Abstract
The vasoconstrictor substance named serotonin was identified as 5-hydroxytryptamine (5-HT) by Maurice Rapport in 1949. In 1951, Rapport gave Gaddum samples of 5-HT substance allowing him to develop a bioassay to both detect and measure the amine. Gaddum and colleagues rapidly identified 5-HT in brain and showed that lysergic acid diethylamide (LSD) antagonized its action in peripheral tissues. Gaddum accordingly postulated that 5-HT might have a role in mood regulation. This review examines the role of UK scientists in the first 20 years following these major discoveries, discussing their role in developing assays for 5-HT in the CNS, identifying the enzymes involved in the synthesis and metabolism of 5-HT and investigating the effect of drugs on brain 5-HT. It reviews studies on the effects of LSD in humans, including Gaddum's self-administration experiments. It outlines investigations on the role of 5-HT in psychiatric disorders, including studies on the effect of antidepressant drugs on the 5-HT concentration in rodent and human brain, and the attempts to examine 5-HT biochemistry in the brains of patients with depressive illness. It is clear that a rather small group of both preclinical scientists and psychiatrists in the UK made major advances in our understanding of the role of 5-HT in the brain, paving the way for much of the knowledge now taken for granted when discussing ways that 5-HT might be involved in the control of mood and the idea that therapeutic drugs used to alleviate psychiatric illness might alter the function of cerebral 5-HT.
Keywords: 5-hydroxytryptamine, lysergic acid diethylamide, depression, brain, antidepressant drugs, monoamines, history, platelets, monoamine oxidase inhibitors, tricyclic antidepressants
Introduction
The history of recent science can be evaluated in two ways, not necessarily exclusively. One approach examines the published literature, primarily journals, to give an accurate time line of the primacy of presentation of new knowledge to the world, although this does not always indicate primacy of discovery. Consequently, the information gained does not always give a clear idea of how the investigator became involved in a specific research project or the way in which ideas developed. The other approach is to talk to the scientists involved in the research, or to colleagues or friends of those involved in the research, to obtain their personal views of events, people and priority. This latter approach has been used recently in the area of psychopharmacology (Healy, 1996, 1998, 1999) and is of course the basis of many personal reviews published in major journals. However, such reviews can be biased by personal memory and even, it has to be said, personal prejudice. Goodfield, a scientific historian, has commented that ‘however charming the personal recollections of scientists after retirement might be, by then they are often quite unreliable historically'. Mark Twain recognized this problem in a more general way, stating that ‘the older I get the more vivid is my recollection of things that never happened'. However, without some insight into the personalities and professional lives of the scientists involved in the original investigations, a history based on published work alone can become a very dry affair. Therefore, although I am going to focus on the publications of UK scientists involved in the early neuropharmacology research on 5-hydroxytryptamine (5-HT), I hope also to give a flavour of some of the people involved. Obviously, research on aspects of 5-HT biochemistry and function outside the brain markedly influenced research on CNS pharmacology, and this is also detailed where relevant.
Why did I want to examine this area of science history? Primarily, I think, because of being closely involved with the Serotonin Club since its formation in 1987, which resulted in my meeting Maurice Rapport, the man who identified the vasoactive substance circulating in blood and already named serotonin as being 5-HT (Rapport, 1949). To meet and talk at length to this distinguished scientist, who discovered the neurotransmitter that I have spent much of my professional life investigating, was a true privilege and made me realize that I had started studying the pharmacology of cerebral 5-HT less than 15 years after it was first identified in the brain (see later). Shortly after this meeting I was asked to write a review of the neuropharmacology of 5-HT for the special issue of the British Journal of Pharmacology, which celebrated 75 years of the British Pharmacological Society (Green, 2006). This task made me also appreciate what a major role UK scientists had played in the early days of 5-HT research and made me want to examine this in more detail, as most of the key players active in this explosive period of neuropharmacology and psychopharmacology research on 5-HT have now retired.
The enthusiasm of most active researchers naturally (and rightly) rests on current research findings, but it would be a tragedy if we forgot the early work that led to current ideas. I have sometimes observed that findings are erroneously presented as novel because the investigators (and presumably also the editors) were unaware of a much earlier paper reporting similar or even near identical data. I therefore firmly believe that we should all be aware of the history of our particular branch of science, so that we do not ‘reinvent the wheel'. Nevertheless, I was amused to read recently a comment by Professor Gerald Curzon (Figure 1), my PhD supervisor and the man who initiated my lifetime interest in 5-HT, who stated: ‘It is a pity that scientists tend to get interested in the history of their subject only as they approach retirement' (Curzon, 1998), as this fits me only too well.
Figure 1.
Montage of pictures of (a) Sir John Gaddum, (b) Marthe Vogt, (c) Hugh Blaschko, (d) Gerald Curzon, (e) David Grahame-Smith, (f) Phillip Bradley, (g) Alec Coppen and (h) Merton Sandler. (a–c) Copyright of Godfrey Argent; The Royal Society, (d–e) author's collection and (f–h) Copyright of British Association for Psychopharmacology.
The period I have limited myself to is 1950–1970. This is a period referred to by Sjoerdsma and Palfreyman (1989) in their short article on the history of 5-HT research as ‘the renaissance', and for good reasons. Although it had been known for nearly a century that a vasoconstrictive compound existed in blood, this compound was only identified as being 5-HT in 1949. From then until 1970, papers on this compound, reporting major new findings, appeared at a dizzy rate. After 1970 research became rather quieter, until the very end of the decade when receptor subtypes started to be identified and characterized, and research again entered a much more active phase (see Green, 2006).
The objective of the current exercise was not to indulge in nationalistic boasting. Science advances by developing work and ideas produced by scientists worldwide and it is often difficult to ascribe a finding as being the results of the effort of any one scientist or group. All that I desire is to show how active UK scientists were in these early years, producing so much of the neuropsychopharmacology knowledge we now take for granted. To avoid producing a daunting list of references, I have sometimes named non-UK investigators, but not provided a full journal citation for the information being discussed. However, I have tried to detail all the major UK-based studies. I hope overseas readers will forgive me for doing this. It is particularly hard on researchers from the USA and Sweden; both countries produced so many major discoveries in the period under discussion. Indeed, I remain astounded at the amount of novel work on 5-HT metabolism and pharmacology that poured out from the National Heart Institute (NHI), part of the National Institutes of Health (NIH) complex in Bethesda, MD, USA, and on neuroanatomy and neuropharmacology from the Karolinska Institute in Stockholm and the Department of Pharmacology at Gothenburg University in Sweden in the period under review.
The characterization and identification of 5-HT
The fact that defibrinogenated blood increased vascular tone in perfused dog muscle was first reported by Ludwig and Schmidt (1868), and Page (1954) also quoted a paper by LT Stevens and FS Lee, who in 1884 reported the existence of a vasoconstrictive substance in clotted blood. This finding was supported by the later work of TG Brodie, the Director of the combined laboratories of the Royal College of Physicians and Royal College of Surgeons in London, who observed that preventing blood from clotting, by adding sodium citrate, also prevented the action of the vasoconstrictive substance (Brodie, 1900, 1903). Subsequent attempts were made to identify the substance further by examining whether it was heat stable and dialysable, but many of the data were conflicting and have been reviewed in detail elsewhere (Page, 1954).
The key period for its final identification occurred between 1945 and 1950. Irvine H Page had been researching the problem of hypertension in nephritic patients since 1931 and had assumed that a humoral substance was responsible. The vasoconstrictive substance appearing in clotted blood interfered with the investigation of other humoral substances involved in hypertension (the primary substance finally being identified as angiotensin). When he moved to the Cleveland Clinic in 1945 as Research Director, he continued these investigations with Arda A Green, a physical biochemist, and Maurice M Rapport who had recently completed his PhD in organic chemistry at Cal Tech. This trio then continued to work on the characterization problem of the vasoconstrictive substance towards its final solution (Rapport et al., 1948a, 1948b, 1948c). However, it was Maurice Rapport alone who finally identified the substance they had isolated as the indolealkylamine 5-HT (Rapport, 1949). The story of this work has been detailed elsewhere by both Page (1957, 1976) and Rapport (1997), although it is notable that Page in his 1957 review avoided giving full credit to Rapport for the final identification; particularly as identification can only be considered absolute when the compound has been synthesized and its activity compared with the isolated substance.
The work on serotonin in the USA was not the only major investigation proceeding on the compound that finally proved to be 5-HT. Vittorio Erspamer and his colleagues had been working in Italy from the early 1930s on a substance present in enterochromaffin cells of the gastric mucosa of mammals and salivary glands of octopuses. This substance was identified as an indole in 1946. Erspamer named it enteramine and produced prodigious amounts of information on its distribution in many different tissues (see Erspamer, 1954). The final confirmation that enteramine was 5-HT was published by Erspamer and Asero (1952). It is fascinating that the groups of Page and Erspamer worked independently and in ignorance of each other's work until both serotonin and enteramine had been identified as 5-HT.
So why were the names serotonin and enteramine chosen? It was Arthur C Corcoran, a colleague of Page, who coined the name serotonin for the vasoconstricting substance, as this describes one of its functions in blood. Erspamer suggested the name enteramine, as this encompasses both the location of 5-HT in the gut and his realization that the substance was an amine. Recognizing the limitations of both names, Bacq (1952) suggested that both should be discarded. Although recognizing the chemical logic of the name enteramine, he noted that it could not be applied to all known locations, whereas serotonin was inaccurate in terms of both specificity of location and action. Bacq therefore suggested that 5-hydroxytryptamine should be the preferred name (abbreviated to 5-HT). Although this proposal was accepted immediately by Erspamer (1954), it is clear that Page remained unhappy about this, as many years later he mentioned that he had chided Gaddum for using 5-HT, saying that ‘5-HTogenic was an abomination' (Page, 1976). What has happened is that serotonin is still generally used for 5-HT in all its functional guises in the USA, although 5-HT is primarily used in the UK, and both terms are used interchangeably elsewhere. Serotonin is almost exclusively used by the popular press in its many articles about the brain, drugs and mood.
Identification of the vasoconstrictive substance as the chemical substance 5-HT by Rapport (1949) allowed him to persuade Ed Maynert, a chemist in the Pharmacology Department at Columbia University, New York, to synthesize 5-HT, to confirm the identification of the isolated substance. He was then able to convince chemists at both Upjohn and Abbott to devise methods for synthesizing 5-HT (Hamlin and Fisher, 1951; Speeter et al., 1951). This synthesis allowed both companies to distribute 5-HT to interested investigators, which doubtless produced the explosive growth in publications on the amine. Hans Heller, Professor of Pharmacology at Bristol University, asked Rapport for a sample of 5-HT for his studies in the kidney and received a 1 mg sample from him in April 1950. Significantly, Maurice Rapport also supplied a small sample (0.65 mg) to John (subsequently Sir John) Gaddum (Figure 1) when they initially met in the USA and subsequently sent him a much larger sample (25 mg) in 1951 to allow him to continue his studies (Figure 2).
Figure 2.
Letter from John Gaddum to Maurice Rapport. Collection of the author, now deposited in the archives of the Royal Society.
On the measurement of 5-HT in tissues
Comparison of the characteristics of the unknown active substance in tissues with the newly available pure 5-HT substance allowed identification of 5-HT in tissues and organs and also the elimination of various other active compounds such as histamine from further consideration. All such early work relied on bioassay, a staple pharmacological tool. This was a technique used by John Gaddum in some of his earliest work and one that he always had a significant interest in, commenting in 1964: ‘the pharmacologist has been the ‘jack of all trades' borrowing from physiology, biochemistry, pathology, microbiology and statistics—but he has developed one technique on his own and that is bioassay' (Gaddum, 1964). His expertise with this technique resulted in several major discoveries that proved crucial for subsequent 5-HT neuropharmacology research, and the value of bioassay in early research on 5-HT cannot be overestimated.
