Abstract
KIT is a member of the tyrosine kinase family of growth factor receptors which is expressed on a variety of haematopoietic cells including mast cells. Stem cell factor (SCF)-dependent activation of KIT is critical for mast cell homeostasis and function. However, when KIT is inappropriately activated, accumulation of mast cells in tissues results in mastocytosis. Such dysregulated KIT activation is a manifestation of specific activating point mutations within KIT, with the human D816V mutation considered as a hallmark of human systemic mastocytosis. A number of other activating mutations in KIT have recently been identified and these mutations may also contribute to aberrant mast cell growth. In addition to its role in mast cell growth, differentiation and survival, localized concentration gradients of SCF may control the targeting of mast cells to specific tissues and, once resident within these tissues, mast cell activation by antigen may also be amplified by SCF. Thus, KIT inhibitors may have potential application in multiple conditions linked to mast cells including systemic mastocytosis, anaphylaxis, and asthma. In this review, we discuss the role of KIT in the context of mast cells in these disease states and how recent advances in the development of inhibitors of KIT activity and function may offer novel therapies for the treatment of these disorders.
Keywords: mast cells, KIT, mastocytosis, anaphylaxis, allergy, tyrosine kinase inhibitors
Introduction
The human c-KIT oncogene, which is mapped to the W locus in the mouse, encodes for a protein, KIT (CD117), which is a member of the transmembrane receptors with tyrosine kinase activity superfamily. This family also includes other growth factor receptors, namely FMS-like tyrosine kinase 3 (FLT3), the platelet-derived growth factor receptor (PDGFR), and the macrophage colony stimulating factor (M-CSF) receptor (Broudy, 1997; Patnaik et al., 2007). Expression of human KIT is primarily restricted to melanocytes, germ cells and cells of haematopoietic lineage, including bone marrow progenitor cells, mast cells, megakaryocytes (Kitamura et al., 1995, 2006; Roskoski, 2005a, 2005b; Alexeev and Yoon, 2006) and, to a lesser extent, basophils and eosinophils (Yuan et al., 1997; Heinemann et al., 2005). It has also been reported to be expressed in other cell types such as epithelial cells and vascular smooth muscle cells (Al-Muhsen et al., 2004; Hollenbeck et al., 2004). KIT is expressed as several alternatively spliced isoforms, which, following glycosylation, give rise to proteins with multiple molecular weights around 145 kDa (Yarden et al., 1987). The ligand for KIT, stem cell factor (SCF), also known as steel factor based on its generation by the Steel locus (Sl) in mouse (Yarden et al., 1987; Huang et al., 1990), exists in two forms that are produced by alternative splicing: a soluble form of approximately 31 kDa and a membrane-bound form of approximately 32 kDa, which lacks the proteolytic site for processing into the soluble form (Flanagan and Leder, 1990). In both human and mouse, there is evidence that KIT may respond differentially to these individual forms of SCF (Toksoz et al., 1992; Kapur et al., 1998; Trieselmann et al., 2003). SCF is expressed in fibroblasts, thymus tissue, spleen, testes, placenta and mast cells (reviewed by Broudy, 1997; Reber et al., 2006) and, under normal conditions, in humans it is present in the plasma at concentrations of approximately 1–3 ng mL−1 (∼0.2 nM) (Kojima et al., 1997). However, local concentrations near the sites of origin and localized membrane-associated KIT concentrations are likely to be significantly higher.
KIT has five immunoglobulin-like regions within its extracellular domain that contain the binding site for SCF (Figure 1). The Kd for the binding of SCF to human KIT is reported to be 4.2 pM for high-affinity binding and 1.7 nM for lower-affinity binding (Broudy et al., 1994). Thus, reasonable increases in localized concentrations of SCF would be sufficient for optimal activation of the receptor. As with other receptors with tyrosine kinase activity, KIT-mediated cellular responses follow ligand-induced dimerization of the receptor and resulting activation of its inherent tyrosine kinase activity. This activity is associated with a split catalytic domain contained within its cytosolic domain (Mol et al., 2003) (Figure 1). The KIT kinase activity targets specific tyrosine residues also contained within the cytosolic domain of KIT. Thus, once ligated, KIT undergoes auto/transphosphorylation (Mol et al., 2003). The Src kinase, Lyn, may also contribute to phosphorylation of specific sites within KIT (Shivakrupa and Linnekin, 2005). Following phosphorylation, sequences containing these phosphotyrosines become docking sites for associating critical signalling molecules. These molecules include the Src kinases Lyn and Fyn, phospholipase Cγ, phosphoinositide 3-kinase (PI3K) and the adaptor molecules Grb2 and Shc (Lev et al., 1992; Serve et al., 1994; Herbst et al., 1995; Linnekin et al., 1997; Price et al., 1997; Thommes et al., 1999; Gilfillan and Tkaczyk, 2006; Samayawardhena et al., 2006) (Figure 2). The specific residues responsible for these interactions have been mapped by site-directed mutagenesis, revealing that, in the case of human KIT, phospholipase Cγ binds to pY936 (Herbst et al., 1995), PI3K to pY721 (Herbst et al., 1995), Grb2 to pY703 and pY936 (Herbst et al., 1995; Thommes et al., 1999), and Lyn and Fyn to pY568 and pY570 (Linnekin et al., 1997; Samayawardhena et al., 2006). By recruiting these molecules, a receptor-signalling molecule complex is established which coordinates the critical downstream signalling events leading to the diverse cellular responses attributable to KIT. Also recruited are a number of signalling molecules whose function is to terminate an ongoing reaction. These include the phosphoinositide phosphatase SHIP (van Dijk et al., 2000), which reverses the actions of PI3K, the protein phosphatase SHP1 (Kozlowski et al., 1998), which reverses the phosphorylation events initiated by KIT, the tyrosine kinase Chk (Price et al., 1997), which inactivates Src kinases, and SOCS6 (Bayle et al., 2004), which downregulates signalling pathways leading to cytokine gene expression. As it is not the purpose of this review to discuss in length the downstream signalling processes that account for the biological responses mediated by KIT, readers are referred to several excellent reviews that contain in-depth discussions of these events (Broudy, 1997; Linnekin, 1999; Lennartsson et al., 2005; Roskoski, 2005a, 2005b; Reber et al., 2006) and to Figure 2, which summarizes these process.
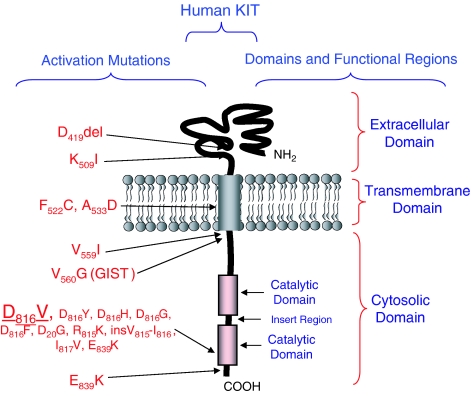
Figure 1.
Structure of human KIT and selected mutations associated with specific human disease states.
Figure 2.
Binding of specific signalling proteins to phosphorylated tyrosine residues on KIT following receptor activation and the subsequent signalling events leading to mast cell responses. For clarity, only one receptor is shown in the figure. SCF-induced KIT dimerization enhances the tyrosine kinase activity associated with the split catalytic domain. This results in phosphorylation of specific tyrosine residues within the cytosolic domain of KIT. The tyrosine kinase, Lyn, may also contribute to this response. The consequential recruitment of critical signalling molecules such as the tyrosine kinases, Lyn and Fyn, the adaptor molecules, SHC and Grb2 and the signalling enzymes, PI3K and phospholipase Cγ, results in the activation of the Ras–Raf–mitogen-activated protein kinase (MAPK) pathway, the enhancement of intracellular calcium levels, and the activation of transcription factors leading to mast cell growth, differentiation and survival; enhanced cell migration and chemotaxis; and cytokine production. Activation of the JAK2 and STAT1/3/5 likely contributes to these responses. Concurrent with these events, KIT induces activation of the Tec kinase Btk and the adaptor molecule NTAL (non-T-cell activation linker), resulting in an enhancement of antigen-mediated degranulation and cytokine production. (For further details, please refer to Broudy, 1997; Linnekin, 1999; Tkaczyk et al., 2004; Iwaki et al., 2005; Lennartsson et al., 2005; Roskoski, 2005a, 2005b; Gilfillan and Tkaczyk, 2006; Reber et al., 2006.)
