Abstract
Animal life is controlled by neurons and in this setting cholinergic neurons play an important role. Cholinergic neurons release ACh, which via nicotinic and muscarinic receptors (n- and mAChRs) mediate chemical neurotransmission, a highly integrative process. Thus, the organism responds to external and internal stimuli to maintain and optimize survival and mood. Blockade of cholinergic neurotransmission is followed by immediate death. However, cholinergic communication has been established from the beginning of life in primitive organisms such as bacteria, algae, protozoa, sponge and primitive plants and fungi, irrespective of neurons. Tubocurarine- and atropine-sensitive effects are observed in plants indicating functional significance. All components of the cholinergic system (ChAT, ACh, n- and mAChRs, high-affinity choline uptake, esterase) have been demonstrated in mammalian non-neuronal cells, including those of humans. Embryonic stem cells (mice), epithelial, endothelial and immune cells synthesize ACh, which via differently expressed patterns of n- and mAChRs modulates cell activities to respond to internal or external stimuli. This helps to maintain and optimize cell function, such as proliferation, differentiation, formation of a physical barrier, migration, and ion and water movements. Blockade of n- and mACHRs on non-innervated cells causes cellular dysfunction and/or cell death. Thus, cholinergic signalling in non-neuronal cells is comparable to cholinergic neurotransmission. Dysfunction of the non-neuronal cholinergic system is involved in the pathogenesis of diseases. Alterations have been detected in inflammatory processes and a pathobiologic role of non-neuronal ACh in different diseases is discussed. The present article reviews recent findings about the non-neuronal cholinergic system in humans.
Keywords: non-neuronal cholinergic system; ACh; ChAT; nicotinic and muscarinic receptors; epithelial, endothelial, mesenchymal cells; immune cells; signal transduction; phenotype cell functions; inflammation
Introduction
ACh is regarded as a classical neurotransmitter. Nicotinic ACh receptors (nAChRs) are recognized as binding and effector proteins to mediate chemical neurotransmission at neurons, ganglia, interneurons and the motor endplate. Muscarinic ACh receptors (mAChRs) are recognized as binding and effector proteins to mediate chemical neurotransmission at neurons and effector organs such as heart, smooth muscle fibres and glands. This traditional view of ACh acting solely as neurotransmitter has to be revised based on the findings published both early and late in the last century, demonstrating the non-neuronal cholinergic system. Cholinergic communication and regulation have been established from the beginning of life; that is, in primitive uni- and multicellular organisms such as bacteria, algae, protozoa, sponge and primitive plants and fungi (Wessler et al., 1999; 2001a, 2003a; Horiuchi et al., 2003). The ubiquitous synthesis of ACh and the expression of n- and mAChRs in mammalian cells give an impressive example of the complexity of biological systems. All receptor subtypes and signal-transduction pathways used by cholinergic neurons are also used by single non-neuronal cells to communicate among each other and to maintain their phenotypic functions and thus organ homoeostasis. The present review will focus on the following topics, with particular emphasis on the situation in humans:
Synthesis of ACh outside the nervous system;
expression of n- and mAChRs on non-neuronal cells;
release mechanisms;
- cellular functions of non-neuronal ACh;
- signal transduction;
- regulation of phenotype cell functions;
- epithelial cells;
- endothelial cells;
- immune cells and the cholinergic anti-inflammatory pathway;
- mesenchymal cells;
non-neuronal cholinergic system involved in the pathophysiology of diseases.
Synthesis of ACh in non-neuronal cells
In the last decades, several reviews have focused on this topic (Sastry and Sadavongvivad, 1978; Grando, 1997; Wessler et al., 1998, 1999, 2001a, 2003a; Kawashima and Fujii, 2000, 2004; Eglen, 2006; Grando et al., 2006, 2007; Kurzen et al., 2007). ACh is synthesized by practically all living cells and can play an intermediary role in the interactions of non-neuronal cells with the external environment, hormones, growth factors, cytokines and also the neural system. On the other hand, all of these factors can also affect the expression and function of the non-neuronal cholinergic system. Table 1 gives a summary of the synthesis of ACh in various human, non-neuronal cells. Evidence for ACh synthesis is not only provided by positive anti-ChAT immunoreactivity, but ChAT enzyme activity and/or ACh content have also been determined in the majority of the cells indicated in Table 1 (epithelial cells in airways, intestine, skin, urothelium, vagina, placenta, cornea; granulosa cells; endothelial and immune cells; subcutaneous fat). In addition, it is noteworthy that embryonic stem cells (mice) also synthesize considerable amounts of ACh (Paraoanu et al., 2007). In the urothelium (mice and humans), positive ChAT immunoreactivity using a polyclonal antibody has been demonstrated, but this polyconal anti-ChAT antibody could be successfully preabsorbed with carnitine acetyltransferase (CarAT), another enzyme generating ACh (Lips et al., 2007b). In this latter paper, ChAT-specific mRNA was not detected, whereas in rat urothelial cells both ChAT- and CarAT-mRNA have been demonstrated (Hanna-Mitchell et al., 2007). Moreover it is known that monoclonal antibodies, also available for anti-ChAT immunoreactivity studies, operate more specifically than polyclonal antibodies.
Table 1.
Positive anti-ChAT immunoreactivity or HPLC detection of ACh in human cellsa
| Epithelial cells | |
| Airways | Basal, ciliated and secretory cells |
| Alimentary tract | Buccal mucosa, oesophagus, stomach, jejunum, ileum, colon, sigmoid and gall bladder |
| Skin | Keratinocytes, eccrine and sebaceous glands |
| Kidney | Tubuli |
| Urogenital tract | Urothelium, vaginal mucosa and granulosa cells |
| Placenta | Trophoblast |
| Glandular tissue | Female breast and thymus |
| Eye | Cornea |
| Endothelial cells | |
| Skin, umbilical vein and pulmonary vessels | |
| Immune cells | |
| Mononuclear leukocytes, bone marrow-derived dendritic cells, macrophages, skin mast cells | |
| Mesothelial cells | |
| Pleura, pericardium | |
| Mesenchymal cells | |
| Adipocytes (skin) | |
| Smooth muscle fibres (skin, airways) | |
| Fibroblasts (airwaysb) | |
| Tendon (tenocytes) | |
| Brain | |
| Astrocytesb | |
Data from Sastry and Sadavongvivad, 1978; Grando et al., 1993; Grando, 1997; Klapproth et al., 1997; Kawashima et al., 1998; Kawashima and Fujii, 2000, 2004; Wessler et al., 1998, 1999, 2001a; Wessler and Kirkpatrick, 2001a, 2001b; Danielson et al., 2007).
Unpublished observation (Wessler and Kirkpatrick, 2007).
The widespread synthesis of ACh beyond the nervous system has changed the paradigm of ACh acting merely as a neurotransmitter. Consequently, it has been repeatedly questioned whether ACh present in non-neuronal cells can be explained by neuronal contamination. This possibility can be categorically ruled out on the basis of several findings:
ChAT mRNA and ChAT protein have been demonstrated in isolated human epithelial and immune cells (Grando et al., 1993; Klapproth et al., 1997; Fujii et al., 1998; Ogawa et al., 2003; Kawashima and Fujii, 2004).
Multiple papers (for references, see review articles indicated above) have described specific labelling of human non-neuronal cells by anti-ChAT antibodies.
Isolated human airway epithelial cells or cultured human keratinocytes show ChAT enzyme activity and contain ACh (Grando et al., 1993; Reinheimer et al., 1996; Klapproth et al., 1997).
The human placenta—free of cholinergic neurons—synthesizes, stores and releases ACh (Olubadewo and Rama Sastry, 1978; Wessler et al., 2001b).
Human blood cells (mononuclear cells, lymphocytes) and human leukaemic cell lines as well as embryonic stem cells (mice) show ChAT enzyme activity and/or contain ACh, even under culture conditions (Fujii et al., 1998; Wessler et al., 2003a; Kawashima and Fujii, 2004; Neumann et al., 2007; Paraoanu et al., 2007).
In vivo release of ACh from the human skin as measured by dermal microdialysis maintained after pretreatment with botulinum toxin, which is known to block the exocytotic release of neuronal ACh (Kao et al., 1976; Schlereth et al., 2006).
Taken together, there exists overwhelming evidence that cells outside the cholinergic neuronal network synthesize, contain and release ACh. This property has been identified not only for the abundant majority of human cells but also in other mammals (rat), lower invertebrates (sponge, coral, sea squirt, sea urchin, tubellaria), protozoa, plants, fungi, blue-green algae and even in bacteria (Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Lactobacillus plantarum; for references, see Wessler et al., 1999; Horiuchi et al., 2003).
It should also be considered that both precursors for ACh synthesis—choline and acetyl-CoA—are present in nearly all cells. Acetyl-CoA is the major product of carbohydrate, protein and lipid catabolism in aerobic organisms, and is thus present in more or less all cells. Choline originates from the intracellular breakdown of choline-containing phospholipids or from the uptake of extracellular choline via low- or high-affinity choline transporter (CHT1). The latter has been demonstrated in the rat and human epithelial, urothelial, endothelial and vascular smooth muscle cells (Haberberger et al., 2002; Lips et al., 2003; Pfeil et al., 2003; Hanna-Mitchell et al., 2007). Thus, the equipment for ACh synthesis corresponds to that of neurons, even with respect to different ChAT mRNA species. In the human brain, R, N0, N1, N2 and M types have been found and most of these subtypes are detected, for example, in human T-lymphocytes (Oda, 1999; Ogawa et al., 2003). Likewise, AChE has been detected as nuclear protein very recently in both neuronal and non-neuronal cells (Santos et al., 2007).
Expression of nicotinic and muscarinic receptors on non-neuronal cells
Table 2 gives an overview of the expression of n- and mAChRs on non-neuronal cells. It is evident that most cell types—not innervated by cholinergic neurons at all—express n- and mAChRs that are part of the auto- and paracrine regulatory loop of non-neuronal ACh released from these cells. The specificity of antibodies raised against subtype-specific n- and mAChRs has recently been questioned (Moser et al., 2007; Zarghooni et al., 2007). When commercial antibodies directed against α3, α4, α7, β2 and β4 nAChR subtypes were applied, the respective immunoreactivity in the brain did not differ between knockout and wild-type mice (Moser et al., 2007). However, in this context it has to be considered that in most of the references given in Table 2 more than one method had been applied to demonstrate the expression of n- and mAChRs. The different methods used are indicated in the legend of Table 2.
Table 2.