In a key study, Gaddum and Hameed (1954) investigated the action of 5-HT on a range of tissues before examining the effects of putative antagonists. 5-HT caused contractions of the uterus, duodenum and colon of rats, the uterus, duodenum and jejunum of rabbits, and the uterus, duodenum, jejunum and ileum of guinea pigs, and also caused vasoconstriction of the perfused rabbit ear. The rat oestrus uterus technique used by Gaddum had originally been used by Erspamer in his early studies (Erspamer, 1942, 1952), and the guinea pig ileum had also previously been used by both Gaddum (1953) and Rocha e Silva et al. (1953). The ileum preparation was also used by Gaddum and Picarelli (1957) in their seminal paper on tryptamine receptor subtypes, which I discuss later.
Rapport et al. (1948a, 1948b) had used the rabbit ear preparation in their earliest studies on the identification of the vasoconstrictive substance, because it was already being used by Arda Green (see Rapport, 1997), and it is interesting to note that the preparation had originally been developed by Gaddum and Kwiatkowski (1938) when Gaddum was at University College, London. JH Burn had said of this method: ‘every young pharmacologist should learn to use this preparation as part of his training' (see Feldberg, 1967).
John (later Sir John) Vane also developed a novel bioassay technique for assaying 5-HT, which used a strip of rat fundus (Vane, 1957), although he later commented that Brendan Whittle had subsequently told him it was actually the corpus, not the fundus (Vane, 1997). However, this preparation became generally known as the rat stomach strip and was also used to examine the potency of various tryptamine analogues (Vane, 1959).
It is important in these days of modern analytical techniques not to underestimate the power of bioassay as an analytical tool. Extract of tissue containing low nanogram amounts of 5-HT could be reliably measured with the rat uterus method and Vane (1957) claimed that the stomach strip could respond to 1 ng of 5-HT.
The collaboration of Betty Twarog with Page in Cleveland resulted in a very different type of bioassay. Twarog was very interested in invertebrate pharmacology (Whitaker-Azmitia, 1999) and she consequently used the isolated heart of the mollusc Venus mercenaria as her bioassay tissue. It was this technique that allowed identification of 5-HT in mammalian brain (Twarog and Page, 1953). Gaddum was also interested in mollusc preparations, and together with Paasonen, a Finnish visitor to his laboratory in Edinburgh, he examined various mollusc heart preparations for their suitability in estimating 5-HT. The study required Paasonen to spend some time at the Gatty Marine Laboratories at St Andrews in Scotland, and despite the fact that they reported on the suitability of the bivalve Spisula solida heart, because ‘the animal is hardy and its heart beats constantly for several days in isolation' (Gaddum and Paasonen, 1955), the approach did not supersede the use of mammalian tissues in Edinburgh, judging by later published studies.
Although bioassay is very sensitive and allows identification of the amine in tissue extracts, and indeed quantitative measurement of tissue 5-HT, it is nevertheless a time-consuming technique that is not amenable to the fast throughput of samples required when examining the effects of drugs on cerebral 5-HT content, a problem that Gaddum was happy to acknowledge, stating of bioassays: ‘Their main disadvantages are that they are not very accurate and comparatively slow; it is generally not possible to make more than about ten estimates in one day' (Gaddum, 1959).
This problem of speed was answered with a major methodological leap forward at the NIH in Bethesda, MD, USA. At the NIH there was a formidable group of scientists in the National Heart Institute, including Bernard B Brodie, Sidney Udenfriend, Parkhurst Shore, Herbert Weissbach, Albert Sjoerdsma, Erminio Costa, Julius Axelrod, and many others, including both domestic and international visiting scientists, such as Arvid Carlsson, Alfred Pletscher, Leslie Iversen and Solomon Snyder, all of whom would make a major impact on our understanding of monoamine pharmacology.
In 1955, Robert Bowman from that division, working with the American Instrument Company, developed a fluorescence spectrophotometer (the Aminco-Bowman), which could activate (induce) and measure emitted fluorescence over a wide spectral range (Bowman et al., 1955). This was then used to develop a method for analysing 5-HT extracted from brain tissue (Bogdanski et al., 1956). This method involved measuring the native fluorescence of 5-HT in strong acid, and it was used extensively until Snyder et al. (1965) modified the method by reacting the 5-HT with ninhydrin to produce a fluorophore, thereby increasing sensitivity considerably. However, it is worth noting that this advance was based on work performed many years earlier at the Middlesex Hospital, London, by Jepson and Stevens. These researchers had reported that ninhydrin produced a specific highly fluorescent derivative with 5-HT, which could be used to identify the amine when used in conjunction with paper chromatography (Jepson and Stevens, 1953).
In the late 1960s, Maickel et al. (1968) reported an even more sensitive method that involved reacting 5-HT with o-phthaldialdehyde in strong acid. I tried this method when undertaking my PhD with Gerald Curzon, but we were disappointed with its sensitivity. However, Korf in Holland then published a method for measuring 5-hydroxyindole acetic acid (5-HIAA) in urine, using o-phthaldialdehyde, and showed that adding cysteine enhanced the sensitivity of the assay. Curzon therefore asked me to try the 5-HT assay again, but with the addition of cysteine. He also suggested that the extraction of 5-HT should leave the metabolite 5-HIAA in the aqueous phase and that this could therefore also be extracted and reacted with o-phthaldialdehyde, thereby allowing both 5-HT and its metabolite 5-HIAA to be measured in the same tissue sample. We found that this method did allow these two indoles to be measured in discrete regions of a single rat brain. This had not previously been possible using the ninhydrin method, and we duly published the work as a short paper (Curzon and Green, 1970). We were somewhat surprised by the enthusiasm with which the method was adopted, and it was used by others extensively over the next decade, until HPLC replaced it in the late 1970s. The use of fluorescence to quantify the major 5-HT metabolite also provided another advance over bioassay, as the metabolite cannot be measured by that technique.
Curzon had also been responsible for some key early work on the measurement of 5-HIAA, albeit not in cerebral tissue, when he developed a paper chromatography method for its detection in urine (Curzon, 1955). He then went on, with others, to examine urinary 5-HIAA excretion in patients with the carcinoid syndrome.
The pathways of 5-HT synthesis and metabolism
UK scientists can lay claim to having been major players in the elucidation of the pathways of 5-HT synthesis and metabolism, starting with the fact that tryptophan, the amino-acid precursor of 5-HT, was discovered by Sir Frederick Gowland Hopkins, the first Professor of Biochemistry at the University of Cambridge (see Curzon, 1987).
The likelihood that 5-HT was formed from tryptophan, by hydroxylation followed by decarboxylation was first proposed and partially demonstrated at the National Heart Institute, Bethesda, MD, USA, in a series of papers in J Am Chem Soc in 1953 (see Udenfriend et al., 1953). The race was then on to characterize fully the enzymes involved.
Tryptophan hydroxylase
Udenfriend et al. (1956) demonstrated the existence of the hydroxylation step by administering [14C]tryptophan orally to toads for 5 days and extracting their venom glands on day 7. Labelled 5-hydroxytryptophan (5-HTP) was then identified. Other studies in this paper showed conversion in mammals of labelled tryptophan to 5-HT and 5-HIAA, thereby establishing the probable chain of biochemical events. However, full identification of the hydroxylase enzyme took many more years, during which time there was discussion as to whether tryptophan was hydroxylated by a specific tryptophan hydroxylase enzyme or whether phenylalanine hydroxylase was utilized, as it is in the liver. The first absolute identification of the hydroxylase enzyme in brain was made by David Grahame-Smith (Figure 1), working at St Mary's Hospital, London (Grahame-Smith, 1964). This preliminary paper was followed by a report of the full characterization of the enzyme (Grahame-Smith, 1967). It is noteworthy that the latter paper also demonstrated the presence of the enzyme in synaptosomes, the pinched-off presynaptic nerve endings first prepared from brain homogenates by Whittaker (1959) at the Agricultural Research Council Laboratories at Babraham in Cambridge. Synaptosomes had been shown to contain both 5-HT (Michaelson and Whittaker, 1962; Michaelson et al., 1964) and 5-HTP decarboxylase (Rodriguez de Lores Arnaiz and de Robertis, 1964). This allowed Grahame-Smith to conclude correctly that brain 5-HT was formed by biosynthesis from tryptophan at the nerve ending and that this formation was sufficient for its needs.
The availability of a specific inhibitor for tryptophan hydroxylase, namely para-chlorophenylalanine, from the Pfizer Laboratories in the USA (Koe and Weissman, 1966), markedly assisted subsequent studies on 5-HT biosynthesis, although the data produced initially caused considerable unease in those working on the function of 5-HT in the brain, as para-chlorophenylalanine produced a profound loss in rodent cerebral 5-HT content with little overt change in behaviour. It took a while before the idea could be confirmed that para-chlorophenylalanine could produce a major loss of 5-HT in the brain without also altering the small functional pool of the amine (see Green, 2006).
5-HTP decarboxylase
The synthesis of 5-HTP by Ek and Witkop (1954) allowed Udenfriend and colleagues both to purify and to characterize the enzyme not long after the initial identification of 5-HT (Clark et al., 1954). Gaddum was also working in this area, undertaking a study with Giarman while he was in Edinburgh on leave of absence from the Pharmacology Department at Yale University. They reported the presence of decarboxylase activity not only in peripheral tissues but also in the brain, and they concluded that the fact that there was activity in the CNS was ‘supporting the theory that 5-HT plays a physiological role [in the brain]' (Gaddum and Giarman, 1956).
Monoamine oxidase
In 1910, researchers at the Wellcome Laboratories (under the then Director Sir Henry Dale) observed that cats perfused with tryptamine produced indole acetic acid (Ewins and Laidlaw, 1910). The characterization of the enzyme responsible for this step was performed by a graduate student, Mary Hare (Bernheim), in the Cambridge laboratory of Sir Frederick Gowland Hopkins (Hare, 1928). A few years later, Hermann (Hugh) Blaschko (Figure 1) and colleagues showed that this enzyme also oxidized adrenaline (Blaschko et al. 1937), and in the same year a paper from the Biochemical Laboratory at the Cardiff City Mental Hospital demonstrated that this enzyme, which we now call monoamine oxidase (MAO) was present in the brain (Pugh and Quastel, 1937). With all this knowledge available, even before the discovery of 5-HT, it is not surprising that MAO was rapidly acknowledged and confirmed as being the mechanism by which the amine was degraded to the aldehyde (Blaschko, 1952).
Recognition that MAO existed in multiple forms came in the late 1960s with work in the laboratory of Merton Sandler (Figure 1) in London. The group identified multiple enzyme forms in both rat (Youdim et al., 1969) and human brains (Youdim et al., 1972), and by the early 1970s it was accepted that there were clearly two functional forms with differing substrate specificities (types A and B, generally now referred to as MAOA and MAOB). Type A has tyramine and 5-HT as its major substrates, whereas type B oxidizes dopamine well but 5-HT poorly. However, it was subsequently shown that achieving a functional increase in cerebral 5-HT function in vivo by selective inhibition of MAOA was problematic (Green and Youdim, 1975). These discoveries were markedly assisted by synthesis of the selective MAOA inhibitor clorgyline at the May and Baker laboratories at Dagenham, Essex (Johnston, 1968). Some of this work has recently been reviewed elsewhere (Youdim and Bakhle, 2006).
Aldehyde dehydrogenase and aldehyde reductase
The 5-hydroxyindoleacetaldehyde that is produced by the action of MAO on 5-HT is short-lived and cannot normally be detected in the brain, unless a trapping agent is used (Udenfriend et al., 1956). In the liver, the aldehyde can then be metabolized to either the acid, 5-hydroxyindoleacetic acid (5-HIAA) or the alcohol, 5-hydroxytryptophol (5-HTOH). In humans, administration of ethanol shifts metabolism towards 5-HTOH (Feldstein et al., 1967).
Donald Eccleston and colleagues at the Medical Research Council (MRC) Brain Metabolism Unit in Edinburgh demonstrated that both human and rat brains could form 5-HTOH (Eccleston et al., 1966), although a subsequent study showed that ethanol did not shift aldehyde formation towards 5-HTOH in the brain (Eccleston et al., 1969). Shortly thereafter, aldehyde dehydrogenase was purified from pig brain and its properties were studied in the Biochemistry Department of Cambridge University (Duncan and Tipton, 1971).
