I think it's remarkable that only a few months after Banting introduced insulin to the world of diabetes, on the heels of one of medicine's greatest discoveries, Elliot Joslin wrote, “insulin is not a cure for diabetes, but a potent preparation alike . . . for evil and for good.” Today, 85 years later, with all the advances in insulin therapy, there is still a dark side to this potent preparation, and it is hypoglycemia. Hypoglycemia is the major barrier preventing insulin from achieving its full, therapeutic promise.
My story begins 30 years ago. Bill Tamborlane came to me with a portable, battery-powered pump being used in pediatrics to infuse the iron-chelating drug desferrioxamine continuously via the subcutaneous route into children with thalassemia major. He hoped to use it to treat children with glycogen storage disease, a study we never performed. This relatively small pump, the Autosyringe Model AS2C, was at that time relatively unique. It had an “instant dose” button that allowed for bolus dose administration as well as the ability to deliver solutions continuously. In short, it was a “perfect” vehicle to infuse insulin in diabetes. Improved glucose control had been demonstrated earlier in the inpatient setting in type 1 diabetic patients given continuous intravenous insulin delivered at preprogrammed basal rates with increments before meals. Such systems were not practical outside of a controlled environment. This device, however, was small enough to be used for continuous subcutaneous insulin delivery.
My enthusiasm for this idea was based on experiments Luigi Sacca and I performed 2 years earlier in the mid 1970s. We demonstrated that overnight basal insulin infusion in doses sufficient to normalize fasting glucose the next morning restored the ability of hyperglycemia to suppress hepatic glucose production in type 1 diabetic patients, despite their failure to release insulin (1). Those studies suggested that if insulin were delivered at more physiological basal rates (something that was not easy to achieve with the intermediate-acting insulin available at the time), it would not only provide better regulation of fasting glucose but also minimize postprandial hyperglycemia after a breakfast meal. The second study made me think that open-loop continuous insulin delivery might be relatively safe. In that study, we infused insulin to “conventionally” (which at that time was poorly) treated type 1 diabetic subjects in doses that approached 2 units/h (2). Only mild hypoglycemia (59 ± 5 mg/dl) was produced; glucose stabilized at levels comparable with those seen in nondiabetic control subjects. Since these doses of insulin exceed usual basal requirements overnight, I assumed that if insulin were delivered continuously by the subcutaneous route in relatively small basal doses, even if dosage errors occurred and plasma glucose fell during sleep, the patient would still be able to muster adequate counterregulatory defenses to protect against severe hypoglycemia.
Our initial inpatient insulin pump studies conducted by Bill Tamborlane and our team of dedicated nurses were highly successful and were trumpeted in the New York Times. The “insulin pump” produced near normalization of blood glucose throughout the day and night in youngsters with type 1 diabetes that had been difficult to control outside of the hospital (3). The improvement in glucose regulation with the pump was achieved without an increase in daily insulin doses and was associated with improved lipid and protein metabolism as well as growth potential (4,5). We saw these studies as confirming the idea that such an approach would not promote excessive hypoglycemia and said so in our report in 1979 (3): “Noteworthy, was the fact that mean concentrations, as well as fluctuations in plasma glucose, were reduced without the development of chemical, or clinical hypoglycemia . . . . The failure to observe hypoglycemia during treatment with the infusion pump, may be related to the physiologic nature of between-meal basal insulin administration.”
This conclusion, unfortunately, proved incorrect. After our patients left the hospital, while this “more physiological” insulin delivery system markedly improved glycemic control (6) and prompted longer-term collaborative clinical trials (7), it did not take long for the dark side of insulin, hypoglycemia, appeared. Contrary to our optimistic predictions, patients had frequent hypoglycemia, and to our surprise, frequently they were totally unaware of it. As with many “new” clinical observations, they are often not as new as they seemed. Fredrick Banting in his 1925 Nobel Laureate lecture stated, “The level at which hypoglycemic symptoms occur, is slightly higher in the diabetic with marked hyperglycemia . . . . As a patient becomes accustomed to a normal blood glucose, the threshold of these reactions, becomes lower.”
The importance of hypoglycemia, as a complication of intensive insulin therapy, became fully apparent when the benefits of such treatment in the Diabetes Control and Complications Trial (DCCT) were announced, more than a decade later (8). The DCCT follow-up analysis (9) showed that one-half of severe hypoglycemic events occurred while patients were asleep. In those patients who were awake at the time of the hypoglycemic event, one-half were not aware of impeding hypoglycemia and therefore could not take protective action. It is for this reason that, for many patients, the immediate fear of severe hypoglycemia exceeds the fear of long-term diabetes complications. This reduces the commitment of patients, their families, and their physicians to intensive insulin treatment.
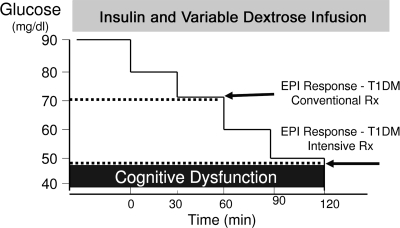
The reason for the higher rate of hypoglycemia became evident when we adapted the euglycemic-hyperinsulinemic clamp for studies of hypoglycemia. This technique, developed a decade earlier when I worked as a postdoctoral fellow in the laboratory of Reuben Andres (10), allowed us to produce a standardized hypoglycemic stimulus, providing a means to compare responses between nondiabetic and diabetic subjects (11,12). These studies taught us that nondiabetic subjects are exquisitely sensitive to very small reductions in circulating glucose. When glucose falls below 80 mg/dl, endogenous insulin secretion is nearly totally suppressed. A further decline below 70 mg/dl provokes glucagon and epinephrine secretion, the two hormones most critical for the rapid restoration of blood glucose, whereas a decline to below 55–60 mg/dl produces symptoms. This multihormonal hypoglycemia defense system is severely compromised in type 1 diabetes, even in the absence of intensive insulin treatment. These patients, by definition, are unable to shut off the delivery of endogenous insulin into the portal circulation, and more importantly, they fail to secrete glucagon specifically when they need it most, during hypoglycemia (at other times glucagon secretion may be inappropriately excessive). This poorly understood defect, originally reported by Jack Gerich (13), makes type 1 diabetic patients totally dependent on epinephrine for their defense against hypoglycemia. Unexpectedly, we found that this alternative defense is compromised in many patients within a short time after initiating intensive insulin therapy. Epinephrine release, and the appearance of symptoms, only occurred when plasma glucose reached dangerously low levels, commonly below 50 mg/dl (Fig. 1). Thus, loss of symptom awareness was linked to a lowering of the glucose level required to trigger epinephrine secretion.
FIG. 1.
Intensive insulin treatment alters the glucose threshold for epinephrine (EPI) release in response to insulin-induced hypoglycemia in patients with type 1 diabetes (T1DM).
Complementary experiments by Stephanie Amiel, then a postdoctoral fellow in the lab, showed that the impairment of glucose counterregulation induced by intensive insulin treatment interfered with the ability of patients to effectively counteract even relatively small doses of infused insulin (14). As we observed earlier (2), when insulin was infused continuously in relatively low doses, conventionally treated type 1 diabetic patients were indeed able to defend against severe hypoglycemia. However, after insulin pump therapy, their response changed. They developed severe hypoglycemia due to the combined effects of inadequate release of epinephrine and improved insulin sensitivity.
It is noteworthy that the levels of glucose bathing the brain are very different from those we measure in the systemic circulation. Remarkably, glucose levels in human brain interstitial fluid during hypoglycemia are only ∼25% of those measured in plasma (15). As a result, brain glucose may fall to concentrations that are rate limiting for glucose metabolism in areas of brain in which activity is locally increased. For glucose, there really is a blood-brain barrier.