Expression of n- and mAChRs on non-neuronal cells
| Cell type | Tissue | Muscarinic | Nicotinic | References |
|---|---|---|---|---|
| Epithelial cells | Airway (human) | |||
| Surface epithelium | M1 (small airways) M3 (M2; BEAS-2B cell line) | α1, α3, α5, α7, α9, β1, β2, β4, δ, ɛ | 1–11 | |
| Alveolar type 2 cells | α4, α7, β1, β2 (rhesus monkey) | |||
| Glands | M1, M3 | α4 (rhesus monkey) | ||
| Skin (human) | ||||
| Keratinocytes | M1, M2, M3, M4, (M5 mRNA) | α3, α5, α7, α9, α10, β1, β2, β4 | ||
| Pilosebaceous unit | All subtypes | α3, α4, α5, α7, α9, α10, β1, β2, β4 | 12–17 | |
| Sweat glands | ||||
| Myoepithelial | M2–M5 | α3, α4, α5, α7 | ||
| Acinar cells | M1, M3, M4 | α9, β2 | ||
| Melanocytes | All subtypes | α1, α3, α5, α7, β1, β2, γ, δ | ||
| Intestine | ||||
| Surface epithelium | M1, M3 | α3 | 18–25 | |
| Colonic epithelial cell line | α4, α5, α7, β1 | |||
| Glands (salivary cells, gastric cells, pancreatic acinar cells) | M1, M3 | α2, α3, α4, α5, α7, β2 (beta cell line, rat) | ||
| Ovary | ||||
| Granulosa (human) | M1, M3, M5 | 26,27 | ||
| Urothelium | ||||
| Human | M1, M2, M3, M4, M5 | α7, α9, α10 | ||
| Rat, mouse | M1, M2, M3, M4, M5 | α2, α4, α5, α6, α7, α9, α10, β3, β4 | 28–30 | |
| Endothelial cells | Aorta/pulmonary vessels | |||
| Human | M1, M2, M3, M2, M4, M5 (corneal endothelium) | α3, α5, α7, β2, β4, β4 | ||
| Rat | α2, α3, α4, α5, α6, α7, α10, β2, β4 | 6,7,31–37 | ||
| Mouse | M3, M5 (brain vessels) | α3, α5, α7, β2 (cerebral microvasculature) | ||
| Bovine | m1, m2, m3 (mRNA) | |||
| Immune cells | ||||
| MNLs (human) | M1–M5 (variable expression) | α2, α4, α5, α6, α7, α9, α10, β2, β4 (variable expression with dominant expression of α2, α5, α7) | ||
| Eosinophiles (human) | α3, α4, α7 | |||
| Macrophages human, airways | M2, M3 | α1, α7, α10 | 38–43 | |
| Mouse | M1–M5 | α2, α4, α5, α6, α7, α10, β2, β4 | ||
| DC cells (mouse) | M1–M5 | α2, α5, α6, α7, α10, β2, β4 | ||
| Mast cells (human) mucosal-type skin | M1 | α3, α5, α10 | ||
| Mesenchymal cells | Fibroblasts | |||
| Sclera (human) | m3>m2>m4>m5>m1 (mRNA) | |||
| Lung (human) | M2>M1>M3>M4 | α1, α3, α4, α6, α7, α9 | ||
| Skin (human) | M2, M4, M5 | β1, β2, β3, β4, δ, ɛ | ||
| Tenocytes | M2 (human) | α7 (rat) | ||
| Adipocytes (rat) | α1–7, α9, α10, β1–4, δ, ɛ | 5,10,36,44–53 | ||
| Smooth muscle fibres | ||||
| Lung | M2, M3 (human) | α6, α7, β2, β4 (rhesus monkey); α4, α7 (mouse) | ||
| Vasculature | M3 (human) | α2–7, α10 (rat); α6, β2, β4 (rhesus monkey) | ||
| Mesothelial cells | Mesothelioma | α7 | 54 | |
| Others | Insulinoma cells (mouse) | α3, α4, β4 | 55 | |
Abbreviations: B, binding experiments; F, functional experiments with agonists and antagonists; I, immunoreactivity; KO, knockout mice; R, detection of subtype-specific mRNA.
(1) Zia et al. (1997) (F, I); (2) Wessler and Kirkpatrick (2001b) (F, I, R); (3) Metzen et al. (2003) (F); (4) Gosens et al. (2006) (F, I, R); (5) Carlisle et al. (2004, 2007) (F, R); (6) Maus et al. (1998) (F); (7) Wang et al. (2001) (R); (8) Proskocil et al. (2004) (I, R); (9) Plummer et al. (2005) (R); (10) Sekhon et al. (2005) (I); (11) Gwilt et al. (2007) (F, I, R); (12) Grando (1997) (B, F, I, R); (13) Buchli et al. (2001) (B, R); (14) Grando et al. (2006) (F, KO, I, R); (15,16) Kurzen et al. (2004, 2007) (F, KO, I, R); (17) Hagforsen (2007) (I); (18) Richardson et al. (2003) (I, R); (19) Hirota and McKay (2006) (F, KO); (20) Gautam et al. (2006) (KO); (21) Haberberger et al. (2006) (F, I, R); (22, 23) Gautam et al. (2004, 2005) (I, KO, R); (24) Xie et al. (2005) (KO); (25) Yoshikawa et al. (2005) (B, F); (26) Fritz et al. (2001) (F, R); (27) Mayerhofer and Fritz (2002) (F, I); (28) Beckel et al. (2006) (F, R); (29) Bschleipfer et al. (2007) (I, R); (30) Zarghooni et al. (2007)(I, R); (31) Grueb et al. (2006) (I); (32) Tracey and Peach (1992) (R); (33) Khurana et al. (2004) (KO); (34) Walch et al. (2001) (F); (35) Yamada et al. (2001) (KO); (36) Bruggmann et al. (2003) (I, R); (37) Hawkins et al. (2005) (F, I); (38) Kawashima and Fujii (2003) (B, F, R); (39) Kawashima et al. (2007) (R); (40) Reinheimer et al. (2000) (F); (41) Profita et al. (2005) (F, I); (42) Wang et al. (2003) (F, KO, R); (43) Blanchet et al. (2007) (F, I, R); (44) Qu et al. (2006) (R); (45) Roman et al. (2004) (F); (46) Sekhon et al. (2002) (B, F, I, R); (47) Racké et al. (2006) (F, R); (48) Matthiesen et al. (2006) (F, R); (49) Danielson et al. (2007) (I, R); (50) Romano et al. (1997) (I, R); (51) Liu et al. (2004) (F, R); (52) Dorion et al. (2005) (F, I); (53) Buchli et al. (1999) (F); (54) Trombino et al. (2004) (B, R); (55) Ohtani et al. (2006) (B, F).
The expression pattern of the nicotinic ion channel complex and of the metabotrophic mAChRs varies according to phenotypic cell functions, as well as internal and external environmental conditions. For example, immature basal keratinocytes express predominantly α3β2/4-nAChRs and M2 and M3 receptors, transitional keratinocytes contain more α5, α9 subtypes and M4 and M5 receptors, whereas the mature keratinized surface keratinocyte expresses mainly α7-nAChRs and M1 receptors (Grando et al., 2006). Steroids (glucocorticosteroids, oestrogen) modify the expression of n- and mAChRs (Batra, 1990; Carrasco-Serrano and Criado, 2004). Thus, cholinergic input varies with cell type, state of cell differentiation and activity, as well as with cell environmental conditions.
Release mechanisms
In contrast to the release of neuronal ACh via exocytosis, our knowledge about the release mechanisms of non-neuronal ACh is scarce. Most details have been obtained from release studies using the human placenta as a model of the non-neuronal cholinergic system (Wessler et al., 2001d). Inhibitors (quinine, corticosterone) of organic cation transporters (OCT)) markedly suppressed ACh release, as well as substrate inhibitors (amiloride, cimetidine, verapamil, noradrenaline) and antisense oligonucleotides directed against subtypes 1 and 3. Experiments with transfected ooyctes (Xenopus laevis) showed ACh transport by OCT1 and OCT2 but not by OCT3 (Lips et al., 2007b). This latter finding differed from the results in the human placenta where OCT3 contributes to the release of ACh (Wessler et al., 2001d). Most likely, the subtypes involved differ between cells and organs. In the airway epithelium, OCT subtypes 1 and 2 appear to mediate the release of non-neuronal ACh (Lips et al., 2005; Kummer et al., 2006). The content of epithelial ACh was substantially enhanced in OCT1/2 double knockout mice compared with wild type (Kummer et al., 2006). These transporter proteins (OCTs) are very widely expressed on more or less every cell and therefore represent appropriate candidates to mediate the release of non-neuronal ACh. In addition, packing of ACh into secretory vesicles or endosomes may occur for intermediate intracellular storage and subsequent release of non-neuronal ACh, when these organelles fuse with the cell membrane. With the technique of immunogold electron microscopy the synthesizing enzyme ChAT has been found in endosomes of the human placenta (Wessler et al., 2001c).
Cellular functions of non-neuronal ACh
Signal transduction
Non-neuronal ACh is released from living cells, for example, from the human skin (Schlereth et al., 2006) and binds to n- and mAChRs of its source and neighbouring cells to mediate auto- and paracrine regulatory loops. Additional endogenous compounds can stimulate cholinergic receptors, for example, choline stimulates the α9 subtype and bile acid M3 receptors (Raufman et al., 2003; Alexander et al., 2006). In this way, cells may receive cholinergic input not only by local but also by hormone-like pathways. Moreover, endogenous allosteric modulators of n- and mAChRs play an essential role as an extremely sophisticated tool to fine-tune the cholinergic input to a cell. For example, progesterone operates as an allosteric modulator on the neuronal α4 subtype (Valera et al., 1992). SLURP (secreted mammalian Ly-6/urokinase plasminogen activator receptor-related protein 1 and 2), a modulator of nAChRs, has recently been found to be co-expressed with nAChRs not only in the brain but also on immune cells and epithelial cells in the skin and lung (Sekhon et al., 2005; Grando et al., 2006; Kawashima et al., 2007).
The most detailed analysis of the signal-transduction machinery involved has been evaluated for keratinocytes of the human skin (Grando et al., 2006). Table 3 summarizes some examples of the biochemical signal-transduction pathways that are triggered by auto- and paracrine actions or applied ACh on non-neuronal cells. It has to be considered that ACh can modify more or less all known signalling pathways via n- and mAChRs. Classical ionic channels as well as non-selective cation channels transporting Na+ and Ca2+ inside the cells can be affected. On granulosa cells ACh activates the calcium-dependent potassium channels (BKCa) and inhibits outward potassium channels such as the six transmembrane potassium (KCNQ) channels (Kunz et al., 2007), an effect that corresponds to the so-called M-current in neurons. In the heart, ACh causes hyperpolarization due to the activation of the inward-rectifier current. Proliferation and differentiation of lymphocytes are strongly associated with the expression and activation of potassium channels (Lee et al., 1986; DeCoursey et al., 1987) and the regulatory role of non-neuronal ACh on lymphocytes may be linked to its action on potassium channels. Recently, it has been shown that the α7 subtype on T cells fails to form a typical ligand-gated Ca2+ channel but requires functional TCR/CD3 (T-cell receptor complex) and leukocyte-specific tyrosine kinase (Razani-Boroujerdi et al., 2007). Thus, via auto-paracrine loops ACh may represent a co-stimulatory pathway for T-cell activation.
Table 3.