Metabolic control of 5-HT
Tryptophan availability
Tryptophan hydroxylase is the rate-limiting enzyme for the formation of 5-HT in the brain and is unsaturated with its substrate (Grahame-Smith, 1967). This means that any increase in brain tryptophan content will increase the rate of 5-HT synthesis. This was demonstrated by administering a diet rich in tryptophan or, as demonstrated in Edinburgh, by intraperitoneal injection of tryptophan (Ashcroft et al., 1965; Moir and Eccleston, 1968).
Although it was first reported in 1958 that most plasma tryptophan is protein bound (and thus not available for transport into the brain), the full import of this fact was not realized for at least another 10 years. Although the work of Curzon in London, Gessa in Italy and Wurtman in the USA, was crucial in clarifying the ways that alterations in food availability, carbohydrates and free fatty acids also altered tryptophan availability for the brain, most of this work was performed in the early to mid-1970s and so will not be considered further here.
However, Curzon was developing some of his ideas on the role of tryptophan availability on cerebral 5-HT concentration in the mid-1960s, and this work formed the basis of my PhD studies with him. There was then much interest in the possible role of corticosteroids in depression and as they were known to induce the main enzyme metabolizing tryptophan, liver tryptophan 2,3-dioxygenase (then called tryptophan pyrrolase), he suggested I should see if hydrocortisone, by depleting tryptophan, would also decrease brain 5-HT. Hydrocortisone produced exactly that effect in rats (Green and Curzon, 1968), and we subsequently performed other studies using stress as the corticosteroid inducer, with similar consequences (Curzon and Green, 1969). These studies resulted in a hypothesis that linked steroids, tryptophan pyrrolase, tryptophan availability, brain 5-HT concentration and depression (Curzon (1969). A very similar hypothesis was proposed by two scientists in Russia at much the same time (Lapin and Oxenkrug, 1969), although they had apparently reached their proposal without any direct experimental work and without seeing any of our supporting experimental work (at least according to their reference list).
Later work we performed suggested that the depletion of cerebral 5-HT content might be due, at least in part, to the increase in metabolites formed down the tryptophan pyrrolase pathway (kynurenine, 3-OH kynurenine), as these compounds are amino acids and compete with tryptophan for entry into cerebral tissues (Green and Curzon, 1970). Again, parallel independent studies were being published, supporting the idea that certain amino acids compete with tryptophan for transport into synaptosomes (Grahame-Smith and Parfitt, 1970).
Tryptophan transport into brain
Although studies that clarified the role of free versus bound tryptophan in the blood, the effect of changes in the concentrations of large neutral amino acids in plasma and the roles of carbohydrates and insulin on tryptophan transport into the CNS were generally not published until the early 1970s, the work of Grahame-Smith and Parfitt (1970) did point the way by defining how tryptophan entered synaptosomes. These studies showed not only that there was competition for the transport of tryptophan by the neutral amino acids (leucine, tyrosine, methionine and phenylalanine) but also that exchange diffusion occurred.
Overall, these findings on the role of free (unbound) tryptophan in blood and the involvement of competing amino acids, insulin and carbohydrates pointed the way to an understanding as to how tryptophan availability for the brain could be altered in vivo and thus alter 5-HT synthesis. Nevertheless, the work of Grahame-Smith and others cast doubt on tryptophan availability as being a crucial determinant in the control of 5-HT function as is now discussed.
Control of synthesis and turnover
The whole concept of measuring synthesis and turnover was due primarily to Ermino Costa, Norton Neff and others in the USA, and UK science had little involvement with it. However, what can be shown is how the idea of turnover (a term much abused by many who do not fully understand it) helped provoke other new ideas on the importance of synthesis and turnover in terms of controlling the functional activity of 5-HT in the brain. While the main work was published after 1970, the ideas were being developed in the late 1960s, and were so influential to further 5-HT research that I shall mention them here. Grahame-Smith (1971) showed that although a tryptophan load markedly increased cerebral 5-HT synthesis rate and an MAO inhibitor increased the cerebral 5-HT concentration 2–3 times, animals treated with either drug alone showed few overt behavioural changes. However, administration of an MAO inhibitor together with tryptophan resulted in a complex behavioural syndrome, often called the ‘serotonin syndrome', with several clear behaviours, including head weaving and reciprocal forepaw treading. From these observations he deduced that 5-HT function was probably controlled, at least in part, by intracellular metabolism of 5-HT by MAO. Crucially, he proposed that this indicated that measuring the cerebral 5-HT concentration or 5-HT synthesis rate was not sufficient to deduce whether function is altered. He also realized that we now had a method for examining how psychoactive drugs alter 5-HT function, by examining the effect of drugs on the syndrome. This led to a series of investigations on psychoactive drugs in his laboratory in Oxford in the 1970s and beyond (Green and Grahame-Smith, 1976).
The paper that suggested to Grahame-Smith that raising cerebral 5-HT function would lead to a series of behavioural changes in rats was that of Hess and Doepfner (1961), who had shown that administration of tryptophan or 5-HTP with an MAO inhibitor increased the concentration of both 5-HT and tryptamine in the brain and also led to a series of complex behavioural changes. They also noted that the severity of the behaviour did not correlate with the increase in brain amine concentration.
This paper had also intrigued a group of scientists working at the Parke Davis Laboratories in Hounslow. They examined whether distinct behavioural changes could also be induced in mice by raising cerebral 5-HT content. This was achieved by administering high doses of the precursor 5-HTP. In an elegant and comprehensive study, they showed that 5-HTP injection produced a characteristic head twitch behaviour, which increased dose-dependently. They also showed that the number of twitches was related to the cerebral 5-HT content and that the response was blocked by a decarboxylase inhibitor (Corne et al., 1963). They were able to antagonize the response with drugs recognized as 5-HT antagonists, such as lysergic acid diethylamide (LSD), cyproheptadine and methysergide, and concluded that the behavioural response ‘can be used to study potentiators and antagonists of 5-HT in vivo'. However, the importance of this work and its implications were not really recognized at the time, and the model was little used for this purpose for many years afterwards. It was perhaps only when it was realized that the head twitch was a 5-HT2 receptor-mediated response that the model became fully exploited.
5-HT and platelets
Although superficially it might seem odd to have a section devoted to platelet 5-HT in a review of neuropharmacology, the fact that the platelet takes up and stores 5-HT gives an analogy to the 5-HT nerve ending (with the immediate and major proviso that platelets do not synthesize the amine). The platelet has therefore been used extensively in studies on 5-HT, as I shall review later.
The identification of the vasoconstrictive substance in blood as 5-HT naturally led to studies examining whether the substance was present in plasma or in specific organelles, a question that, when previously addressed, had led to conflicting answers (see Page, 1954). However, Reid in Melbourne, Australia, was another scientist who received an early sample of pure 5-HT from Rapport, which assisted him in showing that essentially all the amine found in blood was present in the platelet (Rand and Reid, 1951).
Subsequent studies in Brodie's laboratory at the NIH and Stacey's laboratory at St Thomas's Hospital in London (Hardisty et al., 1956) showed that reserpine depleted platelet 5-HT, and Humphrey and Toh (1954), working at the National Institute for Medical Research (NIMR) at Mill Hill in London, reported that dog platelets would accumulate 5-HT. This was confirmed in human platelets the next year by Hardisty and Stacey (1955) who stated that 5-HT was ‘taken up' by platelets by an ‘active concentrating mechanism' against the concentration gradient. Other UK scientists subsequently reported that platelets had a high-affinity uptake system (Born and Gillson, 1959; Stacey, 1961; Sneddon, 1969). Crucially, this was several years before it was demonstrated that the brain possessed a high-affinity uptake system (Ross and Renyi, 1967). However, it is interesting to note that the concept that amines are taken up and stored in tissues had first been proposed about 30 years earlier by JH Burn, another distinguished UK pharmacologist, who investigated ephedrine and tyramine while working in London at the pharmacological laboratories of the Pharmaceutical Society of Great Britain (Burn, 1932).
5-HT receptor subtypes
We are now familiar with the concept that 5-HT has multiple receptor subtypes. However, relatively few people realize that the proposal that 5-HT could act on more than one receptor type was first made by Gaddum and Hameed (1954) in their studies on the effect of 5-HT on various mammalian tissues. The definitive investigation on receptor subtypes followed 3 years later (Gaddum and Picarelli, 1957). Gaddum's classification was that of D and M receptors, names that he chose because the receptors were blocked by dibenzyline and morphine, respectively. However, this classification was considered to be applicable only to peripheral receptors, and it was not until the mid-1970s that 5-HT receptor subtypes were identified and characterized in CNS tissue (see Green, 2006). However, the correctness of Gaddum's original work is exemplified by the fact that the D receptor was finally categorized as the 5-HT2 receptor and the M receptor as the 5-HT3 site.
5-HT in the CNS
Introduction
The realization that serotonin was not unique to blood, nor enteramine to the gut, coupled with the ability to identify 5-HT and measure its concentration by bioassay, resulted in substantial efforts by several groups to examine extracts from a multitude of tissues. It is clear that the existence of 5-HT in the brain was discovered independently, and at much the same time, by Twarog and Page in Cleveland, OH and by Gaddum and colleagues in Edinburgh. Gaddum presented the results orally to a meeting of the British Pharmacological Society in July 1952 (the full paper appearing 2 years later: Amin et al., 1954), and the full paper of Twarog and Page (1953) was submitted in June of that year. The Edinburgh group not only identified the amine in the brain but also published data on its regional distribution in dog brain, using bioassay preparations involving the rat uterus (Amin et al., 1954) and S. solida mollusc (Paasonen and Vogt, 1956). Both studies showed a high concentration of 5-HT in caudate and hypothalamus and a low concentration in cerebellum. Paasonen and Vogt (1956) also reported on the amine-depleting effect of reserpine in dog brain.
5-HT, LSD and mental state
The first work to suggest that a tryptaminergic compound could have CNS-stimulatory effects was performed at the Wellcome Laboratories in Herne Hill, London, almost a century ago. Laidlaw examined ‘the amine corresponding to the amino acid tryptophane [sic]' and observed that the effect of injection of indolethylamine (tryptamine) in cats was that of brief behavioural excitation (Laidlaw, 1912).
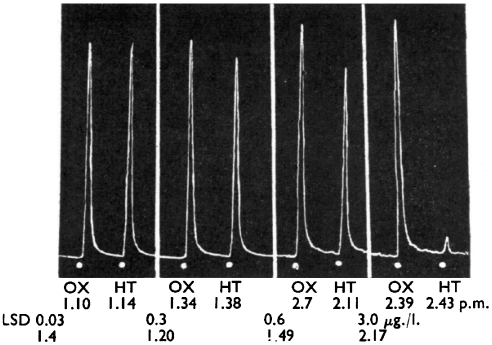
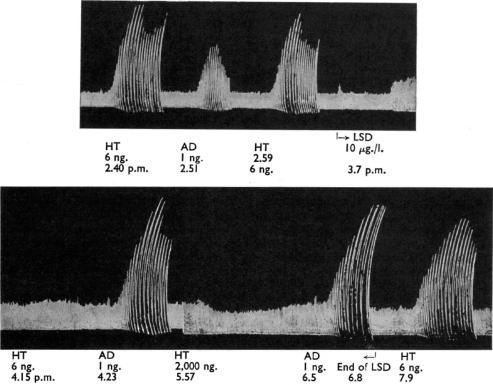
Identification of 5-HT in the brain by Gaddum and Hameed (1954) in Edinburgh was accompanied by the major discovery that LSD had an antagonistic action on the effect of 5-HT in both the rat uterus bioassay system (Figure 3) and in the rabbit ear preparation (Figure 4). It is not surprising that Gaddum investigated ergot compounds in his preparations. He had trained under Sir Henry Dale at the National Institute for Medical Research, and Dale had been interested in ergot compounds from his days at the Wellcome Laboratories at Herne Hill near London (where Gaddum's career had begun when he worked with JW Trevan). While at the Wellcome Laboratories Dale had investigated the actions of ergot (Dale, 1906), and Laidlaw, in the same laboratories, had even demonstrated that ergotoxin antagonized the action of tryptamine (Laidlaw, 1912). Gaddum had also extended the finding of Heymans in 1932 that ergotamine would antagonize the vasoconstrictor substance found in defibrinogenated blood by observing that dihydroergotamine had the same effect when using the rabbit ear preparation (Gaddum et al., 1949).
