q/F (mL h−1) | 849 | — | — |
| VP/F (mL) | 3120 | — | — |
| t1/2,PCA (h) | 16 | — | — |
| EC50,S (ng mL−1) | 397 | 52% | 21% |
| PCA0 (%) | 89 | 10% | — |
| aRatio for cranberry on EC50,S | 0.85 | — | — |
| aRatio for garlic on EC50,S | 1.04 | — | — |
| Residual variability |
σexp, S-warfarin (CV %) |
σadd, S-warfarin (s.d.) (ng mL−1) |
σadd, PCA (s.d.) (%) |
| 15% | 6 | 7% | |
Abbreviations: CL, clearance; CL/F, apparent clearance; CV, coefficient of variation; EC50,S, concentration of S-warfarin that produces 50% inhibition of PCA; F, bioavailability; ka, absorption rate constant; PCA, prothrombin complex activity; PCA0, PCA in the absence of warfarin; Q, distributional clearance between the compartments; t1/2,PCA, half-life of prothrombin complex activity; VC, volume of distribution in central compartment; VP, volume of distribution in peripheral compartment; σadd, PCA, variance for PCA residual variability of additive model; σadd, S-warfarin, variance for S-warfarin residual variability of additive model; σexp, S-warfarin, variance for S-warfarin residual variability of exponential model.
The impact of herbal medicine treatment was evaluated as a ratio of the treatment to control on specific parameters (e.g., EC50,S); —, not evaluated.
Geometric mean ratio intervention to control.
Impact of CYP2C9 and VKORC1 genotype
Subjects with the CT and TT allele for the VKORC1 gene exhibited significantly lower S-warfarin EC50 values (derived from posterior Bayesian estimates) compared with people with the CC genotype when warfarin was administered alone or in combination with either cranberry or garlic (Table 4). Subject numbers are smaller but it is apparent that people with the CT and TT genotypes had a significant reduction in S-warfarin EC50 when warfarin was co-administered with cranberry juice extract but no effect was noted during garlic treatment compared with warfarin only (genomic mean ratios). In contrast, subjects with the VKORC1 wild-type genotype showed an increase in the S-warfarin EC50 when warfarin was administered with garlic (Table 4).
Table 4.
EC50 of S-warfarin and VKORC1 genotype
|
EC50 (95% CI) |
Geometric mean ratio (90% CI) |
||||
|---|---|---|---|---|---|
| Warfarin only | Warfarin and cranberry | Warfarin and garlic | Warfarin and cranberry/warfarin only | Warfarin and garlic/warfarin only | |
| CC (n=4, 33%) | 648 (511–785) | 611 (551–671) | 791 (572–1010) | 0.96 (0.85–1.06) | 1.22* (1.11–1.34) |
| CT (n=6, 50%) | 379± (239–519) | 276.8± (178–375) | 379± (150–608) | 0.78* (0.63–0.92) | 1.01 (0.94–1.08) |
| TT (n=2, 17%) | 228± (174–281) | 202± (168–236) | 194± (72–316) | 0.89* (0.84–0.94) | 0.81 (0.53–1.10) |
Abbreviations: CI, confidence interval; VKORC1, vitamin K epoxide reductase subunit 1.
*Statistically and clinically significant difference between treatment arm and control arm for subjects of particularly genotype.
±Statistically different from the CC genotype (P<0.03, Student's unpaired t-test) in a particular treatment arm.
The CL/F of S-warfarin tended to be higher in people with the CYP2C9 wild-type when compared with people with CYP2C9*1/*2 variant. There was no CYP2C9 genotype–herb–drug interaction for the CL/F of S-warfarin when subjects received warfarin with either cranberry or garlic (Table 5).
Table 5.
S-warfarin apparent clearance (mL h−1) and CYP2C9 genotype
| S-warfarin apparent clearance (95% CI) |
Geometric mean ratio (90% CI) |
||||
|---|---|---|---|---|---|
| Warfarin only | Warfarin and cranberry | Warfarin and garlic | Warfarin and Cranberry/warfarin only | Warfarin and garlic/warfarin only | |
| *1/*1 (n=9, 75%) | 220 (197–244) | 208 (182–234) | 215 (187–244) | 0.94 (0.88–1.01) | 0.97 (0.91–1.03) |
| *1/*2 (n=3, 25%) | 189 (170–209) | 190 (167–212) | 164 (116–212) | 1.00 (0.91–1.10) | 0.85 (0.62–1.07) |
Abbreviations: CI, confidence interval; CYP2C9, cytochrome P450 2C9 gene.