The reason that severe hypoglycemia most commonly occurred during sleep in the DCCT became apparent when Tim Jones performed hypoglycemic clamp experiments during the day and in the middle of the night (16). At night, nondiabetic and diabetic subjects were studied both while awake and while asleep. As expected, hypoglycemia provoked epinephrine release when subjects were awake, independent of the time of day. In striking contrast, the same hypoglycemic stimulus had little effect on epinephrine release when either group of subjects was asleep. It took a much greater glucose fall to generate symptoms of sufficient intensity to awaken them.
Why intensively treated patients released epinephrine and experienced symptoms at lower glucose levels became apparent when Simon Heller and Philip Cryer reported that the body adapts to acute hypoglycemia by lowering the glucose level at which counterregulatory hormones are released and symptoms appear the next day (17). This scenario predicts that recurrent insulin-induced hypoglycemia would create a vicious cycle, progressively magnifying the defect in counterregulation and symptom awareness. They called the phenomenon “hypoglycemia-associated autonomic failure” (HAAF). Subsequently, Geri Bolli's group showed that HAAF could, over time, be reversed by scrupulous avoidance of hypoglycemia (18). This is, of course, not easy to achieve in clinical practice.
This counterintuitive observation raised the question, why would the body adapt to hypoglycemia by suppressing its defense against it? My search for answers led me to a little-explored path, away from the conventional view of glucose metabolism—one that led to territory first explored by Claude Bernard long before the discovery of insulin. Bernard proposed, one and a half centuries ago, that the control center for glucose homeostasis resides in the brain (19). A more current stimulus for my change in direction was a study published by Alan Cherrington's laboratory (20). Insulin-induced hypoglycemia was performed in two groups of dogs; in one group glucose was delivered directly into the vessels perfusing the brain to prevent glucose from falling in the brain. In these dogs the rise in epinephrine and glucagon secretion was nearly abolished, despite hypoglycemia in the rest of the body, suggesting that the brain is the primary site for hypoglycemia detection. This prompted me to ask three basic questions: 1) Where in the brain is the glucose sensor? 2) What mechanisms does it use to activate hypoglycemic counterregulation? 3) Why does hypoglycemia beget hypoglycemia?
WHERE IN THE BRAIN IS THE GLUCOSE SENSOR?
To address this question, I turned to studies performed by Oomura and coworkers, nearly 40 years ago (21). Oomura demonstrated the existence of specialized neurons in the brain with the unique ability to alter their firing rate when glucose levels changed. These neurons were predominantly located in the hypothalamus, a critical brain region for many basic survival functions such as reproduction, energy metabolism, and feeding. In particular, they were concentrated in the ventromedial hypothalamus (VMH), a brain area that encompasses the ventromedial and arcuate nuclei. To determine if the VMH was the location of the brain glucose sensor, Jerry Shulman and I joined forces to adapt the hypoglycemic clamp, originally developed in humans for studies in awake, chronically catheterized rodents. This allowed us to test the effect of selective bilateral VMH lesions on counterregulatory responses under identical hypoglycemic conditions in awake, freely moving rats (22). Hypoglycemia increased glucagon and epinephrine secretion in sham-operated control rats as well as in rats with lesions elsewhere in brain. In contrast, VMH lesions caused a striking reduction in hormonal responses to hypoglycemia, indicating that the VMH was a place to explore in more detail. The clinical relevance of these observations in rodents was subsequently supported in a 1999 case report of a patient with the same dramatic suppression of hormonal responses to hypoglycemia in which sarcoid lesions had destroyed the VMH (23).
The complexity and small size of the VMH meant we needed a new way to navigate—one that allowed us to selectively alter the local environment of the VMH. For this purpose, Walter and Monica Borg conducted experiments in which guide cannulae were inserted in close proximity to the VMH. After the animals recovered, microdialysis probes were inserted to deliver test substances directly to the VMH. Hypoglycemic clamp studies could then be performed while simultaneously manipulating the local microenvironment of the VMH. In one study, VMH glucopenia was selectively produced by local delivery of 2-deoxy-glucose (24). This caused glucose to rise in conjunction with a marked increase in counterregulatory hormone secretion. Next, d-glucose (metabolically active) and l-glucose (a metabolically inactive control) were delivered directly to the VMH to selectively prevent hypoglycemia in the VMH, while the rest of the brain and extra-cerebral tissues were exposed to hypoglycemia (25). VMH perfusion with d-glucose markedly suppressed both glucagon and epinephrine release, while inactive l-glucose control had no effect. Interestingly, VMH perfusion with l-lactate (but not metabolically inactive d-lactate) also abolished the hormone response to hypoglycemia (26). Taken together, the data suggest VMH neurons are critical for the activation of glucose counterregulation and that they may act as fuel and not simply as glucose sensors.
It should be emphasized that our data do not mean that glucose sensing is restricted to neurons in the VMH. The system is undoubtedly much more complex. Glucose sensors have been identified in peripheral tissues, such as the portal vein and carotid body (27,28), as well as elsewhere in the brain (29–31). The VMH may act as a central integrator of glucose-sensing signals that are both locally generated and come from a network of sensors widely distributed throughout the body during hypoglycemia. Its function is to integrate this information and to activate downstream signals to rapidly restore and maintain glucose homeostasis.
We next tested whether the capacity of the VMH to sense hypoglycemia becomes impaired in rats after exposure to antecedent hypoglycemia; rats, like humans, develop defective glucose counterregulation under these conditions (32). Delivery of 2-deoxy-glucose to block VMH glucose metabolism in rats exposed to recurrent insulin-induced hypoglycemia failed to activate glucagon and epinephrine secretion, whereas in control animals, 2-deoxy-glucose, as expected, provoked counterregulatory hormone secretion (33). These findings prompted us to examine the mechanisms used by the VMH to sense glucose and to determine if they are altered by antecedent hypoglycemia.
WHAT MECHANISMS DOES THE VMH USE TO ACTIVATE COUNTERREGULATION?
In vitro electrophysiology studies (34–36) have identified two distinct types of glucose-sensing VMH neurons: 1) glucose-excited (GE) neurons that increase their activity with a rise in glucose and are suppressed by a fall in glucose and 2) glucose-inhibited (GI) neurons that decrease their activity when glucose rises and increase their activity when glucose falls. Interestingly, these glucose-sensing neurons appear to employ mechanisms to detect glucose excess and deficiency that are very similar to those used in peripheral tissues, such as β-cells and muscle. Current data suggest that GE neurons may sense glucose via its effects on ATP-sensitive K+ channel (KATP channel) activity, much like pancreatic β-cells (34–36). Interestingly, VMH neurons express the same SUR1 KIR-6.2 KATP channel isoform as the pancreatic β-cell (37). VMH GI neurons, on the other hand, may use the AMP kinase to sense fuel deficit (38,39). This enzyme is regulated by the relative cellular concentrations of AMP and ATP and functions as a fuel gauge in and outside the brain (e.g., muscle) to protect against energy depletion (40). These in vitro findings prompted us to explore if these glucose-sensing mechanisms were used by the VMH in vivo.
KATP channel activity.