Roles of KIT and normal and dysregulated control of mast cell function
Stem cell factor-dependent ligation of KIT, and the subsequent activation of KIT kinase activity, is essential for mast cell homeostasis. Mice that are defective for functional KIT expression (W/Wv and W/Wsh) (Kitamura et al., 1978) and those defective for SCF (Sl/Sld) (Kitamura and Go, 1979) are thus mast cell deficient. In humans, expansion and differentiation of mast cells from their CD34+/CD117+ progenitor cells is also dependent on SCF, as demonstrated in ex vivo culture systems (Kirshenbaum et al., 1999). Once mature, the continued survival of human mast cells is also dependent on the presence of SCF, as cells deprived of SCF display signs of apoptosis within 24–48 h (Metcalfe et al., 1995). A number of activating mutations in KIT are associated with the dysregulated growth of mast cells associated with mastocytosis (Akin and Metcalfe, 2004). These activating mutations will be discussed later. Under experimental conditions, in vitro, SCF has been demonstrated to be a potent chemotactic agent for mast cells (Nilsson et al., 1994; Dastych et al., 1998) and mast cell precursors (Taylor et al., 2001), and to induce mast cell adhesion to basement membrane proteins (Dastych and Metcalfe, 1994; Lorentz et al., 2002). Thus, in vivo, in addition to its role in mast cell homeostasis, SCF likely contributes to the processes that regulate the homing of mast cells to their sites of residence within tissues/organs such as skin, intestinal mucosa and submucosa, alveolar walls, nasal mucosa, bronchial subepithelium and tonsils (Okayama and Kawakami, 2006). However, the extent to which SCF contributes to this process in vivo is currently unknown.
In addition to its ability to regulate mast cell homeostasis and tissue distribution, SCF is also recognized as a potent modifier of mast cell activation. When triggered, mast cells release an array of inflammatory mediators that contribute to the initiation of anaphylaxis and the inflammatory reactions associated with the asthmatic response (Metcalfe et al., 1997). These mediators include granule-associated bioactive amines such as histamine and 5-hydroxytryptamine (serotonin), eicosanoids such as prostaglandin D2 and leukotriene C4, chemokines such as CCL2, CCL3, CCL5 and cytokines such as tumour necrosis factor-α (TNF-α), granulocyte macrophage colony-stimulating factor, interleukins (ILs) 3, 4, 5, 6, 10 (Galli et al., 2005; Gilfillan and Tkaczyk, 2006). These events generally occur following antigen-dependent aggregation of IgE-occupied high-affinity receptors for IgE (FcɛRI) on the mast cell surface (Metcalfe et al., 1997; Galli et al., 2005; Rivera and Gilfillan, 2006; Tkaczyk et al., 2006). FcɛRI-mediated mast cell activation, however, can be dramatically enhanced by the concurrent activation of other receptors on mast cells, including KIT, and those for adenosine, sphingosine-1-phosphate, complement component C3a, PGE2 and lysophosphatidic acid (Gilfillan and Tkaczyk, 2006; Kuehn and Gilfillan, 2007), although, in cultured human mast cells, adenosine and PGE2 can also inhibit antigen-induced mast cell degranulation (Kuehn and Gilfillan, 2007). Owing to its unique influence on mast cell homeostasis (Kitamura et al., 2006), however, of these receptors, KIT is likely the major modifier of antigen-mediated mast cell responses in a physiological setting. The evidence supporting the role of KIT as a co-activator of mast cells, however, has primarily emerged from studies conducted in both rodent and human mast cell cultures where it has been demonstrated that the threshold for antigen-mediated degranulation is lowered and the magnitude of this response is synergistically enhanced by SCF (Gilfillan and Tkaczyk, 2006). These studies have also demonstrated that antigen-mediated cytokine production in mast cells is also dramatically enhanced by SCF. Thus, as with the role of KIT on mast cell homing, the precise contribution of KIT activation on mast cell activation in vivo has yet to be determined.
KIT and mast cell disorders
Although KIT is a critical molecule in haematopoesis, gametogenesis, and mast cell development, activating mutations resulting in ligand-independent autophosphorylation may lead to dysregulated growth of the affected cells, thereby inducing tumourogenesis (Akin and Metcalfe, 2004). Diseases such as gastrointestinal stromal tumours, systemic mastocytosis, germ cell tumours and core factor binding acute myeloid leukaemias carry mutations in KIT (Patnaik et al., 2007). However, in terms of mast cell disorders, the two principal conditions where aberrant KIT activation may play a role are mastocytosis and anaphylaxis.
Mastocytosis
Pathologic constitutive activation of KIT is associated with the mast cell proliferative disorder, mastocytosis (Valent et al., 2001, 2003b), as originally described by Nagata et al. (1995). More than 90% of patients with systemic mastocytosis have the D816V KIT point mutation, resulting in SCF-independent autophosphorylation (Akin, 2006; Garcia-Montero et al., 2006). The aspartic acid produced by Codon 816 of KIT is located in the tyrosine kinase domain and is critically involved in ATP binding and subsequent phosphotransferase activity of the receptor (Mol et al., 2003; Vendome et al., 2005). Mutations resulting in replacement of this aspartic acid with valine stabilize the kinase in its active conformation (Mol et al., 2004; Vendome et al., 2005), thus obviating the need for binding SCF for autophosphorylation (Furitsu et al., 1993). Although D816V is by far the most common mutation detected in mastocytosis, mutations involving other KIT domains (such as in the juxtamembrane, transmembrane and extracellular regions) have also been described (Figure 1).
The exact role of D816V KIT in pathogenesis of mastocytosis remains to be identified. In vitro studies showed that D816V KIT was able to confer growth factor independence to transformed haematopoietic cell lines (Kitayama et al., 1995) as well as murine haematopietic progenitor cells (Kitayama et al., 1996). Human mast cells carrying D816V KIT were more resistant to apoptosis induced by SCF withdrawal (Akin et al., 2003) and migrated more vigorously to SCF (Taylor et al., 2004). Animal studies showed some, but not all (8 out of 28), transgenic mice carrying D816V c-KIT mutation under the control of chymase promoter develop a disease state resembling mastocytosis with infiltration of tissues such as skin and spleen (Zappulla et al., 2005). On the other hand, mice transplanted with haematopoietic stem cells retrovirally transduced with D814V KIT, as well as mice transgenic for this mutation, develop acute leukaemias and lymphomas but not mastocytosis (Kitayama et al., 1996). In humans, activating KIT mutations are seen not only in mastocytosis, but also in core factor binding acute leukaemias (Beghini et al., 2000), gastrointestinal stromal tumours (Hirota et al., 1998), germ cell tumours (Tian et al., 1999) and some lymphoproliferative disorders (Hongyo et al., 2000). The presence of KIT mutations do not appear to correlate with aggressiveness or prognosis of mastocytosis in humans. Overall, these observations suggest that, although KIT mutations (particularly D816V) are important pathogenic factors in mastocytosis, additional mutations or polymorphisms are needed to determine the final disease phenotype.
Anaphylaxis
In addition to a role for KIT mutations in the development of mastocytosis, it has been proposed that there is a link between aberrant KIT activation and anaphylaxis, although this conclusion is somewhat more controversial. Certainly, based on in vitro studies, it would be logical to assume that an activating mutation in KIT would result in exaggerated antigen-mediated mast cell activation. Surprisingly, one study using mismatch amplification real-time PCR assay found a relatively high occurrence of the D816V mutation (2 out of 9, 22%) in subjects without a history of atopy or anaphylaxis (Lawley et al., 2005). The same study identified the mutation in 9 out of 21 (43%) patients with anaphylaxis, although this apparently increased detection rate in anaphylaxis was not statistically significant (Lawley et al., 2005).
