Abstract
Background and purpose:
Screening of 12 000 compounds for binding affinity to the synaptic vesicle protein 2A (SV2A), identified a high-affinity pyrrolidone derivative, brivaracetam (ucb 34714). This study examined its pharmacological profile in various in vitro and in vivo models of seizures and epilepsy, to evaluate its potential as a new antiepileptic drug.
Experimental approach:
The effects of brivaracetam and levetiracetam on epileptiform activity and seizure expression were examined in rat hippocampal slices, corneally kindled mice, audiogenic seizure–susceptible mice, maximal electroshock and pentylenetetrazol seizures in mice, hippocampal-kindled rats, amygdala-kindled rats and genetic absence epilepsy rats.
Key results:
Brivaracetam and levetiracetam reduced epileptiform responses in rat hippocampal slices, brivaracetam being most potent. Brivaracetam also differed from levetiracetam by its ability to protect against seizures in normal mice induced by a maximal electroshock or maximal dose of pentylenetetrazol. In corneally kindled mice and hippocampal-kindled rats, brivaracetam induced potent protection against secondarily generalized motor seizures and showed anti-kindling properties superior to levetiracetam. In amygdala-kindled rats, brivaracetam induced a significant suppression in motor-seizure severity and, contrary to levetiracetam, reduced the after-discharge at a higher dose. Audiogenic seizure–susceptible mice were protected more potently against the expression of clonic convulsions by brivaracetam than by levetiracetam. Brivaracetam induced a more complete suppression of spontaneous spike-and-wave discharges in genetic absence epilepsy rats than levetiracetam.
Conclusions and implications:
Brivaracetam has higher potency and efficacy than levetiracetam as an anti-seizure and anti-epileptogenic agent in various experimental models of epilepsy, and a wide therapeutic index.
Keywords: brivaracetam, levetiracetam, antiepileptic effect, anticonvulsive effect, safety profile, synaptic vesicle protein, SV2A
Introduction
Up to one-third of patients on current antiepileptic drug (AED) treatment experience seizures or unacceptable medication-related side effects, although several new AEDs have been introduced since 1978 (Meldrum and Rogawski, 2007). Therefore, there still is an urgent need for new AEDs with improved efficacy and/or adverse effect profiles.
Marketed AEDs predominantly act by blocking voltage-gated Na+ channels, blocking T-type voltage-gated Ca2+ channels or by enhancing GABA-ergic neurotransmission. In addition to these targets, several novel potential molecular targets for AED action, including the synaptic vesicle protein 2A (SV2A), have recently been identified (Meldrum and Rogawski, 2007). Levetiracetam ((S)-α-ethyl-2-oxopyrrolidine acetamide), acts as a ligand for SV2A (Lynch et al., 2004; Gillard et al., 2006), and provides significant seizure protection in various animal models mimicking both partial and primary generalized epilepsy (Klitgaard et al., 1998). Among newer and established AEDs, levetiracetam was unique by its molecular mechanism, targeting the SV2A protein in the brain (Lynch et al., 2004) and its novel profile in animal models of seizures and epilepsy (Klitgaard, 2001).
The pharmacological properties of levetiracetam indicated a promising potential as AED by a combination of a broad spectrum of efficacy and minimal adverse effects (Klitgaard et al., 1998). However, the levetiracetam molecule is not an optimized ligand regarding the interaction with the SV2A protein. The correlation between the affinity of levetiracetam analogues for SV2A and their anticonvulsant potencies (Noyer et al., 1995; Lynch et al., 2004) therefore triggered a drug discovery programme aiming at the identification of high-affinity SV2A ligands with antiepileptic properties potentially superior to those of levetiracetam.
In this search, approximately 12 000 compounds were screened in vitro for SV2A binding affinity, 900 compounds were examined in an in vivo model of protection against audiogenic seizures in mice and 30 compounds were more widely characterized in animal models of seizures and epilepsy. As a first outcome of these drug discovery efforts, a pyrrolidone derivative, brivaracetam ((2S)-2-[(4R)-2-oxo-4-propylpyrrolidinyl]butanamide; Figure 1), was identified. Brivaracetam (ucb 34714) had a markedly higher binding affinity (pKi=7.1) than levetiracetam (pKi=6.1) for SV2A (Kenda et al., 2004; Lynch et al., 2004). This paper reports the pharmacological profile of brivaracetam in several in vitro and in vivo models of seizures and epilepsy.
Figure 1.
Chemical structures of brivaracetam (BRV) and levetiracetam (LEV).
Methods
All experiments were performed in accordance with the Guidelines of the local Ethical Committee for Animal Experimentation.
Epileptiform responses in hippocampal slices
Levetiracetam reduces epileptiform responses induced in rat hippocampal slices by high-K+/low-Ca2+ concentrations in the perfusion fluid (Margineanu and Klitgaard, 2000) and induced by bicuculline (Birnstiel et al., 1997; Margineanu et al., 1998). The effect of brivaracetam on epileptiform responses induced by high-K+/low-Ca2+ concentrations or by bicuculline was examined in transverse hippocampal slices prepared from Sprague–Dawley rats according to previously reported standard procedures (Margineanu and Klitgaard, 2000; Margineanu and Klitgaard, 2001). The epileptiform responses were induced by passing from a normal perfusion of artificial cerebrospinal fluid (ACSF) (K+ 3 mM; Ca2+ 2.4 mM) to either high-K+/low-Ca2+ fluid (HKLCF) (K+ 7.5 mM; Ca2+ 0.5 mM) or to 5 μM bicuculline methiodide (BMI)-containing ACSF.
Extracellular field potentials (FPs) were recorded in the CA3 area of the slices with 2 M NaCl-filled glass microelectrodes. The evoked FPs were recorded at 10-min intervals in response to fimbrial stimulation with constant current rectangular pulses that elicit a single population spike (PS) of 50–75% of the maximal amplitude when the slice is in ACSF. In the HKLCF model, 2 min of spontaneous activity were also recorded, in the middle of each 10-min interval between the recordings of evoked responses.
Either brivaracetam or levetiracetam was added to the bathing fluid of the slices 20 min before shifting from ACSF to either HKLCF or 5 μM BMI-containing ACSF, and was kept in the perfusion fluid throughout the experiment.