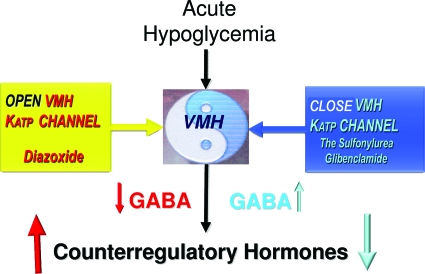
To evaluate whether KATP channel activity of VMH neurons alters glucose counterregulation, KATP channel openers (KCOs) (activators), KATP channel closers (blockers), or vehicle (control) were bilaterally microinjected directly into the VMH of awake, chronically catheterized nondiabetic rats before a hypoglycemic clamp study (41,42). VMH microinjection of diazoxide, a KCO, reduced by 50% the glucose infusion rate needed to maintain hypoglycemia and increased the response of both epinephrine and glucagon to hypoglycemia threefold (P < 0.05). Similar enhancement of glucose counterregulation was observed when a SUR1-specific KCO was delivered to the VMH instead. Conversely, closure of VMH KATP channels with the sulfonylurea glibenclamide suppressed counterregulatory hormone secretion during hypoglycemia. Thus, the activity of KATP channels in VMH neurons, much like in β-cells, modulates hormone responses during hypoglycemia (Fig. 2).
FIG. 2.
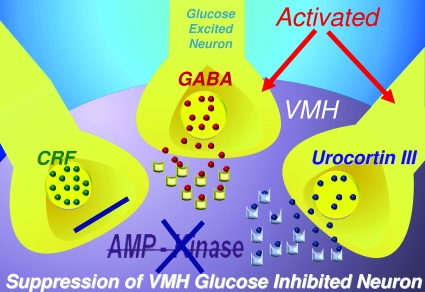
Changes in VMH KATP channel activity modulate the counterregulatory hormone response to hypoglycemia via the local release of GABA.
HOW DO CHANGES IN VMH KATP CHANNEL ACTIVITY MODULATE GLUCOSE COUNTEREGULATION?
To address this issue, we focused on the inhibitory neurotransmitter γ-aminobutyric acid (GABA), based upon the link between intra-islet GABA inhibitory tone and the secretion of glucagon (43). Initial studies showed that acute hypoglycemia rapidly reduced GABA levels in VMH interstitial fluid, implying that a fall of this inhibitory neurotransmitter might activate a VMH signal. To test this hypothesis further, Owen Chan (Yale University, New Haven, CT) measured GABA levels in VMH interstitial fluid while we altered VMH KATP channel activity during acute hypoglycemia (44). Opening KATP channels with diazoxide produced a much more pronounced reduction in GABA levels in VMH interstitial fluid and at the same time augmented hormone release. Conversely, closure of KATP channels by local delivery of a sulfonylurea to the VMH caused a paradoxical increase in VMH GABA levels in conjunction with suppression of the counterregulatory hormone response to hypoglycemia (Fig. 2). Furthermore, modulation of VMH GABA tone using GABAA receptor agonists suppressed, whereas GABAA receptor antagonists augmented, glucagon and epinephrine responses to hypoglycemia (45). Taken together, it would appear that KATP channels in glucose-sensing VMH neurons modulate local GABAergic inhibitory tone, which in turn modulates the magnitude of the counterregulatory response.
AMP kinase activity.
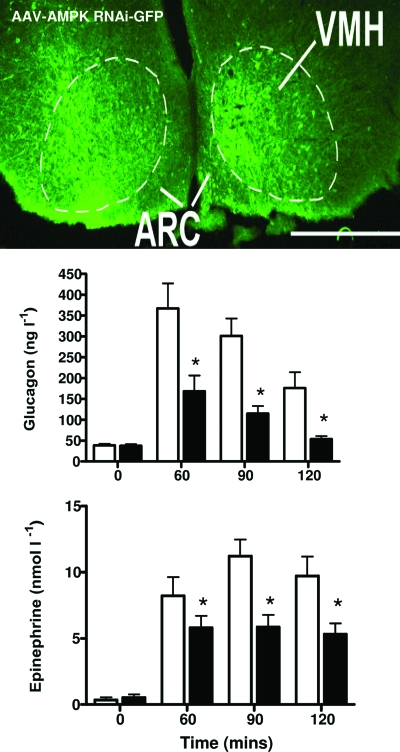
Rory McCrimmon explored the role of AMP kinase in VMH glucose sensing. For this purpose, AICAR (5-aminoimidazole-4-carboxamide) was delivered into the VMH to locally stimulate the enzyme. This increased glucose counterregulation (46). Conversely, he selectively reduced ΑΜPΚα2 gene expression by ∼40% in the VMH using a selective short hairpin RNA (shRNA) processed in an adeno-associated virus (AAV) (47). This caused a marked reduction in epinephrine and glucagon release during hypoglycemia (Fig. 3), suggesting that VMH AMP kinase activity has an important influence on the magnitude of the counterregulatory response, as well. This conclusion is also supported by in vitro data from Vanessa Routh's laboratory demonstrating that the activation of VMH glucose-inhibited neurons is linked to the activation of AMP kinase within these neurons (48).
FIG. 3.
Inhibition of AMPKα2 gene expression in the VMH with AAV RNA interference (AMPK shRNA) reduces glucagon and epinephrine release during hyperinsulinemic hypoglycemia. The upper panel shows the distribution of the virus following bilateral VMH microinjections of a green fluorescent protein (GFP)-tagged AAV-shAMPK; most GFP-positive neurons are located in the VMH rather than arcuate nucleus (ARC). (Please see http://dx.doi.org/10.2337/db08-9023 for a high-quality digital representation of this figure.).
HOW DO THESE STUDIES ALL FIT TOGETHER?
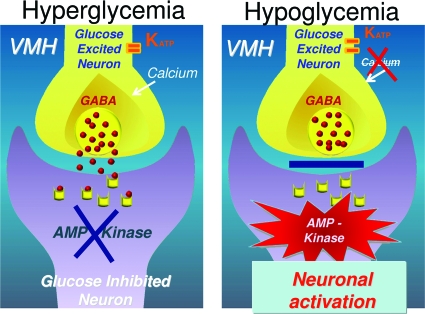
I propose a working model of VMH glucose sensing in which a downstream VMH signal to promote counterregulatory hormone secretion is driven by the simultaneous activation of AMP kinase in GI neurons as well as by GE neuron–driven disinhibition of GABA neurons (Fig. 4). Based on recent data implicating ventromedial nucleus SF1 glutamate neurons in activating counterregulatory responses (49), it is intriguing to speculate that ventromedial nucleus SF1 glutamate neurons may be responsible for generating this downstream signal.
FIG. 4.
Working model of VMH glucose sensing. Reciprocal changes in glucose-excited and glucose-inhibited neurons act in concert to stimulate or inhibit glucose counterregulation when blood glucose rises or falls.
The model predicts that changes in VMH glucose-sensing activity in GI and GE neurons work in concert to stimulate or inhibit glucose counterregulation. In response to hyperglycemia, AMP kinase in VMH GI neurons is suppressed, reducing neuronal activity, and at the same time KATP channels in GE neurons close and calcium enters the neuron, stimulating the release of the inhibitory neurotransmitter GABA, further suppressing the GI neuron. Conversely, hypoglycemia stimulates AMP kinase activity, stimulating VMH GI neurons, and at the same time KATP channels open in GE neurons and calcium entry is reduced, reducing GABA tone, which in turn causes disinhibition of VMH GI neurons. Remarkably, this picture bears a striking resemblance to the crosstalk found in the islet when blood glucose levels change. The β-cell and glucose-excited neurons have much in common, as do the α-cell and glucose-inhibited neurons.
Corticotropin-releasing factor receptors.