Discussion
This study investigated the pharmacodynamic and pharmacokinetic interaction of warfarin with cranberry juice concentrate and enteric-coated garlic tablets. However, cranberry did not alter the pharmacokinetics of either S-warfarin or R-warfarin nor did 2 weeks of pretreatment with cranberry juice extract affect platelet aggregation.
The lack of a pharmacokinetic interaction between warfarin and cranberry is in agreement with the findings of the study by Greenblatt et al. (2006). They demonstrated that cranberry did not affect CYP2C9 enzyme activity in vivo (assessed by flurbiprofen metabolism). However, these researchers only used a single dose of cranberry juice. Lilja et al. (2007) also investigated the effect of pretreatment with cranberry juice for 5 days on warfarin pharmacokinetics and found no clinically significant change in the AUC of S-warfarin. This lack of an effect of cranberry on drug metabolism is supported by the study by Grenier et al. (2006), who found no pharmacokinetic interaction between a single dose of cranberry juice and cyclosporine. The hepatic enzyme CYP3A4 is known to be involved in the metabolism of cyclosporine and in the metabolism of R-warfarin (Wittkowsky, 2003).
The results of the present study on the pharmacodynamic interaction between warfarin and cranberry are in contrast to the recent studies performed by Li et al. (2006b) and Ansell et al. (2008). In these studies no significant change in the warfarin response was observed during concomitant administration of cranberry juice. However, these studies have a number of limitations including a relatively small sample size, Li et al. (2006b) included 7 patients with atrial fibrillation, whereas Ansell et al. (2008) included 14 patients with diverse medical indications in intervention group. Details regarding patient characteristics, concomitant medicines taken by patients and the nature and strength of the cranberry juice were omitted by Ansell et al. (2008). Lilja et al. (2007) concluded that there were no pharmacodynamic interactions between warfarin and cranberry juice, but they employed a low dose of warfarin (10 mg), which made this end point difficult to assess. The studies by Li et al. (2006b) and Lilja et al. (2007) also include a relatively short duration of cranberry pretreatment (7 days or less). The data available from case reports suggest that a longer pretreatment period with cranberry is needed to detect changes in INR (Grant, 2004). Despite these negative findings, case reports on interactions between cranberry and warfarin continue to be published (Paeng et al., 2007). We believe the randomized crossover design, the higher (25 mg) single warfarin dose, the 2-week pretreatment with herbal medicines of known quality along with rigorous investigation of pharmacokinetic and pharmacodynamic end points give this study advantages over previous studies in this regard.
In this study, we used a population PKPD modelling approach to elucidate further the nature of the interaction between warfarin and cranberry. The findings of this analysis suggested that subjects were more sensitive to the effects of warfarin when it was co-administered with cranberry. This confirmed that the interaction between cranberry and warfarin was pharmacodynamic in nature. To explore the pharmacodynamic interaction between cranberry and warfarin further, we investigated the effect of the herbal treatment on the activities of the clotting Factor II, Factor VII and Factor X. Previous studies have suggested that Factor II and Factor X significantly contribute to the clinical effects of warfarin (Mahe et al., 2006; Sarode et al., 2006). Each of the clotting factors investigated displayed a trend consistent with increased sensitivity to warfarin when administered during cranberry treatment. This finding is also consistent with the INR observations during this phase of the study.
Case reports of warfarin and cranberry have been reviewed and some authors have concluded lack of causality of these cases (Pham and Pham, 2007). Some authors have suggested that the interaction may be dose or dose form dependent (as most patients in case reports ingested cranberry juice) (Aston et al., 2006). The cranberry juice concentrate used in this study has been shown to exhibit chemical and therapeutic similarities with cranberry juice (Stothers, 2002; Upton et al., 2002). However, there is a lack of consistency in the dose of cranberry used in clinical trials, and the equivalence between cranberry juice and concentrate has not been established (Jepson and Craig, 2008). The dose of cranberry used in this study is equivalent to 57 g of dry fruit per day, which is less than the dose employed by a randomized, double-blind, placebo control study conducted on 153 elderly women to evaluate the effect of cranberry juice on the risk of bacteriuria (Avorn et al., 1994).