Figure 3.
The effect of 5-HT (HT; 16 μg L−1), oxytocin (OX; 30 μg L−1) on a rat uterus preparation in a 3 mL bath and the action of various concentrations of LSD. Reproduced from Gaddum and Hameed (1954), with permission of the British Pharmacological Society and Nature Publishing Group.
Figure 4.
The effect of 5-HT (HT) and adrenaline (AD) in the rabbit ear preparation. LSD (10 μg L−1) specifically inhibited the response to 5-HT. Reproduced from Gaddum and Hameed (1954), with permission of the British Pharmacological Society and Nature Publishing Group.
Gaddum found that LSD was the most potent of the eight ergots examined for their ability to antagonize 5-HT in the rat uterus and rabbit ear preparations (Gaddum and Hameed, 1954). A later study by these two scientists, in collaboration with others from Glaxo Laboratories in Greenford, Middlesex, examined a range of indole-containing compounds and confirmed, using pA2 calculations, that LSD was the most potent compound available and also that its effects became insurmountable (Gaddum et al., 1955).
LSD was by then also known to produce clear psychedelic effects. These had originally been experienced by the medicinal chemist Albert Hofmann, who had first synthesized the compound in the Sandoz laboratories in Basel (see Hofmann, 1979). For his own description of the effects of ingesting LSD, see http://www.flashback.se/archive/my_problem_child. The paper reporting on these effects was first published by Stoll (1947), and Gaddum and colleagues (Amin et al., 1954) specifically concluded that the psychoactive effects of LSD could be due to an action on 5-HT in the brain.
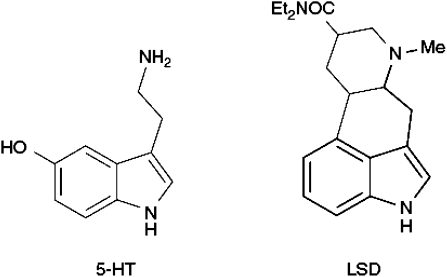
Similar ideas were being developed simultaneously on the other side of the Atlantic by Woolley and Shaw (1953), who investigated the antagonistic action of various indole-containing compounds using in vitro tissue preparations, and subsequently pointed out that some mood-altering ergot compounds, including LSD, ‘contained' within their structure the 5-HT structure (Figure 5) (Woolley and Shaw, 1954). They referred to LSD as being a ‘serotonin antimetabolite' and also proposed that antagonism of 5-HT might be the reason that such compounds were what came to be called psychotomimetic.
Figure 5.
The structure of LSD compared with that of 5-HT.
Gaddum and Vogt (1956) used the new approach of intraventricular injection of drugs in conscious cats, a method that had recently been developed by Feldberg and Sherwood (1954), to investigate the effects of LSD. Although low intraventricular doses of LSD (200 μg) appeared to have little effect on behaviour, it was found that when a higher dose (800 μg) was given cats that had been quiet and friendly ‘became for a time unreasonable and intolerant', hissed fiercely and bared their teeth. The non-hallucinogen brom-LSD had no effect.
However, buried in this report of a major preclinical study is a quite amazing section, simply subheaded ‘methylamphetamine and LSD'. This states that a few observations were made in man and goes on to say: ‘one of us took, on four occasions [my italics], 36–86 μg LSD by the mouth and experienced some of the known effects of this drug, such as a feeling of irresponsibility and euphoria, increased awareness of shapes and colours, shimmering of peripheral vision, and a dreamy feeling that sensations did not represent real objects'. Which of these two distinguished pharmacologists took the drug? No clue is given. However, perusal of his laboratory notebook in the archives of the Royal Society reveals that it was in fact Gaddum who took the hallucinogen. RB (Dick) Barlow, who later joined the Edinburgh department, told me that he had heard that Marthe Vogt (Figure 1) had been very worried about what ensued.
The initial self-ingestion of LSD occurred on Good Friday, 3 April 1953, when Gaddum took 30 μg. Few mental changes occurred, other than his reporting that ‘everything looks a little blue, like a picture of a ghost by Stanley Spencer'. He therefore took a further dose of 40 μg on Saturday 4 April, when he also took methedrine (methamphetamine; 5 mg) to observe whether the amine oxidase-inhibitory action of the amphetamine might increase cerebral 5-HT and thereby antagonize the effect of the LSD. He concluded that methedrine had the effect of making him feel more normal. Two further experiments were performed on 9 May and 1 June, using much higher doses (85 and 86 μg, respectively) and with a variety of tests, including repeating numbers, drawing a geometric shape, threading a needle, touching his nose with the finger and dictating passages of a book. In the last study, he also took methedrine (10 mg). It is clear that the mood-altering effects of LSD were much more profound than in the initial studies, with changes in body ideation, colour changes, feelings of irresponsibility and loss of concentration (Figure 6). The methedrine appeared to diminish some of the intoxicant effects of LSD but not the subjective effects. These results were reported only very briefly by Gaddum and Vogt (1956).
Figure 6.
From the laboratory notebook of JH Gaddum written during self-administration of LSD; held in archives of the Royal Society; Copyright of Royal Society.
This clinical part of the study of Gaddum and Vogt (1956) perhaps reflects the fact that there was some enthusiasm in the UK for investigating the effect of LSD, not only in animals but also humans—both healthy volunteers and psychiatric patients. Mayer-Gross et al. (1951) at Crighton Royal Hospital, Dumfries, gave LSD to 20 volunteer hospital staff and examined mood. They also examined glucose metabolism by use of plasma measures because, as they explained: ‘normally the only substance metabolised in the brain cell is glucose'. Not surprisingly, they found little change. Several years later LSD was still being given to volunteers and the change in psychological state was assessed. For example, it was reported that LSD induced some schizophrenia-like effects and that these effects were reduced by administration of 5-HTP (Brengelmann et al., 1958). However, the same investigators subsequently concluded that LSD did not induce a schizophrenia-like state, as 5-HTP had little therapeutic effect in people with schizophrenia (Brengelmann et al., 1959). Liddell and Weil-Malherbe (1953) gave LSD intravenously in doses up to 60 μg to a mixed group of psychiatric patients in Runwell Hospital, Essex, and examined the subsequent behavioural changes. Although some patients had relatively benign LSD-induced ‘trips', one female depressive patient clearly experienced a severe adverse response—she screamed continuously for over 2 h and was reported as having a ‘sense of terror'. There was an even more serious adverse event in the Brengelmann et al. (1958) study, in which one volunteer, a Maudsley Hospital junior doctor, also suffered a bad trip. This event has been described elsewhere by Merton Sandler, who with CMB (Mike) Pare was one of the investigators involved in this study (Sandler, 1996). Needless to say Ethics Committees overseeing clinical investigations in either healthy volunteers or patients were unknown in the 1950s.
Phillip Bradley (Figure 1) undertook his PhD studies in the University of Birmingham laboratory of Joel Elkes, where both preclinical and clinical studies on LSD were being conducted. He also studied the effects of LSD on the electrical activity of the brain of healthy volunteers (Bradley et al., 1953) and showed that the EEG changes observed after LSD were paralleled by the changes in electrical activity seen in unrestrained cats given the drug (Bradley and Elkes, 1957). Bradley's studies were reported in a series of presentations to the Physiological Society during 1952–1954, and in subsequent full papers (Elkes et al., 1954; Bradley and Elkes, 1957). These investigations induced in Bradley a career-long interest in the electrophysiological effects of 5-HT (Vane et al., 1961), and he was still publishing major papers on the interaction of LSD with 5-HT neurons in the brains of experimental animals almost 20 years later, although many of the later studies used iontophoretic techniques (Boakes et al., 1969, 1970).
Another link between 5-HT and mental state was firmly established in 1955. The year before, Woolley and Shaw (1954) had noted that reserpine produced a change not only in blood pressure but also in mental state and also observed that reserpine contained the indole moiety in its structure. Brodie and his colleagues at NIH then reported in a series of papers that reserpine was released from 5-HT from its stores and thereby depleted brain 5-HT content (Pletscher et al., 1956) and they concluded that 5-HT was required for normal brain function. However, this was just speculation and great efforts were made to strengthen the hypothesis by examining other mood-altering drugs, to determine whether they also changed the 5-HT concentration in the brain. Such work is discussed later in this article.
The role of 5-HT in temperature regulation
As well as studies on the role of 5-HT in mood regulation, the work of Wilhelm Feldberg and others on the role of cerebral 5-HT and thermoregulation should not be forgotten. In 1964, while working with Myers, an American visitor to his laboratory, Feldberg demonstrated that intraventricular 5-HT raised the body temperature of cats, whereas adrenaline and noradrenaline had the opposite effect (Feldberg and Myers, 1964). It was suggested that the hypothalamus was the site of action of the amines, as this region contained a substantial amount of endogenous amine. This proposal was supported by a subsequent study that showed that microinjections of amines into the hypothalamus had a similar effect (Feldberg and Myers, 1965). However, Cooper et al. (1965) working in Oxford found that, in contrast, 5-HT had a hypothermic action when injected into the cerebral ventricles or hypothalamus of rabbits. The same effect of 5-HT was subsequently reported in mice by Brittain and Handley (1967) working in the Glaxo Laboratories. Such studies prompted many subsequent studies by others, particularly Myers following his return to the USA (see Myers, 1973).
5-HT and psychiatric disorders
The reasons why UK scientists should have focused primarily on 5-HT when investigating both the pharmacology and the aetiology of affective disorders (Pare, 1965; Coppen, 1967; Curzon, 1969), whereas American scientists focused more on noradrenaline (Schildkraut, 1965) remains unclear, but it is certainly the impression gained by examining the literature (see below) and crucially when reviewing the opinion of prominent UK scientists active in this period (Coppen, 1996; Sandler, 1996; Eccleston, 1999).
5-HT and antidepressant drugs: preclinical studies
Following the proposal that 5-HT might be involved in the control of mood, the logical progression of thought was that therapeutic drugs used to alleviate psychiatric illness might alter the function of 5-HT in the brain. The word serendipity is often used when discussing the development of this research area, and although serendipity undoubtedly did play a role, the major influence was good clinical observation. As stated above, a major initiating factor was the discovery that reserpine, which was known to produce depression in some susceptible people taking it for hypertension (Achor et al., 1955), depleted 5-HT in the brain. Analogously, it was observed that the antituberculosis drug iproniazid caused behavioural excitation in some patients, and this compound was found to inhibit MAO activity in vitro (Zeller et al., 1952). As iproniazid increased the cerebral content of 5-HT following its administration to rats (Udenfriend et al., 1957), the idea gained ground that ‘depression' and ‘excitation' could be related to the lack or excess of 5-HT in the brain. This simple hypothesis was further strengthened by studies on tricyclic antidepressants, when they were subsequently shown to inhibit high-affinity 5-HT and noradrenaline reuptake (Axelrod and Inscoe, 1963) and potentiate the action of these amines at the postsynaptic receptor.
These areas will only be examined briefly, as most major preclinical discoveries were not made in the UK.
Studies on the mechanism of action of the MAO inhibitors on 5-HT biochemistry were primarily conducted outside the UK, although, as pointed out earlier, the discovery of the enzyme occurred in Cambridge, and one of the UK's great biochemical pharmacologists, Hugh Blaschko, was responsible for much of our knowledge on its involvement in the metabolism of catecholamines.