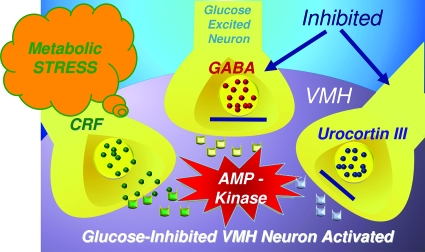
As with most things in biology, the story turns out to be more complex. VMH neurons are regulated during hypoglycemia by a second system, namely the stress response. Hypoglycemia provokes stress signals from brain regions outside of the VMH that modulate its activity via two types of corticotropin-releasing factor receptors that oppose each other. The primary stress peptide hormone corticotrophin-releasing factor (CRF) predominately stimulates VMH CRF1 receptors and, in turn, counterregulatory responses (50). Local VMH delivery of a CRF1 receptor antagonist during hypoglycemia suppresses both epinephrine and glucagon responses, whereas local VMH microinjection of CRF stimulates the release of these hormones during acute hypoglycemia. The stress response also provokes a counterbalancing stress inhibitory signal urocortin III, a member of the CRF family of neuropeptides identified by Wylie Vale's laboratory a decade ago (51). Urocortin III is a selective CRFR2 receptor agonist that is generated in the medial amygdula and released locally into the VMH, where it acts to suppress counterregulatory hormone responses to hypoglycemia. This effect is reversed by the selective CRFR2 antagonist antisauvagine (52). These observations are consistent with whole cell–current clamp recordings in brain slices showing that CRF2 receptor activation directly lowers the glucose concentration required to trigger activation of VMH GI neurons. In short, hypoglycemic stress provokes dual modulation of the neuroendocrine response via stimulation of endogenous CRF2 (inhibitory) and CRF1 (stimulatory) receptors. Based on these data, I have expanded my working model to incorporate the additional neuronal input to the VMH generated by the stress pathway during hypoglycemia. This postulated multistep process allows for the full activation of the VMH downstream neuronal signal stimulating glucose counterregulation (Fig. 5). How might such a model help us to understand why hypoglycemia begets hypoglycemia?
FIG. 5.
Expanded VMH model for activation of glucose counterregulation to include stimulatory and inhibitory input from the CRF family of neuropeptides. The model posits that hypoglycemia provokes the activation of AMP kinase in VMH glucose-inhibited neurons. Simultaneously, the inhibitory neuronal inputs, urocortin III and GABA, are suppressed, and the CRF metabolic stress pathway is activated. Together, these changes act in concert to fully activate downstream neural pathways to restore glucose homeostasis.
WHY DOES HYPOGLYCEMIA BEGET MORE HYPOGLYCEMIA?
The proposed model of VMH glucose sensing may serve as a starting point to help us understand why hypoglycemia begets hypoglycemia. Several putative mechanisms emerge. Recurrent antecedent hypoglycemia might reduce VMH KATP channel activity or increase VMH GABA or urocortin III inhibitory input or suppress VMH AMP kinase activity. To address these possibilities, we developed an animal using nondiabetic rats exposed to recurrent antecedent insulin-induced hypoglycemia for 3 h for 3 days. Typically, this protocol reduces hormonal responses during a hypoglycemic clamp study by ∼50%, much as it does in humans (17).
Reduced VMH KATP channel activity.
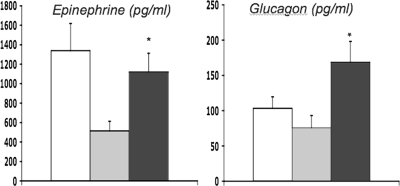
To determine whether KCO delivery to the VMH could reverse defective glucose counterregulation, rodents exposed to antecedent hypoglycemia were given bilateral microinjections of either diazoxide or vehicle into the VMH, after which they underwent a hypoglycemic clamp study (41). VMH delivery of diazoxide or a SUR1-specific KCO totally reverses the animal's defective counterregulatory response (Fig. 6). Preliminary studies suggest that the systemic delivery of KCO has similar beneficial effects on hormonal responses to hypoglycemia in animals with impaired glucose counterregulation and thus might have therapeutic benefit in patients with type 1 diabetes.
FIG. 6.
Delivery of KCOs into the VMH reverses the suppressive effect of antecedent recurrent hypoglycemia on glucagon and epinephrine secretion. Three groups of rats were studied: control (open bars), recurrent hypoglycemic (shaded bars), and recurrent hypoglycemic plus bilateral VMH KCO microinjections (black bars).
Increased VMH GABAergic inhibitory tone.
To assess whether increased VMH GABA tone contributes to the development of defective counterregulation, we quantified the GABA synthetic enzymes GAD65 and GAD67 within the VMH of rats exposed to recurrent hypoglycemia (53). These rodents demonstrated a specific increase in GAD65 gene expression and a five- to sixfold increase in GAD65 protein expression within the VMH. We also measured GABA levels in VMH interstitial fluid in rats exposed to antecedent hypoglycemia. In keeping with the GAD65 protein findings, basal levels of GABA in VMH interstitial fluid were threefold higher in the basal state and during acute hypoglycemia. Furthermore, delivery of a GABAA receptor antagonist into the VMH of these rats reversed their defective counterregulatory hormone response (53). These data suggest that antecedent hypoglycemia may enhance VMH GABAergic inhibitory tone and in turn impair glucose counterregulation.
Increased VMH CRF2 receptor activity.
The role of altered VMH CRF receptor activity in the pathogenesis of impaired counterregulation after antecedent hypoglycemia remains to be explored. The possibility that hypoglycemia-induced increases in plasma cortisol might be the driving force (54) led us to study nondiabetic human subjects in whom antecedent hypoglycemia was produced with and without concurrent blockade of endogenous cortisol production using metyrapone (55). We observed that antecedent hypoglycemia suppressed the epinephrine response to hypoglycemia the next day regardless of whether endogenous cortisol production was selectively suppressed with metyrapone. These observations are most consistent with rodent data (50,52,56), suggesting that hypoglycemia-induced alterations in brain CRH receptor activity rather than increases in plasma cortisol are more likely to contribute to the pathogenesis of defective counterregulation following antecedent hypoglycemia.
Suppressed VMH AMP kinase activity.
To test the potential role of suppressed VMH AMPK activity in defective counterregulation, we injected the chemical activator of AMP kinase, AICAR, into the VMH of rats exposed to recurrent hypoglycemia (57). This totally reversed the impaired hormonal response to acute hypoglycemia seen in these animals, findings consistent with data demonstrating impaired hypothalamic AMPK activation in this rodent model (58). Similar results were also observed in diabetic BB rats, a model of type 1 diabetes that exhibits a nearly absent glucagon response to hypoglycemia (59). Chemical activation of AMP kinase within the VMH increased epinephrine secretion three- to fourfold during hypoglycemia and unexpectedly stimulated glucagon release, implying that this defect in type 1 diabetes might be reversible as well (unpublished data, R. McCrimmon).
WHY DOES HYPOGLYCEMIA BEGET HYPOGLYCEMIA?
Lessons learned from my journey.
One might speculate, based on our VMH model of glucose counterregulation, that recurrent hypoglycemia impairs VMH glucose sensing and signaling by multiple complementary adaptations (Fig. 7), including suppressed VMH AMP kinase activity and increased activation of inhibitory neuronal circuits, mediated by GABA or CRF receptors. It is noteworthy that these adaptive responses appear to be reversible, as has been reported clinically. Most importantly, the proposed model offers a number of potential therapeutic targets to reverse the phenomenon in patients with type 1 diabetes. Of course, we are undoubtedly dealing with a much more complex system. This should not be a surprise, given that maintenance of glucose homeostasis is so important to survival.
FIG. 7.
Potential adaptive mechanisms for the development of defective glucose counterregulation based on the proposed VMH glucose-sensing model, including diminished AMP kinase activity in VMH glucose-inhibited neurons as well as increased activation of GABA and CRF2 receptor inhibitory neuronal circuits.
WHY WOULD THE BRAIN ADAPT TO RECURRENT HYPOGLYCEMIA BY BECOMING MORE VULNERABLE TO IT?