Signal-transduction pathways triggered by n- and mAChRs on non-neuronal cells
| Cell type | Nicotinic receptor | Muscarinic receptor | References |
|---|---|---|---|
| Epithelial cells keratinocytes | Increase in Cain (for example, mediated by α7): Ras/Raf/MEK/ERG CaMKII, PKC, PI3K α9: PLC, Src, PKC, Rac, Rho |
M1: increase in Cain Ras/Raf/MEK/ERG CaMKII, PKC, PI3K M3: Gq/11/guanylyl cyclase/cGMP/PKG; increase in Cain M4: Gi/o/adenylyl cyclase/cAMP/PKA; decrease in Cain |
Grando et al. (2006); Chernyavsky et al. (2007) |
| Lung cell line (BEAS2B) | Muscle type (α1, β1, δ, ɛ): increase in Cain/PKC/MAPK(p38) | Carlisle et al. (2004) | |
| NSLC | MAPK-ERK1/2, PKC, PKA, PI3K, Bad | Jin et al. (2004) | |
| Skin fibroblasts | α3β2: increase in Cain p21, cyclin D1, PCNA, Ki67, caspase 3 | M2/M4: Gi/o/MAPK-ERK1/2 (p42/p44) | Kurzen et al. (2007); Matthiesen et al. (2007) |
| Lung fibroblasts | Muscle type (α1, β1, δ, ɛ) or α7: increase in CainPKC/MAPK (p42/44) | M2: Gi/o/MAPK-ERK1/2 (p42/p44) | Carlisle et al. (2004); Matthiesen et al. (2007) |
| Lung smooth muscle fibres (cultured) | M2, M3: MAPK (p42/p44) increase in Cain | Gosens et al. (2007) | |
| Immune cells (T and B cells) | α3, α7 subtypes: increase in Cain Jak2-STAT3 | M3, M5: Gq/11/guanylyl cyclase/cGMP/PKG/PLC/DAG/IP3/increase in Cain/cfos | Kawashima and Fujii (2003); De Jonge et al. (2005) |
| Endothelial cells | α7 subtype: increase in Cain PI3K/Akt/MAPK-ERK1/2/p38 | Heeschen et al. (2002); Wang et al. (2006) | |
| Differentiating embryoid cells | MAPK-ERK1/2 (p42/p44) | Serobyan et al. (2007) |
Abbreviations: Akt, protein kinase B (signalling molecule in the phosphatidyl inositol 3-kinase pathway regulating energy metabolism, apoptosis, proliferation and migration); BAD, pro-apoptotic protein; Cain, intracellular Ca; CaMKII, calmodulin-dependent protein kinase; DAG, diacylglycerol; ERG, extracellular signal-regulated kinase; IP3, inositol 1,4,5-trisphosphate; Jak/STAT, signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; NSLC, non-small lung cancer cells; PI3K, phospatidylinositol-3-kinase; PKA, protein kinase A; PKC, protein kinase C; PKG, protein kinase G; PCNA, proliferating cell nuclear antigen; PLC, phospholipase C; p21, inducer of cell arrest; Rac and Rho, small GTPases; Src, family of tyrosine kinases.
In conclusion, a large body of evidence indicates that all elements of the cholinergic system (ChAT and ACh synthesis, release mechanisms, receptors) are functionally expressed independently of cholinergic innervation and can modify or even control phenotypic cell functions.
Regulation of phenotype cell functions
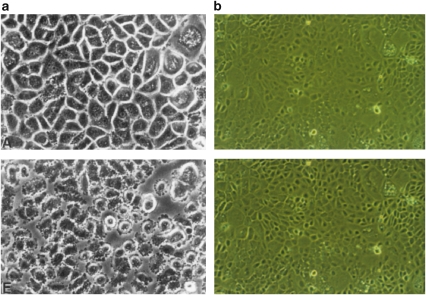
Primary cultures of human keratinocytes or bronchial epithelial cells are excellent examples to demonstrate the regulatory effect of auto- and paracrine ACh. Grando (1997) showed profound morphological alteration induced by n- and mAChR antagonists, that is, within a couple of minutes the cells lose their polygonal shape, retract their intermediate filament bundles, show substantial shrinkage and detach from each other (see Figure 1a, from Grando, 1997). A comparable but less extensive effect has been reported with 10μM tubocurarine and human bronchial epithelial cells, showing shrinkage of the cells and separation from neighbouring cells, thus increasing the intercellular space (see Figure 1b; Klapproth et al., 1994). Cultured gingival keratinocytes responded to 0.1mM mecamylamine in a comparable manner (Nguyen et al., 2000). Importantly, these alterations are reversible either by waiting for recovery, by washing or by the application of agonists (Grando, 1997). The difference in the degree of cell alterations between skin and airway cells may be linked to the more than 100-fold higher ACh content in keratinocytes (Reinheimer et al., 1996, Klapproth et al., 1997) and to the considerable differences in the concentrations of the applied antagonists. All these culture preparations are free of cholinergic neurons, enabling the following conclusions to be drawn:
The effects of the antagonists indicate an endogenous tone, that is, non-neuronal ACh is released and exerts auto-/paracrine effects via stimulation of n- and mAChRs.
These receptors are strongly involved in the control of the cytoskeleton and cell–cell contact. In transfected cells, it has already been shown that M3 receptors regulate cytoplasmic myosin via the small GTPase RhoA and PKC (Strassheim et al., 1999).
Epithelial cells establish a ‘cholinergic' micromilieu to control their homoeostasis. In the present experiments, blockade of the non-neuronal cholinergic system induces profound, but reversible cellular alterations.
Figure 1.
(a) Confluent keratinocyte monolayer; effect of 1mM atropine after 5min. Upper figure: cells immediately before atropine (Grando, 1997). (b) Confluent monolayer of human airway epithelial cells; effect of 10μM tubocurarine after 20min. Upper figure: cells immediately before tubocurarine (Klapproth et al., 1994).
In the following sections, further regulatory mechanisms are presented to demonstrate cellular effects mediated by non-neuronal ACh. Some examples mediated by identified subtypes of n- or mAChRs on distinct specialized cells are indicated in Table 4. Some caution is addressed to the interpretation of the results from knockout experiments. Co-segregating background genes may contribute to the observed effects unless a 100% backcrossing of the knockout mice onto isogenic backgrounds has been achieved and demonstrated.
Table 4.
Examples of cellular effects mediated by identified subtypes of n- and mAChRs (↑ increase; ↓ decrease)
| Cell type | Receptor | Functions | References |
|---|---|---|---|
| Epithelial cells | |||
| Skin | α7 | Terminal differentiation, apoptosis ↑, random migration ↓, directed migration ↑ | Grando et al. (2006) |
| α3 | Cell–cell contact ↑, random migration ↑ | Tournier et al. (2006) | |
| α9 | Cornification, cell shape, cytoplasm mobility, cell adhesion | Metzen et al. (2003); Chernyavsky et al. (2007) | |
| M3 | Random migration ↓, wound healing ↓ | ||
| Airways | M4 | Random migration ↑, wound healing ↑ | |
| M4 | Regulation of hair follicle cycle | ||
| α3,α5,β2 | Wound healing ↑ | ||
| M1 | Proliferation ↑ | ||
| Macrophages, mononuclear cells | α7 | Anti-inflammatory effects, release of proinflammatory cytokines ↓ | Shytle et al. (2004); Pavlov and Tracey (2005) |
| T cells | α7 | Interaction with TRC, Cain ↑ | Razani-Boroujerdi et al. (2007) |
| B cells | α7 | Regulation of antibody production | Kawashima et al. (2007) |
| Mucosal mast cells | M1 | Histamine release ↓ | Reinheimer et al. (2000) |
| Endothelial cells | α7 | Angiogenesis ↑ | Heeschen et al. (2002); Cooke (2007) |
| M1 | Release of NO ↑ | Evora et al. (2007) | |
| Fibroblasts (airway) | α7 | Collagen gene expression ↑ | Sekhon et al. (2002); Matthiesen et al. (2006) |
| M2 | Proliferation ↑ | Racké et al. (2008) |
Epithelial cells
Epidermis
The most detailed analysis of the regulatory role of non-neuronal ACh on cellular functions has been established for the epidermis by the investigation of intact skin, wound healing and cultured keratinocytes using different techniques (knockout mice, anti-sense RNA, selective agonists and antagonists; Grando et al., 1995, 2006; Chernyavsky et al., 2003, 2004, 2005; Kurzen et al., 2004, 2007).
Stimulation of mAChRs on isolated human gingival keratinocytes induces proliferation markers such as PCNA (proliferating cell nuclear antigen), Ki67 (nuclear antigen) and cyclin D1. Elimination of α7-nAChRs signalling slowed down terminal differentiation and reduced the amounts of proapoptotic proteins Bad and Bax (Grando et al., 2006). Thus, nicotinic pathways appear to facilitate the progress of cell cycle and differentiation. In organotypic epidermal co-cultures, combined blockade of n- and mAChRs completely prevented epidermal proliferation and differentiation (Kurzen et al., 2007). Physiological skin regeneration, as well as wound healing, is strongly dependent on proliferation, cell–cell detachment with assembling and disassembling processes involving metalloproteases, migratory and linker proteins, locomotion, migration and finally, reassembling of first adherens junctions and subsequently desmosomal junctions (Grando et al., 2006). M4 and α3-nAChRs appear to facilitate random migration (chemokinesis), whereas M3 and α7-nAChRs mediate the opposite effects. It has also been published that ACh and carbachol stimulate wound healing (see Grando et al., 2006). Wound healing is impaired in M4−/− knockout mice but enhanced in M3−/− (Grando et al., 2006), an observation that is consistent with the role of muscarinic receptor subtypes on chemokinesis. M4−/− knockout mice also showed an impaired hair follicle cycling with a prolonged telogen phase and failed to produce pigmented hair shafts (Hasse et al., 2007). Directed migration (chemotaxis) is promoted by α7-nAChRs. Moreover, keratinocytes exposed in a chamber to an electrical field re-orientated with their leading edge (lamellipodium) to the cathode and increased their expression of α7-nAChRs and M1 receptors (Chernyavsky et al., 2005). Long-term blockade of α3-, α9-nAChRs and M3 receptors by antisense oligonucleotides induces cell–cell detachment and changes in the expression of E-cadherin and catenins (Grando et al., 2006). Cadherin–catenin complexes are involved in cell–cell aggregation and essential morphoregulatory processes, and a change in their expression or structure is associated with cell migration and invasion. All these findings indicate that the non-neuronal cholinergic system is intimately involved in numerous cell functions including cell cycle, control of differentiation, apoptosis, organization of the cytoskeleton, cell–cell contact and migration (see also Figure 1).