Although rather little groundbreaking preclinical research on MAO inhibitor drugs can be claimed by UK scientists during the early 1960s, the synthesis and preclinical evaluation of clorgyline (Johnston, 1968) markedly assisted our understanding of the two enzyme subtypes (see earlier). The push to develop subtype-specific enzyme inhibitors was occasioned by a severe adverse reaction seen in some patients taking MAO inhibitors, which had been detailed in papers from the Maudsley Hospital, London. Although Blackwell (1963), a junior doctor at the hospital, was not the first person to report on the problem of a hypertensive crisis in some patients taking this type of drug, he was the first to show a clear association with food, and primarily cheese. He suggested that the problem was the presence in cheese of a pressor substance, normally destroyed by MAO, or one involved in the release of catecholamines. He extended his work in two impressive preclinical studies with Marley showing that both cheese and yeast extracts contained pressor substances and that administration of either cheese or yeast extract by intraduodenal injection to cats pretreated with an MAO inhibitor resulted in the detection of tyramine in the plasma (Blackwell and Marley, 1966a, 1966b). A full review of the pharmacology and clinical problem of foodstuffs and MAO inhibitors was presented to psychiatrists shortly thereafter (Blackwell et al., 1967). There is a significant number of foodstuffs that are contraindicated with MAO inhibitors (Table 1), which makes prescribing such drugs hazardous.
Table 1.
Efficacy of various treatments for depression as observed in the Medical Research Council (1965) trial, adapted from data presented in that publication
| Treatment | n (M/F) |
% improved |
||
|---|---|---|---|---|
| Men | Women | Total | ||
| Placebo | 51 (15/36) | 53 | 42 | 45 |
| Phenelzine | 50 (15/35) | 60 | 29 | 38 |
| Imipramine | 58 (22/36) | 82 | 67 | 72 |
| ECT | 58 (21/37) | 71 | 92 | 84 |
Abbreviation: ECT, electroconvulsive therapy.
Although the so-called ‘cheese reaction' did much to deter psychiatrists from prescribing MAO inhibitors, it was another major study in the UK that increased their antipathy towards this class of drug. This was a multicentre study on the efficacy in depressed patients of imipramine versus the MAO inhibitor phenelzine versus electroconvulsive therapy, which was conducted under the auspices of the Medical Research Council. The data suggested that phenelzine was no better than placebo (Table 2). The publication (Medical Research Council, 1965) was much criticized by some psychiatrists, who questioned not only the dose used but also the patient selection, arguing that MAO inhibitors were better in patients with ‘reactive' rather than ‘endogenous' depression, although such terms were contentious even then. The end result was that many felt that it was much safer to prescribe a tricyclic antidepressant for their depressed patients rather than a drug carrying with it a problem associated with ingestion of several foodstuffs and with questionable efficacy. The market for MAO inhibitors never recovered. The food interaction problem led in part to the suggestion that an MAO inhibitor that did not inhibit the subtype responsible for metabolizing tyramine (MAOA) might provide an antidepressant effect without the food-induced adverse events. However, the failure of the subtype-selective MAO inhibitors to demonstrate a high degree of clinical efficacy has resulted in MAO inhibitors continuing to have a limited application in the treatment of depression even after the discovery of modern MAOA-selective drugs such as moclobemide.
Table 2.
Some common foods that can precipitate a hypertensive crisis in patients taking monoamine oxidase inhibitors
| Cheese (particularly camembert) |
| Wines (particularly red wine) |
| Bovril and marmite (yeast extracts) |
| Chicken livers |
| Broad beans |
| Avocado pears |
| Pickled herrings |
| Beers |
| Yoghurt |
| Flavoured textured vegetable protein |
The tricyclic antidepressant drugs were evolved from structural modifications of the phenothiazine type of anti-schizophrenic drugs, such as chlorpromazine. These compounds came into clinical use following the observation that they had antidepressant rather than antipsychotic activity and with little if any knowledge of their possible mechanism of action. A major indication of their possible mechanism came from the laboratory of Todrick in Dumfries, Scotland, who found that the 5-HT concentration in the platelets of depressed patients increased when they were treated with MAO inhibitors, but decreased when they were given tricyclic antidepressants. It was suggested that imipramine depletion of 5-HT might involve competition with the uptake process rather than ‘destruction' of the stored amine, as with reserpine (Marshall et al., 1960). However, by 1964 the same group were clearly persuaded that tricyclic antidepressants inhibited 5-HT uptake and were reporting on the structure–activity relationships of the tricyclic compounds using inhibition of 5-HT uptake by the platelet (Yates et al., 1964).
The role of uptake by tissues on amine inactivation, and indeed on its possible effect in terms of potentiating the action of amines at the postsynaptic receptor, was just beginning to be understood in the 1960s. Brown and Gillespie (1957) in Oxford suggested that noradrenaline could be taken up by nerve endings during their studies on the spleen, and subsequently Axelrod, working with Weil-Malherbe (who by then had left the UK to work at the NIH), studied the fate of labelled noradrenaline in animals (Whitby et al., 1961). These early studies by Axelrod and colleagues paved the way for major investigations conducted by Leslie Iversen at Cambridge University, which did so much to clarify the uptake systems of catecholamines by nerve endings (Iversen, 1971).
However, a fuller understanding of 5-HT uptake and its functional consequences had to await the activities of a dynamic group of Swedish scientists (pharmacologists, biochemists and histologists) working at the Karolinska Institute in Stockholm and the Department of Pharmacology at the University of Gothenburg. Major names included Arvid Carlsson, Nils Hillarp, Hans Corrodi, Kjell Fuxe, Tomas Hökfelt, Urban Ungerstedt, Gösta Jonsson and Nils-Erik Ánden. The output from these neuroscientists was prodigious, and from the mid- to late 1960s they demonstrated that 5-HT had its own selective uptake system, and that this resulted in increased synaptic concentrations of the monoamine and resultant feedback inhibition of 5-HT synthesis.
It was many of these same scientists who, during the 1960s, also performed the first full mapping of the 5-hydroxytryptaminergic pathways in the brain by the use of fluorescent histochemical techniques.
5-HT and antidepressant drugs: clinical studies
The major early studies on the therapeutic effects of putative antidepressant compounds were conducted before or very soon after the formation of the Dunlop Committee, which was the forerunner of the Committee on Safety of Medicines (CSM), the UK equivalent of the American Food and Drug Administration (FDA). Studies in those days were not performed in accordance with current experimental criteria such as blinding or randomization and with no apparent understanding of the patient numbers required to produce a result with some statistical validity. A good example is the first major paper on imipramine (Kuhn, 1958), which reported on studies in 500 patients but did so in a purely qualitative way, with meaningless statements such as ‘side effects are relatively slight' and ‘often a feeling of dryness of mouth' and with no mathematical evaluation of efficacy anywhere in the paper. Against that one must remember that in the early 1950s there were no effective treatments for the major psychiatric illnesses (apart from electroconvulsive therapy). Consequently, experienced psychiatrists were familiar with patients with long-term intractable illness, and any treatment that produced a positive effect was reasonably likely to be real. As Donald Eccleston remarked about the lack of a placebo group in many early studies: ‘But if someone has been ill for—the average was 7 years—then in some ways they act as their own control' (Eccleston, 1999). The lack of improvement seen in many patients before the advent of psychoactive drugs has also been commented on by Alec Coppen (Figure 1). In remarks about the introduction of the antidepressant and antipsychotic drugs he stated: ‘when I go to West Park Hospital now I find about 400 patients suffering from dementia. What a contrast to 40 years ago when there were 2000 very disturbed young and middle aged patients, many of whom are now leading ordinary and rewarding lives thanks to these advances' (Coppen, 1996). It is also worth mentioning that it was the bold experimental clinical approaches used in the 1950s and early 1960s that produced many important advances in our understanding. Such studies would now be impossible to conduct.
It was Coppen who was responsible for several major findings when experimenting on new therapeutic approaches to depressive illness. Noting that the paper of Hess and Doepfner (1961) demonstrated that administration of an MAO inhibitor plus tryptophan increased the concentrations of 5-HT and tryptamine in the brain, and conscious of the albeit limited evidence that 5-HT might be deficient in depression, Coppen et al. (1963) gave tranylcypromine to depressed subjects and then administered either placebo or DL-tryptophan (214 mg kg−1) in divided doses. The patients given the amino acid were reported to show a greater improvement than those given only tranylcypromine. This result was rapidly confirmed by Pare (1965). Coppen also examined the effect of tryptophan alone and reported an antidepressant effect, but again noted a greater effect if an MAO inhibitor had also been given (Coppen et al., 1967).
These studies naturally helped focus thought on the role of 5-HT in depression and raised the possibility that there might be a lowered concentration of the amine in the brain of the depressed patient.
Studies on 5-hydroxyindoles in human post-mortem tissue
If, as much of the data then being generated suggested, alteration of the cerebral 5-HT concentration was the mechanism by which antidepressant drugs achieved their therapeutic effect, the obvious corollary was that there might be an abnormality in 5-HT concentrations in the brains of depressed patients, which could be detected post mortem. Furthermore, it might be possible to detect the effects of antidepressant treatments on 5-hydroxyindoles post mortem. A major observation indicating that it might be possible to undertake meaningful post-mortem investigations was reported by Daphne Joyce while she was working at the Institute of Neurology, Queen Square, London. She found that 5-HT loss in both rodent and human brain was slow, provided that the brain remained in situ; but that following its removal from the cranium the 5-HT concentration decreased rapidly unless the tissue was immediately frozen (Joyce, 1962).
Much of the pioneering clinical work was undertaken under the leadership of Mike Pare, a psychiatrist at St Bartholomew's Hospital, London, and RS (Sam) Stacey, Professor of Pharmacology and Therapeutics at St Thomas' Hospital, London. They first confirmed that tissue 5-HT loss was modest if the body was stored at 2 °C until autopsy, when cerebral tissue was taken, divided into regions and frozen (Maclean et al., 1965). They then examined cerebral 5-HT content in controls and patients who had been ingesting MAO inhibitors up until death. The major finding was that brain 5-HT content did not rise appreciably until the patient had been taking an MAO inhibitor for more than 2 weeks, with a maximum and consistent raised concentration after 4 weeks. This, as they pointed out, corresponded closely to the time required for the drugs to produce a clear antidepressant effect (Maclean et al., 1965). These data therefore provided a basis for believing that antidepressant drugs acted via a change in cerebral 5-HT concentration and function.
The next step they took was to examine if there was an abnormality in cerebral 5-HT in depressed patients. In this later study, they examined brain 5-HT concentrations in patients who had committed suicide. Both 5-HT and 5-HIAA were measured. No difference in 5-HIAA was detected between controls and suicides and although a modest loss in 5-HT was observed this was proposed to be, at least in part, an age-related effect (Pare et al., 1969). Other UK groups were also reporting decreased levels of either 5-HT or 5-HIAA in the hindbrain of suicides (Shaw et al., 1967; Bourne et al., 1968). Later studies generally failed to confirm these findings, which may have been influenced by age, sampling or post-mortem conditions. Nevertheless, one should not underestimate the novel approaches being used by the investigators at the time the studies were performed.
Studies on 5-HIAA in human CSF
Another clinical approach to the question as to whether there was a defect in monoamine function in the brain was developed in Edinburgh. This involved examination of monoamine metabolites in CSF and resulted to some extent from the excellent links between preclinical and clinical psychiatrists working at this university.
The initial impetus for the studies came from the work of Dennis Sharman, who in 1960 was completing his PhD studies. He had obtained one of the first Aminco-Bowman spectrophotofluorometers in the UK and used it to measure 5-HIAA in human CSF (Ashcroft and Sharman, 1960). He showed that depressed patients had a lowered concentration of 5-HIAA in the CSF. This initiated a major series of both preclinical (Ashcroft et al., 1965) and clinical (Ashcroft et al., 1966) studies, which continued through the 1960s.