The brain is not as vulnerable as it seems. It may have adapted to this recent challenge (from an evolutionary perspective) the best way it can, namely, by becoming more efficient metabolically, by increasing the transport of glucose across the blood-brain barrier, and by using alternative fuels to glucose. This view is supported by several observations. Antecedent insulin-induced hypoglycemia increases brain glucose transport and utilization in rodents (60–62) and brain glucose uptake in nondiabetic and diabetic humans rendered hypoglycemic for several days (63,64). Under conditions of more prolonged glucose deprivation, the brain most likely adapts to use a number of alternative fuels to generate ATP, such as the monocarboxylic acids lactate, ketone bodies, and acetate. All are potential energy sources. In starvation, humans can tolerate severe hypoglycemia (1 mmol/l) without an impairment of cognitive function by increasing the use of ketone bodies as an energy source for the brain (65). In keeping with this scenario, the brain utilization of monocarboxylic acids appears to be increased in type 1 diabetic patients receiving intensive insulin therapy (66). Thus, the brain exposed to antecedent hypoglycemia may adapt, much as it did long before the discovery of insulin, i.e., when glucose declined during prolonged starvation.
The adaptation to recurrent hypoglycemia is likely to serve two basic survival functions, serving initially to protect the body from the negative consequences of recurrent stress and serving long term to protect the brain from fuel deprivation, much like the phenomenon of preconditioning. The problem is that these beneficial adaptations also act to limit the brain's ability to detect reductions in blood glucose. As a result, when it does sense hypoglycemia, it may be too little, too late.
DO HYPOGLYCEMIA-INDUCED ADAPTATIONS IN BRAIN FUEL METABOLISM PROTECT IT FROM INJURY?
To examine this question, Ewan McNay tested spatial memory performance and brain glucose metabolism in nondiabetic and streptozotocin-induced diabetic rats exposed to hypoglycemia for 3 consecutive days (67). Remarkably, memory performance improved in both groups of rats when they were studied at euglycemia the next day, most likely due to an increase in glucose transport across the blood-brain barrier. The capacity of antecedent hypoglycemia to enhance cognition was, however, limited. When these animals were exposed to moderately severe hypoglycemia, the improvement in cognitive performance could not be sustained. Thus, a point may be reached when there is need to preserve basic functions (e.g., hypothalamic and brainstem activity) at the expense of more complex functions (e.g., spatial memory). This view is supported by studies demonstrating that antecedent hypoglycemia facilitates brainstem neural transmission during acute hypoglycemia (68).
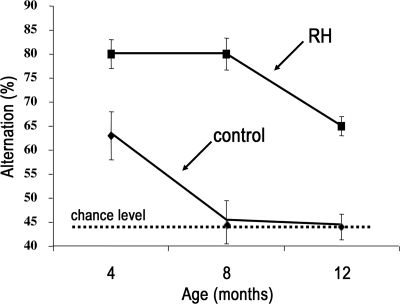
A similar picture was observed in long-term studies using a more clinically relevant protocol (69). Hypoglycemia was induced in nondiabetic rats for 3 h once each week, a pattern similar to that seen in insulin-treated diabetic patients. Weekly bouts of insulin-induced hypoglycemia were initiated at 1 month of age and continued until the animals were 1 year of age (Fig. 8). Spatial working memory performance was tested 1 week after the animal's last bout of hypoglycemia when they were euglycemic. At 4 months of age the rats exposed to weekly hypoglycemia, like their counterparts in the short-term study, performed better during the memory task. After 1 year, while the control animals showed the expected steep decline in memory performance, this failed to occur in rats exposed to hypoglycemia. Indeed, they performed like a 4-month-old control animal. These data suggest that there are marked differences in the immediate consequences of acute hypoglycemia and the long-term effects of recurrent hypoglycemia on higher brain function. This conclusion is consistent with the follow-up analysis of DCCT patients, nearly two decades after the start of the study (70). Detailed neuropsychiatric testing (when these patients were not hypoglycemic) failed to detect cognitive dysfunction in the intensively treated patients with more frequent severe hypoglycemia. Although these data are not definitive, they suggest that insulin, as we give it today, really has two dark sides. From an acute prospective it is the serious immediate consequences of hypoglycemia, but over the long haul, for most patients, it is hyper- rather than hypoglycemia.
FIG. 8.
Influence over time of once weekly bouts of insulin-induced hypoglycemia (3 h) on memory performance in nondiabetic rats. Animals exposed to recurrent hypoglycemia (RH) and those given saline injections (controls) were tested 1 week after their last injection of insulin or saline when they were euglycemic.
VIEW OF GLUCOSE HOMEOSTASIS FROM ACROSS THE BLOOD-BRAIN BARRIER
For the 85 years since Banting's landmark discovery, the regulation of glucose homeostasis has been viewed by the diabetes world from an islet-centric perspective. Bernard's vision of the brain as the master control site of glucose homeostasis has, for the most part, been abandoned. My journey across the blood-brain barrier has taught me that Bernard's vision should not be forgotten. I believe we evolved dual control centers for the regulation of glucose homeostasis, the islet and the brain, and that they are wired together and connected with the rest of the body to more precisely regulate blood glucose as fuel demands change.
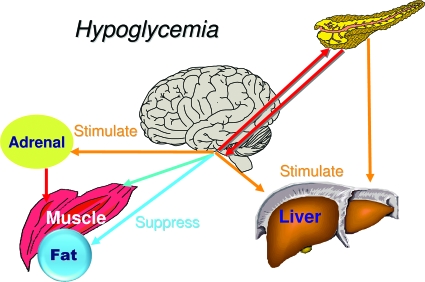
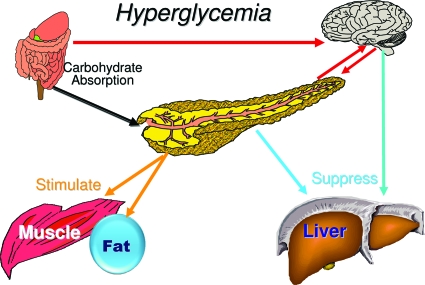
I believe the brain, and the VMH in particular, is the dominant control center initiating counterregulatory responses when blood glucose falls below baseline. This view is consistent with the brain's immediate dependence on glucose as an energy source and the marked differences in the concentration of glucose within brain interstitial fluid compared with levels elsewhere in the body. However, dual control is more effective. The islet supports the VMH by reducing its secretion of insulin and by working in concert with the VMH to increase glucagon secretion (Fig. 9). On the other hand, the islet serves as the dominant sensor when blood glucose rises, by virtue of its ability to increase its secretion of insulin and to reduce its secretion of glucagon. However, once again dual control is more effective in restoring glucose homeostasis (Fig. 10). The brain receives neural and hormonal signals from the gut that promote insulin secretion as well as glucose storage in the liver (71,72). The brain may also receive a signal from the islet to facilitate the suppression of hepatic glucose production (73) as well as glucagon secretion. Again, the islet and VMH are working synergistically.
FIG. 9.
The brain and islet work in concert to regulate glucose homeostasis. During hypoglycemia the brain serves as the dominant control center.
FIG. 10.
The brain and islet work in concert to regulate glucose homeostasis. During hyperglycemia the islet serves as the dominant control center.
How did such a dual glucose sensor system evolve? There are lessons we can learn from the fruit fly. The cells that produce insulin in the fruit fly, like other invertebrates, are located in the brain (74). These insulin-secreting neurons communicate directly with cells that secrete an anti-insulin hormone that has effects similar to glucagon (75). Thus, the ancestral home of the islet and the regulation of glucose homeostasis most likely resided in the brain. One can imagine that, as we evolved, it was more advantageous for the islet to move from the brain to an area closer to the liver, the site where glucose is stored. Nevertheless, one can imagine that it would have been crucial for survival to retain glucose-sensing neurons with characteristics similar to β- and α-cells in the VMH to directly monitor minute-to-minute changes in brain glucose availability more closely. This might be particularly important given that the levels of glucose in brain interstitial fluid are only a small fraction (∼25%) of those bathing peripheral tissues. Thus, I believe it was essential to retain glucose sensors in the VMH; only a site within the brain can provide a truly accurate assessment of its energy supply. A peripheral glucose sensor exposed to much higher glucose levels during hypoglycemia probably couldn’t do the job as well. This is crucial since the brain is the only organ that must have glucose to meet its immediate needs. As a result, insulin-induced hypoglycemia is a brain-specific complication.