The acquisition software averaged three successive samples of the FPs that were evoked at rates of about 0.1 Hz, calculated the amplitudes of PSs from the negative peak to a tangent drawn between the preceding and the following maxima and counted the number of PSs up to an amplitude of no less than 10% of the largest one. Drug effects on the evoked FPs were quantified through ΔPS1, the increase in amplitude of the first (and largest) population spike (PS1) observed in ACSF perfusion and through the number of repetitive PSs per evoked burst. In the HKLCF model, drug effects were also quantified from the number of spontaneous bursts per 2-min recording.
Corneal kindling in mice
Kindling was induced in male NMRI mice (20–25 g) by twice-daily corneal stimulations (3 mA, 3 s) with a minimum of 4 h between each stimulation, according to a previously described method (Matagne and Klitgaard, 1998). The animals were considered kindled after the appearance of four consecutive generalized seizures, observed as clonic convulsions in the forelimbs with or without rearing, falling and loss of balance (Matagne and Klitgaard, 1998). Fully kindled mice (n=10 per group) were pretreated with saline in the morning, then stimulated and observed for convulsive behaviour. The animals were treated similarly in the afternoon after pretreatment with saline, brivaracetam (i.p., 30 min) or levetiracetam (i.p., 60 min). The proportion of mice protected against generalized seizures was used as end point to evaluate anticonvulsant activity. The pretreatment time of 30 min for brivaracetam in mice was chosen based upon preliminary studies as the optimal pretreatment time giving maximal protection against acute seizures induced by methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (data not shown).
Corneal kindling development in mice
Kindling was induced by twice-daily corneal stimulations (3 mA, 3 s) in groups of 20 male NMRI mice (20–25 g) pretreated with saline, brivaracetam (i.p., 30 min) or levetiracetam (i.p., 60 min) before each stimulation. After 19 days of twice-daily stimulations, the drug treatment was terminated and a wash-out period of 2 days without stimulation was applied. Then the animals were restimulated twice daily for an additional period of 5 days without drug treatment. The appearance of generalized seizures was used as end point to assess the development of corneal kindling.
Audiogenic seizures in mice
Genetically sound-sensitive male mice (16–28 g; n=10 per group), responding with wild running, clonic and tonic convulsions to an acoustic stimulation, were used. These mice were originally derived from a DBA strain by Dr Lehmann (Laboratory of Acoustic Physiology, Paris) and subsequently bred in the UCB animal husbandry unit since 1978. Audiogenic seizures were induced by an acoustic stimulus (90 dB, 10–20 kHz) applied for 30 s. The mice were pretreated with either saline, brivaracetam (i.p., 30 min) or levetiracetam (i.p., 60 min), and the proportion of mice protected against clonic convulsions was used as the end point to assess anticonvulsant activity.
Chemically induced seizures in mice
Pentylenetetrazol, 83 mg kg−1 s.c., was used to evaluate the anticonvulsant properties of brivaracetam. The dose was selected based on dose–effect curves in saline-treated animals as the convulsive dose inducing clonic convulsions of all four extremities in 97% of the animals. Immediately after administration of the chemoconvulsant, the mice were placed individually in small plastic cages (25 × 13 × 8 cm) and observed for the presence of clonic convulsions in all four extremities, for 60 min. The occurrence of tonic convulsions (hindlimb extension) and mortality was also recorded during this interval. The proportion of mice protected against clonic convulsions was calculated and used as the end point for anticonvulsant activity.
Maximal electroshock seizures in mice
Maximal electroshock seizures were induced by a stimulator (WITT IndustrieElektronik, Berlin, Germany) using a current intensity of 50 mA delivered with a constant pulse frequency of 50 Hz for a duration of 0.2 s through corneal electrodes. After stimulation, the mice were observed for 10 s, and the incidence of tonic hindlimb extension was noted. The proportion of mice protected against tonic hindlimb extension was calculated and used as the end point for anticonvulsant activity.
Hippocampal kindling in rats
Male Sprague–Dawley rats (250–350 g) were implanted with a bipolar stimulation/recording electrode of stainless steel into the right dorsal hippocampus (coordinates=AP-3.5, L-2.5 and V-2.5) according to the procedure described by Paxinos and Watson (1982), under pentobarbital anaesthesia. Kindling was induced by twice-daily stimulations, 5 days per week, with 600 μA, 1-s monophasic square wave pulses, 50 Hz. A rat was defined as kindled and used for drug experiments after the appearance of at least three consecutive stage 4 or 5 seizures according to the scale of Racine (Racine, 1972). Fully kindled rats (n=8 per group) were stimulated once with the same stimulation parameters as above 60 min after oral administration of water. Two days later, this protocol was repeated with the same animals, after oral administration of either water, brivaracetam or levetiracetam (60 min). The behavioral effect of stimulation was scored according to the scale of Racine.
Amygdala kindling in rats
Male Sprague–Dawley rats (250–350 g) were implanted with a bipolar stimulation/recording electrode into the right basolateral amygdala (AP-2.3, L-4.8, V-8.5) under pentobarbital anaesthesia (Paxinos and Watson, 1982). Kindling was induced by once-daily stimulation, 5 days per week, with 500-μA monophasic square wave pulses, 50 Hz for 1 s (Löscher et al., 1986). The animals were considered kindled after the appearance of at least 10 consecutive stage 4 or 5 seizures according to the scale of Racine (Racine, 1972). Fully kindled rats (N=8 per group) were stimulated once with the same stimulation parameters as above after administration of saline (i.p., 60 min). The animals were treated similarly 2 days later, after pretreatment with either saline, brivaracetam or levetiracetam (i.p., 60 min). The behavioral effect of stimulation, expressed by a ‘seizure severity score,' was assessed according to the scale of Racine. The electroencephalographic effect of the stimulation was determined by measuring the duration of the stimulation-induced after-discharge. An after-discharge was defined as an EEG activity with an amplitude of at least twice the amplitude of the prestimulus recording and a frequency greater that 1 Hz. The pretreatment time of 60 min for brivaracetam in rats was selected based upon preliminary studies in fully amygdala-kindled rats as the optimal pretreatment time giving maximal protection against generalized motor seizures (data not shown).