Cytochrome P450 2C9 and VKORC1 genotype account for the major proportion of the variability in warfarin response (Sconce et al., 2005). However, relatively little is known about the significance of genotype in interactions with other drugs or herbal medicines. This study found that subjects with less functional VKORC1 variants (CT and TT alleles) have significantly lower S-warfarin EC50 values derived from the PKPD modelling (Li et al., 2006a). Furthermore, this study provides the first evidence that both cranberry and garlic interactions with warfarin are dependent on VKORC1 genotype. Subjects who carry the VKORC1 variant type (CT and TT alleles) were more prone to interactions with warfarin and cranberry; cranberry significantly increased the effects of warfarin (decrease in EC50 suggesting the need to lower warfarin dose). Furthermore, there is preliminary evidence to suggest that people with the VKORC1 wild-type genotype are more prone to interactions between warfarin and garlic in that garlic significantly decreased the effects of warfarin.
In this study, garlic had no effect on warfarin pharmacokinetics or pharmacodynamics and we found no effect on platelet aggregation induced by ADP, arachidonic acid, collagen or ristocetin. This result is consistent with a recent randomized, placebo-controlled study conducted by Macan et al. (2006) in patients receiving an aged-garlic extract and warfarin. Whereas these researchers used a different garlic formulation to that employed in the present study, they also found no change in clinical effects of warfarin between patients receiving garlic and warfarin and patients on placebo and warfarin (Macan et al., 2006).
Evidence from four subjects with the wild-type VKORC1 genotype suggests that people with this genotype exhibit a pharmacodynamic interaction with garlic that induces a significant decrease in warfarin response. Garlic remains a widely used remedy by people who are also taking warfarin (Ramsay et al., 2005) with some suggestion that it confers beneficial cardiovascular effects (Kannar et al., 2001), and, hence, INR monitoring is likely to remain a safety initiative in people receiving this combination. This observation warrants further investigation.
This study is the first of its kind to explore VKORC1 gene variation and interactions with warfarin. Relatively small sample size for each genotype is one of the limitations of this study and could be the reason for the variable results. Hence, further future studies are needed to confirm these findings.
In conclusion, this study comprehensively addressed the various possible mechanisms of interactions between warfarin and two commonly used herbal medicines. Treatment with cranberry juice extract for 2 weeks significantly increased the sensitivity of healthy male subjects to warfarin without influencing the pharmacokinetics of warfarin enantiomers. These findings, when taken together with recent case reports, strongly suggest that patients who are co-administered cranberry and warfarin should be closely monitored, or preferably cranberry should be avoided in such patients. By contrast garlic (at least when presented as an enteric-coated tablet dose form) did not have significant effects on platelet aggregation or the pharmacodynamics or pharmacokinetics of warfarin in healthy male subjects. Both herbal medicines showed some evidence of VKORC1 genotype-dependent interactions with warfarin, which is worthy of further investigation.
Acknowledgments
We acknowledge financial support from a National Health and Medical Research Council project grant and clinical support from the staff at the St Vincent's Clinical Trial Centre (Darlinghurst, NSW, Australia). We also acknowledge Dr Heather James (Institute of Medical and Veterinary Science, Adelaide, Australia) for the VKORC1 genotyping.
Conflict of interest
The authors state no conflict of interest.