POSTSCRIPT
I’d like to end my journey across the blood-brain barrier with a postscript. When I began my research career, I was taught that there were specific organs that responded to insulin and that the brain was not one of them. This view is now being challenged on many fronts. Dan Porte, Steve Woods, and Michael Schwartz have established that insulin modulates satiety by acting on the hypothalamus (76). Rossetti and coworkers (73) suggest the existence of an insulin-brain-liver connection that regulates glucose metabolism. Furthermore, our preliminary studies indicate that delivery of small amounts of insulin directly into the hippocampus of nondiabetic rats, a brain region critical for learning and memory and rich in insulin receptors and GLUT4 (77), significantly improves cognitive performance and stimulates local brain glucose metabolism. This effect is impaired in insulin-resistant rats with diet-induced obesity, suggesting a potential link between defective insulin-stimulated glucose metabolism in the hippocampus and the increased risk of cognitive impairment seen in type 2 diabetes.
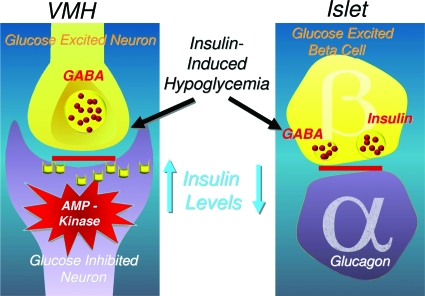
These observations raise another important question, namely, could insulin also act directly on the VMH, altering its capacity to sense fuel deficit? This possibility is supported by evidence that VMH neurons express insulin receptors and that insulin alters the firing rate of VMH glucose-sensing neurons in vitro (78). It is intriguing to speculate that we evolved a dual glucose-sensing system based in the VMH and islet designed to defend against hypoglycemia in the setting in which it originally occurred, namely during prolonged starvation, a time when both glucose and insulin levels fall simultaneously. Thus, we may not have optimally adapted to insulin-induced hypoglycemia, a danger rarely, if ever, encountered before Banting (Fig. 11). During insulin-induced hypoglycemia, the α-cell sees a drop in local islet insulin in spite of the rise in systemic insulin levels, given the suppression of endogenous insulin production. On the other hand, the VMH gets a very different message, namely, a feeding signal: insulin. How this might impact on the homeostatic response to hypoglycemia in nondiabetic and diabetic individuals’ disease warrants careful scrutiny. It is possible that insulin-induced hypoglycemia may limit the capacity of the body to defend against the challenge of acute hypoglycemia. A maximal response to insulin-induced hypoglycemia may require both the islet and the VMH to see a drop in insulin concentration.
FIG. 11.
The islet and brain see very disparate changes in local insulin levels during insulin-induced hypoglycemia. Although insulin levels rise in the VMH, a paradoxical decrease in insulin levels occurs within the islet due to the suppression of endogenous insulin secretion.
Six years ago in my American Diabetes Association president's address, I challenged the diabetes community to think beyond the barriers—beyond glucose. I suggested that our journey would require engaging scientists from many different disciplines, people not currently working in diabetes. I challenge you again to think beyond the islet and to engage the burgeoning world of neuroscience. These efforts will help us unravel not only hypoglycemia but also such problems as obesity, Alzheimer's disease, and perhaps even type 2 diabetes.
Acknowledgments
I would like to end by acknowledging the people who guided me along my research career path. I had the good fortune to begin my journey as an intern in a department headed by the late Solomon Berson, the 1965 Banting Medal recipient for his seminal work on the development of the insulin radioimmunoassay. For me, Dr. Berson defined the important role of the physician scientist in clinical practice. After my residency, I joined the laboratory of Reuben Andres, the father of the glucose clamp. He gave me a unique opportunity to work on the development of the euglycemic clamp to study insulin action in humans (it was only later adapted for animal research). This experience laid the groundwork for my career in diabetes as a clinical investigator and drove my decision to come to Yale to work with one of diabetes’ most outstanding physician scientists, Philip Felig. His research on the regulation of fuel metabolism in humans still has few peers. More recently, when I recognized that some of my questions required that I develop the tools needed to move from the bedside to the bench, an unorthodox path for a clinician, I was given the opportunity by my friend and colleague, the late Charlie Janeway—a scientist's scientist. I am also indebted to Dan Porte, a friend and mentor, and the members of our Brain Glucose-Sensing Club (Barry Levin, Vanessa Routh, Charles Mobbs, Nicole Sanders, Susan Vannucci, Ian Simpson, Diane Latteman, and Lee Beverly) for helping me think beyond the blood-brain barrier.
Research is a team effort, and the Yale team that contributed to the work presented here was large. It included clinical investigators (Gerald Shulman, Sonia Caprio, Tim Jones, David Maggs, Stephanie Amiel, David Kerr, Philip Goldberg, Katie Page, Donald Simonson, Mike Genel, Luigi Sacca, Naomi Dreisen, Doug Rothman, Todd Constable, Mathew During, Idil Cavus, Walid Abi-Saab, and John Gore), laboratory-based scientists (Ewan McNay, Mark Evans, Owen Chan, Monica Borg, Walter Borg, Daniel Flanagan, Raimund Herzog, Tamas Horvath, Sabrina Diano, Hiaying Cheng, Kevin Behar, Ann Williamson, Sachin Paranjape, Barbara Szepietowska, Ligang Zhou, and Margaret Shaw), technical staff (Xioning Fan, Yuyan Ding, Wailing Zhu, Ajin Wang, Ralph Jacob, Jennifer Morgan, Aida Groszman, Andrea Belous, Codruta Todesa, and Mikhail Smolgovsliy, Tamara Dlugos), and nurses (Fran Rife, Mary Walesky, Susan Bates, Joanne Ahern, Patricia Gatcomb, and an outstanding group of general clinical research center nurses). I particularly want to give special thanks to Willliam Tamborlane, my close collaborator on the clinical side, and to Rory McCrimmon, who has been the major contributor to the animal work.
I also thank my many other colleagues in the Yale diabetes community who over the years have enriched my scientific career. They include both faculty (Ralph DeFronzo [winner of the 2008 Banting Award], Richard Flavell, Pietro DeCamilli, Silvio Inzucchi, Li Wen, Werner Gurr, Rosa Hendler, Robert Gelfand, Donald Coustan, Eugene Barrett, Kitt Petersen, Lawrence Young, Michele Solimena, Dennis Spenser, Catherine Yeckel, Johnathan Bogan, and Barbara Gulanski) and a wonderful group of postdoctoral fellows (Harry Shamoon, Susan Wong, Pia Reich, Luciano Rossetti, Ele Ferrannini, Riccardo Bonadonna, Martin Press, V. Koivisto, Judith Fradkin, Rubina Heptulla, David Fryberg, Stefan Enoksson, Gabrieli Rossi, Michael Diamond, Susan Boulware, Ram Weiss, Hermann von Grafenstein, Isabelle Millet, and Daniel Zekzer).
It goes without saying that none of this research could have taken place without the generous support of the groups that paid the journey's tolls: the National Institutes of Health (grants R37 DK20495, R01 DK072409, UL1 RR 024139, and P30 DK 45735), the Juvenile Diabetes Research Foundation, and the American Diabetes Association (Mentor Grant).