To support this finding and confirm that the 5-HIAA concentration in the lumbar CSF reflected the concentration of the ventricular CSF, a series of major studies were initiated using implanted tubes in dog brain to measure ventricular or cisterna magna CSF to determine whether the 5-HIAA concentration in the lumbar CSF reflected the concentration in higher brain centres. Other studies were conducted using probenecid to block acid metabolite transport from the brain. Many of these studies were reviewed by the Edinburgh investigators at the time (Moir et al., 1970) but, despite promising data, the approach gradually fell out of favour. This was partly due to ethical aspects, as it is hard to justify lumbar puncture, which is both painful and unpleasant and also carries a small but definite risk and which furthermore was not proving to have value as either a diagnostic or a therapeutic tool. Results also tended to be too inconsistent for clear conclusions to be drawn. Furthermore, the origin of lumbar CSF 5-HIAA started to be questioned together with its relationship to 5-HT functional activity. The observation by Curzon et al. (1971) that patients with a partial block in CSF flow had normal lumbar CSF values argued for a mainly spinal origin for 5-HIAA. Later data strengthened this proposal and indicated that lowered lumbar CSF probably reflected the low level of motor activity of depressed patients (Curzon et al., 1980).
The monoamine hypothesis of affective disorders: a retrospective glance
The huge amount of data on the action of antidepressant drugs on monoaminergic transmission and the clinical studies on patient tissues were developed during the 1960s into a framework that has often been called the ‘monoamine hypothesis'. At its most basic this stated that depression is caused by a decrease in the concentration or function of monoamines (5-HT and/or noradrenaline) and that antidepressant drugs increased concentrations or function by inhibiting monoamine breakdown (MAO inhibitors) or by increasing the concentrations of monoamines in the synaptic cleft (reuptake inhibitors). While work continued during the 1970s to try and support this hypothesis, with the examination of monoamine receptors in post-mortem tissue from patients who had died during an episode of depression or by suicide, overall the results obtained did not lead to any all-encompassing theory. This is perhaps not surprising, given our present knowledge of neurotransmitter interactions, adaptation and modulation. However, at the time good logic underpinned the studies, and our current position results from the information generated, even if negative. Without these studies we might still be wondering whether some primary abnormality might be detectable in the CNS.
We should also not underestimate the value of the hypothesis in helping us reach our current position of knowledge and it is worth quoting Professor Hermann van Praag on this point: ‘Quite apart from the fact that it probably contains a kernel of truth, the heuristic value of the monoamine hypothesis for psychiatry cannot be overestimated. It has catalysed an impressive amount of research'. Although it is true that none of the data on their own are very impressive, when all the information generated is put together it remains hard to resist the conclusion that monoamines are involved in the pathogenesis of depression and that most, if not all, antidepressant drugs act in a way that involves monoamines. It is notable that in 2000 the top five drugs being sold that act on CNS function all modulate the function of 5-HT (Jones and Blackburn, 2002).
What remains fascinating is how controversial such proposals were at the time of their development. This can be gathered from a sentence in the classic review paper of Alec Coppen, which illustrates the fact that at this time there were many who did not even believe that depression might have a biochemical causation. His review was titled ‘The biochemistry of affective disorders' and it started: ‘The title of this review would be regarded by some psychiatrists as provocative; they would relegate the biochemical concomitants of depression and mania to a secondary position and deny that biochemical changes have any place in the aetiology of these conditions' (Coppen, 1967).
We can be proud of the UK pioneers whose work led to the acceptance of the idea that brain biochemistry is intimately involved in mood disorder and that altering monoamine function can produce clinical improvement in psychiatric disorders. We do, of course, remain in ignorance as to the detailed biological causation of the problem. Monoamine function does appear to be abnormal in depressed patients but how this relates to the clinical syndrome is unclear.
Conclusions
A relatively small group of scientists, initially in Edinburgh (and continuing for 20 years), joined by another loose knit group in London, provided most of the major UK-based neuropharmacology discoveries on 5-HT during the 20 years under evaluation. It is interesting to note how much the London scientists interacted with each other over this time.
It is reasonable to propose that the initial impetus for this flow of information was the work of John Gaddum, who not only detected 5-HT in the brain but also showed that LSD antagonized the action of 5-HT on peripheral tissue. The inspired jump to proposing that 5-HT might therefore be involved in the regulation of mood followed immediately. What is equally interesting is how quickly findings in animal studies were translated into clinical investigations by interested psychiatrists (and then back again for further preclinical investigations). The data produced have provided the basis for most of our current knowledge of antidepressant therapy. Of course, subtypes of 5-HT receptors were not even thought about in the period that has been examined, but would come into their own in the late 1970s and beyond. However, it can be argued plausibly that the advances made in 5-HT neuropharmacology and psychopharmacology in the 1950–1970 period were proportionately much greater than those made after that time. This is unquestionably true if we relate the number of major findings to the modest number of people involved in the research.
As stated earlier, the word serendipity has often used for the growth of observations concerning the proposal that 5-HT might be involved in the control of mood and the idea that therapeutic drugs used to alleviate psychiatric illness might alter the function of 5-HT in the brain. However, we can knock this simplistic notion on the head with a final comment from Sir John Gaddum, the father of 5-HT research in the UK: ‘It is true that many discoveries (in Pharmacology) have been accidents, but these accidents would not have occurred to anyone who was not engaged in a systematic research for new knowledge, and without the techniques and apparatus of modern science they would usually have passed unheeded in the modern world' (Gaddum, 1954).
Acknowledgments
This article has been written following a grant from the WDM Paton Historical Research Fund of the British Pharmacological Society (BPS). For me there was a particular personal pleasure in receiving the award, because of a link with its origin. In 1991, Sir William (Bill) Paton was awarded the Wellcome Gold Medal by the British Pharmacological Society for his outstanding contributions to pharmacology. He decided that his health did not permit travel to London for receiving the medal at the Official Dinner of the winter meeting of the Society. I therefore went to his house in Oxford one Saturday after Christmas to present him with the medal, since I was at that time General Secretary of the British Pharmacological Society. I spent a very pleasant couple of hours with Bill and his wife, chatting about the Society and shared times in Oxford. After receiving the medal he informed me that he really did not need the accompanying cheque, and he asked me to raise with the Committee a proposal that the money be used to found an award to enable applicants to study a historical aspect of pharmacology of their choice. The British Pharmacological Society Committee supported the idea enthusiastically and matched the money he had donated, thereby establishing the Paton Historical Research Fund. At that time I had no idea that I would ever be applying to the fund for support, and I can but hope that Bill would have approved of this article.
Abbreviations
- 5-HIAA
5-hydroxyindole acetic acid
- 5-HTP
5-hydroxytryptophan
- NIH
National Institutes of Health
- MAO
monoamine oxidase
Conflict of interest
The author states no conflict of interest.
References
- Achor RW, Hanson NO, Gifford RW., Jr Hypertension treated with Rauwolfia serpentina (whole root) and with reserpine; controlled study disclosing occasional severe depression. JAMA. 1955;159:841–845. doi: 10.1001/jama.1955.02960260011004. [DOI] [PubMed] [Google Scholar]
- Amin AH, Crawford TBB, Gaddum JH. The distribution of substance P and 5-hydroxytryptamine in the central nervous system of the dog. J Physiol. 1954;125:596–618. doi: 10.1113/jphysiol.1954.sp005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GW, Crawford TBB, Eccleston D, Sharman DF, MacDougall EJ, Stanton JB, et al. 5-Hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet. 1966;288:1049–1052. doi: 10.1016/s0140-6736(66)92028-9. [DOI] [PubMed] [Google Scholar]
- Ashcroft GW, Eccleston D, Crawford TBB. 5-Hydroxyindole metabolism in rat brain: a study of intermediate metabolism using the technique of tryptophan loading. J Neurochem. 1965;12:483–503. doi: 10.1111/j.1471-4159.1965.tb06775.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft GW, Sharman DF. 5-Hydroxyindoles in human cerebrospinal fluids. Nature. 1960;186:1050–1051. doi: 10.1038/1861050a0. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Inscoe JK. The uptake and binding of circulating serotonin and the effect of drugs. J Pharmacol Exp Ther. 1963;141:161–165. [PubMed] [Google Scholar]
- Bacq ZM. Les amines biologiquement intéressantes dérivées des acides aminés. Rapport au Congrès Internat Biochimie: Paris; 1952. pp. 59–74. [Google Scholar]
- Blackwell B. Hypertensive crises due to monoamine inhibitors. Lancet. 1963;281:849–851. doi: 10.1016/s0140-6736(63)92743-0. [DOI] [PubMed] [Google Scholar]
- Blackwell B, Marley E. Interaction of cheese and its constituents with monoamine oxidase inhibitors. Br J Pharmacol. 1966a;26:120–141. doi: 10.1111/j.1476-5381.1966.tb01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell B, Marley E. Interaction of yeast extracts and their constituents with monoamine oxidase inhibitors. Br J Pharmacol. 1966b;26:142–161. doi: 10.1111/j.1476-5381.1966.tb01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell B, Marley E, Price J, Taylor D. Hypertensive interactions between monoamine oxidase inhibitors and foodstuffs. Br J Psychiat. 1967;113:349–365. doi: 10.1192/bjp.113.497.349. [DOI] [PubMed] [Google Scholar]
- Blaschko H. Amine oxidase and amine metabolism. Pharmacol Rev. 1952;4:415–458. [PubMed] [Google Scholar]
- Blaschko H, Richter D, Schlossman H. The oxidation of adrenaline and other amines. Biochem J. 1937;31:2187–2196. doi: 10.1042/bj0312187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RJ, Bradley PB, Briggs I, Dray A. Antagonism by LSD to effects of 5-HT on single neurones. Brain Res. 1969;15:529–531. doi: 10.1016/0006-8993(69)90176-0. [DOI] [PubMed] [Google Scholar]
- Boakes RJ, Bradley PB, Briggs I, Dray A. Antagonism of 5-hydroxytryptamine by LSD 25 in the central nervous system: a possible neuronal basis for the actions of LSD 25. Br J Pharmacol. 1970;40:202–218. doi: 10.1111/j.1476-5381.1970.tb09914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski DF, Pletscher A, Brodie BB, Udenfriend S. Identification and assay of serotonin in brain. J Pharmacol Exp Ther. 1956;117:82–88. [PubMed] [Google Scholar]
- Born GVR, Gillson RE. Studies on the uptake of 5-hydroxytryptamine by blood platelets. J Physiol. 1959;146:472–491. doi: 10.1113/jphysiol.1959.sp006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Bunney WE, Colburn RW, Davis JM, Davis JN, Shaw DM, et al. Noradrenaline, 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in hindbrains of suicidal patients. Lancet. 1968;292:805–808. doi: 10.1016/s0140-6736(68)92459-8. [DOI] [PubMed] [Google Scholar]