Genetic absence epilepsy in rats
Male rats with genetic absence epilepsy from Strasbourg (GAERS) were implanted with four platinum electrodes into the left and right frontal cortex and left and right occipital cortex, under pentobarbital anaesthesia. Cortical, spontaneous spike-and-wave discharges (SWDs) were recorded bilaterally from the rats that were placed into plexiglass boxes and prevented from falling asleep by gentle sensory stimulation. After a 20-min habituation period, the rats were injected i.p. with either saline, brivaracetam or levetiracetam, and the EEG recorded continuously over consecutive 20-min intervals up to 120 min. The cumulative duration of the SWDs was calculated.
Rotarod performance in kindled mice and rats
The adverse effects on motor function were assessed in a rotarod test using a rod with a diameter of 3 cm for mice, 10 cm for rats, rotating at a constant speed of 6 r.p.m. Fully corneally kindled mice (n=10 per group) or amygdala-kindled rats (n=8 per group) were pretrained, and only animals able to remain on the rod for at least 60 s in three consecutive trials were retained. The following day, either saline, brivaracetam (i.p., 30 min for mice; 60 min for rats) or levetiracetam (i.p., 60 min) was administered, and the number of animals unable to remain on the rod for at least 60 s was recorded.
Statistical analysis
ED50 (effective dose protecting 50% of the animals) and TD50 (dose inducing impairment of rotarod performance in 50% of the animals) values were calculated using a log-probit analysis (SAS/STATR Software). The significance of differences between individual control recordings and recordings after drug treatment in hippocampal- and amygdala-kindled rats was calculated by the Wilcoxon sign rank test for paired replicates; level of significance, P<0.05.
Materials
Brivaracetam (ucb 34714) and levetiracetam were synthesized in the chemical laboratories of UCB. Bicuculline methiodide was obtained from Sigma-Aldrich Chemie Gmbh, Steinheim, Germany; pentylenetetrazol from Acros Organics, Geel, Belgium; methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate from Research Biochemicals International, Natick, MA, USA; and pentobarbital from Siegfried AG, Zofingen, Switzerland. NaCl, KCl and CaCl2 were obtained from Vel, Leuven, Belgium.
Results
Epileptiform responses in hippocampal slices
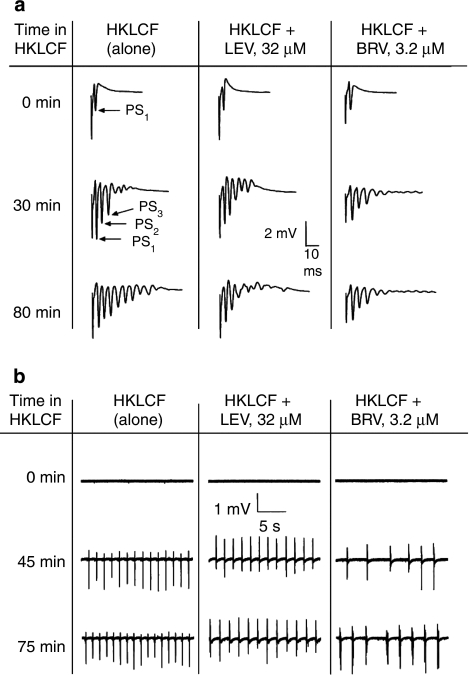
Changing the perfusion of rat hippocampal slices from the normal ACSF to HKLCF produced increasingly epileptiform FPs in the CA3 area in response to constant-current fimbrial stimulation. In control slices exposed to HKLCF alone, the PS1 amplitude progressively increased, reaching plateau values within 20 min (4.25±0.77 mV), nearly twofold higher than those recorded under ACSF perfusion (2.18±0.15 mV; mean±s.d. for n=10 slices). Also, constant-current single stimuli-evoked bursts of repetitive PSs (that is, PS2, PS3 and so on) increased markedly in number in the first 30 min of HKLCF perfusion from the single PS1 to an average of 7.6±2.3 PS per evoked burst, and continued to increase slightly up to the end of the records, reaching an average of 8.8±1.6 PS per evoked burst after 80-min perfusion of HKLCF. Both brivaracetam and levetiracetam reduced these epileptiform responses as illustrated by the typical records shown in Figure 2a. Upon 15-min perfusion of HKLCF, spontaneous field bursts occurred in 4 out of the 10 control slices exposed to HKLCF alone, whereas from 25 min in HKLCF to the end of the records, all control slices presented regular field bursting. Brivaracetam (3.2 μM), but not levetiracetam (32 μM), reduced the rate of this spontaneous bursting (Figure 2b).
Figure 2.
Effect of brivaracetam (BRV) and levetiracetam (LEV) on PS amplitude and repetitive PS number (a) and on rate of spontaneous bursting (b) in the presence of HKLCF (FP tracings) in CA3 area of rat hippocampal slices. FP, field potential; HKLCF, high-K+/low-Ca2+ fluid; PS, population spike.
The control responses recorded under ACSF perfusion, prior to any drug addition, were quite similar in all groups of slices, the average PS amplitude in each group being in a very narrow range between 2.14±0.13 and 2.19±0.12 mV (mean±s.d., for n=10 slices per group). Minor increases in the average amplitude of the responses evoked by constant-current stimuli occurred in all groups of slices in the 20-min interval between the addition of either brivaracetam or levetiracetam to the perfusion fluid and the shift from ACSF to HKLCF perfusion. These minor average increases, arising from a fairly irregular small variation of individual responses, were not obviously correlated with brivaracetam concentration: 0.19±0.20 mV in the group exposed to brivaracetam 0.1 μM, 0.08±0.39 mV for brivaracetam 1 μM, 0.18±0.36 mV for brivaracetam 3.2 μM and 0.12±0.39 mV for brivaracetam 10 μM. Indeed, the slight potentiation of the evoked PS over the 20-min perfusion with ACSF was best expressed in the control group of slices not exposed to any drug; 0.34±0.19 mV. Upon minute adjustments of the stimulation current, immediately prior to shifting from ACSF to HKLCF, all groups of slices had responses of similar amplitude at time ‘0 min in HKLCF', as illustrated by the first rows of traces in Figure 2a.