Mucosa/glandular tissue
ACh stimulates proliferation of human and rat airway epithelial cells via mAChRs (M1 in rat) and nAChRs (Klapproth et al., 1997; Wessler and Kirkpatrick, 2001a, 2001b; Metzen et al., 2003). Nicotine in nanomolar concentrations activates the serine/threonine kinase Akt and at high concentration (100μM) the mitogen-activated protein kinase (MAPK) p38 kinase pathway, thereby affecting the cell cycle and apoptosis (West et al., 2003; Carlisle et al., 2004). These different agonist sensitivities allow an extremely sophisticated modulation of cell cycle and survival by auto- and paracrine effects of non-neuronal ACh. Proliferation, cell spreading and migration are important repair mechanisms also in the airway mucosa. Wound repair of bronchial surface epithelium (in vivo and in vitro) was significantly improved by nicotine and ACh but delayed by nicotinic receptor antagonists such as mecamylamine and κ-bungarotoxin (Tournier et al., 2006). These functional experiments correspond with the enhanced expression of α3α5β2 nAChRs on migrating bronchial epithelial cells involved in wound repair (Tournier et al., 2006).
Stimulatory effects of ACh on ciliary activity, mucociliary clearance and apical chloride secretion are well-established actions mainly mediated via mAChRs (Acevedo, 1994). ACh inhibits apical sodium conductance and stimulates basolateral potassium conductance, thus modifying ion and fluid movements of mucosal and glandular epithelial cells. Similar effects are mediated by ACh in the mammalian intestine, as recently summarized by Hirota and McKay (2006). Baseline activity of ion and water transports remains despite the removal of the myenteric plexus and the application of tetrodotoxin that gives some evidence for local effects of auto- and paracrine ACh. However, the effect of atropine on baseline activities varies among different intestinal regions and species. Therefore, a modulatory role of ACh on baseline activity has been questioned in contrast to the effect of applied ACh on ion transport (Hirota and McKay, 2006).
Permeability of contact surfaces (skin, airways and gastrointestinal tract) is controlled by trans- and paracellular mechanisms. Blockade of n- and mAChRs in cultures of airway epithelial cells and keratinocytes reduces cell–cell contact, increases the intercellular space and therefore enhances permeability and impairs the physical barrier function in these cell layers (see Figures 1a and b). In the pancreas, ACh increases paracellular permeability by interfering with tight junctions (Jansen et al., 1980). mAChRs affect the activity of gap junctions in gastric epithelial cells (rat) and in granulosa cells of the ovary (Ueda et al., 1995; Fritz et al., 2002), most likely an extremely old regulatory target on the evolutionary timescale. Very recently it has been demonstrated that ACh is the transmitter responsible for regulating gap-junction activity of the central synapse of Drosophila (Allen and Murphey, 2007).
Finally, the release of mediators such as prostanoids, leukotrienes and chemokines (granulocyte macrophage-CSF; neutrophil and monocyte chemoattractants, interleukin (IL)-8) from airway epithelial cells is regulated by n- and mAChRs (Koyama et al., 1992, Brunn et al., 1995; Klapproth et al., 1998; Profita et al., 2008). This mechanism of recruiting immune cells together with the effect of ACh on the epithelial barrier function and the stimulation of mucus and mucociliary activity may represent local anti-infectious pathways to protect mucosal boundaries from invasion by pathogens.
With respect to the extremely wide distribution of nAChRs the role of active and passive smoking and nicotine has been re-considered, particularly with respect to the situation on public areas. Passive smoking transfers sufficient amounts of nicotine to stimulate nAChRs on non-neuronal cells. Nicotine induces proliferation of epithelial cells, including tumour cell lines, and angiogenesis (Cattaneo et al., 1997; Heeschen et al., 2002; Cooke, 2007). The underlying molecular mechanisms are comparable to the effect of growth factors, that is, the involvement of Src kinase, Raf-1 kinase, cyclins D and E and proliferative promoters (Dasgupta and Chellappan, 2006). However, the involvement of the non-neuronal cholinergic system in tumorigenesis remains to be elucidated.
Endothelial cells
Convincing evidence has been published in recent years that the cholinergic system is strongly involved in angiogenesis. nAChRs mediate proliferation, survival, migration and tube formation in vitro as well as angiogenesis in vivo (Heeschen et al., 2002; Cooke, 2007). Nicotine and neostigmine facilitate migration of endothelial cells; nicotine promotes the generation of growth factors and autocoids such as endothelin and prostacyclin (Cooke, 2007). Most importantly, capillary network formation in vitro is blocked by specific nicotinic receptor antagonists indicating an endogenous tone by non-neuronal ACh (Heeschen et al., 2002). This effect is mediated by the α7 subtype. nAChR antagonists reduce neovascularization in response to inflammation, ischaemia and neoplasia (Heeschen et al., 2002). Moreover, nicotine stimulates interactions between endothelial cells and monocytes and thereby facilitates arteriogenesis (Heeschen et al., 2003). All these recent findings demonstrate that the non-neuronal cholinergic system is involved in the regulation of angiogenesis, a mechanism which may become impaired by smoking or other disease conditions.
Endothelial cells contribute to the regulation of perfusion. In vascular tissue, ACh via activation of mAChRs (M3 and M1 subtypes) is a well-known mediator for the release of nitric oxide (NO), endothelium-derived hyperpolarizing factor and prostanoids. Blood flow, shear stress, body temperature and local blood pressure may affect endothelial ACh synthesis and release and as a consequence may modulate the release of vasoactive mediators. Gradual hypothermia induces NO-dependent vasodilatation of canine isolated coronary, femoral and renal arteries, an effect which was blocked by 1μM pirenzepine (Evora et al., 2007). This recent finding indicates an endogenous regulatory loop mediated most likely by non-neuronal ACh. Milner et al. (1990) have shown the release of endothelial ACh in response to an increased flow. Immunocompetent cells must penetrate the vascular wall before their migration into inflammatory tissue. Adhesion molecules mediate the cross-talk between immune and endothelial cells. Nicotine at low concentrations did not affect the expression of VCAM (vascular cellular adhesion molecule) and E-selectin, but slightly enhanced that of ICAM-1 (intercellular adhesion molecule-1) (Kirkpatrick et al., 2003). In contrast to these results, it was reported that nicotine substantially stimulated the expression of VCAM1, ICAM and E-selectin in human umbilical vein endothelial cells (HUVECs) via calcium influx, an effect sensitive to mecamylamine and MAPK inhibitors (Albaugh et al., 2004; Wang et al., 2004, 2006). Nicotine increased the blood–brain barrier permeability and paracellular permeability and reduced connexin 43 expression and gap-junctional communication (Abbruscato et al., 2002; Tsai et al., 2004; Hawkins et al., 2005). All these findings open new and highly important insights into the fine-tuning of endothelial homoeostasis by non-neuronal cholinergic mechanisms.
Immune cells
In recent years, several review articles have focused on the expression and biological role of the non-neuronal cholinergic system in immune cells, particularly in T and B cells (Kawashima and Fujii, 2000, 2003, 2004). Activation of the T-cell receptor by phytohaemagglutinin or by anti-CD11a antibodies triggers the expression of ChAT and M5 receptors and enhances the synthesis of ACh. Likewise, stimulation of dendritic cells with lipopolysaccharide causes expression of ChAT (Kawashima et al., 2007). ACh is involved in the induction of CD4+ T-cell maturation as well as in the generation of cytolytic CD8+ T-lymphocytes under in vitro conditions (Kawashima and Fujii, 2000, 2003; Zimring et al., 2005). In addition, it has been demonstrated that ACh can modify immune responses. Thus, for example, nAChRs appear to play a role in the generation of antibodies with a possible inhibitory regulatory role of the α7 subtype (Kawashima et al., 2007; Skok et al., 2007). Stimulation of the same nAChR subtype (α7) by neuronal or non-neuronal ACh inhibits the release of proinflammatory mediators (tumour necrosis factor, IL1-β) from immune cells as part of the so-called cholinergic anti-inflammatory pathway (Borovikova et al., 2000; Pavlov and Tracey, 2005; see below). Most interestingly, a comparable regulatory pathway has been described for brain mononuclear phagocytic cells, the microglia, as stimulation of α7-nAChRs suppresses lipopolysaccharide-induced tumour necrosis factor-α release (Shytle et al., 2004).
All these observations indicate that ACh modulates the activity of immune cells via auto- and paracrine loops. The α7 subtype expressed on T cells requires functional TRC/CD3 to raise [Ca2+]in (Razani-Boroujerdi et al., 2007), suggesting a co-stimulatory function of non-neuronal ACh in T cells. Interestingly, the components of the non-neuronal cholinergic system are expressed very early during fetal haematopoiesis and nicotine affects the colonization of the fetal bone with haematopoietic stem/progenitor cells (Serobyan et al., 2007). Finally, mucosal mast cells in human airways are controlled by a strong M1-mediated inhibitory pathway, thereby limiting the liberation of proinflammatory mediators (Reinheimer et al., 2000). This effect becomes impaired in advanced chronic obstructive pulmonary disease (Wessler et al., 2007a).
The cholinergic anti-inflammatory pathway
Borovikova et al. (2000) described the so-called cholinergic anti-inflammatory reflex or pathway. Stimulation of the vagal nerve during artificial lethal endotoxaemia prevented the development of shock and reduced the release of proinflammatory cytokines such as tumour necrosis factor-α, IL-1β, IL-6 and IL-18 (Borovikova et al., 2000). Meanwhile, the concept of a cholinergic anti-inflammatory pathway has been examined in various models of acute systemic or local inflammation (Tracey, 2002; Pavlov and Tracey, 2005; de Jonge and Ulloa, 2007). Undoubtedly, the vagal nerve plays an important role as a sensory and nociceptive system to communicate the activation state of the immune system to the brain. In addition, efferent fibres mediate regulatory integrative responses. However, it is still questionable whether the anti-inflammatory effect occurring intimate to the immune cells is mediated by vagally released, that is, neuronal ACh. Rather few neuroanatomical studies have been performed to identify the innervation pattern of the immune system by the vagal nerve. In contrast to the innervation by the sympathetic nervous system, there is no convincing neuroanatomical evidence for an innervation of the spleen, thymus, lymph nodes and bone marrow by the vagal nerve, for example in rats (Bulloch and Moore, 1981; Nance et al., 1987; Bellinger et al., 1993; for review, see Nance and Sanders, 2007). Some immune cells show close membrane apposition with neuronal elements, for example, in the area postrema (Goehler et al., 2006). How should a vagally mediated efferent response at macrophages, dendritic and other immune cells migrated in mucosal and parenchymatous tissues operate without a close neuroanatomical substrate? In this context, one has to consider the extremely high effectiveness of AChE at neurons, which prevents neuronal ACh from acting as a diffusing signalling molecule. Nance and Sanders (2007) have suggested that stimulation of the efferent vagal nerve caused activation of the adrenal medulla and the sympathetic nervous system which, in turn, contributes or even mediates the anti-inflammatory mechanism. In addition, the vagal nerve, particularly the subdiaphragmatic portion, modulates ascending impulse transmission projecting to the hypothalamus and is involved in the threshold regulation of the nociceptive system in abdominal visceral organs (Miao et al., 1997a, b). From this point of view, it is not surprising that vagotomy increases lethality of endotoxaemia. Moreover, nicotine induces complex effects depending on the route of application and dose. For example, intrathecal application of nicotine (rat) reduces bradykinin-induced plasma extravasation and this anti-inflammatory effect was substantially potentiated by subdiaphragmatic vagotomy (Miao et al., 1997a). In contrast, local application of a very low dose of nicotine induces proinflammatory effects when the adrenal medulla has been inactivated (Miao et al., 1997b, 2001). All these observations demonstrate extreme complex interactions of applied nicotine at central and peripheral neurons, nociceptors, adrenal medulla and non-neuronal cells.