- Bowman RL, Caulfield PA, Udenfriend S. Spectrophotofluorometric assay in the visible and ultraviolet. Science. 1955;122:32–33. doi: 10.1126/science.122.3157.32. [DOI] [PubMed] [Google Scholar]
- Bradley PB, Elkes C, Elkes J. On some effects of lysergic acid diethylamide (L.S.D. 25) in normal volunteers. J Physiol. 1953;121:50P–51P. [PubMed] [Google Scholar]
- Bradley PB, Elkes J. The effect of some drugs on the electrical activity of the brain. Brain. 1957;80:77–117. doi: 10.1093/brain/80.1.77. [DOI] [PubMed] [Google Scholar]
- Brengelmann JC, Pare CM, Sandler M. Alteration of the psychological effects of LSD in man by 5-hydroxytryptophan. J Ment Sci. 1958;104:1237–1244. doi: 10.1192/bjp.104.437.1237. [DOI] [PubMed] [Google Scholar]
- Brengelmann JC, Pare CM, Sandler M. Effects of 5-hydroxytryptophan on schizophrenia. J Ment Sci. 1959;105:770–776. doi: 10.1192/bjp.105.440.770. [DOI] [PubMed] [Google Scholar]
- Brittain RT, Handley SL. Temperature changes produced by injection of catecholamines and 5-hydroxytryptamine into the cerebral ventricles of the conscious mouse. J Physiol. 1967;192:805–813. doi: 10.1113/jphysiol.1967.sp008332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie TG. Immediate action of intravenous injection of blood serum. J Physiol. 1900;26:48–71. doi: 10.1113/jphysiol.1900.sp000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie TG. Perfusion of surviving organs. J Physiol. 1903;29:266–275. doi: 10.1113/jphysiol.1903.sp000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Gillespie JS. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957;138:81–88. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn JH. The action of tyramine and ephedrine. J Pharmacol Exp Ther. 1932;46:75–95. [Google Scholar]
- Clark CT, Weissback H, Udenfriend S. 5-Hydroxytryptophan decarboxylase: preparation and properties. J Biol Chem. 1954;210:139–148. [PubMed] [Google Scholar]
- Cooper KE, Cranston WI, Honour AJ. Effects of intraventricular and intrahypothalamic injection of noradrenaline and 5-HT on body temperature in conscious rabbits. J Physiol. 1965;181:852–864. doi: 10.1113/jphysiol.1965.sp007801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppen A. The biochemistry of affective disorders. Br J Psychiatry. 1967;113:1237–1264. doi: 10.1192/bjp.113.504.1237. [DOI] [PubMed] [Google Scholar]
- Coppen A.Biological psychiatry in Britain The Psychopharmacologists 1996Chapman and Hall: London; 265–286.In: Healy D (ed). [Google Scholar]
- Coppen A, Shaw DM, Farrell JP. Potentiation of the antidepressive effect of a monoamine-oxidase inhibitor by tryptophan. Lancet. 1963;281:79–81. doi: 10.1016/s0140-6736(63)91084-5. [DOI] [PubMed] [Google Scholar]
- Coppen A, Shaw DM, Herzberg B, Maggs R. Tryptophan in the treatment of depression. Lancet. 1967;290:1178–1180. doi: 10.1016/s0140-6736(67)91894-6. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW, Warner BT. A method for assessing the effect of drugs on the central actions of 5-hydroxytryptamine. Br J Pharmacol. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G. A rapid chromatographic test for high urinary excretion of 5-hydroxy-indole-acetic acid and 5-hydroxytryptamine. Lancet. 1955;266:1361–1362. doi: 10.1016/s0140-6736(55)93161-5. [DOI] [PubMed] [Google Scholar]
- Curzon G. Tryptophan pyrrolase—a biochemical factor in depressive illness. Br J Psychiatry. 1969;115:1367–1374. doi: 10.1192/bjp.115.529.1367. [DOI] [PubMed] [Google Scholar]
- Curzon G.Hopkins and the discovery of tryptophan Progress in Tryptophan and Serotonin Research 1987Walter de Gruyter & Co.: Berlin; 29–38.In: Bender DA (ed). [Google Scholar]
- Curzon G.From neurochemistry to neuroscience The Psychopharmacologists 2 1998Altman: London; 307–323.In: Healy D (ed). [Google Scholar]
- Curzon G, Green AR. Effect of immobilisation on rat liver tryptophan pyrrolase and brain 5-hydroxytryptamine metabolism. Br J Pharmacol. 1969;37:689–697. doi: 10.1111/j.1476-5381.1969.tb08507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G, Green AR. Rapid method for the determination of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in small regions of rat brain. Br J Pharmacol. 1970;39:653–655. doi: 10.1111/j.1476-5381.1970.tb10373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon G, Gumpert EJW, Sharpe DM. Amine metabolites in the lumbar cerebrospinal fluid of humans with restricted flow of cerebrospinal fluid. Nature (New Biol) 1971;231:189–191. doi: 10.1038/newbio231189a0. [DOI] [PubMed] [Google Scholar]
- Curzon G, Kantamanini BD, Van Boxel P, Gillman PK, Barlett JR, Bridges PK. Substances related to 5-hydroxytryptamine in plasma and in lumbar and ventricular fluid of psychiatric patients. Acta Psychiatr Scand Suppl. 1980;280:3–20. [PubMed] [Google Scholar]
- Dale HH. On some physiological actions of ergot. J Physiol. 1906;34:163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RJS, Tipton KF. The purification and properties of the NAD-linked aldehyde dehydrogenase from pig brain. Eur J Biochem. 1971;22:257–262. doi: 10.1111/j.1432-1033.1971.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Eccleston D.The receptor enters psychiatry (2) The Psychopharmacologists 3 1999Hodder Arnold: London; 201–212.In: Healy D (ed). [Google Scholar]
- Eccleston D, Moir ATB, Reading WH, Ritchie IM. Formation of 5-hydroxytryptophol in brain in vitro. Br J Pharmacol. 1966;28:367–377. doi: 10.1111/j.1476-5381.1966.tb01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston D, Reading WH, Ritchie IM. 5-Hydroxytryptamine metabolism in brain and liver slices and the effect of ethanol. J Neurochem. 1969;16:274–276. doi: 10.1111/j.1471-4159.1969.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Ek A, Witkop B. Synthesis and biochemistry of 5- and 7-hydroxytryptophan and derivatives. J Am Chem Soc. 1954;75:500–501. [Google Scholar]
- Elkes J, Elkes C, Bradley PB. The effect of some drugs on the electrical activity of the brain, and on behaviour. J Mental Sci. 1954;100:125–128. doi: 10.1192/bjp.100.418.125. [DOI] [PubMed] [Google Scholar]
- Erspamer V. Pharmacologische Studien über Enteramin IV. Über die Inacktivierung von Enteramin durch tierisches gewebe. Arch Exp Pathol Pharmakol. 1942;200:43–59. [Google Scholar]
- Erspamer V. Enteramina e metossitriptamina. Tossicità. Azione sulla diuresi, sulla pressione del sangue e su alcune organi a muscolatura liscia. Ric Sci. 1952;22:694–702. [Google Scholar]
- Erspamer V. Pharmacology of indolealkylamines. Pharmacol Rev. 1954;6:425–487. [PubMed] [Google Scholar]
- Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxtryptamine. Nature. 1952;169:800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- Ewins J, Laidlaw PP. The fate of parahydroxyphenylethylamine in the organism. J Physiol. 1910;41:78–87. doi: 10.1113/jphysiol.1910.sp001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W. John Henry Gaddum. Biogr Mem Fellows R Soc. 1967;13:57–77. doi: 10.1098/rsbm.1970.0006. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Myers RD. Effects on temperature of amines injected into the cerebral ventricles. A new concept of temperature regulation. J Physiol. 1964;173:226–231. doi: 10.1113/jphysiol.1964.sp007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W, Myers RD. Changes in temperature produced by micro-injections of amines into the anterior hypothalamus of cats. J Physiol. 1965;177:239–245. doi: 10.1113/jphysiol.1965.sp007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W, Sherwood SL. Injection of drugs into the lateral ventricle of the cat. J Physiol. 1954;123:148–167. doi: 10.1113/jphysiol.1954.sp005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein A, Hoagland H, Freeman H, Williamson O. Effect of ethanol ingestion on serotonin-C14 metabolism in man. Life Sci. 1967;6:53–61. doi: 10.1016/0024-3205(67)90361-x. [DOI] [PubMed] [Google Scholar]
- Gaddum JH. Tryptamine receptors. J Physiol. 1953;119:363–368. doi: 10.1113/jphysiol.1953.sp004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH. Discoveries in therapeutics. J Pharm Pharmacol. 1954;6:497–512. doi: 10.1111/j.2042-7158.1954.tb10979.x. [DOI] [PubMed] [Google Scholar]
- Gaddum JH. Bioassay procedures. Pharmacol Rev. 1959;11:241–249. [PubMed] [Google Scholar]
- Gaddum JH.A perspective on pharmacology Drugs in Our Society 1964Johns Hopkins Press: Baltimore, Maryland; 17–26.In: Talalay P, Murnaghan JH (eds). [Google Scholar]
- Gaddum JH, Giarman NJ. Preliminary studies on the biosynthesis of 5-hydroxytryptamine. Br J Pharmacol. 1956;11:88–92. doi: 10.1111/j.1476-5381.1956.tb01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Hameed KA. Drugs which antagonise 5-hydroxytryptamine. Br J Pharmacol. 1954;9:240–248. doi: 10.1111/j.1476-5381.1954.tb00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Hameed KA, Hathway DE, Stephens FF. Quantitative studies of antagonists for 5-hydroxytryptamine. Q J Exp Physiol Cogn Med Sci. 1955;40:49–74. doi: 10.1113/expphysiol.1955.sp001097. [DOI] [PubMed] [Google Scholar]
- Gaddum JH, Kwiatkowski H. The action of ephedrine. J Physiol. 1938;94:87–100. doi: 10.1113/jphysiol.1938.sp003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Paasonen MK. The use of some molluscan hearts for the estimation of 5-hydroxytryptamine. Br J Pharmacol. 1955;10:474–483. doi: 10.1111/j.1476-5381.1955.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Peart WS, Vogt M. The estimation of adrenaline and allied substances in blood. J Physiol. 1949;108:467–481. doi: 10.1113/jphysiol.1949.sp004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Picarelli ZP. Two kinds of tryptamine receptor. Br J Pharmacol. 1957;12:323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Vogt M. Some central actions of 5-hydroxytryptamine and various antagonists. Br J Pharmacol. 1956;11:175–179. doi: 10.1111/j.1476-5381.1956.tb01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith DG. Tryptophan hydroxylation in brain. Biochem Biophys Res Commun. 1964;16:586–592. doi: 10.1016/0006-291x(64)90197-4. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. The biosynthesis of 5-hydroxytryptamine in brain. Biochem J. 1967;105:351–360. doi: 10.1042/bj1050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivity in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971;18:1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG, Parfitt AG. Tryptophan transport across the synaptosomal membrane. J Neurochem. 1970;17:1339–1353. doi: 10.1111/j.1471-4159.1970.tb06869.x. [DOI] [PubMed] [Google Scholar]
- Green AR. Neuropharmacology of 5-hydroxytryptamine. Br J Pharmacol. 2006;147:S145–S152. doi: 10.1038/sj.bjp.0706427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Curzon G. Decrease of 5-hydroxytryptamine in the brain provoked by hydrocortisone and its prevention by allopurinol. Nature. 1968;220:1095–1097. doi: 10.1038/2201095a0. [DOI] [PubMed] [Google Scholar]
- Green AR, Curzon G. Effect of tryptophan metabolites on brain 5-hydroxytryptamine metabolism. Biochem Pharmacol. 1970;19:2061–2068. doi: 10.1016/0006-2952(70)90303-5. [DOI] [PubMed] [Google Scholar]
- Green AR, Grahame-Smith DG. The effects of drugs on the processes regulating the functional activity of brain 5-hydroxytryptamine. Nature. 1976;260:487–491. doi: 10.1038/260487a0. [DOI] [PubMed] [Google Scholar]
- Green AR, Youdim MBH. Effects of monoamine oxidase inhibition by clorgyline, deprenil or tranylcypromine on 5-hydroxytryptamine concentrations in rat brain and hyperactivity following subsequent tryptophan administration. Br J Pharmacol. 1975;55:415–422. doi: 10.1111/j.1476-5381.1975.tb06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin KE, Fisher FE. The synthesis of 5-hydroxytryptamine. J Am Chem Soc. 1951;73:5007. [Google Scholar]
- Hardisty RM, Ingram GIC, Stacey RS. Reserpine and human platelet 5-hydroxytryptamine. Experientia. 1956;12:424–425. doi: 10.1007/BF02157365. [DOI] [PubMed] [Google Scholar]