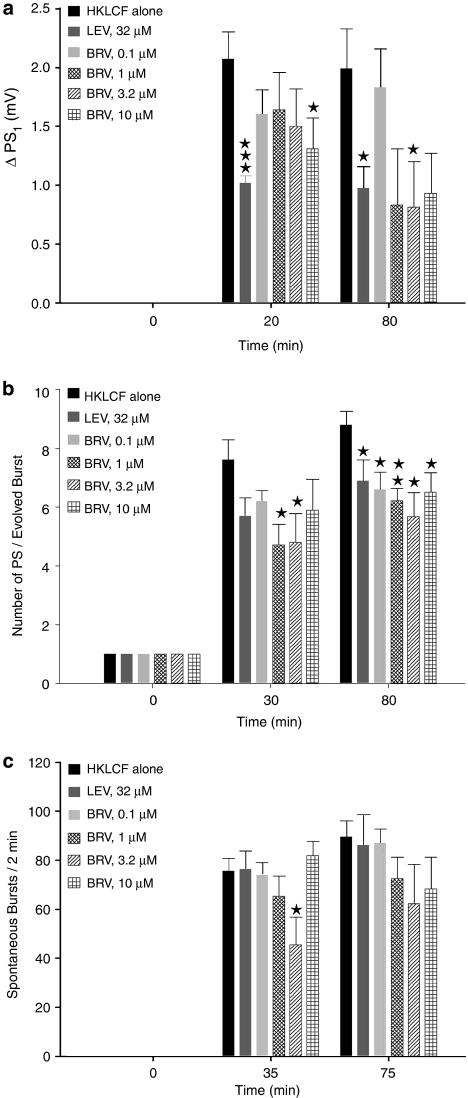
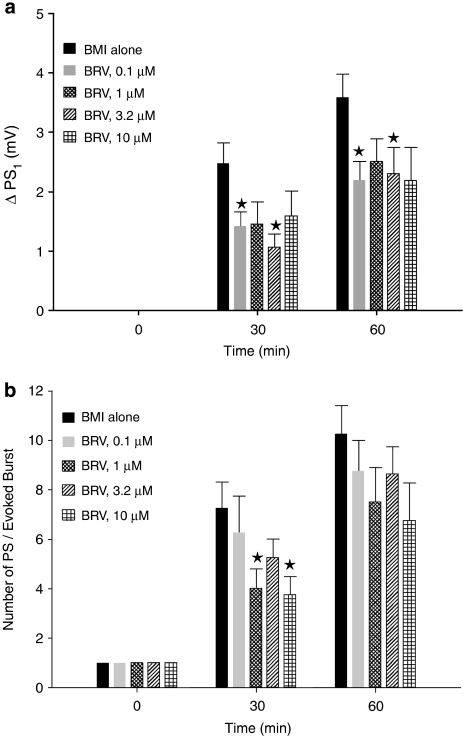
Both brivaracetam and levetiracetam depressed the HKLCF-induced ΔPS1 (Figure 3a) and the number of HKLCF-induced repetitive PSs (Figure 3b). Brivaracetam consistently reduced the number of repetitive PSs (Figure 3b), although without a clear concentration dependence, whereas the effect of levetiracetam was stronger against ΔPS1 than against the number of HKLCF-induced repetitive PSs (Figures 3a and b). The occurrence of spontaneous bursts was reduced with 3.2 μM brivaracetam (Figure 3c), whereas levetiracetam, at the 32 μM concentration previously reported as optimal on evoked responses (Margineanu and Klitgaard, 2000), was without effect on this parameter. The long (∼2 h) duration of these records, imposed by the time course of the epileptiform effect of HKLCF itself on brain slices, along with an occasional late fading of some of the effects of brivaracetam (Figure 3c), prevented a trustworthy assessment of the reversibility upon washout.
Figure 3.
Effect of brivaracetam (BRV) and levetiracetam (LEV) on ΔPS1 (a), repetitive PS number (b) and rate of spontaneous bursting (c) in the presence of HKLCF in rat hippocampal slices. The bars in the three graphs represent mean value±s.e.mean, averaged over the slices in each group (n=10 per group). For the parameters of evoked responses plotted in (a) and (b), the individual value for each slice at every given time point was calculated on line by the acquisition software on the average of three successive samples of field potentials, evoked at about 0.1 Hz. Significant differences with respect to the group exposed to HKLCF alone, assessed with two-tailed t-test, are indicated. ***P<0.0005, **P<0.005, *P<0.05. HKLCF, high-K+/low-Ca2+ fluid; PS, population spike; ΔPS1, increase in amplitude of the first (and largest) population spike.
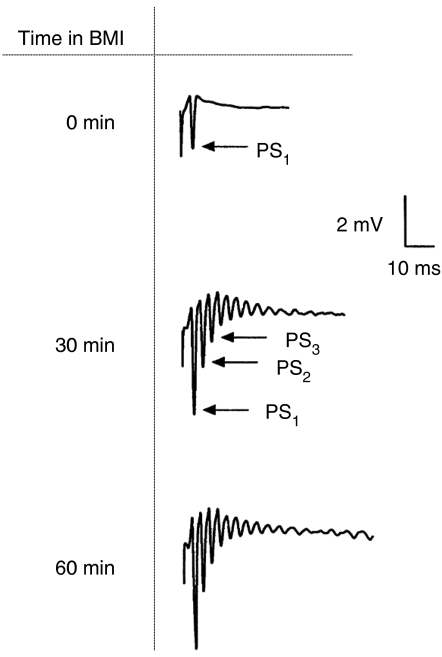
Similar to HKLCF, 5 μM BMI in the perfusion fluid increased the amplitudes of the evoked PSs and produced repetitive PSs in response to single stimuli (Figure 4). However, spontaneous field bursts occurred only occasionally in this milieu, in contrast to those in HKLCF, where brivaracetam depressed both BMI-induced ΔPS1 (Figure 5a) and the number of BMI-induced repetitive PSs (Figure 5b), although without a clear concentration dependence. A previous study from this laboratory (Margineanu et al., 1998) showed that levetiracetam also inhibited these epileptiform markers, with a maximal effect at 32 μM.
Figure 4.
Effect of bicuculline methiodide (BMI) on PS amplitude and repetitive PS number in rat hippocampal slices (FP tracings). FP, field potential; PS, population spike.
Figure 5.
Effect of brivaracetam (BRV) on ΔPS1 (a) and repetitive PS number (b) in rat hippocampal slices in the presence of BMI. As shown in Figures 3a and b, the bars represent mean value±s.e.mean, averaged over the slices in each group (n=8 per group), the individual value for each slice at every given time point being calculated on line on the average of three successive samples of field potentials, evoked at about 0.1 Hz. *P<0.05 compared to control (BMI alone). BMI, bicuculline methiodide; PS, population spike; ΔPS1, increase in amplitude of the first (and largest) population spike.