Nevertheless, one should consider that anti-inflammatory effects are also observed with muscarinic agonists (at higher concentrations) and can be detected with locally applied nicotinic agonists or ACh on isolated immune cells (Borovikova et al., 2000; Tracey, 2002; Pavlov and Tracey, 2005; de Jonge and Ulloa, 2007). Thus, the principle of a cholinergic, anti-inflammatory mechanism mediated, for example, by nAChRs does exist. We suggest that besides the activation of the adrenal medulla, neuronal ACh released from efferent and probably also from afferent fibres is involved and triggers the release of non-neuronal ACh from neighbouring cells, passing the signal such as a wave within mucosal and parenchymatous tissues. In this context, it is noteworthy that the release of non-neuronal ACh is increased in the human placenta by nAChRs (Wessler et al., 2001b).
Mesenchymal cells
Our knowledge about the role of non-neuronal ACh in mesenchymal cells, such as adipocytes, fibroblasts, smooth muscle fibres and tenocytes, is very poor. At best, airway fibroblasts have been examined. All subtypes of mAChRs are expressed with a dominance of the M2 subtype. Stimulation of these receptors increased the proliferation rate and also the incorporation of proline, the latter giving evidence of an enhanced collagen synthesis (Matthiesen et al., 2006; Racké et al., 2008). Whether these effects are involved in the pathogenesis of chronic airway diseases remains to be elucidated. In human neonatal skin fibroblasts, mAChRs stimulate DNA synthesis and CD40 expression (Casanova et al., 2006).
ACh can modify phenotypic functions of the airway smooth muscle fibres (Gosens et al., 2004). For example, the expression of contractile proteins as well as smooth muscle secretory functions can be affected by ACh, and mAChRs potentiate the mitogenic effect of growth factors (Gosens et al., 2004). Acute or chronic inflammation can cause increased levels of ACh (see next section) and this effect may contribute to the remodelling processes induced by inflammation. Recently, the first evidence has been presented that AChE is highly expressed in fibroblasts in which the enzyme protein mediates non-enzymatic morphoregulatory effects (Anderson et al., 2007). AChE is prominently expressed at the leading edge of spreading and migrating fibroblasts. In this context, cell surface AChE has been proposed to contribute as a more general signalling mechanism to protrusion and migration of polarized cells (Anderson et al., 2007).
Non-neuronal cholinergic system involved in the pathophysiology of diseases
All components of the system—synthesis, storage, release, inactivation as well as the expression and function of the various n- and mAChRs—can be affected as a key pathogenetic event or secondary to the disease state. Particularly, the fine-tuning of cellular effects mediated by the different subtypes of n- and mAChRs may become impaired. Subtypes of n- and mAChRs act in a synergistic or antagonistic way (see Tables 3 and 4) and even a small change in the expression pattern may result in cellular stress. The scientific community has just started to investigate the non-neuronal cholinergic system. A systematic analysis of all components of the non-neuronal cholinergic system in different diseases is practically non-existent and should be established as soon as possible. In the final section some pathophysiological conditions are discussed, where substantial alterations of the non-neuronal cholinergic system have already been described.
The effect of inflammation on the non-neuronal cholinergic system and vice versa appears to be regulated in a very complex manner and is as yet only poorly understood. Chronic inflammation may upregulate ACh synthesis. For example, in atopic dermatitis substantially enhanced levels of ACh have been detected within the skin, including the superficial 2mm of epidermis (Scott, 1962; Wessler et al., 2003b). In a model of acute renal allograft rejection in rats, it was found that the ACh-synthesizing machinery was substantially upregulated in intravascular leukocytes (Hecker et al., 2006). The opposite was observed in mice or rat lung using an acute allergic inflammation model (Lips et al., 2007a). Enhanced levels of ACh have been associated with pruritus, thickening of the stratum spinosum, enhanced blood flow and impaired barrier function (Wessler et al., 2003b). In psoriasis, enhanced expression of SLURP-2 has been demonstrated, which binds to nAChRs, thus inhibiting caspase 3 and filaggrin (Grando et al., 2006). AChE activity is lowered in vitiliginous skin. Acantholysis observed in patients with pemphigus is caused by autoantibodies directed against desmosomal cadherins and nAChRs (Grando, 2000). Mustard gases such as sulphur mustard cause dose-dependent injuries of skin and mucosa. These toxins interfere with the cholinergic system, for example with n- and mAChRs and produce similar effects as shown in Figure 1a (Grando, 2003).
Chronic patellar tendon tendinosis is associated with enhanced anti-ChAT and anti-M2 immunoreactivity (Danielson et al., 2007). A downregulation of ChAT and upregulation of M2 receptors have been found in the colon epithelium of patients with ulcerative colitis (Jonsson et al., 2007). As already mentioned, ACh released by the vagal nerve as well as non-neuronal ACh can induce an anti-inflammatory effect via α7-nAChRs in various models of acute systemic inflammation (Pavlov and Tracey, 2005; de Jonge and Ulloa, 2007). Interestingly, muscarinic receptor antagonists appear to mediate anti-inflammatory effects (Profita et al., 2005; Pahl et al., 2006, Bühling et al., 2007).
Very recently it has been shown that ACh content is substantially reduced in blood cells (leukocytes) as well as in bronchi of patients with cystic fibrosis despite a somewhat enhanced ChAT activity (Wessler et al., 2007b). This decrease was not caused by an enhanced esterase activity. Double labelling experiments with anti-ChAT and anti-CFTR (cystic fibrosis transmembrane regulator protein) antibodies showed a colocalization (Wessler et al., 2007b). This observation gives first evidence that ChAT and CFTR—a widely expressed transport and regulator protein—may be linked in a functional way, presumably to regulate the storage and transport of non-neuronal ACh within a cell. In cystic fibrosis, the storage may become impaired and in consequence cells contain less ACh. In the airways of CF patients, this cholinergic dysfunction may contribute to the deleterious alterations in ion and water movements, as ACh is known to stimulate apical Cl− secretion and to inhibit apical Na+ absorption.
It has been reported that ACh inhibits long-term hypoxia-induced apoptosis in mouse stem cells (Kim et al., 2008), demonstrating protective and trophic effects. On endothelial cells, α7-nAChRs are upregulated by hypoxia or ischaemia and nicotinic receptor antagonists block endothelial network formation in vitro (Heeschen et al., 2002). Meanwhile, it has been demonstrated that nicotine and endothelial ACh represent proangiogenic factors. Nicotine promotes the growth of atherosclerotic plaques, potentiates endothelial–monocyte interactions and the incorporation of endothelial progenitor cells into newly established vessels (Heeschen et al., 2003, 2006; Cooke, 2007). Lung cancers express n- and mAChRs; activation of these receptors by applied nicotine or ACh stimulates growth of tumour cell lines (Cattaneo et al., 1997; Song et al., 2003). Small cell lung carcinoma cells utilize ACh as an auto- and paracrine growth factor, and M3 receptor antagonists have been reported to reduce cell growth both in vitro and in vivo (Song et al., 2007). Exposure to tobacco products or nicotine alters cell cycle and can induce squamatization of oral keratinocytes and the formation of squamous cell carcinoma (Arredondo et al., 2008). It has been shown that environmental tobacco smoke or nicotine upregulates the expression of α5- and α7-nAChRs, raising Cain signalling and causing profound pathobiologic effects as described above (Arredondo et al., 2007). In this context, one has to consider differences between acute and chronic exposure as well as high and low concentrations of nicotine producing either pro- or anti-inflammatory responses. For example, smoking appears to exert protective effects against ulcerative colitis and can reduce mucosal inflammation (for references, see de Jonge and Ulloa, 2007).
In conclusion, it is important to analyse systematically the changes of the main components of the non-neuronal cholinergic system (synthesis and release of ACh, expression and function of subtypes of n- and mAChRs, expression and function of esterases) in models of acute and chronic inflammation in different organs and in human diseases. Release can be modified by alterations of synthesis (ChAT expression), intracellular storage and transporter proteins. Expression and function of n- and mAChR subtypes can be modified by various mediators, including inflammatory cytokines and autoantibodies. Numerous interactions occur and this complex scenario needs to be elucidated to learn more about the regulatory role of the non-neuronal cholinergic system in acute and chronic inflammatory processes. This will help in understanding the cholinergic properties of non-neuronal cells and in turn will lead to optimization of drug therapy.
Abbreviations
- mAChRs
muscarinic ACh receptors
- nAChRs
nicotinic ACh receptors
- OCT
organic cation transporter
- TCR/CD3
T-cell receptor complex
- VCAM
vascular cellular adhesion molecule
Conflict of interest
The authors state no conflict of interest.