Mice receiving chronic treatment of SCF do not have an increase in IgE-dependent anaphylaxis (Ando et al., 1993). In contrast, there is evidence suggesting that the SCF–KIT axis is important in the development of non-IgE-dependent anaphylaxis. Anaphylactic-type dermal mast cell degranulation was observed in patients with advanced breast cancer who received subcutaneous injections of SCF in a Phase 1 clinical trial (Costa et al., 1996). More recently, the D816V c-KIT mutation and other markers of clonal mast cell disease such as aberrant surface expression of CD25 by mast cells have been reported in a subgroup of patients with recurrent idiopathic anaphylaxis (Akin et al., 2007). Some of these patients had a mild increase in mast cell numbers in bone marrow, which did not meet the diagnostic criteria for systemic mastocytosis. Such patients who experience anaphylaxis and carry a population of clonal mast cells without meeting the diagnostic criteria for systemic mastocytosis have been termed to have a monoclonal mast cell activation syndrome (Florian et al., 2005; Akin et al., 2007).
The multiple roles that SCF and KIT may play in dysregulated mast cell homeostasis and activation therefore provide a basis for considering inhibitors of KIT activity and function in the treatment of a number of mast cell related disorders including mastocytosis, atopic asthma, and anaphylaxis.
Pharmacological targeting of KIT
It is clear from mutational analysis and from studies conducted in knock out mice that KIT-induced phosphorylation of the tyrosines contained within the cytosolic tail and the subsequent recruitment of signalling molecules are essential events for the biological function of KIT (Broudy, 1997; Linnekin, 1999; Roskoski, 2005a, 2005b; Akin et al., 2007). Thus, pharmacological targeting of these processes, especially the KIT catalytic activity, has been a major strategy for blocking KIT-mediated responses. In the following sections, we will discuss the pharmacology of KIT inhibitors and how the disease states discussed above may be suitable target for potential targeting with KIT inhibitors.
The various tyrosine kinase inhibitors that have been described to inhibit KIT activity are listed in Table 1. The most widely recognized compound that blocks KIT catalytic activity is imatinib mesylate (imatinib) (also known as STI571, Gleevec and Glivec). Imatinib targets KIT at the ATP-binding site, thereby maintaining the receptor in a non-activated state. It is relatively selective as, in addition to KIT, it has been reported to inhibit only Bcr-Abl and the PDGFR. This may explain why imatinib induces relatively few side effects and is well tolerated (Levitzki and Mishani, 2006). Imatinib targets not only wild-type KIT but also KIT carrying the V560G mutation (Heinrich et al., 2000). However, KIT carrying the D816V mutation associated with systemic mastocytosis is resistant to imatinib inhibition, due to the mutation changing the ATP binding site configuration, thereby blocking the binding of imatinib to KIT (Scheinfeld, 2006). Thus, although imatinib can prevent the growth of human mast cells that express wild-type KIT, the dysregulated growth of tumour mast cells linked to the D816V mutation is resistant to imatinib treatment (Zermati et al., 2003). A similar pharmacological profile has been reported for the imatinib mimetics, nilotinib (AMN107) and PD180970, which can inhibit both wild-type KIT and KIT carrying the V560G mutation, but not KIT containing the D816V mutation (Corbin et al., 2004; Verstovsek et al., 2006a; Chow et al., 2007). Nilotinib, in addition to targeting, KIT, Bcr-Abl and the PDGFR, has also been described to be cytotoxic to B cells, due to caspase activation, independently of kinase inhibition (Gleixner et al., 2006). Besides KIT, PD180970 has been described to inhibit only Bcr-Abl and Src (Dorsey et al., 2000). There has therefore been a focus on the development of KIT kinase inhibitors that overcome the drug-resistance associated with the D816V mutation.
Table 1.
KIT inhibitors and their targets
| Inhibitor | Additional names | Kit target | Additional targets | Reference |
|---|---|---|---|---|
| Imatinib | Gleevec | Wild type, V560G | Bcr-Abl, PDGFR | (Jensen et al., 2007) |
| Glivec | (Levitzki et al., 2006) | |||
| STI571 | (Ma et al., 2002) | |||
| Nilotinib | AMN107 | Wild type, V560G | Bcr-Abl, PDGFR | (Chow et al., 2007) |
| (Gleixner et al., 2006) | ||||
| PD180970 | Wild type, V560G | Bcr-Abl, Src | (Corbin et al., 2004) | |
| (Roskoski, 2005a, 2005b) | ||||
| Dasatinib | BMS-354825 | Wild type, V560G, D816V | Src kinases, Tec, Btk | (Shah et al., 2006) |
| (Gleixner et al., 2007) | ||||
| (Hantschel et al., 2007) | ||||
| Midostaurin | PKC412 | Wild type, V560G, D816V | PKC, FLT3, VEGFR2, | (Patnaik et al., 2007) |
| N-benzoyl-staurosporine | PDGFR, FGFR | (Fabbro et al., 2000) | ||
| (Gleixner et al., 2006) | ||||
| Hypothemycin | Wild type, D816V | a | (Schirmer et al., 2006) | |
| EXEL-0862 | Wild type, D816V | STAT3 | (Pan et al., 2007) | |
| MLN518 | Wild type, D816V | STAT3 | (Corbin et al., 2004) | |
| AP23646/AP23848 | Wild type, D816V | STAT3, Akt, ERK | (Patnaik et al., 2007) | |
| Semaxinib | SU5416 | Wild type, D816V | STAT3, Akt, ERK | (Kosmider et al., 2007) |
| Sunitinib | SU11248 | Wild type, V559D, V645A, | VEGFR, PDGFR, FLT3 | (Chow et al., 2007) |
| V559D/T670I, V670I | (Prenen et al., 2006) | |||
| Sorafenib | BAY 43-9006, Nexavar | Wild type | VEGFR 2,3, PDGFR, FLT3, | (Liu et al., 2006) |
| Raf, MEK, ERK | ||||
| Pazapanib | GW786034 | Wild type | VEGFR 1,3, PDGFRa,b | (Sonpavde and Hutson, 2007) |
| 17-AAG | Wild type | HSP90, Akt, STAT3 | (Ramanathan et al., 2005) | |
| (Fumo et al., 2004) | ||||
| MD-0354 | V560G, D816V | NFkB | (Tanaka et al., 2005) |
PKs with a conserved cysteine in the ATP-binding site.
Recently, several compounds have been identified that inhibit the catalytic activity associated with KIT carrying the D816V mutation. These include dasatinib (BMS-354825), midostaurin (PKC412, N-benzoyl-staurosporine), hypothemycin, EXEL-0862, MLN518, AP23646/AP23848 and semaxinib (SU5416). These compounds are all multikinase inhibitors and therefore less specific than imatinib, nilotinib and PD18070. Dasatinib inhibits the growth of both human mast cell line (HMC)-1.1 and HMC-1.2 human mast cell lines, which express the V560G mutation or the V560G and V816D mutations, respectively. The growth inhibitory effect correlates with the compound's inhibitory effect on KIT autophosphorylation found in both of these cell lines (Shah et al., 2006; Gleixner et al., 2007). Dasatinib has also been reported to cooperate with midostaurin, nilotinib, imatinib and 2CdA (2-chloro-deoxy-adenosine) to produce an enhanced inhibition of growth, and the induction of apoptosis, of neoplastic mast cells (Gleixner et al., 2007). In addition to KIT inhibition, however, dasatinib is also described to target Src kinases and the related Tec kinases Bruton's tyrosine kinase (Btk) and Tec. These properties would explain the reported ability of dasatinib to block histamine release from mast cells and basophils (Hantschel et al., 2007): a process dependent on the activation of both Src kinases and Btk (Gilfillan and Tkaczyk, 2006). Midostaurin has a broad antiproliferative activity against various normal and tumour cell lines, including HMC-1.1 and HMC-1.2 cells, where it is also associated with induction of apoptosis (Fabbro et al., 2000; Gleixner et al., 2006). The many different cell types affected by midostaurin may be due to its several targets besides KIT, as this protein kinase inhibitor also targets PKC, FLT3, vascular endothelial growth factor receptor (VEGFR)-2, PDGFR and FGFR (Fabbro et al., 2000).