In vivo studies
Brivaracetam displayed a more potent protection than did levetiracetam against secondarily generalized motor seizures in fully corneally kindled mice and hippocampal-kindled rats (Table 1).
Table 1.
Effect of brivaracetam and levetiracetam against secondary generalized motor seizures
| Brivaracetam | Levetiracetam | |
|---|---|---|
| ED50 (mg kg−1 i.p.), corneally kindled mice (mean value and range) | 1.2 (0.6–2.4) | 7.3 (1.8–10.0) |
| MAD (mg kg−1 p.o.), hippocampal-kindled rats | 0.21 | 54 |
MAD, minimal active dose, the first dose inducing a significant reduction in seizure severity score vs predrug value (P<0.05).
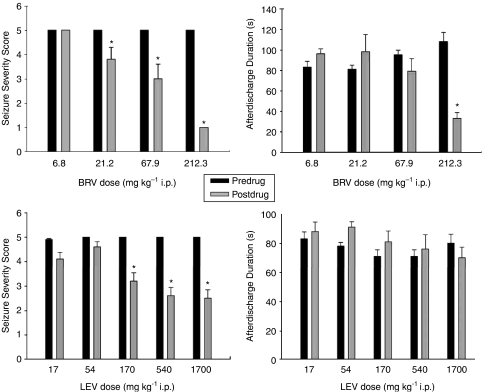
In fully amygdala-kindled rats, brivaracetam induced a significant suppression in motor-seizure severity from a dose of 21.2 mg kg−1, whereas levetiracetam induced a similar effect from a dose of 170 mg kg−1 (Figure 6). Brivaracetam also significantly reduced the after-discharge duration at the highest dose tested (212.3 mg kg−1), whereas levetiracetam was inactive on this parameter up to 1700 mg kg−1 (Figure 6).
Figure 6.
Effect of brivaracetam (BRV) and levetiracetam (LEV) on seizure parameters in amygdala-kindled rats. *P<0.05, predrug compared with postdrug recordings. Mean value±s.e.mean (n=8 per group for brivaracetam, 22 per group for LEV). Predrug recordings were performed 2 days before the drug testing.
Audiogenic seizure-susceptible mice were protected against the expression of clonic convulsions by brivaracetam and levetiracetam; ED50 values are shown in Table 2. Brivaracetam, administered i.p. 30 min before seizure induction in mice, also protected against clonic convulsions induced by pentylenetetrazol and against tonic hindlimb extension induced by a maximal electroshock in mice, although with higher ED50 values (Table 2).
Table 2.
Effect of brivaracetam (BRV) and levetiracetam (LEV) against clonic convulsions in sound-sensitive mice and of brivaracetam against chemically induced and maximal electroshock-induced seizures in mice
| Seizure induction |
ED50 (mg kg−1 i.p.) (mean value and range) |
|
|---|---|---|
| BRV | LEV | |
| Audiogenic | 2.4 (1.4–4.0) | 30 (24–48) |
| Pentylenetetrazol | 30 (10–87) | — |
| Maximal electroshock | 113 (89–136) | — |
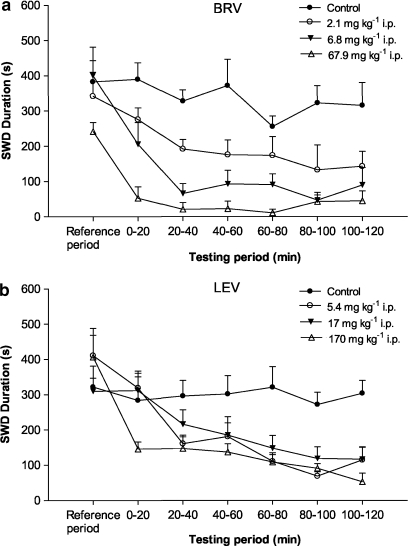
Brivaracetam significantly suppressed spontaneous SWDs in GAERS rats from a dose of 2.1 mg kg−1 with complete inhibition appearing at the highest dose tested (67.9 mg kg−1) (Figure 7a). Levetiracetam, on the other hand, induced significant suppression of SWDs from a dose of 5.4 mg kg−1 but never produced complete inhibition, even at the highest dose tested (170 mg kg−1) (Figure 7b).
Figure 7.
Effect of brivaracetam (BRV in (a)) and levetiracetam (LEV in (b)) on spike-and-wave discharge (SWD) duration in GAERS. Mean value±s.e.mean (n=5 per group). All drug-treated groups were significantly (P<0.05) different from the control group. Data for levetiracetam were adapted from Gower et al. (1995). GAERS, genetic absence epilepsy rats from Strasbourg.
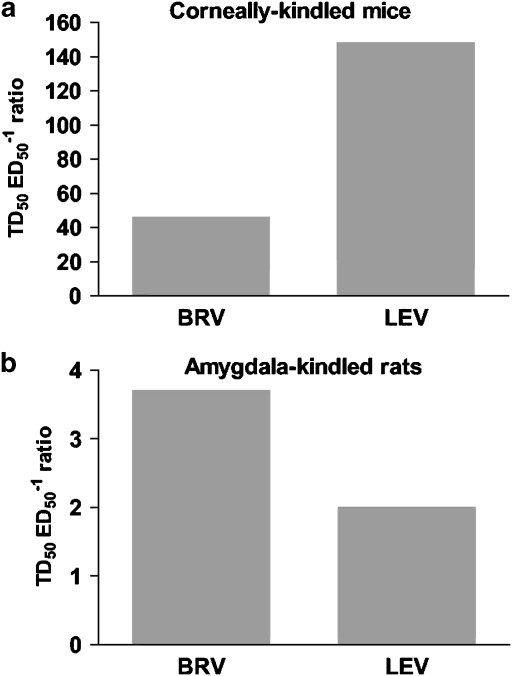
The therapeutic index between impairment of rotarod performance and protection against kindled seizures was defined as the ratio between the TD50 value, determined in the rotarod test in fully kindled animals, and the protective ED50 value obtained against generalized motor seizures in the same animals. Previous studies in corneally kindled mice have revealed a large TD50:ED50 ratio (148) for levetiracetam, compared with similar ratios for other classical or newer AEDs in the range of 2–21. This demonstrates an unusually high separation between the doses of levetiracetam that induce significant rotarod impairment and doses inducing seizure protection in corneally kindled mice. The TD50:ED50 ratio for brivaracetam was 46 as shown in Figure 8a. In a similar evaluation in amygdala-kindled rats, the ratios for brivaracetam and levetiracetam were 4 and 2, respectively (Figure 8b).