References
- Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91:2525–2538. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- Acevedo M. Effect of acetylcholine on ion transport in sheep tracheal epithelium. Pflugers Arch. 1994;427:543–546. doi: 10.1007/BF00374272. [DOI] [PubMed] [Google Scholar]
- Albaugh G, Bellavance E, Strande L, Heinburger S, Hewitt CW, Alexander JB. Nicotine induces mononuclear leukocyte adhesion and expression of adhesion molecules, VCAM and ICAM, in endothelial cells in vitro. Ann Vasc Surg. 2004;18:302–307. doi: 10.1007/s10016-004-0030-9. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels. Br J Pharmacol (Suppl) 2006;147:S85. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Murphey RK. The chemical component of the mixed GF-TTMn synapse in Drosophila melanogaster uses acetylcholine as its neurotransmitter. Eur J Neurosci. 2007;26:439–445. doi: 10.1111/j.1460-9568.2007.05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AA, Ushakov DS, Ferenczi MA, Mori R, Martin P, Saffell JL. Morphoregulation by acetylcholinesterase in fibroblasts and astrocytes. J Cell Physiol. 2007;215:82–100. doi: 10.1002/jcp.21288. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: acceleration of sequential expression of α5 and α7 nicotinic receptor subunits in oral keratinocytes exposed to cigarette smoke. FASEB J. 2008;22:1356–1368. doi: 10.1096/fj.07-9965.com. [DOI] [PubMed] [Google Scholar]
- Batra S. Influence of chronic oestrogen treatment on the density of muscarinic cholinergic receptors and calcium channels in the rabbit uterus. J Endocrinol. 1990;125:185–189. doi: 10.1677/joe.0.1250185. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2006;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- Blanchet MR, Langlois A, Israël-Assayag E, Beaulieu MJ, Ferland C, Laviolette M, et al. Modulation of eosinophil activation in vitro by a nicotinic receptor agonist. J Leukoc Biol. 2007;81:1245–1251. doi: 10.1189/jlb.0906548. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bruggmann D, Lips KS, Pfeil U, Haberberger RV, Kummer W. Rat arteries contain multiple nicotinic acetylcholine receptor alpha-subunits. Life Sci. 2003;72:2095–2099. doi: 10.1016/s0024-3205(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Brunn G, Wessler I, Racke K. Mucosa-dependent muscarinic liberation of prostaglandins from rat isolated trachea. Br J Pharmacol. 1995;116:1991–1998. doi: 10.1111/j.1476-5381.1995.tb16403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, et al. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci. 2007;80:2303–2307. doi: 10.1016/j.lfs.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Buchli R, Ndoye A, Arredondo J, Webber RJ, Grando SA. Identification and characterization of muscarinic acetylcholine receptor subtypes expressed in human skin melanocytes. Mol Cell Biochem. 2001;228:57–72. doi: 10.1023/a:1013368509855. [DOI] [PubMed] [Google Scholar]
- Buchli R, Ndoye A, Rodriguez JG, Zia S, Webber RJ, Grando SA. Human skin fibroblasts express m2, m4, and m5 subtypes of muscarinic acetylcholine receptors. J Cell Biochem. 1999;74:264–277. [PubMed] [Google Scholar]
- Buhling F, Lieder N, Kuhlmann UC, Waldburg N, Welte T. Tiotropium suppresses acetylcholine-induced release of chemotactic mediators in vitro. Respir Med. 2007;101:2386–2394. doi: 10.1016/j.rmed.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Moore RY. Innervation of the thymus gland by brain stem and spinal cord in mouse and rat. Am J Anat. 1981;162:157–166. doi: 10.1002/aja.1001620207. [DOI] [PubMed] [Google Scholar]
- Carlisle DL, Hopkins TM, Gaither-Davis A, Silhanek MJ, Luketich JD, Christie NA, et al. Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respir Res. 2004;5:27. doi: 10.1186/1465-9921-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle DL, Liu X, Hopkins TM, Swick MC, Dhir R, Siegfried JM. Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells. Pulm Pharmacol Ther. 2007;20:629–641. doi: 10.1016/j.pupt.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Carrasco-Serrano C, Criado M. Glucocorticoid activation of the neuronal nicotinic acetylcholine receptor alpha7 subunit gene: involvement of transcription factor Egr-1. FEBS Lett. 2004;566:247–250. doi: 10.1016/j.febslet.2004.04.049. [DOI] [PubMed] [Google Scholar]
- Casanova M, Furlán C, Sterin-Borda L, Borda ES. Muscarinic cholinoceptor activation modulates DNA synthesis and CD40 expression in fibroblast cells. Auton Autacoid Pharmacol. 2006;26:293–301. doi: 10.1111/j.1474-8673.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo MG, D'atri F, Vicentini LM. Mechanisms of mitogen-activated protein kinase activation by nicotine in small-cell lung carcinoma cells. Biochem J. 1997;328:499–503. doi: 10.1042/bj3280499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Karlsson E, Wessler I, Grando SA. The Ras/Raf-1/MEK1/ERK signaling pathway coupled to integrin expression mediates cholinergic regulation of keratinocyte directional migration. J Biol Chem. 2005;280:39220–39228. doi: 10.1074/jbc.M504407200. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Marubio LM, Grando SA. Differential regulation of keratinocyte chemokinesis and chemotaxis through distinct nicotinic receptor subtypes. J Cell Sci. 2004;117:5665–5679. doi: 10.1242/jcs.01492. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Vetter DE, Grando SA. Central role of alpha9 acetylcholine receptor in coordinating keratinocyte adhesion and motility at the initiation of epithelialization. Exp Cell Res. 2007;313:3542–3555. doi: 10.1016/j.yexcr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Nguyen VT, Arredondo J, Ndoye A, Zia S, Wess J, et al. The M4 muscarinic receptor-selective effects on keratinocyte crawling locomotion. Life Sci. 2003;72:2069–2073. doi: 10.1016/s0024-3205(03)00085-7. [DOI] [PubMed] [Google Scholar]
- Cooke JP. Angiogenesis and the role of the endothelial nicotinic acetylcholine receptor. Life Sci. 2007;80:2347–2351. doi: 10.1016/j.lfs.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson P, Andersson G, Alfredson H, Forsgren S. Extensive expression of markers for acetylcholine synthesis and of M2 receptors in tenocytes in therapy-resistant chronic painful patellar tendon tendinosis—a pilot study. Life Sci. 2007;80:2235–2238. doi: 10.1016/j.lfs.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Chellappan SP. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- De Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- Decoursey TE, Chandy KG, Gupta S, Cahalan MD. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 1987;89:405–420. doi: 10.1085/jgp.89.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion G, Israel-Assayag E, Beaulieu MJ, Cormier Y. Effect of 1, 1-dimethylphenyl 1, 4-piperazinium on mouse tracheal smooth muscle responsiveness. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1139–L1145. doi: 10.1152/ajplung.00406.2004. [DOI] [PubMed] [Google Scholar]
- Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26:219–233. doi: 10.1111/j.1474-8673.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- Evora PR, Cable DG, Chua YL, Rodrigues AJ, Pearson PJ, Schaff HV. Nitric oxide and prostacyclin-dependent pathways involvement on in vitro induced hypothermia. Cryobiology. 2007;54:106–113. doi: 10.1016/j.cryobiol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Fritz S, Kunz L, Dimitrijevic N, Grunert R, Heiss C, Mayerhofer A. Muscarinic receptors in human luteinized granulosa cells: activation blocks gap junctions and induces the transcription factor early growth response factor-1. J Clin Endocrinol Metab. 2002;87:1362–1367. doi: 10.1210/jcem.87.3.8326. [DOI] [PubMed] [Google Scholar]
- Fritz S, Wessler I, Breitling R, Rossmanith W, Ojeda SR, Dissen GA, et al. Expression of muscarinic receptor types in the primate ovary and evidence for nonneuronal acetylcholine synthesis. J Clin Endocrinol Metab. 2001;86:349–354. doi: 10.1210/jcem.86.1.7146. [DOI] [PubMed] [Google Scholar]
- Fujii T, Yamada S, Watanabe Y, Misawa H, Tajima S, Fujimoto K, et al. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J Neuroimmunol. 1998;82:101–107. doi: 10.1016/S0165-5728(97)00195-1. [DOI] [PubMed] [Google Scholar]
- Gautam D, Duttaroy A, Cui Y, Han SJ, Deng C, Seeger T, et al. M1–M3 muscarinic acetylcholine receptor-deficient mice: novel phenotypes. J Mol Neurosci. 2006;30:157–160. doi: 10.1385/JMN:30:1:157. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Heard TS, Cui Y, Miller G, Bloodworth L, et al. Cholinergic stimulation of amylase secretion from pancreatic acinar cells studied with muscarinic acetylcholine receptor mutant mice. J Pharmacol Exp Ther. 2005;313:995–1002. doi: 10.1124/jpet.105.084855. [DOI] [PubMed] [Google Scholar]
- Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol. 2004;66:260–267. doi: 10.1124/mol.66.2.260. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Erisir A, Gaykema RP. Neural-immune interface in the rat area postrema. Neuroscience. 2006;140:1415–1434. doi: 10.1016/j.neuroscience.2006.03.048. [DOI] [PubMed] [Google Scholar]
- Gosens R, Dueck G, Gerthoffer WT, Unruh H, Zaagsma J, Meurs H, et al. p42/p44 MAP kinase activation is localized to caveolae-free membrane domains in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1163–L1172. doi: 10.1152/ajplung.00471.2006. [DOI] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Grootte Bromhaar M, Nelemans A, Meurs H. Acetylcholine: a novel regulator of airway smooth muscle remodelling. Eur J Pharmacol. 2004;500:193–201. doi: 10.1016/j.ejphar.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grando SA. Biological functions of keratinocyte cholinergic receptors. J Investig Dermatol Symp Proc. 1997;2:41–48. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- Grando SA. Autoimmunity to keratinocyte acetylcholine receptors in pemphigus. Dermatology. 2000;201:290–295. doi: 10.1159/000051540. [DOI] [PubMed] [Google Scholar]
- Grando SA. Mucocutaneous cholinergic system is targeted in mustard-induced vesication. Life Sci. 2003;72:2135–2144. doi: 10.1016/s0024-3205(03)00074-2. [DOI] [PubMed] [Google Scholar]
- Grando SA, Horton RM, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, et al. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105:774–781. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kawashima K, Kirkpatrick CJ, Wessler I. Recent progress in understanding the non-neuronal cholinergic system in humans. Life Sci. 2007;80:2181–2185. doi: 10.1016/j.lfs.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Invest Dermatol. 2006;126:1948–1965. doi: 10.1038/sj.jid.5700151. [DOI] [PubMed] [Google Scholar]
- Grueb M, Reinthal E, Rohrbach JM, Bartz-Schmidt KU. Muscarinic acetylcholine receptor subtypes in human corneal epithelium and endothelium. Graefes Arch Clin Exp Ophthalmol. 2006;244:1191–1195. doi: 10.1007/s00417-006-0263-0. [DOI] [PubMed] [Google Scholar]
- Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation. Pharmacol Ther. 2007;115:208–222. doi: 10.1016/j.pharmthera.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Haberberger R, Schultheiss G, Diener M. Epithelial muscarinic M1 receptors contribute to carbachol-induced ion secretion in mouse colon. Eur J Pharmacol. 2006;530:229–233. doi: 10.1016/j.ejphar.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Haberberger RV, Pfeil U, Lips KS, Kummer W. Expression of the high-affinity choline transporter, CHT1, in the neuronal and non-neuronal cholinergic system of human and rat skin. J Invest Dermatol. 2002;119:943–948. doi: 10.1046/j.1523-1747.2002.00182.x. [DOI] [PubMed] [Google Scholar]