In addition to inhibiting wild-type and D816V KIT, hypothemycin has also been described to inhibit other protein kinases with a conserved cysteine in the ATP-binding site. These include several of the mitogen-activated protein kinases (Schirmer et al., 2006). Studies conducted in mast cells also suggest that hypothemycin can inhibit an upstream regulator of Btk (Jensen et al., 2008); however, hypothemycin does not appear to inhibit Btk directly as assessed in an in vitro kinase assay (Schirmer et al., 2006). The ability of hypothemycin to inhibit the activation of Btk in mast cells would again account for the ability of the compound to inhibit antigen-induced and KIT-enhanced mast cell degranulation and cytokine production (Jensen et al., 2008). EXEL-0862, MLN518 AP23646/AP23848 and semaxinib have been reported to inhibit STAT3 phosphorylation in addition to their effects on wild-type and D816V KIT (Corbin et al., 2004; Kosmider et al., 2007; Pan et al., 2007; Patnaik et al., 2007). Furthermore, AP23646/AP23848 and semaxinib have also been shown to affect Akt and ERK activities (Kosmider et al., 2007; Patnaik et al., 2007).
Certain VEGFR inhibitors have been found to also target KIT; however, their ability to inhibit KIT containing the D816V mutation is unreported. Sunitinib (SU11248), for example, inhibits VEGFR, PDGFR, KIT, FLT3 and has been reported to inhibit KIT containing V559D and V559D/T670I mutations in systemic mastocytosis and imatinib-resistant KIT mutations in gastrointestinal stromal tumours (V645A and T670I) (Prenen et al., 2006; Chow and Eckhardt, 2007; Patnaik et al., 2007). Sorafenib (BAY 43-9006, Nexavar), which targets VEGFR2+3, PDGFR, FLT3 and Raf (Liu et al., 2006), inhibits MAPK/ERK kinase and ERK phosphorylation and downregulates the anti-apoptotic protein Mcl-1 in an MAPK/ERK kinase/ERK independent way and also has been demonstrated to inhibit wild-type KIT. Furthermore, pazopanib (GW786034), which targets VEGFR1, 2 and 3 and PDGFR-α and PDGFR-β, also inhibits wild-type KIT (Sonpavde and Hutson, 2007).
A number of compounds, not primarily considered to be tyrosine kinase inhibitors, have also been reported to inhibit KIT activity or KIT-mediated responses in mast cells. These include the following: (1) The anti-tumour antibiotic 17-allylamino-17-demethoxygeldanamycin, which, in addition to targeting the cytocolic heat shock protein 90 (Ramanathan et al., 2005), was also reported to decrease the phosphorylation of KIT, Akt and STAT3 in both HMC-1 subclones (Fumo et al., 2004); (2) IMD-0354, an nuclear factor-κB inhibitor that has been shown to suppress proliferation of the subtype HMC-1.2 cells but not in cord blood-derived human mast cells expressing wild-type KIT (Tanaka et al., 2005). HMC-1.2 cells have constitutively activated nuclear factor-κB, which mediates upregulation of cyclin D3, resulting in cell cycle progression. Targeting nuclear factor-κB and inhibiting its activation pathway therefore results in suppression of cell proliferation; (3) Rapamycin targets the serine/threonine protein kinase mTOR, which is activated by KIT in a PI3K-dependent manner in mast cells (Kim et al., 2008). The mTOR pathways is critical for optimal mast cell growth, and tumour mast cells expressing the V560G or D816V mutation have constitutively activated mTOR. Rapamycin has been reported to only induce apoptosis in cells carrying D816V mutation, but not in V560G-mutated cells or wild-type mast cells (Gabillot-Carre et al., 2006). However, in a later study, rapamycin was reported to inhibit mast cell growth/survival, irrespective of the presence of a specific mutation (Kim et al., 2008). These results are somewhat in contrast with imatinib, which only affects wild type and mast cells expressing V569G KIT.
The potential use of KIT inhibitors in the treatment of systemic mastocytosis
Cytoreductive therapy of systemic mastocytosis is generally reserved for patients with advanced forms of mastocytosis associated with decreased life expectancy (Valent et al., 2003a). This is because of the concerns about potential mutagenicity, teratogenicity and lack of knowledge about the long-term toxicity of tyrosine kinase inhibitors. Inhibition of KIT as a potential cytoreductive approach in mast cell disease is based on several lines of evidence, perhaps the strongest of which is the high (greater than 90%) detection rate of the presence of the activating D816V KIT mutation in mastocytosis. Transfection of this mutation in haematopoietic cells renders growth factor independence (Kitayama et al., 1995, 1996), thereby confirming the role of the mutation in neoplastic transformation. Moreover, there is a strong correlation between the ability of a given compound to inhibit KIT autophosphorylation and its cytotoxic effects on mast cell lines. Ex vivo experiments performed with mononuclear cells obtained from the bone marrow of patients with systemic mastocytosis showed that tyrosine kinase inhibitors effective against D816V KIT cause preferential cytotoxicity of mast cells carrying this mutation over other mononuclear cells (Akin et al., 2003; Shah et al., 2006). On the other hand, it is not known whether neoplastic mast cells have other survival pathways in addition to KIT. This is especially plausible in patients with advanced forms of mastocytosis such as aggressive systemic mastocytosis. Moreover, the concentrations required to inhibit mast cell growth may be difficult to achieve in vivo without toxicity to other tissues. This problem may be particularly pertinent for non-specific inhibitors with multiple targets.
The proof of concept that a KIT inhibitor can indeed cause regression of mastocytosis driven by a KIT mutation resulted from a study in which a patient with an unusual activating KIT mutation was treated with imatinib, and achieved complete remission (Akin et al., 2004). This patient had a transmembrane mutation involving codon 522 (F522C), which was sensitive to imatinib. Treatment of the patient with imatinib doses up to 400 mg day−1 resulted in a dramatic decrease in serum levels of the mast cell marker, tryptase, and mast cell burden. Imatinib is also effective in the treatment of rare cases of systemic mastocytosis associated with chronic eosinophilic leukaemia, which is characterized by the presence of the FIP1L1–PDGFRA rearrangement (Pardanani et al., 2003a, 2003b). This rearrangement occurs due to an approximately 800 kB interstitial deletion in chromosome 4 (detected by deletion of CHIC2 locus in 4q12 by immunofluorescence in situ hybridization or by reverse transcription-PCR), leading to constitutive activation of the intrinsic tyrosine kinase activity of PDGFRA. These patients, who have a multilineage myeloproliferative disorder that involves mast cell as well as eosinophil progenitors, are generally male, display organ pathology due to eosinophilia and do not have the characteristic D816V KIT mutation observed in other categories of mastocytosis (Klion et al., 2003, 2004; Pardanani et al., 2003c; Maric et al., 2007). In addition, a patient with a rare variant of mastocytosis associated with chronic basophilic leukaemia and a PDGFRB fusion has been shown to respond to imatinib (Lahortiga et al., 2008). The therapeutic effect of imatinib in these disorders is due to its inhibition of PDGFR and not KIT.