Figure 8.
Safety margin of brivaracetam (BRV) and levetiracetam (LEV) in corneally kindled mice (a) and amygdala-kindled rats (b). The safety margin was calculated by dividing the TD50 value for rotarod impairment in kindled animals by the protective ED50 value determined against generalized motor seizures in the same animals. Both brivaracetam and levetiracetam were administered i.p.
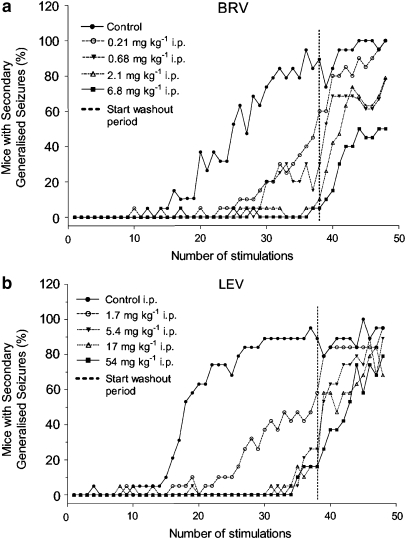
Pretreatment with brivaracetam during corneal kindling of mice resulted in a significant reduction in the incidence of generalized motor seizures, and a similar incidence reduction was observed with levetiracetam at higher doses (Figure 9). Continued corneal stimulations following termination of treatment showed a persistent reduction in the incidence of generalized motor seizures in the group previously treated with the highest dose of brivaracetam. A similar effect was not observed in any of the groups previously treated with levetiracetam.
Figure 9.
Effect of brivaracetam (BRV in (a)) and levetiracetam (LEV in (b)) on the development of corneal kindling in mice. Values given are the percentage of animals with secondarily generalized seizures; brivaracetam and levetiracetam were administered 30 and 60 min, respectively, before each of the twice-daily stimulations. The hatched line indicates a 2-day wash-out period without stimulation. After that period, the animals were restimulated twice daily for an additional period of 5 days without drug treatment.
Discussion
The present study shows that both HKLCF and BMI induced a hypersynchronous firing of a high number of neurons in the CA3 area of rat hippocampal slices, as indicated by time-increasing PS amplitudes and neuronal hyperexcitability assessed by repetitive PSs (Figures 2 and 4). Both brivaracetam and levetiracetam decreased the epileptiform features of the evoked FPs induced by HKLCF and BMI. The concentrations of brivaracetam applied in the perfusion fluid of hippocampal slices in this study are clinically relevant, when compared with human pharmacokinetics data, as they were lower than plasma Cmax values of the maximum tolerated dose of brivaracetam (Sargentini-Maier et al, 2007). It is interesting to note that brivaracetam was particularly active against the repetitive firing, with a maximal effect at 3.2 μM, whereas levetiracetam at 32 μM inhibited mainly the increase in PS amplitude (Figure 2), confirming its previously reported de-synchronizing activity (Margineanu and Klitgaard, 2000). Brivaracetam also presented an interesting tendency to inhibit the HKLCF-induced spontaneous field bursting (that is, spontaneous neuronal synchronization), whereas this epileptiform marker was resistant to treatment with levetiracetam, 32 μM (Figures 2b and 3c). Brivaracetam reduced, but did not abolish, the markers of epileptiform effect of HKLCF, shown in Figure 3, which might suggest a modest effect. However, none of the classical AEDs valproate, clonazepam and carbamazepine produced any decrease of HKLCF-induced ΔPS1, whereas they reduced the firing of repetitive PSs (Margineanu and Klitgaard, 2000). The present study thus demonstrates that brivaracetam possesses higher potency and efficacy than levetiracetam against epileptiform responses in two different in vitro rat hippocampal slice models of epilepsy. The reasons for the lack of concentration dependence in the antiepileptic effects shown in Figures 3 and 5 are not clear. It may reflect the involvement of several drug mechanisms, as in the case of levetiracetam (Klitgaard and Verdru, 2007) and might also be the same for brivaracetam (Zona et al., 2004).
The seizure suppression and the antiepileptogenic properties of brivaracetam in chronic animal models of epilepsy appear to be superior to those of levetiracetam. Brivaracetam also differs from levetiracetam in its ability to protect against seizures in normal animals induced by a maximal electroshock and several chemoconvulsants, albeit at relatively high doses. This could indicate that the mechanism(s) responsible for the pharmacological activity of brivaracetam may be more complicated than might be deduced by simple extrapolation of the properties of levetiracetam, such as the enhanced affinity of brivaracetam for SV2A. It is possible that the interaction of brivaracetam and levetiracetam with SV2A may result in qualitatively different functional responses, and the pharmacological activities of brivaracetam and levetiracetam may also involve mechanisms beyond those related to SV2A.
The latter question has been addressed by several studies exploring to what extent brivaracetam may modulate mechanisms beyond SV2A. Like levetiracetam, brivaracetam was found to be devoid of significant affinity for a number of binding sites associated with different receptor, second messenger systems and ion channels (data not shown). Patch-clamp studies in cultured hippocampal neurons have previously demonstrated that brivaracetam is devoid of any direct action with inhibitory and excitatory neurotransmission, with the exception of a weak minor inhibition of the NMDA receptor current (Rigo et al., 2002, 2004). Brivaracetam was also devoid of effect on high- and low-voltage activated calcium currents (Kostyuk et al., 2004), and on voltage-gated potassium currents (Margineanu et al., 2004) in isolated rat hippocampal neurons. In contrast, brivaracetam has a potent ability, like levetiracetam, to completely oppose the action of negative modulators of currents gated by the two main inhibitory receptor types, GABAA and glycine receptors, which may contribute to its mechanism of action (Rigo et al., 2004). Furthermore, brivaracetam is distinct from levetiracetam by inhibiting voltage-dependent Na+ channels in cortical rat neurons at low micromolar concentrations (Zona et al., 2004). This demonstrates that the mechanism of action of brivaracetam is different from that of levetiracetam and may explain its broader activity profile, in particular the ability to inhibit maximal electroshock seizures.