- Hardisty RM, Stacey RS. 5-Hydroxytryptamine in normal human platelets. J Physiol. 1955;130:711–720. doi: 10.1113/jphysiol.1955.sp005437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare MLC. Tyramine oxidase. 1. A new enzyme in liver. Biochem J. 1928;22:968–979. doi: 10.1042/bj0220968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy D. The Psychopharmacologists 1996Chapman and Hall: London; 633 pp [Google Scholar]
- Healy D. The Psychopharmacologists 2 1998Altman: London; 672 pp [Google Scholar]
- Healy D. The Psychopharmacologists 3 1999Hodder Arnold: London; 580 pp [Google Scholar]
- Hess SM, Doepfner W. Behavioural effects and brain amine content in rats. Arch Int Pharmacodyn Ther. 1961;134:89–99. [PubMed] [Google Scholar]
- Hofmann A. How LSD originated. J Psychedelic Drugs. 1979;11:53–60. doi: 10.1080/02791072.1979.10472092. [DOI] [PubMed] [Google Scholar]
- Humphrey JH, Toh CC. Absorption of serotonin (5-hydroxytryptamine) and histamine by dog platelets. J Physiol. 1954;124:300–304. doi: 10.1113/jphysiol.1954.sp005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen LL. Role of transmitter uptake systems in synaptic neurotransmission. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepson JB, Stevens BJ. A fluorescence test for serotonin and other tryptamines. Nature. 1953;172:772. doi: 10.1038/172772a0. [DOI] [PubMed] [Google Scholar]
- Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17:1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–568. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- Joyce D. Changes in the 5-hydroxytryptamine content of rat, rabbit and human brain after death. Br J Pharmacol. 1962;18:370–380. doi: 10.1111/j.1476-5381.1962.tb01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koe BK, Weissman A. P-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Kuhn R. Treatment of depressive states with G-22355 (imipramine) hydrochloride. Am J Psychiatry. 1958;115:459–464. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- Laidlaw PP. The physiological action of indolethylamine. Biochem J. 1912;6:141–150. doi: 10.1042/bj0060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF. Intensification of the central serotonergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1:132–136. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- Liddell DW, Weil-Malherbe H. The effects of methedrine and of lysergic acid diethylamide on mental processes and on the blood adrenaline level. J Neurol Neurosurg Psychiatry. 1953;16:7–13. doi: 10.1136/jnnp.16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig C, Schmidt A. Das Verhalten der Gase, Welche mit dem Blut durch den reizbaren Säugethiermuskel strömen. Arb Physiol Anstalt Leipzig. 1868;3:1. [Google Scholar]
- Maclean R, Nicholson WJ, Pare CMB, Stacey RS. Effect of monoamine-oxidase inhibitors on the concentrations of 5-hydroxytryptamine in the human brain. Lancet. 1965;286:205–208. doi: 10.1016/s0140-6736(65)90693-8. [DOI] [PubMed] [Google Scholar]
- Maickel RP, Cox RH, Jr, Saillant J, Miller FP. A method for the determination of serotonin and norepinephrine in discrete areas of rat brain. Int J Neuropharmacol. 1968;7:275–281. doi: 10.1016/0028-3908(68)90034-8. [DOI] [PubMed] [Google Scholar]
- Marshall EF, Stirling GS, Tait AC, Todrick A. The effect of iproniazid and imipramine on the blood platelet 5-hydroxytryptamine level in man. Br J Pharmacol. 1960;15:35–41. doi: 10.1111/j.1476-5381.1960.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Gross W, McAdam W, Walker JW. Psychological and biochemical effects of lysergic acid diethylamide. Nature. 1951;168:827–828. doi: 10.1038/168827b0. [DOI] [PubMed] [Google Scholar]
- Medical Research Council Report by the Clinical Psychiatry Committee. Clinical trial of the treatment of depressive illness. Br Med J. 1965;i:881–886. doi: 10.1136/bmj.1.5439.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson IA, Whittaker VP. The distribution of 5-hydroxytryptamine in brain fractions. Biochem Pharmacol. 1962;11:505–506. doi: 10.1016/0006-2952(62)90237-x. [DOI] [PubMed] [Google Scholar]
- Michaelson IA, Whittaker VP, Laverty R, Sharman DF. Location of acetylcholine, 5-hydroxytryptamine and noradrenaline within subcellular particles derived from guinea pig subcortical brain tissue. Biochem Pharmacol. 1964;12:1450–1453. doi: 10.1016/0006-2952(63)90221-1. [DOI] [PubMed] [Google Scholar]
- Moir ATB, Ashcroft GW, Crawford TBB, Eccleston D, Guldberg C. Cerebral metabolites in cerebrospinal fluid as a biochemical approach to the brain. Brain. 1970;93:357–368. doi: 10.1093/brain/93.2.357. [DOI] [PubMed] [Google Scholar]
- Moir ATB, Eccleston D. The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. J Neurochem. 1968;15:1093–1108. doi: 10.1111/j.1471-4159.1968.tb06827.x. [DOI] [PubMed] [Google Scholar]
- Myers RD.The role of hypothalamic serotonin in thermoregulation Serotonin and Behavior 1973Academic Press: New York; 293–302.In: Barchas J, Usdin E (eds). [Google Scholar]
- Paasonen MK, Vogt M. The effect of drugs on the amounts of substance P and 5-hydroxytryptamine in mammalian brain. J Physiol. 1956;131:617–626. doi: 10.1113/jphysiol.1956.sp005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page IH. Serotonin (5-hydroxytryptamine) Physiol Rev. 1954;34:563–588. doi: 10.1152/physrev.1954.34.3.563. [DOI] [PubMed] [Google Scholar]
- Page IH. Serotonin. Sci Am. 1957;197:52–56. [Google Scholar]
- Page IH. The discovery of serotonin. Perspect Biol Med. 1976;20:1–8. doi: 10.1353/pbm.1976.0058. [DOI] [PubMed] [Google Scholar]
- Pare CMB. Potentiation of monoamine-oxidase inhibitors by tryptophan. Lancet. 1965;282:527–528. doi: 10.1016/s0140-6736(63)90271-x. [DOI] [PubMed] [Google Scholar]
- Pare CMB, Yeung DPH, Price K, Stacey RS. 5-Hydroxytryptamine, noradrenaline and dopamine in brainstem, hypothalamus and caudate nucleus of controls and of patients committing suicide by coal gas poisoning. Lancet. 1969;294:133–135. doi: 10.1016/s0140-6736(69)92442-8. [DOI] [PubMed] [Google Scholar]
- Pletscher A, Shore PA, Brodie BB. Serotonin as a mediator of reserpine action in brain. J Pharmacol Exp Ther. 1956;116:84–89. [PubMed] [Google Scholar]
- Pugh CEM, Quastel JH. Oxidation of amines by animal tissues. Biochem J. 1937;31:2306–2321. doi: 10.1042/bj0312306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M, Reid G. Source of ‘serotonin' in serum. Nature. 1951;168:385. doi: 10.1038/168385b0. [DOI] [PubMed] [Google Scholar]
- Rapport MM. Serum vasoconstrictor (serotonin). V. The presence of creatinine in the complex: a proposed structure of the vasoconstrictor principle. J Biol Chem. 1949;180:961–969. [PubMed] [Google Scholar]
- Rapport MM. The discovery of serotonin. Perspect Biol Med. 1997;40:260–273. doi: 10.1353/pbm.1997.0040. [DOI] [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Partial purification of the vasoconstrictor in beef serum. J Biol Chem. 1948a;174:735–741. [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor (serotonin). III Chemical inactivation. J Biol Chem. 1948b;176:1237–1241. [PubMed] [Google Scholar]
- Rapport MM, Green AA, Page IH. Serum vasoconstrictor (serotonin). IV. Isolation. J Biol Chem. 1948c;176:1243–1251. [PubMed] [Google Scholar]
- Rocha e, Silva M, Valle JR, Picarelli ZP. A pharmacological analysis of the mode of action on serotonin (5-hydroxytryptamine) upon the guinea-pig ileum. Br J Pharmacol. 1953;8:378–388. doi: 10.1111/j.1476-5381.1953.tb01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Lores Arnaiz G, de Robertis E. 5-Hydroxytryptophan decarboxylase activity in nerve endings of rat brain. J Neurochem. 1964;11:213–219. doi: 10.1111/j.1471-4159.1964.tb06133.x. [DOI] [PubMed] [Google Scholar]
- Ross SB, Renyi AL. Accumulation of tritiated 5-hydroxytryptamine in brain slices. Life Sci. 1967;6:1407–1415. doi: 10.1016/0024-3205(67)90188-9. [DOI] [PubMed] [Google Scholar]
- Sandler M.The place of chemical pathology in psychopharmacology The Psychopharmacologists 1996Chapman and Hall: London; 381–400.In: Healy D (ed). [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Shaw DM, Camps FE, Eccleston EG. 5-Hydroxytryptamine in the hind-brain of depressive suicides. Br J Psychiatry. 1967;113:1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A, Palfreyman MG. History of serotonin and serotonin disorders. Ann NY Acad Sci. 1989;600:1–7. doi: 10.1111/j.1749-6632.1990.tb16869.x. [DOI] [PubMed] [Google Scholar]
- Sneddon JM. Sodium-dependent accumulation of 5-hydroxytryptamine by rat blood platelets. Br J Pharmacol. 1969;37:680–688. doi: 10.1111/j.1476-5381.1969.tb08506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Axelrod J, Zweig M. A sensitive and specific assay for tissue serotonin. Biochem Pharmacol. 1965;14:831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- Speeter ME, Heinzelmann RV, Weisblatt DI. The synthesis of the blood serum vasoconstrictor principle serotonin creatinine sulphate. J Am Chem Soc. 1951;73:5514–5515. [Google Scholar]
- Stacey RS. Uptake of 5-hydroxytryptamine in platelets. Br J Pharmacol. 1961;16:284–295. doi: 10.1111/j.1476-5381.1961.tb01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll WA. Lysergsäure-diäthyl-amid, ein phantastikum aus der Mutterkorngruppe. Schweiz Arch Neurol Psychiatrie. 1947;60:279–280. [Google Scholar]
- Twarog BM, Page IH. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;75:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Clark CT, Titus E. 5-Hydroxytryptophan decarboxylase: a new route of metabolism for tryptophan. J Am Chem Soc. 1953;75:501–502. [Google Scholar]
- Udenfriend S, Titus E, Weissbach H, Peterson RE. Biogenesis and metabolism of 5-hydroxyindole compounds. J Biol Chem. 1956;219:335–344. [PubMed] [Google Scholar]
- Udenfriend S, Weissbach H, Bogdanski DF. Effect of iproniazid on serotonin metabolism in vivo. J Pharmacol Exp Ther. 1957;120:255–260. [PubMed] [Google Scholar]
- Vane JR. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol. 1957;12:344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. The relative activities of some tryptamine analogues on the isolated rat stomach preparation. Br J Pharmacol. 1959;14:87–98. doi: 10.1111/j.1476-5381.1959.tb00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR. A sensitive method for the assay of 5-hydroxytryptamine. Commentary. Br J Pharmacol. 1997;120 Suppl:140–141. doi: 10.1111/j.1476-5381.1997.tb06791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane JR, Collier HO, Corne SJ, Marley E, Bradley PB. Tryptamine receptors in the central nervous system. Nature. 1961;191:1068–1069. doi: 10.1038/1911068a0. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. The discovery of serotonin and its role in neuroscience. Neuropsychopharmacology. 1999;21 Suppl 2:25–85. doi: 10.1016/S0893-133X(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Whitby LG, Axelrod J, Weil-Malherbe H. The fate of 3H-norepinephrine in animals. J Pharmacol Exp Ther. 1961;132:193–201. [PubMed] [Google Scholar]
- Whittaker VP. The isolation and characterization of acetylcholine containing particles from brain. Biochem J. 1959;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. Antimetabolites of serotonin. J Biol Chem. 1953;203:69–79. [PubMed] [Google Scholar]
- Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc Natl Acad Sci USA. 1954;40:228–231. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CM, Todrick A, Tait AC. Effect of imipramine and some analogues on the uptake of 5-hydroxytryptamine by human blood platelets in vitro. J Pharm Pharmacol. 1964;16:460–463. doi: 10.1111/j.2042-7158.1964.tb07494.x. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol. 2006;147:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MBH, Collins CGS, Sandler M. Multiple forms of rat brain monoamine oxidase. Nature. 1969;223:626–628. doi: 10.1038/223626a0. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Collins CGS, Sandler M, Bevan-Jones AB, Pare CMB, Nicholson WJ. Human brain monoamine oxidase: multiple forms and selective inhibitors. Nature. 1972;236:225–227. doi: 10.1038/236225b0. [DOI] [PubMed] [Google Scholar]
- Zeller EA, Barsky J, Berman JR, Fouls JR. Action of isonicotinic acid hydrazide and related compounds on enzymes involved in the autonomic nervous system. J Pharmacol Exp Ther. 1952;106:427–432. [Google Scholar]