Imatinib is currently approved by the US Food and Drug Administration for the indication of treatment of adult patients with aggressive systemic mastocytosis without the D816V c-Kit mutation or with c-Kit mutational status unknown. The bulk of the clinical evidence forming the basis of this recommendation appears to have come from patients with chronic eosinophilic leukaemia associated with systemic mastocytosis and FIP1L1–PDGFRA fusion gene as discussed above. However, as opposed to its beneficial effects in rare variants of systemic mastocytosis without codon 816 KIT mutations, or those with PDGFR rearrangements, imatinib is not effective in inhibiting codon 816 KIT mutations carried by the great majority of patients with systemic mastocytosis (Ma et al., 2002; Akin et al., 2003; Zermati et al., 2003). As discussed earlier, this is thought to be due to the inability of the drug to bind the enzymatic pocket of KIT, whose structure is altered by the point mutation in codon 816. In apparent contrast to these findings, a recent study on 14 patients with mastocytosis reported a modest beneficial effect of imatinib on patients with D816V KIT who received the drug, as measured by decreased mast cell mediator levels, bone marrow mast cell numbers and symptomatic improvement (Droogendijk et al., 2006). However, imatinib in this study was administered with glucocorticoids, complicating the interpretation of the response to imatinib. Interestingly, imatinib has been shown to decrease spontaneous histamine release in HMC-1.2 human leukaemic mast cells carrying the D816V mutation (Lober et al., 2008), although it is not cytotoxic to this cell line, which may partially explain the symptomatic improvement observed in the study. However, because imatinib preferentially inhibits wild-type KIT, long-term use of this drug can potentially confer a growth advantage to the neoplastic clones with D816V KIT and therefore theoretically worsen the disease course. On the basis of in vitro data mentioned above as well as our personal experience (Cem Akin), our current practice is not to treat patients with codon 816 KIT mutations with imatinib.
Clinical trials with other KIT inhibitors have largely yielded disappointing results. In a phase-2 trial of nilotinib, among 60 patients (83% positive for D816V KIT) treated with 400 mg twice daily dose of nilotinib, only 2 showed complete remission (Hochhaus et al., 2006). The overall response rate was 20% and two patients died due to disease progression. Nausea and headaches were the most frequently reported side effects. These results are consistent with the lack of in vitro activity of nilotinib on D816V KIT (Verstovsek et al., 2006a). Dasatinib has been evaluated for its therapeutic effect on mastocytosis in a recent clinical trial. Thirty patients with systemic mastocytosis received dasatinib at a dose of 70 mg twice daily (Verstovsek et al., 2006b). There were two complete remissions, both observed in patients without D816V KIT mutation and low tryptase levels. There was a high incidence of systemic toxicity: the drug was stopped in 10 patients and dose reductions were necessary in 12. Six patients developed pleural effusions. These results may be due to the non-specific targeting of KIT by dasatinib, resulting in toxicity in concentrations required to inhibit D816V KIT.
An ongoing clinical trial with midostaurine (100 mg twice daily) has yielded more promising results in an interim analysis (Gotlib et al., 2007). Eleven of 15 (73%) patients with advanced systemic mastocytosis have shown a response. Although there were no complete remissions, 5 patients displayed a major response and 6 had a partial response. Nausea and vomiting were the most frequently observed non-haematologic side effects. Midostaurine has also shown a partial, although temporary, mast cell cytoreductive effect in a patient with mast cell leukaemia with associated myelodysplasia (Gotlib et al., 2005).
Combination therapy of mastocytosis with regimens incorporating tyrosine kinase inhibitors is yet to be explored in clinical trials, although in vitro data obtained so far appear promising. Combination of dasatinib with midostaurine or cladribine yielded synergistic effects in HMC-1.2 mast cells carrying the D816V c-KIT mutation (Gleixner et al., 2007). Another study employing antisense mcl-1 oligonucleotides in combination with midostaurine similarly showed a synergistic growth inhibition in the same cell line (Aichberger et al., 2007).
Potential use of KIT inhibitors in other disorders
As discussed earlier, mast cells play a central role in the allergic reactions following antigen-dependent aggregation of IgE-occupied FcɛRI, a response that, at least under experimental conditions in both mouse and human mast cells, can be strongly potentiated by SCF-induced KIT activation. This can occur even at antigen concentrations that produce minimal degranulation (Tkaczyk et al., 2004). Thus, in addition to the primary targeting of the IgE-response as a therapeutic strategy for the treatment of allergic reactions, as discussed in a recent review article, targeting SCF and/or KIT in addition to FcɛRI may have therapeutic relevance in the treatment of these disorders (Jensen et al., 2007). However, use of KIT inhibitors in non-neoplastic conditions should be contemplated in clinical trials only after a careful consideration of risk-to-benefit ratio of the compounds to be investigated, including demonstration that such compounds do not increase the risk for developing secondary malignancies.
The concept of concurrent inhibition has been explored using hypothemycin, as this molecule in both human and mouse mast cells targets FcɛRI signalling in addition to KIT (Jensen et al., 2008). In addition to its ability to block FcɛRI-mediated and KIT-enhanced degranulation and cytokine production in mast cells in culture, hypothemycin significantly reduced in vivo passive cutaneous anaphylaxis in mice. These results demonstrate the potential of a combined KIT/FcɛRI inhibition in the treatment of allergic diseases.
Mast cells have been implicated in the pathogenesis of rheumatoid arthritis (Malone et al., 1986, 1987; Malone and Metcalfe, 1988), and a recent study (Juurikivi et al., 2005) revealed that imatinib effectively induces mast cell apoptosis and reduces TNF-α production in human synovial tissue cultures. This could lead to attenuation of the inflammation in arthritic joints (Juurikivi et al., 2005). Other diseases where mast cells have been proposed to play a role may also benefit from targeting KIT in a manner similar to that described above, although this has yet to be investigated. Such disorders include migraine headaches, inflammatory bowel disease, multiple sclerosis, artherosclerosis and angiogenesis associated with tumour progression. With regard to migraine headaches, degranulation of rat mast cells from the duramater induced by compound 48/80 was shown to result in excitation of meningal nociceptors, which was accompanied by phosphorylation of the intracellular proteins ERK and c-fos, resulting in a long-lasting activation leading to the migraine headache (Levy et al., 2007). Mast cell mediators have also been shown to be major regulators of the severity of inflammatory bowel disease. In this respect, histamine can induce the smooth muscle and endothelial cell contractions, increased vascular permeability, cytokine production and chemotaxis of inflammatory cells associated with this condition (Fogel et al., 2005). In addition, patients with Crohn's disease have also been found to have an increased number of mast cells in the submucosal layer and muscularis propria of the gut (Oshitani et al., 2006). Therefore, eliminating histamine release by silencing mast cells present in the gastrointestinal tract, in a KIT/SCF dependant way, could contribute to a better prognosis of these conditions.
A role for mast cells in multiple sclerosis has been suggested by their ability to be activated by the main multiple sclerosis protein, myelin basic protein, which results in the release of IL-8 and TNF-α. Furthermore, mast cells appear to markedly enhance T-cell activation by cell-to-cell contact and by TNF-α (Theoharides et al., 2007). The ability of imatinib and hypothemycin to block antigen-mediated IL-8 and TNF-α release from human and mouse mast cells and to block the SCF enhancement of this response (Jensen et al., 2008) may provide the basis for considering KIT inhibitors in the treatment of this disease. A role of mast cells in the progression of atherosclerosis may be due to release of the inflammatory cytokines IL-6 and interferon-γ following mast cell activation as demonstrated in the mouse (Sun et al., 2007). Again, as imatinib and hypothemycin has been shown to inhibit IL-6 release from murine bone marrow-derived mast cells (Jensen et al., 2008), these drugs may be able to dampen the role of mast cells in atherosclerosis. Finally, human tumours are often surrounded by mast cells, and this infiltration is associated with a poor prognosis (Theoharides and Conti, 2004). Using a oncogene mouse model carrying a mutation in the gene coding for RAS, it was shown that not only did these mice have more mast cells in dermis before development of skin tumours, compared with controls, but a decrease in KIT function inhibited the progress of carcinogenesis in the skin (Muto et al., 2007). This indicates that, even though an increase in mast cell number exist, if the KIT response is inhibited, a better prognosis might be obtained.