The enhanced affinity of brivaracetam for SV2A compared to that of levetiracetam was accompanied by a superior antiseizure and antiepileptogenic action in the present study. This confirms previous reports describing a rank order of binding affinity for (S) stereoisomer analogues of levetiracetam to synaptic plasma membranes in the CNS correlating with their potency for seizure protection (Noyer et al., 1995). However, this also suggests that a similar correlation may exist for antiepileptogenic effects in kindling models. This apparent association between the antiseizure and antiepileptogenic properties of levetiracetam and brivaracetam on the one hand and their SV2A affinity on the other hand highlights the promise of the SV2A protein as a novel target for AED discovery.
Although the medical management of epilepsy has been successful by focusing on suppression of seizure activity, an important need remains for drugs with antiepileptogenic properties that may prevent epilepsy or alter its natural course. The main preclinical studies of epileptogenesis have been performed in the kindling model of temporal lobe epilepsyTLE, although it is difficult to identify a human correlate to this process (Walker et al., 2002). Only a small number of AEDs have been shown to delay the kindling process and for all these, the antiepileptogenic efficacy disappears after cessation of drug treatment (Silver et al., 1991). Particular attention was therefore devoted to the discovery (Löscher et al., 1998) and later confirmation (Stratton et al., 2003) that levetiracetam possesses an ability to permanently counteract the kindling-induced increase in after-discharge duration in the rat amygdala-kindling model of temporal lobe epilepsy, also when amygdala stimulations were continued in the absence of the drug. This persisting effect on kindling development suggests that levetiracetam may provide a different potential for antiepileptogenic effects, compared to currently available AEDs.
This highlights the importance of the findings in the mice corneal kindling model employed by the present study. Brivaracetam showed a more potent ability than levetiracetam to inhibit kindling acquisition during the drug treatment period. However, following cessation of drug treatment and a wash-out period, continued corneal stimulations induced kindling development in all groups previously treated with either levetiracetam or brivaracetam. But only the latter groups revealed a marked and dose-dependent reduction in the proportion of animals reaching the kindling criteria of four consecutive generalized seizures in this latter stimulation period, devoid of drug treatment. To fully assess the antiepileptogenic potential of brivaracetam in the kindling model, it will be important to evaluate whether this effect is also present on the duration of epileptiform after-discharges in animal models of focal, electrical kindling. However, the reduced proportion of seizing animals in previously brivaracetam-treated groups can certainly not be due to retarded elimination of brivaracetam or its metabolites, as these are rapidly eliminated in mice (data not shown). Instead, it may relate to the enhanced affinity of brivaracetam for SV2A, compared to that of levetiracetam. It appears likely that the improved antiseizure and antiepileptogenic effects of brivaracetam compared to those of levetiracetam are not reflecting a chance association, but rather a molecular correlate to SV2A involved in mechanisms that are relevant both for the acute genesis of epileptic seizures and for the underlying epileptogenic process. This is supported by recent data showing accelerated kindling acquisition in SV2A(+/−) heterozygous knockout mice (Leclercq et al., 2006).
The clinical utility of new AEDs depends upon their therapeutic index, which expresses the margin between the doses producing therapeutic and those producing adverse effects. This emphasizes the importance of appropriately assessing the adverse potential of new AED candidates during preclinical testing (Klitgaard et al., 2002a). The validity of using normal animals for adverse effect predictions in epilepsy patients is questionable as the dysfunction of the epileptic brain may render patients more susceptible to adverse effects imposed by NMDA antagonists and certain AEDs (Löscher and Schmidt, 1994). This appears to be compatible with the corneal kindling (Matagne and Klitgaard, 1998) and amygdala kindling (Löscher and Hönack, 1991; Hönack and Löscher, 1995) models of temporal lobe epilepsy that produce kindled animals that are more sensitive than normal animals to behavioral alterations caused by these agents. Thus, it appears important to involve animals with a chronic brain dysfunction, mimicking temporal lobe epilepsy, in preclinical testing of adverse effects, particularly when evaluating AED candidates with novel mechanisms of action (Klitgaard et al., 2002b).
This highlights the importance of the observation in the present study that brivaracetam, like levetiracetam (Klitgaard et al., 1998), is devoid of any adverse effect reactions, specifically in fully corneally kindled mice and amygdala-kindled rats. However, brivaracetam was more potent in inducing behavioral alterations than previously observed with levetiracetam (Klitgaard et al., 1998). When quantifying adverse effects by taking the TD50 value in the rotarod test in amygdala-kindled rats and dividing it by the ED50 for protection against generalized seizures in the same animals, brivaracetam showed a higher safety margin (4) than did levetiracetam (2) (Figure 8). The similar safety margin for brivaracetam in corneally kindled mice was lower (46) than the one (148) previously reported for levetiracetam (Klitgaard et al., 1998). Under similar experimental conditions, classical and newer AEDs have therapeutic indices between 2 and 21 in corneally kindled mice (Klitgaard et al., 1998). Together, these preclinical studies show that brivaracetam, like levetiracetam, displays a substantial separation between doses inducing antiseizure/antiepileptogenic effects and doses inducing adverse effects in kindled animals. This suggests that brivaracetam has a potential for an excellent tolerability profile in man.
The present study demonstrated that the new high-affinity SV2A ligand, brivaracetam, induced a more potent and complete suppression of seizures and kindling acquisition than did levetiracetam in various in vitro and in vivo models of epilepsy. The antiseizure activities observed with brivaracetam in animal models that are thought to mimic partial-onset (kindled animals) and generalized (audiogenic seizure-susceptible mice and GAERS) epilepsy in humans were superior to those observed with levetiracetam. Pretreatment with brivaracetam during the corneal kindling process in mice revealed a potent and persistent ability to inhibit kindling development, which was superior to that of levetiracetam. Brivaracetam also showed a wide therapeutic index in kindled animals, similar to that of levetiracetam. Taken together, these results demonstrated that brivaracetam possessed properties superior to levetiracetam as an antiepileptic and antiepileptogenic agent in various experimental models of epilepsy.