- Hagforsen E. The cutaneous non-neuronal cholinergic system and smoking related dermatoses: studies of the psoriasis variant palmoplantar pustulosis. Life Sci. 2007;80:2227–2234. doi: 10.1016/j.lfs.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci. 2007;80:2298–2302. doi: 10.1016/j.lfs.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasse S, Chernyavsky AI, Grando SA, Paus R. The M4 muscarinic acetylcholine receptor plays a key role in the control of murine hair follicle cycling and pigmentation. Life Sci. 2007;80:2248–2252. doi: 10.1016/j.lfs.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Egleton RD, Davis TP. Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. Am J Physiol Heart Circ Physiol. 2005;289:H212–H219. doi: 10.1152/ajpheart.01210.2004. [DOI] [PubMed] [Google Scholar]
- Hecker A, Lips K, Pfeil U, Kummer W, Zakrzewics A, Padberg W, et al. Up-regulation of high-affinity choline transporter (CHT1) and choline acetyltransferase (CHAT) gene expression by intravascular leukocytes during acute rejection of renal allografts. Eur Surg Res (Suppl 1) 2006;38:23. [Google Scholar]
- Heeschen C, Chang E, Aicher A, Cooke JP. Endothelial progenitor cells participate in nicotine-mediated angiogenesis. J Am Coll Cardiol. 2006;48:2553–2560. doi: 10.1016/j.jacc.2006.07.066. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- Hirota CL, McKay DM. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006;149:463–479. doi: 10.1038/sj.bjp.0706889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi Y, Kimura R, Kato N, Fujii T, Seki M, Endo T, et al. Evolutional study on acetylcholine expression. Life Sci. 2003;72:1745–1756. doi: 10.1016/s0024-3205(02)02478-5. [DOI] [PubMed] [Google Scholar]
- Jansen JW, Fleuren-Jakobs AM, De Pont JJ, Bonting SL. Blocking by 2, 4, 6-triaminopyrimidine of increased tight junction permeability induced by acetylcholine in the pancreas. Biochim Biophys Acta. 1980;598:115–126. doi: 10.1016/0005-2736(80)90269-2. [DOI] [PubMed] [Google Scholar]
- Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosis. J Biol Chem. 2004;279:23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Norrgard O, Forsgren S. Presence of a marked nonneuronal cholinergic system in human colon: study of normal colon and colon in ulcerative colitis. Inflamm Bowel Dis. 2007;13:1347–1356. doi: 10.1002/ibd.20224. [DOI] [PubMed] [Google Scholar]
- Kao I, Drachman DB, Price DL. Botulinum toxin: mechanism of presynaptic blockade. Science. 1976;193:1256–1258. doi: 10.1126/science.785600. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its biological function. Life Sci. 2003;72:2101–2109. doi: 10.1016/s0024-3205(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T, Watanabe Y, Misawa H. Acetylcholine synthesis and muscarinic receptor subtype mRNA expression in T-lymphocytes. Life Sci. 1998;62:1701–1705. doi: 10.1016/s0024-3205(98)00131-3. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Khurana S, Chacon I, Xie G, Yamada M, Wess J, Raufman JP, et al. Vasodilatory effects of cholinergic agonists are greatly diminished in aorta from M3R−/− mice. Eur J Pharmacol. 2004;493:127–132. doi: 10.1016/j.ejphar.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kim MO, Heo JS, Kim JS, Han HJ. Acetylcholine inhibits long-term hypoxia-induced apoptosis by suppressing the oxidative stress-mediated MAPKs activation as well as regulation of Bcl-2, c-IAPs, and caspase-3 in mouse embryonic stem cells. Apoptosis. 2008;13:295–304. doi: 10.1007/s10495-007-0160-y. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CJ, Bittinger F, Nozadze K, Wessler I. Expression and function of the non-neuronal cholinergic system in endothelial cells. Life Sci. 2003;72:2111–2116. doi: 10.1016/s0024-3205(03)00069-9. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Racke K, Wessler I. Modulation of the airway smooth muscle tone by mediators released from cultured epithelial cells of rat trachea. Naunyn Schmiedebergs Arch Pharmacol (Suppl) 1994;349:R72. [Google Scholar]
- Klapproth H, Racke K, Wessler I. Acetylcholine and nicotine stimulate the release of granulocyte-macrophage colony stimulating factor from cultured human bronchial epithelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:472–475. doi: 10.1007/pl00005195. [DOI] [PubMed] [Google Scholar]
- Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, et al. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- Koyama S, Rennard SI, Robbins RA. Acetylcholine stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Physiol. 1992;262:L466–L471. doi: 10.1152/ajplung.1992.262.4.L466. [DOI] [PubMed] [Google Scholar]
- Kummer W, Wiegand S, Akinci S, Wessler I, Schinkel AH, Wess J, et al. Role of acetylcholine and polyspecific cation transporters in serotonin-induced bronchoconstriction in the mouse. Respir Res. 2006;7:65–72. doi: 10.1186/1465-9921-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz L, Roggors C, Mayerhofer A. Ovarian acetylcholine and ovarian KCNQ channels: insights into cellular regulatory systems of steroidogenic granulosa cells. Life Sci. 2007;80:2195–2198. doi: 10.1016/j.lfs.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, et al. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol. 2004;123:937–949. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. doi: 10.1055/s-2007-961816. [DOI] [PubMed] [Google Scholar]
- Lee SC, Sabath DE, Deutsch C, Prystowsky MB. Increased voltage-gated potassium conductance during interleukin 2-stimulated proliferation of a mouse helper T lymphocyte clone. J Cell Biol. 1986;102:1200–1208. doi: 10.1083/jcb.102.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips KS, Luhrmann A, Tschernig T, Stoeger T, Alessandrini F, Grau V, et al. Down-regulation of the non-neuronal acetylcholine synthesis and release machinery in acute allergic airway inflammation of rat and mouse. Life Sci. 2007a;80:2263–2269. doi: 10.1016/j.lfs.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Reiners K, Rimasch C, Kuchelmeister K, Braun-Dullaeus RC, et al. Expression of the high-affinity choline transporter CHT1 in rat and human arteries. J Histochem Cytochem. 2003;51:1645–1654. doi: 10.1177/002215540305101208. [DOI] [PubMed] [Google Scholar]
- Lips KS, Volk C, Schmitt BM, Pfeil U, Arndt P, Miska D, et al. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol. 2005;33:79–88. doi: 10.1165/rcmb.2004-0363OC. [DOI] [PubMed] [Google Scholar]
- Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, et al. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol. 2007b;51:1042–1053. doi: 10.1016/j.eururo.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Liu RH, Mizuta M, Matsukura S. The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther. 2004;310:52–58. doi: 10.1124/jpet.103.065037. [DOI] [PubMed] [Google Scholar]
- Matthiesen S, Bahulayan A, Holz O, Racke K. MAPK pathway mediates muscarinic receptor-induced human lung fibroblast proliferation. Life Sci. 2007;80:2259–2262. doi: 10.1016/j.lfs.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Matthiesen S, Bahulayan A, Kempkens S, Haag S, Fuhrmann M, Stichnote C, et al. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am J Respir Cell Mol Biol. 2006;35:621–627. doi: 10.1165/rcmb.2005-0343RC. [DOI] [PubMed] [Google Scholar]
- Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, et al. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol. 1998;54:779–788. doi: 10.1124/mol.54.5.779. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Fritz S. Ovarian acetylcholine and muscarinic receptors: hints of a novel intrinsic ovarian regulatory system. Microsc Res Tech. 2002;59:503–508. doi: 10.1002/jemt.10228. [DOI] [PubMed] [Google Scholar]
- Metzen J, Bittinger F, Kirkpatrick CJ, Kilbinger H, Wessler I. Proliferative effect of acetylcholine on rat trachea epithelial cells is mediated by nicotinic receptors and muscarinic receptors of the M1-subtype. Life Sci. 2003;72:2075–2080. doi: 10.1016/s0024-3205(03)00086-9. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Benowitz NL, Heller PH, Levine JD. Contribution of adrenal hormones to nicotine-induced inhibition of synovial plasma extravasation in the rat. Br J Pharmacol. 1997b;120:298–304. doi: 10.1038/sj.bjp.0700884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Benowitz NL, Levine JD. Endogenous opioids suppress activation of nociceptors by sub-nanomolar nicotine. Br J Pharmacol. 2001;133:23–28. doi: 10.1038/sj.bjp.0704031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao FJ, Jänig W, Levine JD. Vagal branches involved in inhibition of bradykinin-induced synovial plasma extravasation by intrathecal nicotine and noxious stimulation in the rat. J Physiol. 1997a;498:473–481. doi: 10.1113/jphysiol.1997.sp021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P, Kirkpatrick KA, Ralevic V, Toothill V, Pearson J, Burnstock G. Endothelial cells cultured from human umbilical vein release ATP, substance P and acetylcholine in response to increased flow. Proc Biol Sci. 1990;241:245–248. doi: 10.1098/rspb.1990.0092. [DOI] [PubMed] [Google Scholar]
- Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, et al. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem. 2007;102:479–492. doi: 10.1111/j.1471-4159.2007.04498.x. [DOI] [PubMed] [Google Scholar]
- Nance DM, Hopkins DA, Bieger D. Re-investigation of the innervation of the thymus gland in mice and rats. Brain Behav Immun. 1987;1:134–147. doi: 10.1016/0889-1591(87)90016-x. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Razen M, Habermehl P, Meyer CU, Zepp F, Kirkpatrick CJ, et al. The non-neuronal cholinergic system in peripheral blood cells: effects of nicotinic and muscarinic receptor antagonists on phagocytosis, respiratory burst and migration. Life Sci. 2007;80:2361–2364. doi: 10.1016/j.lfs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Hall LL, Gallacher G, Ndoye A, Jolkovsky DL, Webber RJ, et al. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–949. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Fujii T, Watanabe Y, Kawashima K. Expression of multiple mRNA species for choline acetyltransferase in human T-lymphocytes. Life Sci. 2003;72:2127–2130. doi: 10.1016/s0024-3205(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Oka T, Badyuk M, Xiao Y, Kellar KJ, Daly JW. Mouse beta-TC6 insulinoma cells: high expression of functional alpha3beta4 nicotinic receptors mediating membrane potential, intracellular calcium, and insulin release. Mol Pharmacol. 2006;69:899–907. doi: 10.1124/mol.105.014902. [DOI] [PubMed] [Google Scholar]
- Olubadewo JO, Rama Sastry BV. Human placental cholinergic system: stimulation–secretion coupling for release of acetylcholine from isolated placental villus. J Pharmacol Exp Ther. 1978;204:433–445. [PubMed] [Google Scholar]
- Pahl A, Bauhofer A, Petzold U, Cnota PJ, Maus J, Brune K, et al. Synergistic effects of the anti-cholinergic R, R-glycopyrrolate with anti-inflammatory drugs. Biochem Pharmacol. 2006;72:1690–1696. doi: 10.1016/j.bcp.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Paraoanu LE, Steinert G, Koehler A, Wessler I, Layer PG. Expression and possible functions of the cholinergic system in a murine embryonic stem cell line. Life Sci. 2007;80:2375–2379. doi: 10.1016/j.lfs.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pfeil U, Lips KS, Eberling L, Grau V, Haberberger RV, Kummer W. Expression of the high-affinity choline transporter, CHT1, in the rat trachea. Am J Respir Cell Mol Biol. 2003;28:473–477. doi: 10.1165/rcmb.2002-0190OC. [DOI] [PubMed] [Google Scholar]