Conclusions
In summary, in this review, we have discussed how specific mast cell disorders are linked to dysregulated control of the kinase activity associated with KIT and how tyrosine kinase inhibitors targeting KIT show promise in the therapeutic management of such disease states. Future advances in the development of more selective tyrosine kinase inhibitors that would target both normal and mutated KIT may thus offer hope in the effective treatment of mastocytosis and potentially anaphylaxis, and other allergic responses. Furthermore, the potential role of mast cells in other clinical conditions may provide a basis for the investigation of the efficacy of KIT inhibitors in these disorders.
Acknowledgments
Research in the authors' laboratories is supported by the NIAID Intramural Program within the National Institutes of Health, USA (AMG), and the University of Michigan Department of Medicine Funds (CA). Due to the restricted space available, not all pertinent literature could be referenced in this article. This does not imply that studies not quoted are of lesser merit.
Abbreviations
- Btk
Bruton's tyrosine kinase
- FcɛRI
high-affinity receptor for IgE
- FLT3
FMS-like tyrosine kinase 3
- HMC
human mast cell line
- IL
interleukin
- M-CSF
macrophage colony stimulating factor
- PDGFR
platelet-derived growth factor receptor
- PI3K
phosphoinositide 3-kinase
- SCF
stem cell factor
- TNF-α
tumour necrosis factor-α
- VEGFR
vascular endothelial growth factor receptor
Conflict of interest
The authors state no conflict of interest.
References
- Aichberger KJ, Mayerhofer M, Gleixner KV, Krauth MT, Gruze A, Pickl WF, et al. Identification of MCL1 as a novel target in neoplastic mast cells in systemic mastocytosis: inhibition of mast cell survival by MCL1 antisense oligonucleotides and synergism with PKC412. Blood. 2007;109:3031–3041. doi: 10.1182/blood-2006-07-032714. [DOI] [PubMed] [Google Scholar]
- Akin C. Molecular diagnosis of mast cell disorders: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn. 2006;8:412–419. doi: 10.2353/jmoldx.2006.060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ, et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol. 2003;31:686–692. doi: 10.1016/s0301-472x(03)00112-7. [DOI] [PubMed] [Google Scholar]
- Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103:3222–3225. doi: 10.1182/blood-2003-11-3816. [DOI] [PubMed] [Google Scholar]
- Akin C, Metcalfe DD. The biology of Kit in disease and the application of pharmacogenetics. J Allergy Clin Immunol. 2004;114:13–19. doi: 10.1016/j.jaci.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with ‘idiopathic' anaphylaxis. Blood. 2007;110:2331–2333. doi: 10.1182/blood-2006-06-028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Muhsen SZ, Shablovsky G, Olivenstein R, Mazer B, Hamid Q. The expression of stem cell factor and c-kit receptor in human asthmatic airways. Clin Exp Allergy. 2004;34:911–916. doi: 10.1111/j.1365-2222.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol. 2006;126:1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- Ando A, Martin TR, Galli SJ. Effects of chronic treatment with the c-kit ligand, stem cell factor, on immunoglobulin E-dependent anaphylaxis in mice. Genetically mast cell-deficient Sl/Sld mice acquire anaphylactic responsiveness, but the congenic normal mice do not exhibit augmented responses. J Clin Invest. 1993;92:1639–1649. doi: 10.1172/JCI116749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle J, Letard S, Frank R, Dubreuil P, De Sepulveda P. Suppressor of cytokine signaling 6 associates with KIT and regulates KIT receptor signaling. J Biol Chem. 2004;279:12249–12259. doi: 10.1074/jbc.M313381200. [DOI] [PubMed] [Google Scholar]
- Beghini A, Peterlongo P, Ripamonti CB, Larizza L, Cairoli R, Morra E, et al. C-kit mutations in core binding factor leukemias. Blood. 2000;95:726–727. [PubMed] [Google Scholar]
- Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- Broudy VC, Kovach NL, Bennett LG, Lin N, Jacobsen FW, Kidd PG. Human umbilical vein endothelial cells display high-affinity c-kit receptors and produce a soluble form of the c-kit receptor. Blood. 1994;83:2145–2152. [PubMed] [Google Scholar]
- Chow KU, Nowak D, Trepohl B, Hochmuth S, Schneider B, Hoelzer D, et al. The tyrosine kinase inhibitor AMN107 (Nilotinib) exhibits off-target effects in lymphoblastic cell lines. Leuk Lymphoma. 2007;48:1379–1388. doi: 10.1080/10428190701385181. [DOI] [PubMed] [Google Scholar]
- Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- Corbin AS, Griswold IJ, La Rosee P, Yee KW, Heinrich MC, Reimer CL, et al. Sensitivity of oncogenic KIT mutants to the kinase inhibitors MLN518 and PD180970. Blood. 2004;104:3754–3757. doi: 10.1182/blood-2004-06-2189. [DOI] [PubMed] [Google Scholar]
- Costa JJ, Demetri GD, Harrist TJ, Dvorak AM, Hayes DF, Merica EA, et al. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J Exp Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastych J, Metcalfe DD. Stem cell factor induces mast cell adhesion to fibronectin. J Immunol. 1994;152:213–219. [PubMed] [Google Scholar]
- Dastych J, Taub D, Hardison MC, Metcalfe DD. Tyrosine kinase-deficient Wv c-kit induces mast cell adhesion and chemotaxis. Am J Physiol. 1998;275:C1291–C1299. doi: 10.1152/ajpcell.1998.275.5.C1291. [DOI] [PubMed] [Google Scholar]
- Dorsey JF, Jove R, Kraker AJ, Wu J. The pyrido[2,3-d]pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. Cancer Res. 2000;60:3127–3131. [PubMed] [Google Scholar]
- Droogendijk HJ, Kluin-Nelemans HJ, van Doormaal JJ, Oranje AP, van de Loosdrecht AA, van Daele PL. Imatinib mesylate in the treatment of systemic mastocytosis: a phase II trial. Cancer. 2006;107:345–351. doi: 10.1002/cncr.21996. [DOI] [PubMed] [Google Scholar]
- Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A, et al. PKC412—a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des. 2000;15:17–28. [PubMed] [Google Scholar]
- Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Florian S, Krauth MT, Simonitsch-Klupp I, Sperr WR, Fritsche-Polanz R, Sonneck K, et al. Indolent systemic mastocytosis with elevated serum tryptase, absence of skin lesions, and recurrent severe anaphylactoid episodes. Int Arch Allergy Immunol. 2005;136:273–280. doi: 10.1159/000083954. [DOI] [PubMed] [Google Scholar]
- Fogel WA, Lewinski A, Jochem J. Histamine in idiopathic inflammatory bowel diseases—not a standby player. Folia Med Cracov. 2005;46:107–118. [PubMed] [Google Scholar]
- Fumo G, Akin C, Metcalfe DD, Neckers L. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103:1078–1084. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- Furitsu T, Tsujimura T, Tono T, Ikeda H, Kitayama H, Koshimizu U, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabillot-Carre M, Lepelletier Y, Humbert M, de Sepuvelda P, Hamouda NB, Zappulla JP, et al. Rapamycin inhibits growth and survival of D816V-mutated c-kit mast cells. Blood. 2006;108:1065–1072. doi: 10.1182/blood-2005-06-2433. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Garcia-Montero AC, Jara-Acevedo M, Teodosio C, Sanchez ML, Nunez R, Prados A, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C, et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica. 2007;92:1451–1459. doi: 10.3324/haematol.11339. [DOI] [PubMed] [Google Scholar]
- Gotlib J, Berube C, Growney JD, Chen CC, George TI, Williams C, et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood. 2005;106:2865–2870. doi: 10.1182/blood-2005-04-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib J, George TI, Corless C, Linder A, Ruddell A, Akin C, et al. The KIT tyrosine kinase inhibitor midostaurine (PKC412) exhibits a high response rate in aggressive systemic mastocytosis (ASM): interim results of a Phase II trial. Blood. 2007;110:1035a. [Google Scholar]