Abbreviations
- AUC
area under the plasma concentration–time curve
- AUCINR
Area under the INR–time curve
- CI
confidence interval
- CL
clearance
- CL/F
apparent clearance
- Cmax
maximum concentration
- CYP2C9
cytochrome P450 2C9 gene
- F
bioavailability
- INR
international normalized ratio of prothrombin time
- INR0
INR of subjects before administration of warfarin
- INRmax
maximum INR reached in respective treatment
- kel
elimination rate constant
- PCA
prothrombin complex activity
- Q
distributional clearance between the compartments
- tmax
time to Cmax
- VKORC1
vitamin K epoxide reductase subunit 1
References
- Adverse Drug Reactions Advisory Committee (ADRAC) International normalised ratio increased 2004. Report no. 195864 and 194643
- Ansell J, McDonough M, Harmatz JS, Greenblatt DJ. A randomized, double-blind trial of the interaction between cranberry juice and warfarin. J Thromb Thrombolysis. 2008;25:112. doi: 10.1177/0091270009337510. [DOI] [PubMed] [Google Scholar]
- Apitz-Castro R, Cabrera S, Cruz MR, Ledezma E, Jain MK. Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction and platelet ultrastructure. Thromb Res. 1983;32:155–169. doi: 10.1016/0049-3848(83)90027-0. [DOI] [PubMed] [Google Scholar]
- Ariga T, Oshiba S, Tamada T. Platelet aggregation inhibitor in garlic. Lancet. 1981;1:150–151. doi: 10.1016/s0140-6736(81)90729-7. [DOI] [PubMed] [Google Scholar]
- Aston JL, Lodolce AE, Shapiro NL. Interaction between warfarin and cranberry juice. Pharmacotherapy. 2006;26:1314–1319. doi: 10.1592/phco.26.9.1314. [DOI] [PubMed] [Google Scholar]
- Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271:751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- Bailey DT, Dalton C, Joseph DF, Tempesta MS. Can a concentrated cranberry extract prevent recurrent urinary tract infections in women? A pilot study. Phytomedicine. 2007;14:237–241. doi: 10.1016/j.phymed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Banfield C, O'Reilly R, Chan E, Rowland M. Phenylbutazone-warfarin interaction in man: further stereochemical and metabolic considerations. Br J Clin Pharmacol. 1983;16:669–675. doi: 10.1111/j.1365-2125.1983.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal M. Herbs continue slide in mainstream market: sales down 14 percent. HerbalGram. 2003;58:71. [Google Scholar]
- Blumenthal M, Busse WR, American Botanical Council; Integrative Medicine Communications, Germany; Bundesgesundheitsamt; Commission E . The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. American Botanical Council; Integrative Medicine Communications: Austin, TX; Boston; 1998. [Google Scholar]
- Briggs WH, Xiao H, Parkin KL, Shen C, Goldman IL. Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. J Agric Food Chem. 2000;48:5731–5735. doi: 10.1021/jf0004412. [DOI] [PubMed] [Google Scholar]
- Burnham BE. Garlic as a possible risk for postoperative bleeding. Plast Reconstr Surg. 1995;95:213. doi: 10.1097/00006534-199501000-00060. [DOI] [PubMed] [Google Scholar]
- Ernst E. The Desktop Guide To Complementary And Alternative Medicine—An Evidence-Based Approach 2002Morby: FL; 1st edn. [Google Scholar]
- German K, Kumar U, Blackford HN. Garlic and the risk of TURP bleeding. Br J Urol. 1995;76:518. doi: 10.1111/j.1464-410x.1995.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Grant P. Warfarin and cranberry juice: an interaction. J Heart Valve Dis. 2004;13:25–26. [PubMed] [Google Scholar]
- Greenblatt DJ, von Moltke LL, Perloff ES, Luo Y, Harmatz JS, Zinny MA. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: in vitro and clinical studies. Clin Pharmacol Ther. 2006;79:125–133. doi: 10.1016/j.clpt.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Grenier J, Fradette C, Morelli G, Merritt GJ, Vranderick M, Ducharme MP. Pomelo juice, but not cranberry juice, affects the pharmacokinetics of cyclosporine in humans. Clin Pharmacol Ther. 2006;79:255–262. doi: 10.1016/j.clpt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Jepson RG, Craig JC. 2008 10.1002/14651858.CD001321.pub4Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev1: Art. No.: CD001321. DOI [DOI] [PubMed]
- Jiang X, Blair EY, McLachlan AJ. Investigation of the effects of herbal medicines on warfarin response in healthy subjects: a population pharmacokinetic–pharmacodynamic modeling approach. J Clin Pharmacol. 2006;46:1370–1378. doi: 10.1177/0091270006292124. [DOI] [PubMed] [Google Scholar]
- Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BD, Duke CC, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–599. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannar D, Wattanapenpaiboon N, Savige GS, Wahlqvist ML. Hypocholesterolemic effect of an enteric-coated garlic supplement. J Am Coll Nutr. 2001;20:225–231. doi: 10.1080/07315724.2001.10719036. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Brockmoller J. Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin Pharmacol Ther. 2005;77:1–16. doi: 10.1016/j.clpt.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Lawson LD, Ransom DK, Hughes BG. Inhibition of whole blood platelet-aggregation by compounds in garlic clove extracts and commercial garlic products. Thromb Res. 1992;65:141–156. doi: 10.1016/0049-3848(92)90234-2. [DOI] [PubMed] [Google Scholar]
- Li T, Lange LA, Li X, Susswein L, Bryant B, Malone R, et al. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet. 2006a;43:740–744. doi: 10.1136/jmg.2005.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Seeram NP, Carpenter CL, Thames G, Minutti C, Bowerman S. Cranberry does not affect prothrombin time in male subjects on warfarin. J Am Diet Assoc. 2006b;106:2057–2061. doi: 10.1016/j.jada.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Lilja JJ, Backman JT, Neuvonen PJ. Effects of daily ingestion of cranberry juice on the pharmacokinetics of warfarin, tizanidine, and midazolam-probes of CYP2C9, CYP1A2, and CYP3A4. Clin Pharmacol Ther. 2007;81:833–839. doi: 10.1038/sj.clpt.6100149. [DOI] [PubMed] [Google Scholar]
- Macan H, Uykimpang R, Alconcel M, Takasu J, Razon R, Amagase H, et al. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136:793S–795S. doi: 10.1093/jn/136.3.793S. [DOI] [PubMed] [Google Scholar]
- Mahe I, Bertrand N, Drouet L, Bal Dit Sollier C, Simoneau G, Mazoyer E, et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica. 2006;91:1621–1627. [PubMed] [Google Scholar]
- Markowitz JS, Devane CL, Chavin KD, Taylor RM, Ruan Y, Donovan JL. Effects of garlic (Allium sativum L.) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin Pharmacol Ther. 2003;74:170–177. doi: 10.1016/S0009-9236(03)00148-6. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare Products Regulatory Agency (MHRA) and Committee on Safety of Medicines (CSM) Possible interaction between warfarin and cranberry juice. Cur Probl Pharmacovigilance. 2004;30:9. [Google Scholar]
- Mills E, Montori VM, Wu P, Gallicano K, Clarke M, Guyatt G. Interaction of St John's wort with conventional drugs: systematic review of clinical trials. BMJ. 2004;329:27–30. doi: 10.1136/bmj.329.7456.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeng CH, Sprague M, Jackevicius CA. Interaction between warfarin and cranberry juice. Clin Ther. 2007;29:1730–1735. doi: 10.1016/j.clinthera.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Pham DQ, Pham AQ. Interaction potential between cranberry juice and warfarin. Am J Health Syst Pharm. 2007;64:490–494. doi: 10.2146/ajhp060370. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- Ramsay NA, Kenny MW, Davies G, Patel JP. Complimentary and alternative medicine use among patients starting warfarin. Br J Haematol. 2005;130:777–780. doi: 10.1111/j.1365-2141.2005.05689.x. [DOI] [PubMed] [Google Scholar]
- Rindone JP, Murphy TW. Warfarin–cranberry juice interaction resulting in profound hypoprothrombinemia and bleeding. Am J Ther. 2006;13:283–284. doi: 10.1097/01.mjt.0000178908.32892.2f. [DOI] [PubMed] [Google Scholar]
- Sarode R, Rawal A, Lee R, Shen YM, Frenkel EP. Poor correlation of supratherapeutic international normalised ratio and vitamin K-dependent procoagulant factor levels during warfarin therapy. Br J Haematol. 2006;132:604–607. doi: 10.1111/j.1365-2141.2005.05917.x. [DOI] [PubMed] [Google Scholar]
- Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–1562. [PubMed] [Google Scholar]
- Sullivan-Klose TH, Ghanayem BI, Bell DA, Zhang ZY, Kaminsky LS, Shenfield GM, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–349. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Suvarna R, Pirmohamed M, Henderson L. Possible interaction between warfarin and cranberry juice. BMJ. 2003;327:1454. doi: 10.1136/bmj.327.7429.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton R, American Herbal Pharmacopoeia . Cranberry Fruit: Vaccinium Macrocarpon Aiton: Standards of Analysis, Quality Control, and Therapeutics. American Herbal Pharmacopoeia: Santa Cruz, CA; 2002. [Google Scholar]
- Wittkowsky AK. Warfarin and other coumarin derivatives: pharmacokinetics, pharmacodynamics, and drug interactions. Semin Vasc Med. 2003;3:221–230. doi: 10.1055/s-2003-44457. [DOI] [PubMed] [Google Scholar]