- Plummer HK, III, Dhar M, Schuller HM. Expression of the alpha7 nicotinic acetylcholine receptor in human lung cells. Respir Res. 2005;6:29. doi: 10.1186/1465-9921-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profita M, Bonanno A, Siena L, Ferraro M, Montalbano AM, Pompeo F, et al. Acetylcholine mediates the release of IL-8 in human bronchial epithelial cells by a NFkB/ERK-dependent mechanism. Eur J Pharmacol. 2008;582:145–153. doi: 10.1016/j.ejphar.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Profita M, Giorgi RD, Sala A, Bonanno A, Riccobono L, Mirabella F, et al. Muscarinic receptors, leukotriene B4 production and neutrophilic inflammation in COPD patients. Allergy. 2005;60:1361–1369. doi: 10.1111/j.1398-9995.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- Proskocil BJ, Sekhon HS, Jia Y, Savchenko V, Blakely RD, Lindstrom J, et al. Acetylcholine is an autocrine or paracrine hormone synthesized and secreted by airway bronchial epithelial cells. Endocrinology. 2004;145:2498–2506. doi: 10.1210/en.2003-1728. [DOI] [PubMed] [Google Scholar]
- Qu J, Zhou X, Xie R, Zhang L, Hu D, Li H, et al. The presence of m1 to m5 receptors in human sclera: evidence of the sclera as a potential site of action for muscarinic receptor antagonists. Curr Eye Res. 2006;31:587–597. doi: 10.1080/02713680600770609. [DOI] [PubMed] [Google Scholar]
- Racké K, Haag S, Jürgens KU, Warnken M. Muscarinic receptors mediate stimulation of collagen synthesis in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol (Suppl) 2008;377:17. [Google Scholar]
- Racké K, Juergens UR, Matthiesen S. Control by cholinergic mechanisms. Eur J Pharmacol. 2006;533:57–68. doi: 10.1016/j.ejphar.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Raufman JP, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig Dis Sci. 2003;48:1431–1444. doi: 10.1023/a:1024733500950. [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, Nandi JS, Mishra NC, Singh SP, et al. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol. 2007;179:2889–2898. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Bernedo P, Klapproth H, Oelert H, Zeiske B, Racke K, et al. Acetylcholine in isolated airways of rat, guinea pig, and human: species differences in role of airway mucosa. Am J Physiol. 1996;270:L722–L728. doi: 10.1152/ajplung.1996.270.5.L722. [DOI] [PubMed] [Google Scholar]
- Reinheimer T, Mohlig T, Zimmermann S, Hohle KD, Wessler I. Muscarinic control of histamine release from airways. Inhibitory M1-receptors in human bronchi but absence in rat trachea. Am J Respir Crit Care Med. 2000;162:534–538. doi: 10.1164/ajrccm.162.2.9911094. [DOI] [PubMed] [Google Scholar]
- Richardson CE, Morgan JM, Jasani B, Green JT, Rhodes J, Williams GT, et al. Effect of smoking and transdermal nicotine on colonic nicotinic acetylcholine receptors in ulcerative colitis. QJM. 2003;96:57–65. doi: 10.1093/qjmed/hcg007. [DOI] [PubMed] [Google Scholar]
- Roman J, Ritzenthaler JD, Gil-Acosta A, Rivera HN, Roser-Page S. Nicotine and fibronectin expression in lung fibroblasts: implications for tobacco-related lung tissue remodeling. FASEB J. 2004;18:1436–1438. doi: 10.1096/fj.03-0826fje. [DOI] [PubMed] [Google Scholar]
- Romano SJ, Corriveau RA, Schwarz RI, Berg DK. Expression of the nicotinic receptor alpha 7 gene in tendon and periosteum during early development. J Neurochem. 1997;68:640–648. doi: 10.1046/j.1471-4159.1997.68020640.x. [DOI] [PubMed] [Google Scholar]
- Santos SC, Vala I, Miguel C, Barata JT, Garção P, Agostinho P, et al. Expression and subcellular localization of a novel nuclear acetylcholinesterase protein. J Biol Chem. 2007;282:25597–25603. doi: 10.1074/jbc.M700569200. [DOI] [PubMed] [Google Scholar]
- Sastry BV, Sadavongvivad C. Cholinergic systems in non-nervous tissues. Pharmacol Rev. 1978;30:65–132. [PubMed] [Google Scholar]
- Schlereth T, Birklein F, an Haack K, Schiffmann S, Kilbinger H, Kirkpatrick CJ, et al. In vivo release of non-neuronal acetylcholine from the human skin as measured by dermal microdialysis: effect of botulinum toxin. Br J Pharmacol. 2006;147:183–187. doi: 10.1038/sj.bjp.0706451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. Acetylcholine in normal and diseased skin. Br J Dermatol. 1962;74:317–322. doi: 10.1111/j.1365-2133.1962.tb13518.x. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER. Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol. 2002;26:31–41. doi: 10.1165/ajrcmb.26.1.4170. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Song P, Jia Y, Lindstrom J, Spindel ER. Expression of lynx1 in developing lung and its modulation by prenatal nicotine exposure. Cell Tissue Res. 2005;320:287–297. doi: 10.1007/s00441-005-1077-9. [DOI] [PubMed] [Google Scholar]
- Serobyan N, Jagannathan S, Orlovskaya I, Schraufstatter I, Skok M, Loring J, et al. The cholinergic system is involved in regulation of the development of the hematopoietic system. Life Sci. 2007;80:2352–2360. doi: 10.1016/j.lfs.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Skok MV, Grailhe R, Agenes F, Changeux JP. The role of nicotinic receptors in B-lymphocyte development and activation. Life Sci. 2007;80:2334–2336. doi: 10.1016/j.lfs.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Lu A, Arredondo J, Sauer D, Gravett C, et al. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007;67:3936–3944. doi: 10.1158/0008-5472.CAN-06-2484. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Proskocil B, Blusztajn JK, Mark GP, Spindel ER. Synthesis of acetylcholine by lung cancer. Life Sci. 2003;72:2159–2168. doi: 10.1016/s0024-3205(03)00078-x. [DOI] [PubMed] [Google Scholar]
- Strassheim D, May LG, Varker KA, Puhl HL, Phelps SH, Porter RA, et al. M3 muscarinic acetylcholine receptors regulate cytoplasmic myosin by a process involving RhoA and requiring conventional protein kinase C isoforms. J Biol Chem. 1999;274:18675–18685. doi: 10.1074/jbc.274.26.18675. [DOI] [PubMed] [Google Scholar]
- Tournier JM, Maouche K, Coraux C, Zahm JM, Cloez-Tayarani I, Nawrocki-Raby B, et al. Alpha3alpha5beta2-nicotinic acetylcholine receptor contributes to the wound repair of the respiratory epithelium by modulating intracellular calcium in migrating cells. Am J Pathol. 2006;168:55–68. doi: 10.2353/ajpath.2006.050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey WR, Peach MJ. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ Res. 1992;70:234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- Trombino S, Cesario A, Margaritora S, Granone P, Motta G, Falugi C, et al. Alpha7-nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res. 2004;64:135–145. doi: 10.1158/0008-5472.can-03-1672. [DOI] [PubMed] [Google Scholar]
- Tsai CH, Yeh HI, Tian TY, Lee YN, Lu CS, Ko YS. Down-regulating effect of nicotine on connexin43 gap junctions in human umbilical vein endothelial cells is attenuated by statins. Eur J Cell Biol. 2004;82:589–595. doi: 10.1078/0171-9335-00348. [DOI] [PubMed] [Google Scholar]
- Ueda F, Ban K, Ishima T. Irsogladine activates gap-junctional intercellular communication through M1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther. 1995;274:815–819. [PubMed] [Google Scholar]
- Valera S, Ballivet M, Bertrand D. Progesterone modulates a neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1992;89:9949–9953. doi: 10.1073/pnas.89.20.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L, Brink C, Norel X. The muscarinic receptor subtypes in human blood vessels. Therapie. 2001;56:223–226. [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, et al. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–1209. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Ai X, Zhao J, Hao X, Lu Y, et al. Nicotine could augment adhesion molecule expression in human endothelial cells through macrophages secreting TNF-alpha, IL-1beta. Int Immunopharmacol. 2004;4:1675–1686. doi: 10.1016/j.intimp.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Z, Zhou Y, Liu L, Zhao Y, Yao C, et al. Nicotine stimulates adhesion molecular expression via calcium influx and mitogen-activated protein kinases in human endothelial cells. Int J Biochem Cell Biol. 2006;38:170–182. doi: 10.1016/j.biocel.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Wessler I, Bittinger F, Kamin W, Zepp F, Meyer E, Schad A, et al. Dysfunction of the non-neuronal cholinergic system in the airways and blood cells of patients with cystic fibrosis. Life Sci. 2007a;80:2253–2258. doi: 10.1016/j.lfs.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Wessler I, Deutsch C, Brockerhoff P, Bittinger F, Kirkpatrick CJ, Kilbinger H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br J Pharmacol. 2001d;134:951–956. doi: 10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler I, Holper B, Kortsik C, Buhl R, Kilbinger H, Kirkpatrick CJ. Dysfunctional inhibitory muscarinic receptors mediate enhanced histamine release in isolated human bronchi. Life Sci. 2007b;80:2294–2297. doi: 10.1016/j.lfs.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H, Bittinger F, Kirkpatrick CJ. The biological role of non-neuronal acetylcholine in plants and humans. Jpn J Pharmacol. 2001a;85:2–10. doi: 10.1254/jjp.85.2. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003a;72:2055–2061. doi: 10.1016/s0024-3205(03)00083-3. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ. The non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001a;14:423–434. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ.Role of non-neuronal and neuronal acetylcholine in the airways Musacrinic Receptors in the Airways 2001bBirkhäuser Verlag: Basel, Boston, Berlin; 25–62.In: Zaagsma J, Meurs H, Roffel AF (eds). [Google Scholar]
- Wessler I, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, Racke K. The cholinergic ‘pitfall': acetylcholine, a universal cell molecule in biological systems, including humans. Clin Exp Pharmacol Physiol. 1999;26:198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Panter H, Bittinger F, Kriegsmann J, Kirkpatrick CJ, Kawashima K, et al. Subcellular location of choline acetyltransferase (ChAT) and acetylcholine (ACh) in human placenta. Naunyn Schmiedebergs Arch Pharmacol (Suppl) 2001c;363:R23. [Google Scholar]
- Wessler I, Reinheimer T, Kilbinger H, Bittinger F, Kirkpatrick CJ, Saloga J, et al. Increased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci. 2003b;72:2169–2172. doi: 10.1016/s0024-3205(03)00079-1. [DOI] [PubMed] [Google Scholar]
- Wessler I, Roth E, Schwarze S, Weikel W, Bittinger F, Kirkpatrick CJ, et al. Release of non-neuronal acetylcholine from the human placenta: difference to neuronal acetylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2001b;364:205–212. doi: 10.1007/s002100100445. [DOI] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Drachenberg C, Yamada M, Wess J, Raufman JP. Cholinergic agonist-induced pepsinogen secretion from murine gastric chief cells is mediated by M1 and M3 muscarinic receptors. Am J Physiol Gastrointest Liver Physiol. 2005;289:G521–G529. doi: 10.1152/ajpgi.00105.2004. [DOI] [PubMed] [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, et al. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Hellstrom-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism. 2005;54:247–254. doi: 10.1016/j.metabol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, et al. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci. 2007;80:2308–2313. doi: 10.1016/j.lfs.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol. 1997;97:243–262. [PubMed] [Google Scholar]
- Zimring JC, Kapp LM, Yamada M, Wess J, Kapp JA. Regulation of CD8+ cytolytic T lymphocyte differentiation by a cholinergic pathway. J Neuroimmunol. 2005;164:66–75. doi: 10.1016/j.jneuroim.2005.03.018. [DOI] [PubMed] [Google Scholar]