- Hantschel O, Rix U, Schmidt U, Burckstummer T, Kneidinger M, Schutze G, et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA. 2007;104:13283–13288. doi: 10.1073/pnas.0702654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann A, Sturm GJ, Ofner M, Sturm EM, Weller C, Peskar BA, et al. Stem cell factor stimulates the chemotaxis, integrin upregulation, and survival of human basophils. J Allergy Clin Immunol. 2005;116:820–826. doi: 10.1016/j.jaci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- Herbst R, Shearman MS, Jallal B, Schlessinger J, Ullrich A. Formation of signal transfer complexes between stem cell and platelet-derived growth factor receptors and SH2 domain proteins in vitro. Biochemistry. 1995;34:5971–5979. doi: 10.1021/bi00017a026. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Ottmann OG, Lauber S, Hughes T, Verhoef G, Schwarer AP, et al. A phase II study of nilotinib, a novel inhibitor of c-kit, PDGFR, and Bcr-Abl, administered to patients with systemic mastocytosis. Blood. 2006;108:764a. [Google Scholar]
- Hollenbeck ST, Sakakibara K, Faries PL, Workhu B, Liu B, Kent KC. Stem cell factor and c-kit are expressed by and may affect vascular SMCs through an autocrine pathway. J Surg Res. 2004;120:288–294. doi: 10.1016/j.jss.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Hongyo T, Li T, Syaifudin M, Baskar R, Ikeda H, Kanakura Y, et al. Specific c-kit mutations in sinonasal natural killer/T-cell lymphoma in China and Japan. Cancer Res. 2000;60:2345–2347. [PubMed] [Google Scholar]
- Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, et al. Btk plays a crucial role in the amplification of Fc epsilonRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcepsilonRI-mediated signaling: coordinated suppression of mast cell activation. J Pharmacol Exp Ther. 2008;324:128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BM, Metcalfe DD, Gilfillan AM. Targeting kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflamm Allergy Drug Targets. 2007;6:57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- Juurikivi A, Sandler C, Lindstedt KA, Kovanen PT, Juutilainen T, Leskinen MJ, et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann Rheum Dis. 2005;64:1126–1131. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur R, Majumdar M, Xiao X, ndrews-Hill M, Schindler K, Williams DA. Signaling through the interaction of membrane-restricted stem cell factor and c-kit receptor tyrosine kinase: genetic evidence for a differential role in erythropoiesis. Blood. 1998;91:879–889. [PubMed] [Google Scholar]
- Kim MS, Kuehn HS, Metcalfe DD, Gilfillan AM. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- Kitamura Y, Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979;53:492–497. [PubMed] [Google Scholar]
- Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006;26:387–405. doi: 10.1016/j.iac.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Tsujimura T, Jippo T, Kasugai T, Kanakura Y. Regulation of development, survival and neoplastic growth of mast cells through the c-kit receptor. Int Arch Allergy Immunol. 1995;107:54–56. doi: 10.1159/000236929. [DOI] [PubMed] [Google Scholar]
- Kitayama H, Kanakura Y, Furitsu T, Tsujimura T, Oritani K, Ikeda H, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85:790–798. [PubMed] [Google Scholar]
- Kitayama H, Tsujimura T, Matsumura I, Oritani K, Ikeda H, Ishikawa J, et al. Neoplastic transformation of normal hematopoietic cells by constitutively activating mutations of c-kit receptor tyrosine kinase. Blood. 1996;88:995–1004. [PubMed] [Google Scholar]
- Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J, et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood. 2003;101:4660–4666. doi: 10.1182/blood-2003-01-0006. [DOI] [PubMed] [Google Scholar]
- Klion AD, Robyn J, Akin C, Noel P, Brown M, Law M, et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome. Blood. 2004;103:473–478. doi: 10.1182/blood-2003-08-2798. [DOI] [PubMed] [Google Scholar]
- Kojima S, Matsuyama T, Kodera Y. Plasma levels and production of soluble stem cell factor by marrow stromal cells in patients with aplastic anaemia. Br J Haematol. 1997;99:440–446. doi: 10.1046/j.1365-2141.1997.4163223.x. [DOI] [PubMed] [Google Scholar]
- Kosmider O, Denis N, Dubreuil P, Moreau-Gachelin F. Semaxinib (SU5416) as a therapeutic agent targeting oncogenic Kit mutants resistant to imatinib mesylate. Oncogene. 2007;26:3904–3908. doi: 10.1038/sj.onc.1210159. [DOI] [PubMed] [Google Scholar]
- Kozlowski M, Larose L, Lee F, Le DM, Rottapel R, Siminovitch KA. SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain. Mol Cell Biol. 1998;18:2089–2099. doi: 10.1128/mcb.18.4.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcepsilonRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahortiga I, Akin C, Cools J, Wilson TM, Mentens N, Arthur DC, et al. Activity of imatinib in systemic mastocytosis with chronic basophilic leukemia and a PRKG2-PDGFRB fusion. Haematologica. 2008;93:49–56. doi: 10.3324/haematol.11836. [DOI] [PubMed] [Google Scholar]
- Lawley W, Hird H, Mallinder P, McKenna S, Hargadon B, Murray A, et al. Detection of an activating c-kit mutation by real-time PCR in patients with anaphylaxis. Mutat Res. 2005;572:1–13. doi: 10.1016/j.mrfmmm.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- Lev S, Givol D, Yarden Y. Interkinase domain of kit contains the binding site for phosphatidylinositol 3′ kinase. Proc Natl Acad Sci USA. 1992;89:678–682. doi: 10.1073/pnas.89.2.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem. 2006;75:93–109. doi: 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-Kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- Lober K, Alfonso A, Escribano L, Botana LM. STI571 (Glivec) affects histamine release and intracellular pH after alkalinisation in HMC-1560, 816. J Cell Biochem. 2008;103:865–876. doi: 10.1002/jcb.21458. [DOI] [PubMed] [Google Scholar]
- Lorentz A, Schuppan D, Gebert A, Manns MP, Bischoff SC. Regulatory effects of stem cell factor and interleukin-4 on adhesion of human mast cells to extracellular matrix proteins. Blood. 2002;99:966–972. doi: 10.1182/blood.v99.3.966. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- Malone DG, Irani AM, Schwartz LB, Barrett KE, Metcalfe DD. Mast cell numbers and histamine levels in synovial fluids from patients with diverse arthritides. Arthritis Rheum. 1986;29:956–963. doi: 10.1002/art.1780290803. [DOI] [PubMed] [Google Scholar]
- Malone DG, Metcalfe DD. Demonstration and characterization of a transient arthritis in rats following sensitization of synovial mast cells with antigen-specific IgE and parenteral challenge with specific antigen. Arthritis Rheum. 1988;31:1063–1067. doi: 10.1002/art.1780310820. [DOI] [PubMed] [Google Scholar]
- Malone DG, Wilder RL, Saavedra-Delgado AM, Metcalfe DD. Mast cell numbers in rheumatoid synovial tissues. Correlations with quantitative measures of lymphocytic infiltration and modulation by antiinflammatory therapy. Arthritis Rheum. 1987;30:130–137. doi: 10.1002/art.1780300202. [DOI] [PubMed] [Google Scholar]
- Maric I, Robyn J, Metcalfe DD, Fay MP, Carter M, Wilson T, et al. KIT D816V-associated systemic mastocytosis with eosinophilia and FIP1L1/PDGFRA-associated chronic eosinophilic leukemia are distinct entities. J Allergy Clin Immunol. 2007;120:680–687. doi: 10.1016/j.jaci.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Mekori JA, Rottem M. Mast cell ontogeny and apoptosis. Exp Dermatol. 1995;4:227–230. doi: 10.1111/j.1600-0625.1995.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Mol CD, Dougan DR, Schneider TR, Skene RJ, Kraus ML, Scheibe DN, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J Biol Chem. 2004;279:31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- Mol CD, Lim KB, Sridhar V, Zou H, Chien EY, Sang BC, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–31464. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- Muto S, Katsuki M, Horie S. Decreased c-kit function inhibits enhanced skin carcinogenesis in c-Ha-ras protooncogene transgenic mice. Cancer Sci. 2007;98:1549–1556. doi: 10.1111/j.1349-7006.2007.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J Immunol. 1994;153:3717–3723. [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshitani N, Kamata N, Inagawa M, Yamagami H, Watanabe K, Fujiwara Y, et al. Significance of activated mast cells in submucosa and muscularis propria of patients with Crohn's disease detected by a novel anti-mast cell surface molecule antibody. Aliment Pharmacol Ther. 2006;24:1–4. [Google Scholar]