Finally, I want to thank my children, who have been a source of light and who have brought me the ultimate joy: grandchildren. I am especially grateful to my wife, Leslie, whom I rely on for support and inspiration on life's journey.
REFERENCES
- 1.Saccà L, Hendler R, Sherwin RS: Hyperglycemia inhibits glucose production in man independent of changes in glucoregulatory hormones. J Clin Endocrinol Metab 47 :1160 –1163,1978 [DOI] [PubMed] [Google Scholar]
- 2.Saccà, Sherwin RS, Hendler R, Felig P: Influence of continuous physiologic hyperinsulinemia on glucose kinetics and counterregulatory hormones in normal and diabetic humans. J Clin Invest 63 :849 –857,1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamborlane WV, Sherwin RS, Genel M, Felig P: Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusionpump. N Engl J Med 300 :573 –578,1979 [DOI] [PubMed] [Google Scholar]
- 4.Tamborlane WV, Sherwin RS, Genel M, Felig P: Restoration of normal lipid and amino acid metabolism in diabetic patients treated with a portable insulin pump. Lancet 1 :1258 –1261,1979 [DOI] [PubMed] [Google Scholar]
- 5.Tamborlane WV, Hintz R, Bergman M, Genel M, Felig P, Sherwin RS: Insulin infusion pump treatment of diabetes: influence of improved metabolic control on plasma somatomedin levels. N Engl J Med 305 :303 –307,1981 [DOI] [PubMed] [Google Scholar]
- 6.Tamborlane WV, Sherwin RS, Genel M, Felig P: Outpatient treatment of juvenile onset diabetes with a preprogrammed portable subcutaneous insulin infusion system. Am J Med 68 :190 –196,1980 [DOI] [PubMed] [Google Scholar]
- 7.KROC Collaborative Study Group: Blood glucose control and the evaluation of diabetic retinopathy and albuminuria: a preliminary multicenter trial. N Engl J Med 311 :365 –372,1984 [DOI] [PubMed] [Google Scholar]
- 8.DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329 :977 –986,1993 [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 46 :271 –286,1997 [PubMed] [Google Scholar]
- 10.Sherwin RS, Kramer KJ, Tobin JD, Insel PA, Liljenquist JE, Berman M, Andres R: A model of the kinetics of insulin in man. J Clin Invest 53 :1481 –1492,1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonson DC, Tamborlane WV, DeFronzo RA, Sherwin RS: Intensive insulin therapy reduces counterregulatory hormone responses to hypoglycemia in patients with type I diabetes. Ann Intern Med 103 :184 –190,1985 [DOI] [PubMed] [Google Scholar]
- 12.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV: Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 37 :901 –907,1988 [DOI] [PubMed] [Google Scholar]
- 13.Gerich J, Langlois M, Noacco C, Karam J, Forsham P: Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha-cell defect. Science 182 :171 –173,1973 [DOI] [PubMed] [Google Scholar]
- 14.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS: Defective glucose counterregulation after strict control of insulin-dependent diabetes mellitus. N Engl J Med 316 :1376 –1383,1987 [DOI] [PubMed] [Google Scholar]
- 15.Abi-Saab WM, Maggs DG, Jones T, Jacob R, Srihari V, Thompson J, Kerr D, Leone P, Krystal JH, Spencer DD, During MJ, Sherwin RS: Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious subjects with epilepsy: effects of hyperglycemia and hypoglycemia. J Cerebral Blood Flow Metab 22 :271 –279,2002 [DOI] [PubMed] [Google Scholar]
- 16.Jones TW, Porter P, Sherwin RS, Davis EA, O'Leary P, Frazer F, Byrne G, Stick S, Tamborlane WV: Decreased epinephrine responses during sleep. N Engl J Med 338 :1657 –1662,1998 [DOI] [PubMed] [Google Scholar]
- 17.Heller SH, Cryer PE: Reduced neuroendocrine and symptomatic responses to subsequent hypoglycemia after one episode of hypoglycemia in non-diabetic humans. Diabetes 40 :223 –226,1991 [DOI] [PubMed] [Google Scholar]
- 18.Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Bolli G: Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes 42 :1683 –1689,1993 [DOI] [PubMed] [Google Scholar]
- 19.Bernard MC: Leçons sur la physiologie et la pathologie en système nerveux. Paris, Baillière, 1854, p.549 –555
- 20.Biggers DW, Myers SR, Neal D, Stinson R, Cooper NB, Jaspan JB, Williams PE, Cherrington AD, Frizzell RT: Role of brain in counterregulation of insulin induced hypoglycemia in dogs. Diabetes 38 :7 –16,1989 [DOI] [PubMed] [Google Scholar]
- 21.Oomura Y, Ono H, Ooyama H, Wayner MJ: Glucose and osmosensitive neurons of the rat hypothalamus. Nature 2222 :282 –284,1969 [DOI] [PubMed] [Google Scholar]
- 22.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI: Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest: 93 :1677 –1682,1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Féry F, Plat L, van de Borne P, Cogan E, Mockel J: Impaired Counterregulation of glucose in a patient with hypothalamic sarcoidosis. N Engl J Med 340 :852 –856,1999 [DOI] [PubMed] [Google Scholar]
- 24.Borg WP, Sherwin RS, During MJ, Borg M, Shulman GI: Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44 :180 –184,1995 [DOI] [PubMed] [Google Scholar]
- 25.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI: Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99 :361 –365,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borg MA, Tamborlane WV, Shulman GI, Sherwin RS: Local lactate perfusion of the ventromedial hypothalamus suppresses hypoglycemic counterregulation. Diabetes 52 :663 –666,2003 [DOI] [PubMed] [Google Scholar]
- 27.Hevener AL, Bergman RN, Donovan CM: Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 46 :1521 –1525,1997 [DOI] [PubMed] [Google Scholar]
- 28.Koyama Y, Coyer RH, Stone EE, Lacy BB, Jabbour K, Williams PE, Wasserman DH: Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49 :1434 –1442,2000 [DOI] [PubMed] [Google Scholar]
- 29.Ritter S, Slusser P, Stone S: Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213 :451 –453,1979 [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Kang L, Saunders NM, Dunn-Meynell AA: Role of neuronal glucosensing in the regulation of energy homeostasis. Diabetes 55 :S122 –S130,2006 [Google Scholar]
- 31.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH: Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50 :2673 –2681,2001 [DOI] [PubMed] [Google Scholar]
- 32.Powell AM, Sherwin RS, Shulman GI: Impaired hormonal responses to hypoglycemia in spontaneously diabetic and recurrently hypoglycemic rats. J Clin Invest 92 :2667 –2674,1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borg M, Borg W, Tamborlane WV, Brines MI, Shulman GI, Sherwin RS: Chronic hypoglycemia and diabetes impair counterregulation induced by localized 2-deoxy glucose perfusion of the ventromedial hypothalamus in rats. Diabetes 48 :584 –587,1999 [DOI] [PubMed] [Google Scholar]
- 34.Ashford MLJ, Boden PR, Treherne JM: Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurons by indirect inhibition of ATP-K+ channels. Br J Pharmacol 101 :531 –540,1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X.-J, Kow L-M, Funabashi T, Mobbs CV: Hypothalamic glucose sensor: similarities to and differences from pancreatic β-cell mechanisms. Diabetes 48 :1763 –1772,1999 [DOI] [PubMed] [Google Scholar]
- 36.Routh VH, McArdle JJ, Levin BE: Phosphorylation modulates the activity of the ATP-sensitive K+ channel in the ventromedial hypothalamic nucleus. Brain Res 778 :107 –119,1997 [DOI] [PubMed] [Google Scholar]
- 37.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S: ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis Nat Neurosci 4 :507 –512,2001 [DOI] [PubMed] [Google Scholar]
- 38.Mountjoy PD, Bailey SJ, Rutter GA: Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia 50 :168 –177,2007 [DOI] [PubMed] [Google Scholar]
- 39.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH: Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol 292 :R1418 –R1428,2007 [DOI] [PubMed] [Google Scholar]