Acknowledgments
We are grateful to Z Povegliano, A Gallisz, C Chausée, MC Tordeur, N Leclere, J Feron and K Leclercq for their skilful technical assistance.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AED
antiepileptic drug
- BMI
bicuculline methiodide
- FPs
field potentials
- GAERS
genetic absence epilepsy rats from Strasbourg
- HKLCF
high-K+/low-Ca2+ fluid
- PS
population spike
- ΔPS1
increase in amplitude of the first (and largest) population spike (PS1)
- SV2A
synaptic vesicle protein 2A
- SWD
spike-and-wave discharge
- TD50
dose-inducing performance impairment in 50% of animals
Conflict of interest
The authors are employees of UCB Pharma SA.
References
- Birnstiel S, Wulfert E, Beck SG. Levetiracetam (ucb LO59) affects in vitro models of epilepsy in CA3 pyramidal neurons without altering normal synaptic transmission. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:611–618. doi: 10.1007/pl00005097. [DOI] [PubMed] [Google Scholar]
- Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. Eur J Pharmacol. 2006;536:102–108. doi: 10.1016/j.ejphar.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Hirsch E, Boehrer A, Noyer M, Marescaux C. Effects of levetiracetam, a novel antiepileptic drug, on convulsant activity in two genetic rat models of epilepsy. Epilepsy Res. 1995;22:207–213. doi: 10.1016/0920-1211(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Hönack D, Löscher W. Kindling increases the sensitivity of rats to adverse effects of certain antiepileptic drugs. Epilepsia. 1995;36:763–771. doi: 10.1111/j.1528-1157.1995.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, et al. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem. 2004;47:530–549. doi: 10.1021/jm030913e. [DOI] [PubMed] [Google Scholar]
- Klitgaard H. Levetiracetam: the preclinical profile of a new class of antiepileptic drugs. Epilepsia. 2001;42 Suppl 4:13–18. [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Lamberty Y. Use of epileptic animals for adverse effect testing. Epilepsy Res. 2002a;50:55–65. doi: 10.1016/s0920-1211(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Vanneste-Goemaere J, Margineanu DG. Pilocarpine-induced epileptogenesis in the rat: impact of initial duration of status epilepticus on electrophysiological and neuropathological alterations. Epilepsy Res. 2002b;51:93–107. doi: 10.1016/s0920-1211(02)00099-2. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Verdru P. Levetiracetam: the first SV2A ligand for the treatment of epilepsy. Expert Opin Drug Discov. 2007;2:1537–1545. doi: 10.1517/17460441.2.11.1537. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Lukyanetz EA, Klitgaard H, Margineanu D-G. UCB 34714, a new pyrrolidone derivative, without impact on high- and low-voltage activated calcium currents in rat isolated neurons. Epilepsia. 2004;45 Suppl 7:141. [Google Scholar]
- Leclercq K, Dassesse D, Lambeng N, Klitgaard H, Matagne A. Unaltered Seizure Susceptibility Contrasts Accelerated Kindling Acquisition in Heterozygous SV2A KO Mice. Society for Neurosciences: Washington DC; 2006. [Google Scholar]
- Löscher W, Hönack D. Anticonvulsant and behavioral effects of two novel competitive N-methyl-D-aspartic acid receptor antagonists, CGP 37849 and CGP 39551, in the kindling model of epilepsy. Comparison with MK-801 and carbamazepine. J Pharmacol Exp Ther. 1991;256:432–440. [PubMed] [Google Scholar]
- Löscher W, Hönack D, Rundfeldt C. Antiepileptogenic effects of the novel anticonvulsant levetiracetam (ucb L059) in the kindling model of temporal lobe epilepsy. J Pharmacol Exp Ther. 1998;284:474–479. [PubMed] [Google Scholar]
- Löscher W, Jackel R, Czuczwar SJ. Is amygdala kindling in rats a model for drug-resistant partial epilepsy. Exp Neurol. 1986;93:211–226. doi: 10.1016/0014-4886(86)90160-3. [DOI] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. Strategies in antiepileptic drug development: is rational drug design superior to random screening and structural variation. Epilepsy Res. 1994;17:95–134. doi: 10.1016/0920-1211(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–9866. doi: 10.1073/pnas.0308208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margineanu D-G, Klitgaard H. Inhibition of neuronal hypersynchrony in vitro differentiates levetiracetam from classical antiepileptic drugs. Pharmacol Res. 2000;42:281–285. doi: 10.1006/phrs.2000.0689. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Klitgaard H. Can gap-junction blockade preferentially inhibit neuronal hypersynchrony vs excitability. Neuropharmacology. 2001;41:377–383. doi: 10.1016/s0028-3908(01)00080-6. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Niespodziany I, Wülfert E. Antagonism by levetiracetam (ucb L059) of bicuculline-induced epileptiform activity in rat hippocampus requires activation of corticoid receptors. Soc Neurosci Abs. 1998;24:769.16.1940. [Google Scholar]
- Margineanu D-G, Schlobohm I, Klitgaard H. UCB 34714, a new pyrrolidone anticonvulsant had no effect on voltage-gated potassium currents in cultured mouse hippocampal neurons. Epilepsia. 2004;45 Suppl 3:116. [Google Scholar]
- Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31:59–71. doi: 10.1016/s0920-1211(98)00016-3. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics. 2007;4:18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyer M, Gillard M, Matagne A, Henichart JP, Wülfert E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol. 1995;286:137–146. doi: 10.1016/0014-2999(95)00436-o. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. Academic Press: Australia; 1982. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, et al. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo JM, Nguyen L, Hans G, Belachew S, Moonen G, Matagne A, et al. UCB 34714: effect on inhibitory and excitatory neurotransmission. Epilepsia. 2004;45 Suppl 3:56. [Google Scholar]
- Sargentini-Maier ML, Rolan P, Connell J, Tytgat D, Jacobs T, Pigeolet E, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol. 2007;63:680–688. doi: 10.1111/j.1365-2125.2006.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilespy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- Stratton SC, Large CH, Cox B, Davies G, Hagan RM. Effects of lamotrigine and levetiracetam on seizure development in a rat amygdala kindling model. Epilepsy Res. 2003;53:95–106. doi: 10.1016/s0920-1211(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Walker MC, White HS, Sander JW. Disease modification in partial epilepsy. Brain. 2002;125:1937–1950. doi: 10.1093/brain/awf203. [DOI] [PubMed] [Google Scholar]
- Zona C, Pieri M, Klitgaard H, Margineanu D-G. UCB 34714, a new pyrrolidone derivative, inhibits Na+ currents in rat cortical neurons in culture. Epilepsia. 2004;45 Suppl 7:146. [Google Scholar]