- Pan J, Quintas-Cardama A, Kantarjian HM, Akin C, Manshouri T, Lamb P, et al. EXEL-0862, a novel tyrosine kinase inhibitor, induces apoptosis in vitro and ex vivo in human mast cells expressing the KIT D816V mutation. Blood. 2007;109:315–322. doi: 10.1182/blood-2006-04-013805. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Elliott M, Reeder T, Li CY, Baxter EJ, Cross NC, et al. Imatinib for systemic mast-cell disease. Lancet. 2003a;362:535–536. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Ketterling RP, Brockman SR, Flynn HC, Paternoster SF, Shearer BM, et al. CHIC2 deletion, a surrogate for FIP1L1–PDGFRA fusion, occurs in systemic mastocytosis associated with eosinophilia and predicts response to imatinib mesylate therapy. Blood. 2003b;102:3093–3096. doi: 10.1182/blood-2003-05-1627. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Reeder T, Li CY, Tefferi A. Eosinophils are derived from the neoplastic clone in patients with systemic mastocytosis and eosinophilia. Leuk Res. 2003c;27:883–885. doi: 10.1016/s0145-2126(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Patnaik MM, Tefferi A, Pardanani A. Kit: molecule of interest for the diagnosis and treatment of mastocytosis and other neoplastic disorders. Curr Cancer Drug Targets. 2007;7:492–503. doi: 10.2174/156800907781386614. [DOI] [PubMed] [Google Scholar]
- Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schoffski P, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res. 2006;12:2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- Price DJ, Rivnay B, Fu Y, Jiang S, Avraham S, Avraham H. Direct association of Csk homologous kinase (CHK) with the diphosphorylated site Tyr568/570 of the activated c-KIT in megakaryocytes. J Biol Chem. 1997;272:5915–5920. doi: 10.1074/jbc.272.9.5915. [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Trump DL, Eiseman JL, Belani CP, Agarwala SS, Zuhowski EG, et al. Phase I pharmacokinetic-pharmacodynamic study of 17-(allylamino)-17-demethoxygeldanamycin (17AAG, NSC 330507), a novel inhibitor of heat shock protein 90, in patients with refractory advanced cancers. Clin Cancer Res. 2005;11:3385–3391. doi: 10.1158/1078-0432.CCR-04-2322. [DOI] [PubMed] [Google Scholar]
- Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005a;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005b;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- Samayawardhena LA, Hu J, Stein PL, Craig AW. Fyn kinase acts upstream of Shp2 and p38 mitogen-activated protein kinase to promote chemotaxis of mast cells towards stem cell factor. Cell Signal. 2006;18:1447–1454. doi: 10.1016/j.cellsig.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Scheinfeld N. A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases. J Drugs Dermatol. 2006;5:117–122. [PubMed] [Google Scholar]
- Schirmer A, Kennedy J, Murli S, Reid R, Santi DV. Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc Natl Acad Sci USA. 2006;103:4234–4239. doi: 10.1073/pnas.0600445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serve H, Hsu YC, Besmer P. Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3-kinase activity in COS-1 cells. J Biol Chem. 1994;269:6026–6030. [PubMed] [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- Shivakrupa R, Linnekin D. Lyn contributes to regulation of multiple Kit-dependent signaling pathways in murine bone marrow mast cells. Cell Signal. 2005;17:103–109. doi: 10.1016/j.cellsig.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Hutson TE. Pazopanib: a novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–119. doi: 10.1007/s11912-007-0007-2. [DOI] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, et al. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105:2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Dastych J, Sehgal D, Sundstrom M, Nilsson G, Akin C, et al. The Kit-activating mutation D816V enhances stem cell factor—dependent chemotaxis. Blood. 2001;98:1195–1199. doi: 10.1182/blood.v98.4.1195. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Sehgal D, Raffeld M, Obiakor H, Akin C, Mage RG, et al. Demonstration that mast cells, T cells, and B cells bearing the activating kit mutation D816V occur in clusters within the marrow of patients with mastocytosis. J Mol Diagn. 2004;6:335–342. doi: 10.1016/S1525-1578(10)60529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–241. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Iliopoulou BP. Mast cells, T cells, and inhibition by luteolin: implications for the pathogenesis and treatment of multiple sclerosis. Adv Exp Med Biol. 2007;601:423–430. doi: 10.1007/978-0-387-72005-0_45. [DOI] [PubMed] [Google Scholar]
- Thommes K, Lennartsson J, Carlberg M, Ronnstrand L. Identification of Tyr-703 and Tyr-936 as the primary association sites for Grb2 and Grb7 in the c-Kit/stem cell factor receptor. Biochem J. 1999;341 Part 1:211–216. [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Frierson HF, Jr, Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol. 1999;154:1643–1647. doi: 10.1016/S0002-9440(10)65419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and Fc epsilon RI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26:427–450. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieselmann NZ, Soboloff J, Berger SA. Mast cells stimulated by membrane-bound, but not soluble, steel factor are dependent on phospholipase C activation. Cell Mol Life Sci. 2003;60:759–766. doi: 10.1007/s00018-003-2349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. 2003a;27:635–641. doi: 10.1016/s0145-2126(02)00168-6. [DOI] [PubMed] [Google Scholar]
- Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K, et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol. 2003b;122:695–717. doi: 10.1046/j.1365-2141.2003.04575.x. [DOI] [PubMed] [Google Scholar]
- Valent P, Horny HP, Escribano L, Longley BJ, Li CY, Schwartz LB, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–625. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- van Dijk TB, van Den Akker E, Amelsvoort MP, Mano H, Lowenberg B, von Lindern M. Stem cell factor induces phosphatidylinositol 3′-kinase-dependent Lyn/Tec/Dok-1 complex formation in hematopoietic cells. Blood. 2000;96:3406–3413. [PubMed] [Google Scholar]
- Vendome J, Letard S, Martin F, Svinarchuk F, Dubreuil P, Auclair C, et al. Molecular modeling of wild-type and D816V c-Kit inhibition based on ATP-competitive binding of ellipticine derivatives to tyrosine kinases. J Med Chem. 2005;48:6194–6201. doi: 10.1021/jm050231m. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Akin C, Manshouri T, Quintas-Cardama A, Huynh L, Manley P, et al. Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 c-kit. Leuk Res. 2006a;30:1365–1370. doi: 10.1016/j.leukres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Cortes J, Ravandi-Kashani F, Borthakur G, Nicaise C, et al. Dasatinib (Sprycel (TM)) therapy for patients with systemic mastocytosis. Blood. 2006b;108:1036a. [Google Scholar]
- Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Austen KF, Friend DS, Heidtman M, Boyce JA. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1) J Exp Med. 1997;186:313–323. doi: 10.1084/jem.186.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappulla JP, Dubreuil P, Desbois S, Letard S, Hamouda NB, Daeron M, et al. Mastocytosis in mice expressing human Kit receptor with the activating Asp816Val mutation. J Exp Med. 2005;202:1635–1641. doi: 10.1084/jem.20050807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermati Y, De Sepulveda P, Feger F, Letard S, Kersual J, Casteran N, et al. Effect of tyrosine kinase inhibitor STI571 on the kinase activity of wild-type and various mutated c-kit receptors found in mast cell neoplasms. Oncogene. 2003;22:660–664. doi: 10.1038/sj.onc.1206120. [DOI] [PubMed] [Google Scholar]