- 40.Hardie DG, Carling D: The AMP-activated protein kinase: fuel gauge of the mammalian cell? Eur J Biochem 246 :259 –273,1997 [DOI] [PubMed] [Google Scholar]
- 41.McCrimmon RJ, Evans ML, Fan X, McNay EC, Chan O, Ding Y, Zhu W, Gram DX, Sherwin RS: Activation of ATP-sensitive K+ channels in ventromedial hypothalamus amplifies counterregulatory hormone responses to hypoglycemia in normal and recurrently hypoglycemic rats. Diabetes 54 :3169 –3174,2005 [DOI] [PubMed] [Google Scholar]
- 42.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS: Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53 :2542 –2550,2004 [DOI] [PubMed] [Google Scholar]
- 43.Xu E, Kumar M, Zhang Y, Ju W, Qbata T, hang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q: Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab 3 :47 –58,2006 [DOI] [PubMed] [Google Scholar]
- 44.Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS: ATP-sensitive potassium channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 54 :3163 –3174,2007 [DOI] [PubMed] [Google Scholar]
- 45.Chan O, Patel AB, Zhu W, Behar KL, Sherwin RS: Blockade of GABAA receptors in the ventromedial hypothalamus further stimulates the sympathoadrenal, but does not effect the hypothalamo-pituitary-adrenal (HPA) response to hypoglycemia. Diabetes 55 :1080 –1087,2006 [DOI] [PubMed] [Google Scholar]
- 46.McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS: Activation of AMP-activated protein kinase in the ventromedial hypothalamus stimulates hepatic glucose counterregulation. Diabetes 53 :1953 –1958,2004 [DOI] [PubMed] [Google Scholar]
- 47.McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS: Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes 57 :444 –450,2008 [DOI] [PubMed] [Google Scholar]
- 48.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH: Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol 292 :R1418 –R1428,2007 [DOI] [PubMed] [Google Scholar]
- 49.Tong Q, Ye CP, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB: Synaptic glumatate release by ventromedial hypothalamic (VMH) neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5 :383 –393,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng H, Zhou L, Zhu W, Wang A, Tang C, Chan O, Sherwin RS, McCrimmon RJ: Type 1 corticotrophin releasing factor receptors (CRFR1) in the ventromedial hypothalamus (VMH) promote hypoglycemia-induced hormonal counterregulation. Am J Physiol 292 :E845 –E852,2007 [DOI] [PubMed] [Google Scholar]
- 51.Bale TL, Vale WW: CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44 :525 –527,2004 [DOI] [PubMed] [Google Scholar]
- 52.McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS: Corticotrophin releasing factor (CRF) receptors within the ventromedial hypothalamus (VMH) regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest 116 :1723 –1730,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS: Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory reponses following antecedent hypoglycemia. Diabetes 57 :1363 –1370,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis SN, Shavers C, Costa F, Mosqueda-Garcia R: Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest 98 :680 –691,1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS: Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia associated autonomic failure. Diabetes 55 :1121 –1126,2006 [DOI] [PubMed] [Google Scholar]
- 56.Flanagan DE, Keshavarz T, Evans ML, Flanagan S, Fan X, Jacob RJ, Sherwin RS: Role of corticotrophin-releasing hormone in the impairment of counterregulatory responses to hypoglycemia. Diabetes 52 :605 –613,2003 [DOI] [PubMed] [Google Scholar]
- 57.McCrimmon RJ, Fan X, Cheng H, McNay EC, Chan O, Shaw M, Ding Y, Zhu Fan X, Sherwin RS: Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 55 :1755 –1760,2006 [DOI] [PubMed] [Google Scholar]
- 58.Alquier T, Kawashima J, Tsuji Y, Kahn BB: Role of hypothalamic AMP kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 148 :1367 –1375,2007 [DOI] [PubMed] [Google Scholar]
- 59.Jacob RJ, Dziura J, Morgen JP, Shulman GI, Sherwin RSS: Time course of the defective a-cell response to hypoglycemia in diabetic BB rats. Metabolism 45 :1422 –1426,1996 [DOI] [PubMed] [Google Scholar]
- 60.McCall AL, Fixman LB, Fleming N, Tornheim K, Chick W, Ruderman NB: Chronic hypoglycemia increases brain glucose transport. Am J Physiol 251 :E442 –E447,1986 [DOI] [PubMed] [Google Scholar]
- 61.Simpson I, Appel N, Hokari M, Oki J, Holman G, Maher F, Koehler-Stec E, Vannucci S, Smith Q: Blood-brain barrier glucose transporter: effects of hypo- and hyperglycemia revisited. J Neurochem 72 :238 –247,1999 [DOI] [PubMed] [Google Scholar]
- 62.Kumagai AK, Kang YS, Boado RJ, Pardridge WM: Upregulation of blood-brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 44 :1399 –1404,1994 [DOI] [PubMed] [Google Scholar]
- 63.Boyle PJ, Nagy RJ, O'Connor AM, Kempers SF, Yeo RA, Qualls C: Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A 91 :9352 –9356,1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyle PJ, Kempers SF, O'Connor AM, Nagy RJ: Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 333 :1726 –1731,1995 [DOI] [PubMed] [Google Scholar]
- 65.Cahill GF Jr, Aoki TT: Alternate fuel utilization in brain. In Cerebral Metabolism and Neuronal Function. Passonneau JV, Hawkins RA, Lust WD, Welsh FA, Eds. Baltimore, Maryland, Williams & Wilkins,1980. , p.234 –242
- 66.Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI: Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 55 :929 –934,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNay EC, Sherwin RS: Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes 53 :418 –425,2004 [DOI] [PubMed] [Google Scholar]
- 68.Jacob RJ, Dziura J, Blumberg M, Morgen JP, Sherwin RS: Effects of recurrent hypoglycemia on brainstem function in diabetic BB rats: protective adaptation during acute hypoglycemia. Diabetes 48 :141 –145,1999 [DOI] [PubMed] [Google Scholar]
- 69.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS: Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes 55 :1088 –1095,2006 [DOI] [PubMed] [Google Scholar]
- 70.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT.EDIC) Study Research Group: Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356 :1842 –1852,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cherrington AD: Banting Lecture 1977: Control of glucose uptake and release by the liver in vivo. Diabetes 48 :1198 –1214,1999 [DOI] [PubMed] [Google Scholar]
- 72.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Grémeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R: Brain glucagon-like peptide–1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 115 :3554 –3563,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Obici S, Zhang BB, Karkanias G, Rossetti L: Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8 :1376 –1382,2002 [DOI] [PubMed] [Google Scholar]
- 74.Rulifson EJ, Kim SK, Nusse R: Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296 :1118 –1120,2002 [DOI] [PubMed] [Google Scholar]
- 75.Kim SK, Rulifson EJ: Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431 :316 –320,2004 [DOI] [PubMed] [Google Scholar]
- 76.Schwartz M, Figlewicz D, Baskin D, Woods S, Porte DJ: Insulin in the brain: a hormonal regulator of energy balance. Endocrine Rev 13 :387 –414,2000 [DOI] [PubMed] [Google Scholar]
- 77.McEwen BS, Reagan LP: Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharmacol 490 :13 –24,2004 [DOI] [PubMed] [Google Scholar]
- 78.Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford MLJ: Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci 3 :757 –758,2000 [DOI] [PubMed] [Google Scholar]